1. Introduction
The central principle of molecular biology considers
RNA as the intermediaries between DNA sequences and their encoded
proteins (1). However, due to the
vast amounts and variety of non-coding RNA transcripts uncovered by
advances in RNA sequencing technology and computational methods, it
has been elucidated that numerous non-coding (nc)RNA transcripts
have important roles in a variety of biological processes. ncRNAs
are conventionally divided into two major classes based on
transcript size; small ncRNAs and long (l)ncRNAs (2). Small ncRNAs are represented by the
well-documented miRNAs, which are ~22 nucleotides(nt) in length
(2). By contrast, lncRNAs are
messenger (m)RNA-like transcripts, which range in length from 200
nt to ~100 kilobases (kb) and lack significant open reading frames
(2).
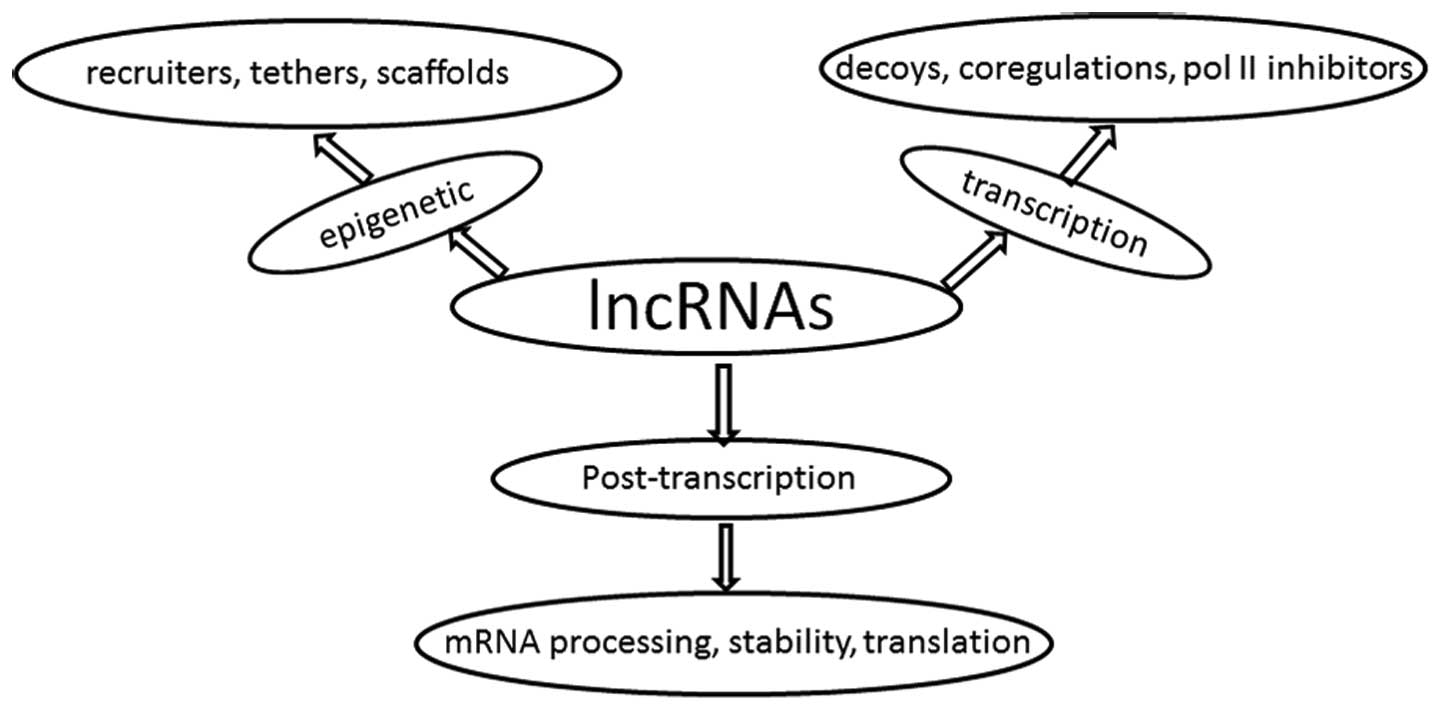
In the last decade, numerous studies have shown that
lncRNAs were able to regulate gene expression at the levels of
epigenetics, transcription and post-transcriptional processes
(3) (Fig. 1). LncRNAs have been reported to
mediate epigenetic changes by recruiting chromatin-modifying
complexes to specific genomic loci or tumor cell-specific promoter
regions (4). For example, Hox
transcript antisense RNA (HOTAIR), which is derived from the HOXC
locus, interacts with the polycomb repressive complex 2 (PRC2) in
order to regulate their target genes in cancer (5); in addition, lncRNAs were shown to
interact with transcription factors or act as transcriptional
co-regulators in order to mediate the process of transcription.
Furthermore, lncRNAs directly interact with RNA polymerase II in
order to regulate transcription (3). LncRNAs have also been recognized as
effective regulators of pre-mRNA splicing, mRNA decay and
translation (6).
