Introduction
Obesity is one of the most prevalent physiological
disorders associated with a variety of conditions, including
hypertension (1), dyslipidemia
(2), atherosclerosis (3), type II diabetes (4), non-alcoholic fatty liver disease
(5), periodontal disease (6), asthma (7), cardiovascular disease (8) and certain types of cancer (9,10).
Additionally, the number of obese individuals is continuing to
increase and is becoming a serious health problem worldwide
(11,12). Therefore, there is a continuing
requirement for novel and safe substances to overcome this problem,
with a view to combating obesity-associated health problems
(13).
Adipogenesis is regulated by a number of
transcription factors, including the CCAAT/enhancer binding
proteins (C/EBPs) and peroxisome proliferator-activated receptor γ
(PPARγ) (14,15).C/EBPβ and C/EBPδ rapidly induce the
expression levels of PPARγ and C/EBPα. The PPARγ and C/EBPα
proteins activate the expression of a number of genes involved in
adipocyte differentiation, including genes responsible for lipid
accumulation and insulin sensitivity (16). In addition, the activation of the
extracellular signal regulated kinase (ERK) and
phosphatidylinositide 3-inase (PI3K)/Akt pathways is necessary for
adipogenesis (16,17). ERKs regulate cell proliferation and
are required for initiating the differentiation process in
pre-adipocytes. The phosphorylation of ERK is increased during the
early stages of adipocyte differentiation in embryonic stem cells.
Several previous studies have reported that inhibition of the ERK
pathway in the early stages of differentiation inhibits
adipogenesis, and there is increasing evidence demonstrating the
importance of the ERK pathway in adipogenesis (18,19).
The involvement of Akt in adipocyte differentiation is better
understood, and inhibition of the PI3K/Akt pathway has been
demonstrated to reduce adipogenesis (20,21).
Zanthoxylum schinifolium, which belongs to
the Rutaceae family of plants, is an aromatic plant widely used as
a pungent condiment and seasoning in Korea and other countries in
east Asia (22). Z.
schinifolium has also been used in traditional Chinese medicine
for the treatment of several symptoms, including the common cold,
diarrhea, toothache and jaundice. Additionally, various
pharmacological activities, including the inhibition of lipid
peroxidation (23), and
anti-oxidant (24), antiplatelet
aggregation (25),
anti-inflammatory (26) and
antitumor (23,27,28)
effects, have been reported by this plant. However, to the best of
our knowledge, the potential anti-obesity activity of Z.
schinifolium remains to be elucidated. Therefore, the aim of
the present study was to determine the inhibitory ability of
ethanol extract from the leaves of Z. schinifolium (EEZS) on
adipocyte differentiation, using the 3T3-L1 murine pre-adipocyte
model, determined by measuring the levels of lipid accumulation and
the expression levels of adipocyte marker genes. The effects EEZS
on the ERK and PI3K/Akt pathways were also examined to investigate
the possible underlying molecular mechanisms.
Materials and methods
Preparation of EEZS
Z. schinifolium was obtained from Dongeui
Oriental Hospital, Dongeui University College of Korean Medicine
(Busan, Republic of Korea). Dried leaves from Z.
schinifolium (40 g) were cut into small pieces, ground into a
fine powder and then soaked with 500 ml 100% ethanol
(Sigma-Aldrich, St. Louis, MO, USA) for 2 days. The extracted
liquid was filtered through Whatman No. 3 filter paper
(Sigma-Aldrich) twice to remove any insoluble materials and was
then concentrated using a rotary evaporator (EYELAN-1000; Rikakikai
Co., Ltd., Tokyo, Japan). The extracts were redissolved in
dimethylsulfoxide (DMSO; Sigma-Aldrich) to a final concentration of
200 mg/ml (extract stock solution) and were subsequently diluted
with Dulbecco’s modified Eagle’s medium (DMEM; WelGENE Daegu,
Korea), to the desired concentration prior to use.
Cell culture and induction of adipocyte
differentiation
The 3T3-L1 cell line was obtained from American Type
Culture Collection (Manassas, VA, USA). The cells were cultured in
DMEM, supplemented with 10% fetal bovine serum (FBS; WelGENE) and
1% penicillin-streptomycin (Sigma-Aldrich) at 37°C in a humidified
atmosphere of 5% CO2. To initiate differentiation of the
3T3-L1 pre-adipocytes into adipocytes, the cells were stimulated
with differentiation inducers, including 0.5 mM
isobuthylmethylxanthine (Sigma-Aldrich), 10 μg/ml insulin
(MDI; Sigma-Aldrich) and 1 μM dexamethasone, which were
added to the DMEM, containing 10% FBS, for 48 h. Following
differentiation, the adipocytes were incubated with
post-differentiation medium, which consisted of DMEM, containing
10% FBS and 10 μg/ml insulin. The medium was replaced every
other day for up to 8 days (29).
Cell viability
To assess the cell viability in the 3T3-L2
adipocytes, the cells were plated into 24-well plates
(1×105 cells/ml) and incubated for 24 h at 37°C until
confluent. The cells were subsequently treated with the indicated
concentrations (100–600 μg/ml) of EEZS for 72 h at 37°C. The
control cells were supplemented with complete medium, containing
0.5% DMSO, as a vehicle control. Subsequent to incubation for 72 h
at 37°C, the medium was removed and the cells were incubated with
0.5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT; Sigma-Aldrich) solution for 3 h at room temperature.
The supernatant was subsequently discarded and the formazan blue,
which had formed in the cells, was then dissolved in 100% DMSO. The
DMSO concentration did not exceed 0.05%. The optical density was
measured at 540 nm using a microplate reader (Dynatech
Laboratories, Chantilly VA, USA) and the effects of EEZS on the
inhibition of cell growth were assessed as the percentage of cell
viability, in which the vehicle-treated cells were considered to be
100% viable.
Oil Red-O staining
The intercellular lipid accumulation within
adipocytes was assessed using Oil Red-O solution. Following the
induction of adipocyte differentiation, the cells were washed twice
with phosphate-buffered saline (PBS; WelGENE) and fixed at room
temperature with 10% formalin (Junsei Chemical Co., Ltd., Tokyo,
Japan) for 1 h. The cells were dried and stained with Oil Red-O
(0.35% Oil Red-O in 100% aqueous 2-isopropanol; Sigma-Aldrich) for
20 min at room temperature. Following staining of the lipid
droplets, the Oil Red-O staining solution was removed, the plates
were rinsed twice with PBS and 60% isopropanol and were then dried.
Images of the stained oil droplets in the 3T3-L1 cells were
captured under light microscopy. To quantify the intracellular
lipids, the stained lipid droplets were dissolved in isopropanol,
containing 4% Nonidet P-40 (NP-40; Sigma-Aldrich). The extracted
dye was transferred into a 96-well plate, and the absorbance was
measured using a microplate reader (MR5000; Dynatech Laboratories,
Chantilly, VA, USA) at 500 nm. The difference in absorbance between
the samples with and without dye solution was calculated (30).
Protein extraction and western blot
analysis
The cells were harvested and washed once with
ice-cold PBS, and were gently lysed for 20 min in ice-cold lysis
buffer containing 40 mM Tris (pH 8.0), 120 mM NaCl, 0.5% NP-40, 0.1
mM sodium orthovanadate, 2 μg/ml leupeptin and 100
μg/ml phenylmethylsulfonyl fluoride (all Sigma-Aldrich). The
supernatants were collected and the protein concentrations were
determined using a Bio-Rad protein assay kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Equal quantities of the protein extracts
were denatured by boiling at 95°C for 5 min in sample buffer
(Bio-Rad Laboratories, Inc.), containing 0.5 M Tris-HCl (pH 6.8),
4% sodium dodecyl sulphate (SDS), 20% glycerol, 0.1% bromophenol
blue and 10% β-mercaptoethanol, at a ratio of 1:1. The samples were
either stored at −80°C or were used immediately for immunoblotting.
Aliquots, containing 30 μg total protein, were separated on
10% SDS-polyacrylamide gels and transferred onto nitrocellulose
membranes (Amersham Life Sciences, Arlington Heights, IL, USA). The
membranes were subsequently blocked with 5% non-fat milk and
incubated overnight at 4°C with primary antibodies, probed with
enzyme-linked secondary antibodies at room temperature for a
further 1 h and then detected using an enhanced chemiluminescence
detection system (Amersham Life Sciences), according to the
manufacturer’s instructions. The primary antibodies were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and Cell
Signaling Technology, Inc. (Danvers, MA, USA) (Table I). The horseradish
peroxidase-conjugated anti-rabbit immunoglobulin G (IgG, sc-2004;
1:1,000), anti-mouse IgG (sc-2005; 1:1,500) and anti-goat IgG
(sc-2350; 1:1,500) were purchased from Santa Cruz Biotechnology,
Inc. β-actin was used as an internal control.
 | Table IList of primary antibodies. |
Table I
List of primary antibodies.
| Antibody | Dilution | Catalog no. | Species of origin
and supplier |
|---|
| C/EBPα | 1:1,000 | 2295s | Rabbit polyclonal;
Cell Signaling Technology, Inc. |
| C/EBPβ | 1:1,000 | 3087s | Rabbit polyclonal;
Cell Signaling Technology, Inc. |
| PPARγ | 1:1,000 | 2430s | Rabbit polyclonal;
Cell Signaling Technology, Inc. |
| ERK | 1:1,000 | sc-154 | Rabbit polyclonal;
Santa Cruz Biotechnology, Inc. |
| p-ERK | 1:500 | 9106S | Mouse monoclonal;
Cell Signaling Technology, Inc |
| PI3K | 1:1,000 | sc-7176 | Rabbit polyclonal;
Santa Cruz Biotechnology, Inc. |
| p-PI3K | 1:500 | sc-293115 | Rabbit polyclonal;
Santa Cruz Biotechnology, Inc. |
| Akt | 1:500 | sc-8312 | Rabbit polyclonal;
Santa Cruz Biotechnology, Inc. |
| p-Akt | 1:500 | sc-101629 | Rabbit polyclonal;
Santa Cruz Biotechnology, Inc. |
| β-actin | 1:1,000 | sc-1616 | Goat polyclonal;
Santa Cruz Biotechnology, Inc. |
Statistical analysis
The data are expressed as the mean ± standard
deviation. Comparisons between the groups were made using analysis
of variance and the significance between differences were analyzed
using Duncan’s multiple range test. Statistical analyses were
performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistical significant
difference.