To date, several techniques have been used for
discovery, identification and detection of lncRNAs. The
predominantly used techniques include microarrays, RNA sequencing
(RNA-seq), Northern blotting, reverse transcription quantitative
polymerase chain reaction (RT-qPCR), in situ hybridization,
bioinformatics prediction and target sequencing (Table I). Scientists have created
databases providing comprehensive annotations of lncRNAs in order
to fully elucidate the functions of lncRNAs in diseases and to
identify potential lncRNAs which may be used as diagnostics,
therapeutics and prognostic markers (Table II).
 | Table IMethods for discovery, identification
and detection of lncRNAs. |
Table I
Methods for discovery, identification
and detection of lncRNAs.
| Method |
Characteristics |
|---|
| Northern
blotting | Verifies the
existence of novel lncRNAs |
| Low sensitivity,
time consuming |
| Requires relatively
large amounts of total RNA |
| RT-qPCR | Validates the
existence of novel lncRNAs with high sensitivity and
specificity |
| Cannot discover
novel lncRNAs |
| In situ
hybridization | Can locate lncRNA
in tissue and cell compartments |
| Low sensitivity and
quantification |
| RNA sequencing | Can be employed for
high throughput discovery of novel lncRNAs and transcriptome
analysis |
| Expensive;
requirement of large number of data for analysis |
| Microarrays | Can be effective
for high-throughput analysis of lncRNA expression |
| Usually the results
rely on the PCR |
| Bioinformatic
prediction | Can give helpful
information for further exploration and reduce experimental
cost |
| Target
sequencing | Combined
multiplexed and target-specific amplification process with a
high-throughput sequencing technology |
 | Table IILong non-coding RNA databases. |
Table II
Long non-coding RNA databases.
Of note, several human diseases have been
demonstrated to be associated with mutated and dysregulated lncRNA
expression, including numerous types of cancer (18–23),
cardiovascular diseases (24–26)
and neurological diseases (27–29).
Candidate lncRNAs, including prostate cancer-associated 3 and
urothelial cancer-associated 1, have been regarded as potential
biomarkers for the diagnosis of prostate and bladder cancer,
respectively (21,30). The present review focuses on the
emerging roles of lncRNAs in lung diseases.
2. LncRNA in lung development
Human lung development may be subdivided into five
distinct stages: Embryonic, pseudoglandular, canalicular, terminal
saccular and alveolar (31).
During the first stage, the lung primordium develops from the
foregut. Thereafter, the original lung buds further branch into a
larger number of smaller areas. The canalicular stage is
characterized by enlargement of the bronchi and vascularization of
the lung tissue. Alveolar ducts and air sacs are established during
the saccular phase. At the final stage, the terminal saccules,
alveolar ducts and alveoli increase in number (31). It has been demonstrated that
microRNAs have important roles during early and late lung
development (32,33). However, the functions of lncRNAs in
lung development remain to be elucidated. Alveolar capillary
dysplasia with misalignment of pulmonary veins (ACD/MPV) is a rare,
congenital lung development malformation, which results in
pulmonary veins adjacent to small pulmonary arteries, medial
thickening of small pulmonary arteries, deficient lobular
development, insufficient alveolar wall capillaries and
occasionally lymphangiectasis (34). ACD/MPV has been associated with
Forkhead box protein F1 (FOXF1) on 16q24.1, which is predominantly
expressed in mesenchymal tissues of the developing lungs (35,36).
Szafranski et al (37)
demonstrated that the loss of a small non-coding gene region at
16q24.1, including lncRNAs, led to the development of ACD/MPV.
Therefore it was proposed that the FOXF1 promoter was regulated by
the interplay between chromatin looping, which may be mediated by
lncRNAs and methylation-controlled glioma-associated oncogene
family zinc finger 2 (GLI2) (37).
Overall, previous studies have indicated that lncRNAs may be
responsible for numerous disorders of human development.
3. LncRNA in lung inflammation
The immune system protects the body against
organisms and foreign substances which may cause infections and
diseases. The immune system is divided into the innate and adaptive
immune systems (38). Inflammation
is one of the first responses of the immune system to infection.
LncRNAs have been demonstrated to have important roles in innate
and adaptive immunity, including regulation of the differentiation
of immune cell subsets and their immunological functions (39–46).
Activation of the innate immune system and
pathological inflammation are the first steps in the protection of
the human body against a vast number of microorganisms (47). The respiratory epithelial surface
is exposed to an enormous number of foreign substances, including
allergens and pathogens. Toll-like receptors (TLRs) are a type of
pattern recognition receptor (PRR) (48). TLR signaling is known to be
involved in pathogen recognition and the activation of innate
immune cell responses following the invasion of microbes across
physical barriers, including the skin and surfaces of other organs
(49–50). Large intergenic non-coding RNA
(lincRNA)-Cox2, which is induced by TLRs, interacts with various
regulatory complexes, including heterogeneous nuclear
ribonucleoproteins (hnRNP)-A/B and A2/B1, in order to regulate
immune genes (39). Rapicavoli
et al (40) reported that
the expression of a pseudogene lncRNA called Lethe increased when
tumor necrosis factor (TNF)-α activated the pro-inflammatory
factory transcription factor nuclear factor (NF)-κB. In addition,
Lethe interacts with the NF-κB subunit RelA in order to prevent DNA
binding and reduce the expression of various inflammatory proteins
(40). Innate immune system
anti-viral host defense is mediated by type I interferon (IFN)
induction and signaling machinery (51). IFN, produced primarily from
dendritic cells, establishes an effective anti-viral state in
cells. It was reported that signal transducer and activator of
transcription factor 1 (STAT1) may be a key modulator of IFN
signaling and have a key role in clearance of severe acute
respiratory syndrome coronavirus (SARS-CoV) in the innate response
(52). Peng et al (41) demonstrated the widespread
differential expression of lncRNA in response to viral infections,
which were found to be involved in innate immunity. These results
were obtained through performing qPCR on lung samples from mice
lacking the IFN (IFNAR-/-) or STAT1 (STAT1-/-), which were infected
with SARS-CoV (41).