Results
Cytotoxic effects of EEZS on 3T3-L1
cells
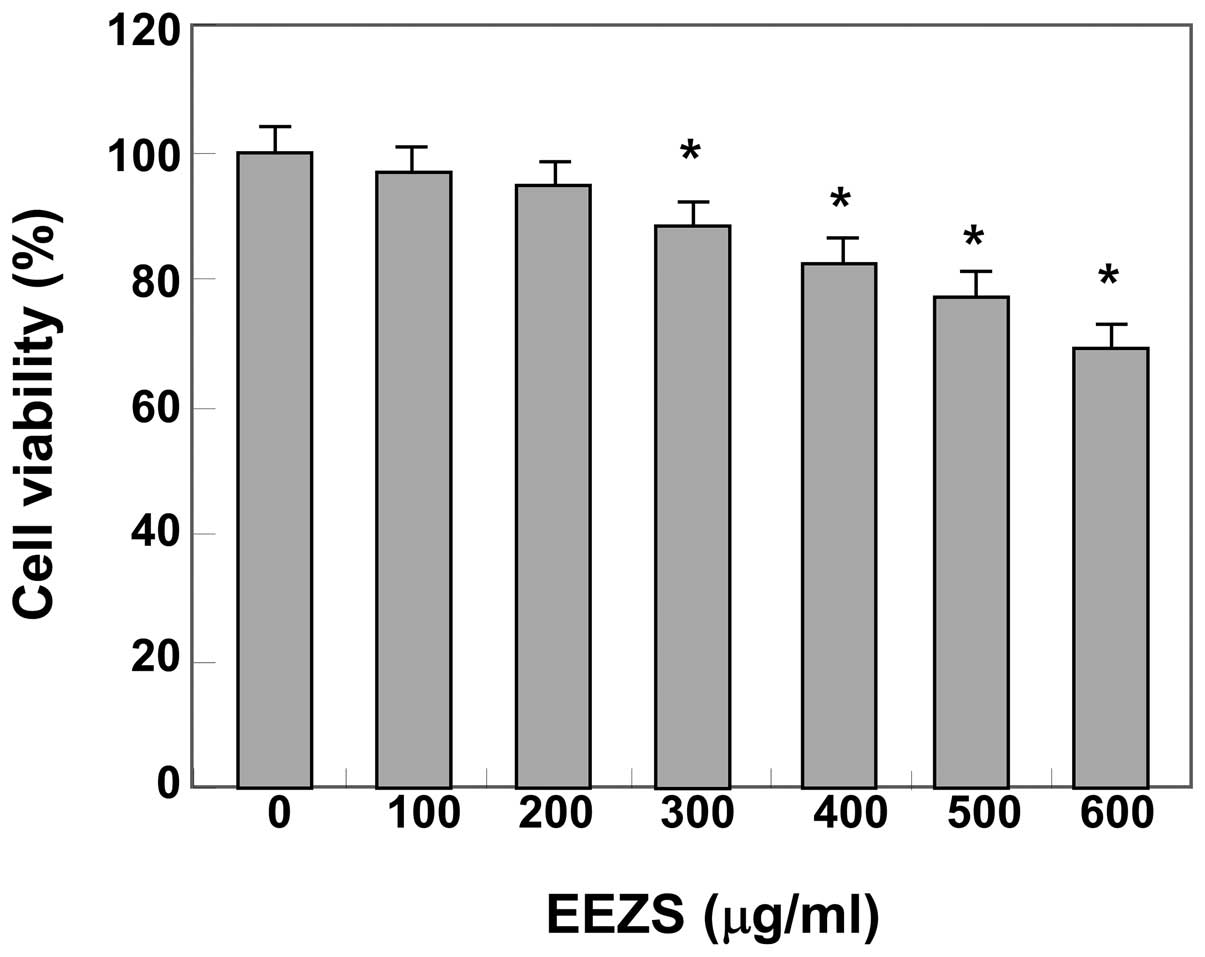
To determine the cytotoxicity of EEZS, the 3T3-L1
cells were treated with various concentrations of EEZS and the cell
viability was assessed using an MTT assay. The data demonstrated
that EEZS exhibited no cytotoxic effects at concentrations ≤200
μg/ml (Fig. 1). Therefore,
concentrations of 50, 100, 150 and 200 μg/ml were selected
for use in the subsequent experiments.
EEZS inhibits adipogenesis in 3T3-L1
cells
As increased lipid accumulation during the
differentiation of pre-adipocytes into adipocytes is a typical
phenomenon, which occurs in 3T3-L1 cells and is used as a marker of
differentiation, the 3T3-L1 pre-adipocytes were treated with
various concentrations of EEZS in the presence of MDI or with MDI
alone for 8 days. Lipid accumulation was subsequently measured in
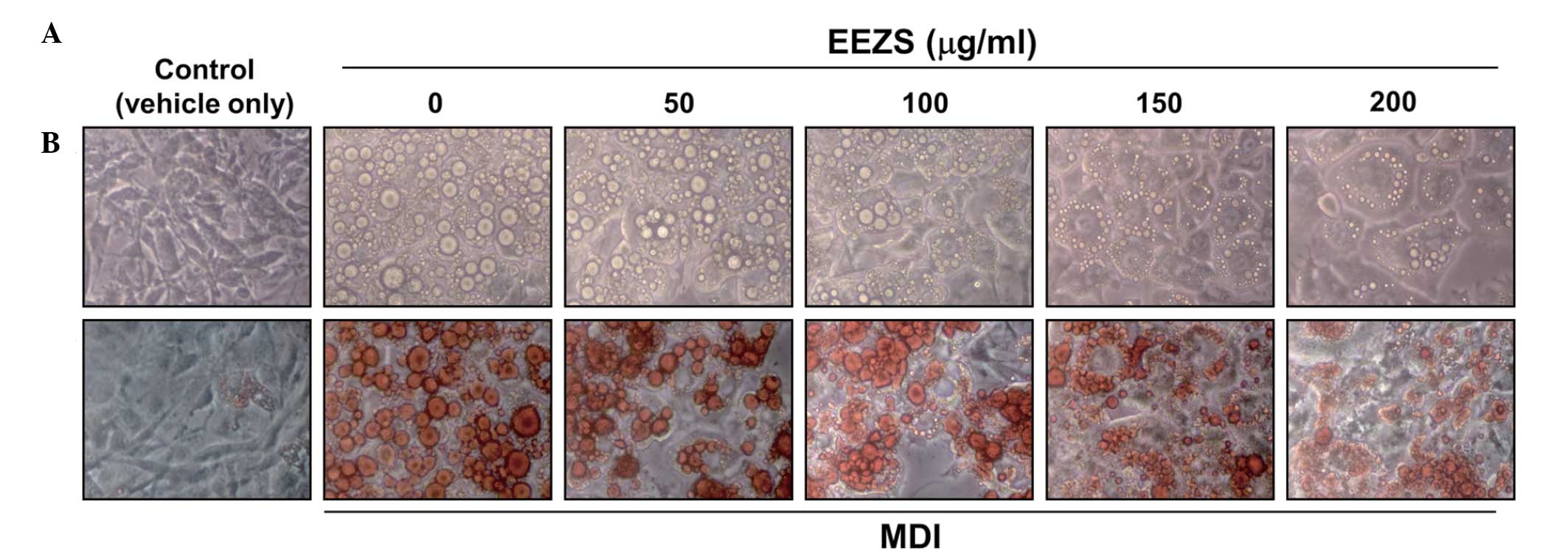
the cells using Oil Red-O staining. As shown in Fig. 2, oil droplets were not visible in
the undifferentiated 3T3-L1 cells, however, several lipid droplets
were visible in the fully differentiated cells treated with MDI.
Microscopic observations of the Oil Red-O staining revealed a
reduction in the number of lipid droplets as the concentrations of
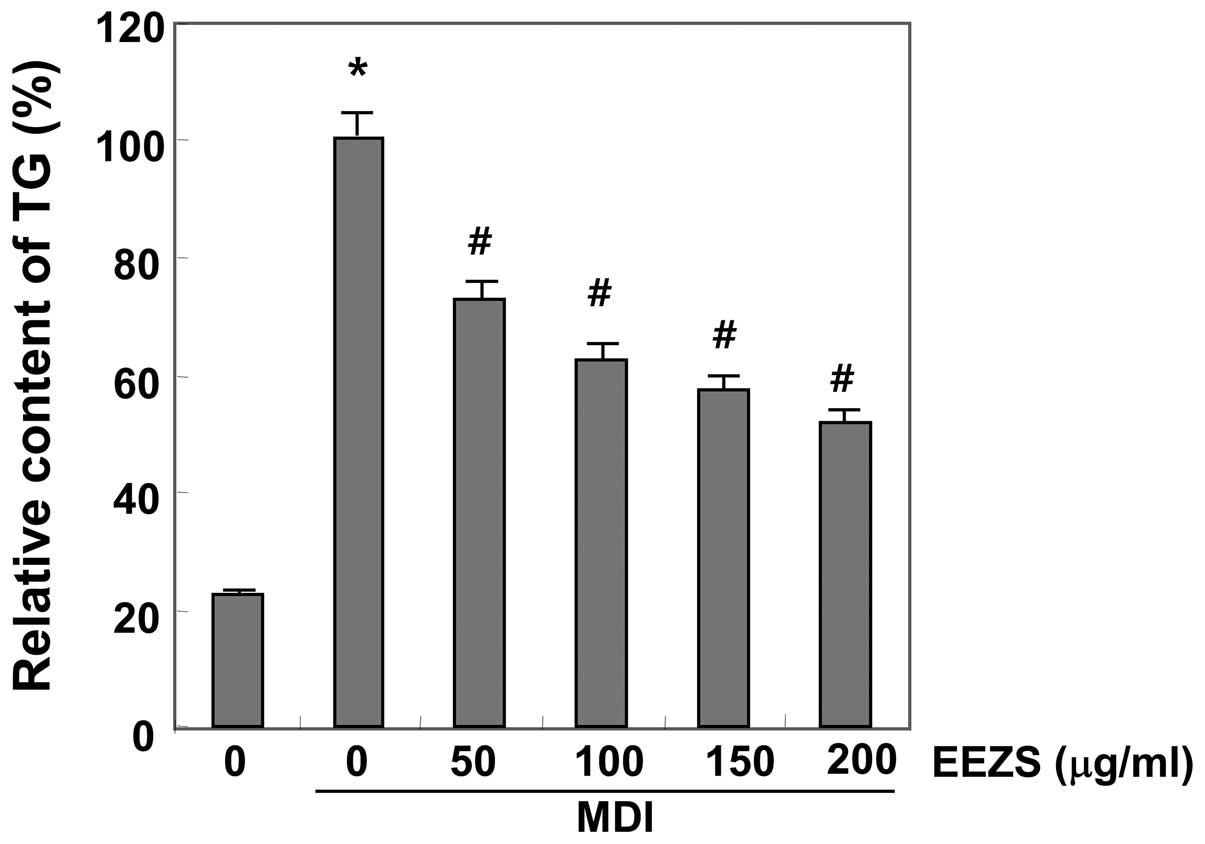
EEZS increased. The inhibitory effects of EEZS on the triglyceride
contents of the differentiated adipocytes were measured, which
revealed that the triglyceride contents of the adipocytes increased
markedly during 8 days incubation with MDI (Fig. 3). However, treatment with EEZS
decreased the triglyceride levels significantly, in a
concentration-dependent manner. These results demonstrated that
EEZS inhibited adipocyte differentiation in the 3T3-L1 cells at
non-cytotoxic concentrations.