LncRNA expression has been identified to be involved
during the development and differentiation of T cells. TMEVPG1, a
novel lincRNA, was first identified using a positional cloning
approach in Theiler’s viral infection (42). Collier et al (43) reported that TMEVPG1 is a type 1
T-heper (Th1)-specific lincRNA which is regulated by STAT4 and
T-box expressed in T cells (T-bet) and was found to be involved in
the transcription of the gene encoding IFN-γ. A further study
demonstrated that TMGVPG1 contributed to histone methylation at the
Ifng locus in CD8+ T cells via interactions with WDR5 (44). In a study by Pang et al
(45), hundreds of lncRNAs were
found to be expressed in mammalian CD8+ T cells, several of which
surrounded or overlapped with the expression of important
protein-coding genes, which indicated their possible function as
regulatory decoy genes. In addition, Hu et al (46) revealed highly dynamic and
cell-specific expression patterns for lincRNAs during T cell
differentiation. LincR-Ccr2-5′AS, a lincRNA regulated by GATA-3,
was reported to be an important component in gene expression
specific to the Th2 subset of T helper (Th) cells as well as the
migration of Th2 cells (46).
4. LncRNA and cigarette smoke
Cigarette smoke is a significant risk factor for the
development of lung diseases, including lung cancer, chronic
obstructive pulmonary disease (COPD) and emphysema (53). Several lncRNAs have been
demonstrated to be differentially expressed between smokers and
non-smokers. One of these lncRNAs was significantly increased in
epithelia of smokers and was also associated with lung cancer
(54). This lncRNA was therefore
named smoke and cancer-associated lncRNA-1 (SCAL1). In addition,
SCAL1 was found to be a key downstream mediator of NF erythroid
2-related factor 2 (Nrf-2) in the regulation of genes responsible
for oxidative stress protection (54). Nrf-2 was demonstrated to be a
transcription factor which protected against the cytotoxic effects
of oxidative stress (55).
Imprinted genes inherit a single allele, while the other allele is
not or only weakly expressed. The H19 gene was reported to be
highly expressed during embryonic development and strongly
down-regulated in the majority of tissues following birth (56). H19 is one of the most highly
conserved imprint genes, which has been shown to have important
roles in normal development as well as oncogenesis (57,58).
Kaplan et al (59)
demonstrated that lncRNA H19 expression was significantly increased
in the bronchial epithelial cells of smokers due to activation of a
H19 single allele, rather than due to loss of imprinting (LOI).
Furthermore, previous studies have shown that LOI of H19 was
associated with lung cancer (60,61).
Therefore, lncRNA H19 and SCAL1 may be potential biomarkers for the
early diagnosis of lung cancer in smokers.
5. LncRNA and lung cancer
Lung cancer is the leading cause of
cancer-associated mortality worldwide. To date, no early detection
mechanisms have been elucidated and the current therapeutic
strategies for lung cancer treatment are ineffective; as a result,
the mortality rate of this disease is high. Recent studies have
indicated that lncRNAs may have an important role in the
development and progression of lung cancer (62,63).
The primary lncRNAs which have been associated with lung cancer to
date include metastasis-associated lung adenocarcinoma transcript
(MALAT)-1, H19, growth arrest-specific gene 6 antisense RNA 1
(GAS-AS1), HOTAIR and MEG3.
Development of lung cancer
LOI refers to the loss of parental-origin-specific
differential allele expression (64). LOI has been considered to be
abundant and precocious in the development of human tumors
(64). Overexpression of H19 has
been observed in lung cancer with LOI of H19 (60,61).
Barsyte-Lovejoy et al (65)
demonstrated that the oncogene c-Myc bound to conserved E-boxes at
the H19 promoter close to the imprinting control region and
upregulated the expression of this lncRNA, which contributed to the
tumorigenic phenotype of lung cancer cells. However, this study
showed that the oncogene c-Myc did not affect the imprinting of
H19, which remained to be monoallelic. In addition, the imprinted
H19 lncRNA is a precursor of micRNA-675 (66), which has been shown to regulate the
tumor suppressor retinoblastoma protein in order to induce
tumorigenesis (67).
Epigenetics refers to the heritable changes in gene
expression without permanent changes to the DNA sequence. These
changes may include DNA methylation, histone modification and
nucleosome positioning. Epigenetic alterations have been recognized
to contribute to several pathological processes, including cancer
(68). MEG3 is a tumor suppressor
lncRNA gene; hypermethylation of the MEG3 promoter has been shown
to contribute to the low expression of MEG3 in lung cancer
(69). In addition, overexpression
of MEG3 may induce reactivated p53 (69), which may indicate another potential
mechanism of MEG3 in tumor suppression.
HOTAIR was proposed to be an oncogene due to its
increased expression in several types of cancers, which was
reported to promote invasion and metastasis (70–72).
Type I collagen (Col-1), a type of interstitial extracellular
matrix (ECM), was found to be abnormally enriched in the tumor
microenvironment and promoted tumor activity (73). Zhuang et al (74) demonstrated that Col-1 induced the
expression of HOTAIR in non-small-cell lung carcinoma (NSCLC)
cells, which indicated that HOTAIR may contribute to the
tumorigenesis of lung cancer.
LncRNA and lung cancer metastasis
MALAT-1 was first identified as a predictive marker
for metastasis development in lung cancer (75); however, its role in metastasis
remains to be elucidated. Tano et al (76) suggested that MALAT-1 promoted cell
motility through transcriptional and post-transcriptional
regulation of motility-associated gene expression. HOTAIR has been
shown to have important roles in the metastasis of several types of
human tumors, including lung cancer (77). HOTAIR was reported to interact with
PRC2 and act as a co-repressor of silencing transcription factors
in order to inhibit tumor metastasis-suppressor gene transcription,
therefore increasing the risk of tumor metastasis (78).
LncRNA in the prognosis and treatment of
lung cancer
A close association has been reported between high
expression of MALAT1 and prognosis of lung cancer patients. Schmidt
et al (79) demonstrated
that the expression of MALAT-1 was associated with the prognosis of
squamous cell carcinoma; however, it was independent of the
prognosis of non-squamous cell carcinoma patients. In addition,
downregulation of MALAT-1 may inhibit the metastasis and invasion
of lung cancer cells (80). NSCLC
patients with low expression of MEG3 were reported to have a poor
prognosis (69). Therefore,
MALAT-1 and MEG3 may be novel diagnostic prognostic markers for
lung cancer. Growth arrest-specific 6 (GAS6) was shown to interact
with the TAM (Axl, Tyro3/Sky and Mer) subfamily of receptor
tyrosine kinases (81). GAS6 was
found to be involved in biological processes, including
proliferation, apoptosis and adhesion. In addition, lncRNA GAS6-AS1
expression was reported to be an independent risk factor for the
overall survival and metastasis in NSCLC patients (82). Furthermore, lncRNA GAS6-AS1 was
shown to be negatively correlated with GAS6 mRNA (82). These studies provided evidence to
suggest that lncRNA GAS6-AS1 may be involved in NSCLC through
regulating or interacting with its host gene GAS6.
Despite novel chemotherapeutic treatments and
targeted drugs which have achieved great improvements in the
treatment of lung cancer, the overall five-year survival rate of
NSCLC has not improved (83).
Chemoresistance is one of the most significant challenges for the
successful treatment of lung cancer. In addition, the correlation
of lncRNAs with chemoresistance has been demonstrated (84–86).
HOTAIR was reported to contribute to cisplatin resistance in human
lung adenocarcinoma cells through affecting apoptosis and cell
cycle distribution via regulation of p21 expression (86). Studies have shown that lung
adenocarcinoma cell resistance to cisplatin was associated with
Nrf-2 as well as its downstream genes (87,88).
Thai et al (54)
demonstrated that Nrf-2 activated the expression of SCAL1 through
binding to the promoter, which suggested the possible role of Nrf-2
in lung cancer chemoresistance. Overall, these studies have
indicated that lncRNAs may be potential drug targets for increasing
the effectiveness of lung cancer treatment.
6. LncRNA and pulmonary hypertension
Pulmonary arterial hypertension (PAH) is a disease
with numerous pathological and physiological factors, which has a
poor prognosis and ineffective treatment options. PAH is
characterized by increasing pulmonary artery pressure and elevated
pulmonary vascular resistance, leading to right heart failure
(89). Studies have revealed the
complex nature of the disorder, including inflammation, hypoxia,
dysregulated pulmonary endothelial cell proliferation and gene
mutations (89–91).
The renin-angiotensin system (RAS) has been shown to
cause endothelial dysfunction and vascular remodeling during the
development of PAH (92). RAS is
primarily composed of angiotensin-converting enzyme (ACE),
angiotensin II (Ang II) and angiotensin II type 1 receptor (AT1R)
(93). A recent study has
identified a novel lncRNA, Lnc-Ang 362, which is differentially
expressed in the response of vascular smooth muscle cells (VSMC) to
Ang II; in addition, this novel lncRNA, as a host transcript for
miR-221 and miR-222, was shown to have a crucial role in cell
proliferation (94). Furthermore,
these two microRNAs were previously reported to be associated with
VSMC proliferation and the regulation of Ang II in endothelial
cells (95,96).
Inflammation may be another important factor which
contributes to PAH due to the release of cytokines, chemokines and
various growth factors that may result in cell proliferation.
LncRNAs have been demonstrated to be involved in the regulation of
inflammation and therefore may have an impact on the pathogenesis
of PAH.
7. LncRNA and lung fibrosis
Idiopathic pulmonary fibrosis (IPF) is defined as
chronic, progressive fibrotic interstitial pneumonia without a
known cause. Until recently there were no effective drug therapies
for the treatment of IPF. The disease is characterized by the
expansion of activated mesenchymal cells and alveolar epithelial
cell injury leading to excessive ECM protein deposition in the
basement membrane and impaired gas exchange (97). Cao et al (98) established a model of
bleomycin-induced lung fibrosis, in which they detected 568
differentially expressed lncRNAs in the bleomycin-treated lung
samples compared with those in the normal control group through
microarray analysis. In addition, levels of lncRNA AJ005396 and
lncRNA S69206 were found to be significantly increased compared
with those in the control animals (98).