EEZS inhibits the expression of
adipogenic transcription factors during adipocyte differentiation
in 3T3-L1 cells
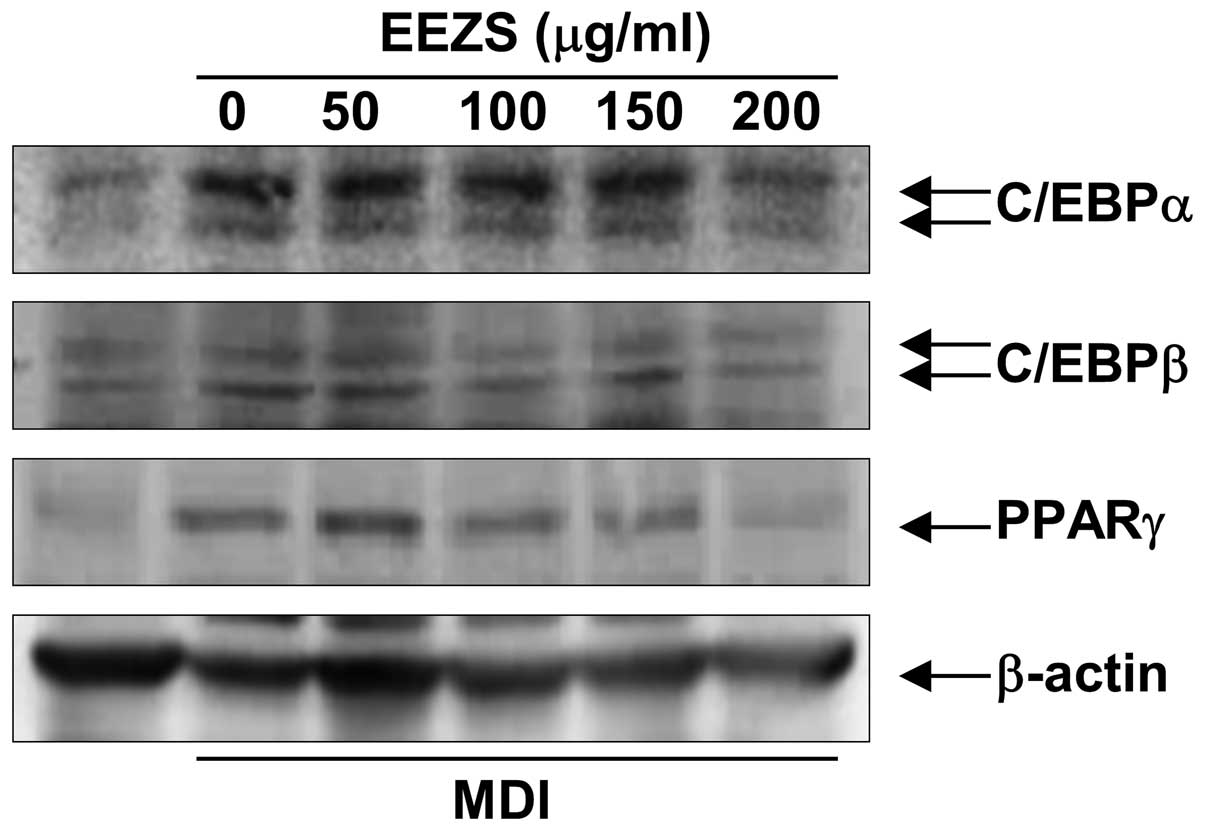
Adipogenesis is accompanied by the increased
expression of adipogenic transcription factors and
adipocyte-specific genes. To investigate the anti-adipogenic
mechanism underlying EEZS, the fully differentiated adipocytes were
treated with different concentrations of EEZS, and the protein
expression levels of PPARγ, C/EBPα and C/EBPβ were determined by
western blot analysis. As expected, the levels of these three
proteins were significantly upregulated during the process of
differentiation (Fig. 4). However,
treatment with EEZS suppressed the expression levels of PPARγ,
C/EBPα and C/EBPβ compared with the fully differentiated control
adipocytes, and this occurred in a concentration-dependent manner.
This result suggested that EEZS inhibited adipogenesis by reducing
the expression of C/EBPβ, which lead to a downregulation in the
expression levels of C/EBPα and PPARγ.
Anti-adipogenic effects of EEZS are
associated with inactivation of the ERK and PI3K/Akt pathways in
3T3-L1 cells
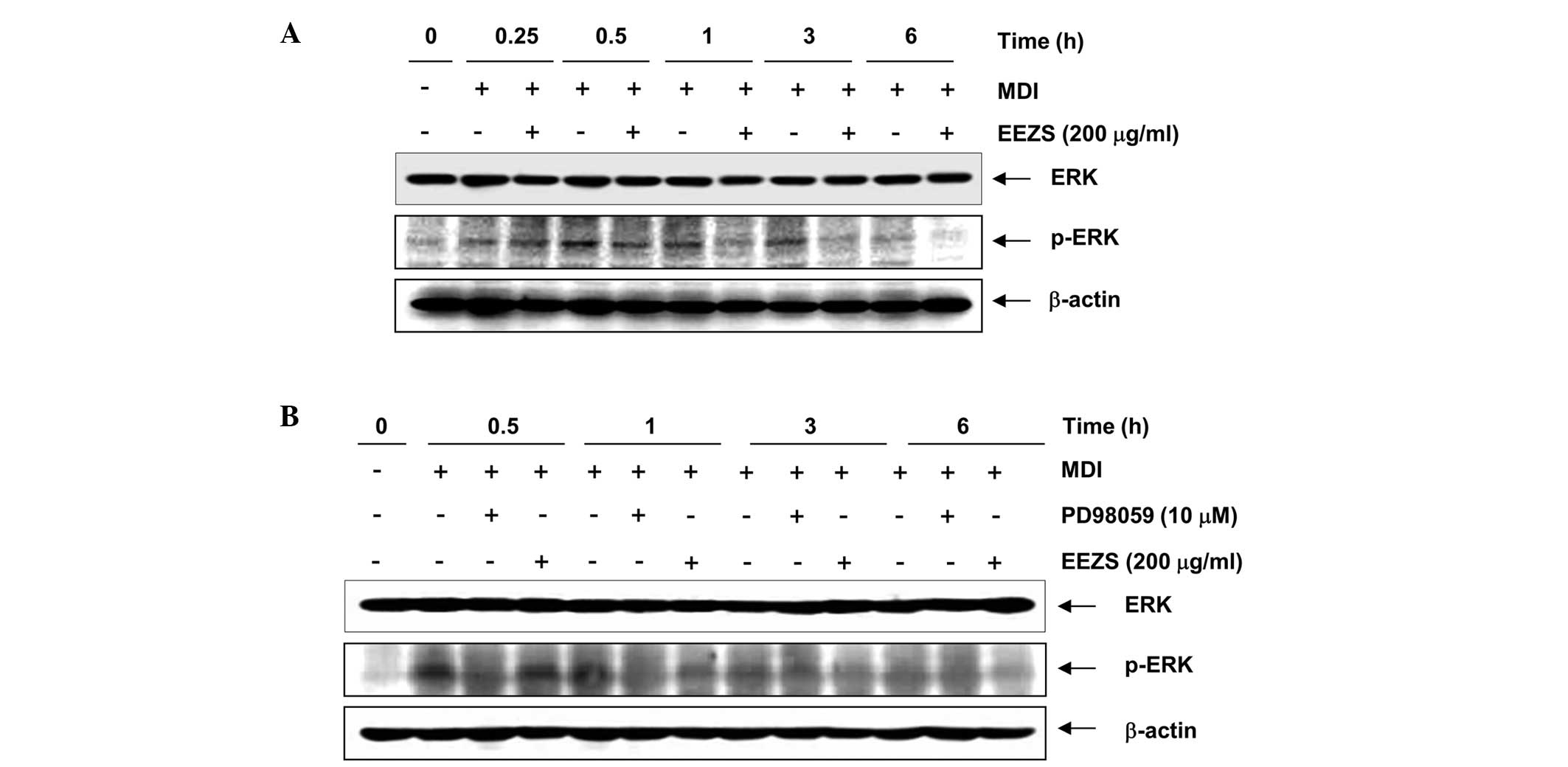
The control of adipogenesis requires two
well-established signaling mechanisms, the ERK and PI3K/Akt
pathways, which are important upstream of adipocyte differentiation
(17). The 3T3-L1 cells were
pretreated with either ERK- or PI3K-specific inhibitors for 1 h
prior to incubation with MDI in the presence or absence of 200
μg/ml EEZS, and the levels of phosphorylated-ERK, PI3K and
Akt were determined at various time-points by western blotting.
Consistent with previous data (16,18),
the expression of phosphorylated-ERK was significantly increased
during the early stages of adipogenesis, and the activation
continued for 3 h following the induction of adipocyte
differentiation by DMI (Fig. 5A).
However, treatment with EEZS effectively suppressed the MDI-induced
phosphorylation of ERK. The specific ERK inhibitor, PD98059,
induced significant inhibition of the phosphorylation of ERK and
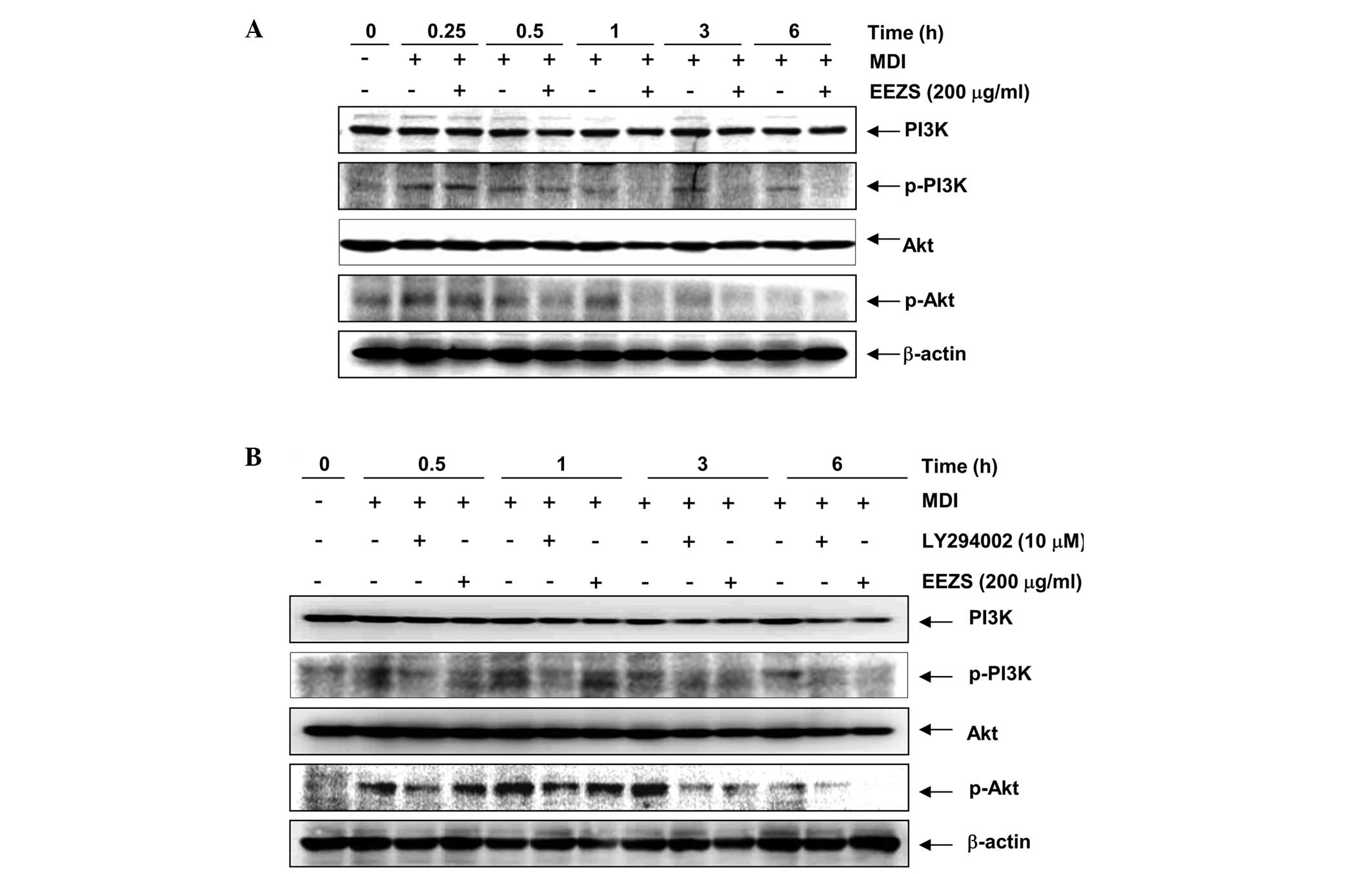
used as a positive control for treatment with EEZS (Fig. 5B). As shown in Fig. 6, treatment with DMI rapidly induced
the phosphorylation of PI3K and Akt, and this continued for 6 h
subsequent to treatment. However, the levels of phosphorylated-PI3K
and Akt were markedly attenuated following treatment with EEZS,
similar to the results, which were obtained using LY294002, a
PI3K-specific inhibitor. These results suggested that the
inhibition of adipocyte differentiation by EEZS was associated with
inactivation of the ERK and PI3K/Akt signaling pathways.
Discussion
Increased consumption of high calorie foods
containing sugars and fats, and lack of physical activity lead to
obesity, which is a major risk factor for serious chronic diseases,
including diabetes, cardiovascular disease and hypertension
(1–11). Adipocyte differentiation is an
adaptive response to excess energy intake, inducing obesity and
metabolic diseases (17,31). Accordingly, adipocytes are a
therapeutic target in treatmenting obesity and investigations are
being performed to prevent obesity through regulating adipogenesis.
During adipogenesis, undifferentiated fibroblast-like
pre-adipocytes become spherical fat cells and accumulate as lipid
droplets (32,33). The present study, as part of an
ongoing investigation to identify anti-obesity agents from
traditional medicine sources, investigated whether EEZS possessed