Studies on lncRNAs associated with the pathogenesis
of IPF are limited; however, the detection of mutated and
dysregulated lncRNAs may elucidate potential molecular targets for
the treatment of lung fibrosis, as lncRNAs have been demonstrated
to have important roles in disease pathogenesis.
8. Conclusion
In conclusion, ncRNAs were previously thought of as
‘noise’; however, following decades of research, evidence has been
provided for the biological functions of ncRNA transcripts. In
recent years, the functions of miRNAs in disease have been well
documented; however, compared with that of studies into the
dysregulation of miRNAs, current knowledge of the role of lncRNAs
in disease is still the tip of the iceberg, as only a small portion
of lncRNA functions have been elucidated. The present review
described the involvement of lncRNAs in respiratory diseases, with
a specific focus on lung cancer, as previous studies have
demonstrated that lncRNAs have key regulatory roles in the
pathogenesis of cancer. An in-depth understanding of the biological
functions of lncRNAs and how they interact with other ncRNAs as
well as target genes in lung cancer may elucidate novel biomarkers
and therapeutic targets for the early diagnosis and treatment of
lung cancer.
References
|
1
|
Crick FH, Barnett L, Brenner S and
Watts-Tobin RJ: General nature of the genetic code for proteins.
Nature. 192:1227–1232. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gutschner T and Diederichs S: The
hallmarks of cancer: a long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kung JT, Colognori D and Lee JT: Long
noncoding RNAs: past, present, and future. Genetics. 193:651–669.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Atkinson SR, Marguerat S and Bähler J:
Exploring long non-coding RNAs through sequencing. Semin Cell Dev
Biol. 23:200–205. 2012. View Article : Google Scholar
|
|
5
|
Shi X, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: a new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon JH, Abdelmohsen K and Gorospe M:
Posttranscriptional gene regulation by long noncoding RNA. J Mol
Biol. 425:3723–3730. 2013. View Article : Google Scholar :
|
|
7
|
Erdmann VA, Szymansk Mi, Hochberg A, Groot
ND and Barciszewski J: Non-coding, mRNA-like RNAs database Y2K.
Nucleic Acids Res. 28:197–200. 2000. View Article : Google Scholar
|
|
8
|
Mituyama T, Yamada K, Hattori E, et al:
The Functional RNA Database 3.0: databases to support mining and
annotation of functional RNAs. Nucleic Acids Res. 37:D89–D92. 2009.
View Article : Google Scholar :
|
|
9
|
Dinger ME, Pang KC, Mercer TR, Crowe ML,
Grimmond SM and Mattick JS: NRED: a database of long noncoding RNA
expression. Nucleic Acids Res. 37:D122–D126. 2009. View Article : Google Scholar :
|
|
10
|
Amaral PP, Clark MB, Gascoigne DK, Dinger
ME and Mattick JS: LncRNAdb: a reference database for long
noncoding RNAs. Nucleic Acids Res. 39:D146–D151. 2011. View Article : Google Scholar :
|
|
11
|
Liao Q, Xiao H, Bu DC, et al: ncFANs: a
web server for functional annotation of long non-coding RNAs.
Nucleic Acids Res. 39:W118–W124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bu DC, Yu KT, Sun SL, et al: NONCODE v3.0:
integrative annotation of long noncoding RNAs. Nucleic Acids Res.
40:D210–D215. 2012. View Article : Google Scholar :
|
|
13
|
Yang JH, Li JH, Jiang S, Zhou H and Qu LH:
ChIPBase: a database for decoding the transcriptional regulation of
long non-coding RNA and microRNA genes from ChIP-Seq data. Nucleic
acids Res. 41:D177–D187. 2013. View Article : Google Scholar :
|
|
14
|
Volders PJ, Helsens K, Wang X, et al:
LNCipedia: a database for annotated human lncRNA transcript
sequences and structures. Nucleic acids Res. 41:D246–D251. 2013.
View Article : Google Scholar :
|
|
15
|
Paraskevopoulou MD, Georgakilas G,
Kostoulas N, Reczko M, Maragkakis M, Dalamagas TM and Hatzigeorgiou
AG: DIANA-LncBase: experimentally verified and computationally
predicted microRNA targets on long non-coding RNAs. Nucleic Acids
Res. 41:D239–D245. 2013. View Article : Google Scholar :
|
|
16
|
Chen G, Wang ZY, Wang DQ, et al:
LncRNADisease: a database for long-non-coding RNA-associated
diseases. Nucleic Acids Res. 41:D983–D986. 2013. View Article : Google Scholar :
|
|
17
|
Bhartiya D, Pal K, Ghosh S, et al:
lncRNome: a comprehensive knowledgebase of human long noncoding
RNAs. Database (Oxford). 2013. pp. bat0342013, View Article : Google Scholar
|
|
18
|
Piao H and Ma L: Non-coding RNAs as
regulators of mammary development and breast cancer. J Mammary
Gland Biol Neoplasia. 17:33–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geng YJ, Xie SL, Li Q, Ma J and Wang GY:
Large intervening non-coding RNA HOTAIR is associated with
hepatocellular carcinoma progression. J Int Med Res. 39:2119–2128.