anti-obesity activity using a 3T3-L1 pre-adipocyte model.
To investigate suppressive effects of EEZS on
adipocyte differentiation, the effects of EEZS on the accumulation
of intracellular lipids were determined using Oil Red-O staining
and triglyceride content analysis in 3T3-L1 pre-adipocytes. The
data demonstrated that EEZS significantly inhibited adipocyte
differentiation and lipid accumulation in a concentration-dependent
manner (Figs. 2 and 3). The concentrations of EEZS used to
inhibit adipocyte differentiation exhibited no affect on cell
viability, as assessed using an MTT assay (Fig. 1), indicating that the promising
anti-obesity potential of EEZS in 3T3-L1 cells was not simply due
to a cytotoxic effect.
Adipocyte differentiation is highly regulated by
several transcription factors. The PPAR and C/EBP families are
important in adipocyte differentiation. More specifically, the
adipocyte marker transcription factors, PPARγ and C/EBPα, have been
reported to be important in differentiation and lipid storage, and
in the coordinated expression of genes involved in creating or
maintaining the phenotype of adipocytes (14,15).
Additionally, C/EBPβ, which is considered to mediate the expression
levels of PPARγ and C/EBPα during adipogenesis, is the first
transcription factor induced following exposure of pre-adipocytes
to differentiation medium and, therefore, may be involved in
directing the differentiation process (32,33).
The present study revealed that EEZS significantly downregulated
the protein expression levels of PPARγ, C/EBPα and C/EBPβ, induced
by differentiation medium, in the 3T3-L1 cells (Fig. 4). Therefore, EEZS appeared to
inhibit adipogenesis, which may be attributable to its ability to
downregulate the expression levels of adipocyte marker
proteins.
It has also been reported that activation of the ERK
and PI3K/Akt pathways is necessary for adipogenesis (18,19).
Activation of these pathways during adipogenesis promotes
differentiation by activating factors that regulate the expression
levels of PPARγ and C/EBPs. Several previous studies have
demonstrated that the activation of ER K and Akt induces
differentiation by activating factors, which regulate the
expression levels PPARγ and C/EBPs during the early-stages of
adipogenesis (14,15). In addition, there is increasing
evidence that the inhibition of the ERK and Akt pathways in
adipocyte differentiation inhibits adipogenesis (16,34,35).