2011. View Article : Google Scholar
|
|
20
|
Kogo R, Shimamura T, Mimori K, et al: Long
noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nilsson J, Skog J, Nordstrand A, Baranov
V, Mincheva- Nilsson L, Breakefield XO and Widmark A: Prostate
cancer-derived urine exosomes: a novel approach to biomarkers for
prostate cancer. Br J Cancer. 100:1603–1607. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ng D, Toure O, Wei MH, et al:
Identification of a novel chromosome region, 13q21.33-q22.2, for
susceptibility genes in familial chronic lymphocytic leukemia.
Blood. 109:916–925. 2007. View Article : Google Scholar
|
|
23
|
Huarte M and Rinn JL: Large non-coding
RNAs: missing links in cancer? Hum Mol Genet. 19(R2): R152–R161.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Holdt LM and Teupser D: Recent studies of
the human chromosome 9p21 locus, which is associated with
atherosclerosis in human populations. Arterioscler Thromb Vasc
Biol. 32:196–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Sanoff HK, Cho H, et al: INK4/ARF
transcript expression is associated with chromosome 9p21 variants
linked to atherosclerosis. PLoS One. 4:e50272009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishii N, Ozaki K, Sato H, et al:
Identification of a novel non-coding RNA, MIAT that confers risk of
myocardial infarction. J Hum Genet. 51:1087–1099. 2006. View Article : Google Scholar
|
|
27
|
Johnson R: Long non-coding RNAs in
Huntington’s disease neurodegeneration. Neurobiol Dis. 46:245–254.
2012. View Article : Google Scholar
|
|
28
|
Qureshi IA, Mattick JS and Mehler MF: Long
non-coding RNAs in nervous system function and disease. Brain Res.
1338:20–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Decourt B and Sabbagh MN: BACE1 as a
potential biomarker for Alzheimer’s disease. J Alzheimers Dis.
24(Suppl 2): 53–59. 2011.
|
|
30
|
Wang XS, Zhang Z, Wang HC, et al: Rapid
identification of UCA1 as a very sensitive and specific unique
marker for human bladder carcinoma. Clin Cancer Res. 12:4851–4858.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zoetis T and Hurtt ME: Species comparison
of lung development. Birth Defects Res B Dev Reprod Toxicol.
68:121–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lü J, Qian J, Chen F, Tang X, Li C and
Cardoso WV: Differential expression of components of the microRNA
machinery during mouse organogenesis. Biochem Biophys Res Commun.
334:319–323. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bhaskaran M, Wang Y, Zhang H, et al:
MicroRNA-127 modulates fetal lung development. Physiol Genomics.
37:268–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bishop NB, Stankiewicz P and Steinhorn RH:
Alveolar capillary dysplasia. Am J Respir Crit Care Med.
184:172–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stankiewicz P, Sen P, Bhatt SS, et al:
Genomic and genic deletions of the FOX gene cluster on 16q24.1 and
inactivating mutations of FOXF1 cause alveolar capillary dysplasia
and other malformations. Am J Hum Genet. 84:780–791. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mahlapuu M, Enerbäck S and Carlsson P:
Haploinsufficiency of the Forkhead gene Foxf1, a target for sonic
hedgehog signaling, causes lung and foregut malformations.
Development. 128:2397–2406. 2001.PubMed/NCBI
|
|
37
|
Szafranski P, Dharmadhikari AV, Brosens E,
et al: Small noncoding differentially methylated copy-number
variants, including lncRNA genes, cause a lethal lung developmental
disorder. Genome Res. 23:23–33. 2013. View Article : Google Scholar :
|
|
38
|
Getz GS: Bridging the innate and adaptive
immune systems. J Lipid Res. 46:619–622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carpenter S, Aiello D, Atianand MK, et al:
A long noncoding RNA mediates both activation and repression of
immune response genes. Science. 341:789–792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rapicavoli NA, Qu K, Zhang JJ, Mikhail M,
Laberge RM and Chang HY: A mammalian pseudogene lncRNA at the
interface of inflammation and anti-inflammatory therapeutics.
ELife. 2:e007622013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peng X, Gralinski L, Armour CD, et al:
Unique signatures of long noncoding RNA expression in response to
virus infection and altered innate immune signaling. mBio.
1:e00206–e00210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vigneau S, Rohrlich PS, Rahic MB and
Bureau JF: Tmevpg1, a candidate gene for the control of Theiler’s
virus persistence, could be implicated in the regulation of
interferon. J Virol. 77:5632–5638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Collier SP, Collins PL, Williams CL,
Boothby MR and Aune TM: Cutting edge: influence of Tmevpg1, a long
intergenic noncoding RNA, on the expression of Ifng by Th1 cells. J
Immunol. 189:2084–2088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
GomeZ JA, Wapinski OL, Yang YW, et al: The
NeST long ncRNA controls microbial susceptibility and epigenetic
activation of the interferon-γ locus. Cell. 152:743–754. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pang KC, Dinger ME, Mercer TR, Malquori L,
Grimmond SM, Chen W and Mattick JS: Genome-wide identification of
long noncoding RNAs in CD8+ T cells. J Immunol. 182:7738–7748.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu G, Tang Q, Sharma S, et al: Expression
and regulation of intergenic long noncoding RNAs during T cell
development and differentiation. Nat Immunol. 14:1190–1198. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Janeway CA Jr, Travers P, Walport M and
Shlomchik M: Principles of innate and adaptive immunity.
Immunobiology: The Immune System in Health and Disease. 5th.
Garland Science; New York, NY: 2001
|
|
48
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: update on
Toll-like receptors. Nature Immunol. 11:373–384. 2010. View Article : Google Scholar
|
|
49
|
Kawai T and Akira S: Toll-like receptor
and RIG-1-like receptor signaling. Ann NY Acad Sci. 1143:1–20.
2008. View Article : Google Scholar
|
|
50
|
Medzhitov R and Horng T: Transcriptional
control of the inflammatory response. Nat Rev Immunol. 9:692–703.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu SY, Sanchez DJ and Cheng G: New
developments in the induction and antiviral effectors of type I
interferon. Curr Opin Immunol. 23:57–64. 2011. View Article : Google Scholar
|
|
52
|
Hogan RJ, Gao G, Rowe T, et al: Resolution
of primary severe acute respiratory syndrome-associated coronavirus
infection requires Stat1. J Virol. 78:11416–11421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rupani H, Sanchez-Elsner T and Howarth P:
MicroRNAs and respiratory diseases. Eur Respir J. 41:695–705. 2013.
View Article : Google Scholar
|
|
54
|
Thai P, Statt S, Chen CH, Liang E,
Campbell C and Wu R: Characterization of a novel long noncoding
RNA, SCAL1, induced by cigarette smoke and elevated in lung cancer
cell lines. Am J Respir Cell Mol Biol. 49:204–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radical Biol
Med. 47:1304–1309. 2009. View Article : Google Scholar
|
|
56
|
Lustig O, Ariel I, Ilan J, Lev-Lehman E,
De-Groot N and Hochberg A: Expression of the imprinted gene H19 in
the human fetus. Mol Reprod Dev. 38:239–246. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gabory A, Jammes H and Dandolo L: The H19
locus: role of an imprinted non-coding RNA in growth and
development. Bioessays. 32:473–480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Matouk IJ, DeGroot N, Mezan S, Ayesh S,
Abu-lail R, Hochberg A and Galun E: The H19 non-coding RNA is
essential for human tumor growth. PloS One. 2:e8452007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kaplan R, Luettich K, Heguy A, Hackett NR,
Harvey BG and Crystal RG: Monoallelic up-regulation of the
imprinted H19 gene in airway epithelium of phenotypically normal
cigarette smokers. Cancer Res. 63:1475–1482. 2003.PubMed/NCBI
|
|
60
|
Kondo M, Suzuki H, Ueda R, Osada H, Takagi
K and Takahashi T: Frequent loss of imprinting of the H19 gene is
often associated with its overexpression in human lung cancers.
Oncogene. 10:1193–1198. 1955.
|
|
61
|
Kondo M and Takahashi T: Altered genomic
imprinting in the IGF2 and H19 genes in human lung cancer. Nihon
Rinsho. 54:492–496. 1996.In Japanese. PubMed/NCBI
|
|
62
|
Xu G, Chen J and Pan Q: Long noncoding RNA
expression profiles of lung adenocarcinoma ascertained by
microarray analysis. PLoS One. 9:e1040442014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
White NM, Cabanski CR, Silva-Fisher JM,
Dang HX, Govindan R and Maher CA: Transcriptome sequencing reveals
altered long intergenic non-coding RNAs in lung cancer. Genome
Biol. 13:4292014. View Article : Google Scholar
|
|
64
|
Jelinic P and Shaw P: Loss of imprinting
and cancer. J Pathol. 221:261–268. 2007. View Article : Google Scholar
|
|
65
|
Barsyte-Lovejoy D, Lau SK, Boutros PC, et
al: The c-Myc oncogene directly induces the H19 noncoding RNA by
allele-specific binding to potentiate tumorigenesis. Cancer Res.
66:5330–5337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cai X and Cullen BR: The imprinted H19
noncoding RNA is a primary microRNA precursor. Rna. 13:313–316.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tsang WP, Ng EK, Ng SS, Jin H, Yu J, Sung
JJ and Kwok TT: Oncofetal H19-derived miR-675 regulates tumor
suppressor RB in human colorectal cancer. Carcinogenesis.
31:350–358. 2010. View Article : Google Scholar
|
|
68
|
Tim W and Feinberg AP: Cancer as a
dysregulated epigenome allowing cellular growth advantage at the
expense of the host. Nat Rev Cancer. 13:497–510. 2013. View Article : Google Scholar
|
|
69
|
Lu KH, Li W, Liu XH, et al: Long
non-coding RNA MEG3 inhibits NSCLC cells proliferation and induces
apoptosis by affecting p53 expression. BMC Cancer. 13:4612013.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gupta RA, Shah N, Wang KC, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yang Z, Zhou L, Wu LM, Lai MC, Xie HY,
Zhang F and Zheng SS: Overexpression of long noncoding RNA HOTAIR
predicts tumor recurrence in hepatocellular carcinoma patients
following liver transplantation. Ann Surg Oncol. 18:1243–1250.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kogo Shimamura T, Mimori K, et al: Long
noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011. View Article : Google Scholar
|
|
73
|
Egeblad M, Rasch MG and Weaver VM: Dynamic
interplay between the collagen scaffold and tumor evolution. Curr
Opin Cell Biol. 22:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhuang Y, Wang X, Nguyen H, et al:
Induction of long intergenic non-coding RNA HOTAIR in lung cancer
cells by type I collagen. J Hematol Oncol. 6:352013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ji P, Diederichs S, Wang W, et al:
MALAT-1, a novel noncoding RNA, and thymosin beta4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tano K, Mizuno R, Okada T, et al: MALAT-1
enhances cell motility of lung adenocarcinoma cells by influencing
the expression of motility-related genes. FEBS Lett. 584:4575–4580.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Nakagawa T, Endo H, YoKoyama M, et al:
Large noncoding RNA HOTAIR enhances aggressive biological behavior
and is associated with short disease-free survival in human
non-small cell lung cancer. Biochem Biophys Res Commun.
436:319–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tsai MC, Manor O, Wan Y, et al: Chang,
Long noncoding RNA as modular scaffold of histone modification
complexes. Science. 329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Schmidt LH, Spieker T, Koschmieder S, et
al: The long noncoding MALAT-1 RNA indicates a poor prognosis in
non-small cell lung cancer and induces migration and tumor growth.
J Thorac Oncol. 6:1984–1992. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gutschner T, Hämmerle M, Eissmann M, et
al: The noncoding RNA MALAT1 is a critical regulator of the
metastasis phenotype of lung cancer cells. Cancer Res.
73:1180–1189. 2013. View Article : Google Scholar :
|
|
81
|
Lee Y, Lee M and Kim S: Gas 6 induces
cancer cell migration and epithelial-mesenchymal transition through
upregulation of MAPK and Slug. Biochem Biophys Res Commun.
434:8–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Han L, Kong R, Yin DD, Zhang EB, Xu TP, De
W and Shu YQ: Low expression of long noncoding RNA GAS6-AS1
predicts a poor prognosis in patients with NSCLC. Med Oncol.
30:6942013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Brody H: Lung cancer. Nature. 513:S12014.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Takahashi K, Yan IK, Kogure T, Haga H and
Patel T: Extracellular vesicle-mediated transfer of long non-coding
RNA ROR modulates chemosensitivity in human hepatocellular cancer.
FEBS Open Bio. 4:458–467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F
and Liu Y: Long non-coding RNA UCA1 increases chemoresistance of
bladder cancer cells by regulating Wnt signaling. FEBS J.
281:1750–1758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liu Z, Sun M, Lu K, et al: The long
noncoding RNA HOTAIR contributes to cisplatin resistance of human
lung adenocarcinoma cells via downregualtion of p21 (WAF1/CIP1)
expression. PloS one. 8:e772932013. View Article : Google Scholar
|
|
87
|
Sing A, Boldin-Adamasky S, Thimmulappa RK,
et al: RNAi-mediated silencing of nuclear factor
erythroid-2-related factor 2 gene expression in non-small cell lung
cancer inhibits tumor growth and increases efficacy of
chemotherapy. Cancer Res. 68:7975–7984. 2008. View Article : Google Scholar
|
|
88
|
Oh S, Kim Y, Kim J, Kwon D and Lee E:
Elevated pressure, a novel cancer therapeutic tool for sensitizing
cisplatin-mediated apoptosis in A549. Biochem Biophys Res Commun.
399:91–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Voelkel NF, Gomez-Arroyo J, Abbate A,
Bogaard HJ and Nicolls MR: Pathobiology of pulmonary arterial
hypertension and right ventricular failure. Eur Respir J.
40:1555–1565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Guignabert C and Dorfmuller P: Pathology
and pathobiology of pulmonary hypertension. Semin Respir Crit Care
Med. 34:551–559. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Caruso P, MacLean MR and Khanin R: Dynamic
changes in lung microRNA profiles during the development of
pulmonary hypertension due to chronic hypoxia and monocrotaline.
Arteriosclerosis Thromb Vasc Biol. 30:716–723. 2010. View Article : Google Scholar
|
|
92
|
Lüscher TF: Endothelial dysfunction: the
role and impact of the renin-angiotensin system. Heart. 84(Suppl
1): i20–i22. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Shenoy V, Qi Y, Katovich MJ and Raizada
MK: ACE2, a promising therapeutic target for pulmonary
hypertension. Curr Opin Pharmacol. 11:150–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Leung A, Trac C, Jin W, et al: Novel long
noncoding RNAs are regulated by angiotensin II in vascular smooth
muscle cells. Circ Res. 113:266–278. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhu N, Zhang D, Chen S, et al: Endothelial
enriched microRNAs regulate angiotensin II-induced endothelial
inflammation and migration. Atherosclerosis. 215:286–293. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Liu X, Cheng Y, Zhang S, Lin Y, Yang J and
Zhang C: A necessary role of miR-221 and miR-222 in vascular smooth
muscle cell proliferation and neointimal hyperplasia. Circ Res.
104:476–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Noble PW and Homer RJ: Back to the future:
historical perspective on the pathogenesis of idiopathic pulmonary
fibrosis. Am J Respir Cell Mol Biol. 33:113–120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Cao G, Zhang J, Wang M, Song X, Liu W, Mao
C and Lv C: Differential expression of long non-coding RNAs in
bleomycin-induced lung fibrosis. Int J Mol Med. 32:355–364.
2013.PubMed/NCBI
|