Therefore, to further assess the effect of EEZS on the upstream
signaling pathways of PPARγ and C/EBPs, the present study
investigated the effects of EEZS on the expression levels of
phosphorylated-ERK and Akt. As the phosphorylation of Akt is
regulated by PI3K, as an upstream kinase of Akt (36,37),
the effects of EEZS on the levels of PI3K were also determined. The
data demonstrated that the phosphorylation of ERK, PI3K and Akt
were significantly activated during the early stages of
adipogenesis, and the activation continued for 3 or 6 h following
the induction of adipocyte differentiation by MDI (Figs. 5 and 6). However, EEZS significantly inhibited
the phosphorylation of ERK, in a time-dependent manner. The protein
expression levels of total PI3K and Akt, as with ERK, remained
unchanged throughout the experiment. EEZS also attenuated the
protein expression levels of phosphorylated-PI3K and Akt (Fig. 6). Although the precise molecular
signaling mechanism underlying EEZS remains to be elucidated, these
results suggested that EEZS inhibited the activation of the ERK and
PI3K/Akt pathways at an early stage and inhibited the expression of
adipogenic transcription factors by modulating the ERK-and
PI3K/Akt-mediated signaling pathways during adipocyte
differentiation.
In conclusion, the present study demonstrated that
EEZS suppressed adipogenesis in the 3T3-L1 cells by downregulating
the expression levels of PPARγ, C/EBPα and C/EBPβ, through
inactivation of the ERK and PI3K/Akt signaling pathways in the
early stages of adipogenesis. These findings suggested the possible
use of EEZS as a therapeutic substance or as a lead in the
development of therapeutic substances for the prevention and
management of obesity.
Acknowledgments
This study was supported by the Blue-Bio Industry
Regional Innovation Center (no. RIC08-06-07) at Dongeui University,
as an RIC program under the Ministry of Trade, Industry &
Energy and Busan city.
References
|
1
|
Dorresteijn JA, Visseren FL and Spiering
W: Mechanisms linking obesity to hypertension. Obes Rev. 13:17–26.
2012. View Article : Google Scholar
|
|
2
|
Franssen R, Monajemi H, Stroes ES and
Kastelein JJ: Obesity and dyslipidemia. Med Clin North Am.
95:893–902. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rocha VZ and Libby P: Obesity,
inflammation and atherosclerosis. Nat Rev Cardiol. 6:399–409. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCarthy MI: Genomics, type 2 diabetes and
obesity. N Engl J Med. 363:2339–2350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Speliotes EK: Genetics of common obesity
and nonalcoholic fatty liver disease. Gastroenterology.
136:1492–1495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pischon N, Heng N, Bernimoulin JP, Kleber
BM, Willich SN and Pischon T: Obesity, infammation and periodontal
disease. J Dent Res. 86:400–409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lloyd CM and Saglani S: Eosinophils in the
spotlight: Finding the link between obesity and asthma. Nat Med.
19:976–977. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tzotzas T, Evangelou P and Kiortsis DN:
Obesity, weight loss and conditional cardiovascular risk factors.
Obes Rev. 12:e282–e289. 2011. View Article : Google Scholar
|
|
9
|
Thompson D and Wolf AM: The medical-care
cost burden of obesity. Obes Rev. 2:189–197. 2001. View Article : Google Scholar
|
|
10
|
Dalla Vecchia CF, Susin C, Rösing CK,
Oppermann RV and Albandar JM: Overweight and obesity as risk
indicators for periodontitis in adults. J Periodontol.
76:1721–1728. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zimmet P, Alberti KG and Shaw J: Global
and societal implications of the diabetes epidemic. Nature.
414:782–787. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bray GA and Tartaglia LA: Medicinal
strategies in the treatment of obesity. Nature. 404:672–677.
2000.PubMed/NCBI
|
|
13
|
Tam CS, Lecoultre V and Ravussin E: Novel
strategy for the use of leptin for obesity therapy. Expert Opin
Biol Ther. 11:1677–1685. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosen ED: The transcriptional basis of
adipocyte development. Prostaglandins Leukot Essent Fatty Acids.
73:31–34. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soukas A, Socci ND, Saatkamp BD, Novelli S
and Friedman JM: Distinct transcriptional profiles of adipogenesis
in vivo and in vitro. J Biol Chem. 276:34167–34174. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prusty D, Park BH, Davis KE and Farmer SR:
Activation of MEK/ERK signaling promotes adipogenesis by enhancing
peroxisome proliferator-activated receptor gamma (PPARgamma) and
C/EBPalpha gene expression during the differentiation of 3T3-L1
preadipocytes. J Biol Chem. 277:46226–46232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosen ED and MacDougald OA: Adipocyte
differentiation from the inside out. Nat Rev Mol Cell Biol.
7:885–896. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith PJ, Wise LS, Berkowitz R, Wan C and
Rubin CS: Insulin–like growth factor–I is an essential regulator of
the differentiation of 3T3-L1 adipocytes. J Biol Chem.
263:9402–9408. 1988.PubMed/NCBI
|
|
19
|
Bost F, Aouadi M, Caron L and Binétruy B:
The role of MAPKs in adipocyte differentiation and obesity.
Biochimie. 87:51–56. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Magun R, Burgering BM, Coffer PJ,
Pardasani D, Lin Y, Chabot J and Sorisky A: Expression of a
constitutively activated form of protein kinase B (c-Akt) in 3T3-L1
preadipose cells causes spontaneous differentiation. Endocrinology.
137:3590–3593. 1996.PubMed/NCBI
|
|
21
|
Peng XD, Xu PZ, Chen ML, Hahn-Windgassen
A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman
KG and Hay N: Dwarfsm, impaired skin development, skeletal muscle
atrophy, delayed bone development and impeded adipogenesis in mice
lacking Akt1 and Akt2. Genes Dev. 17:1352–1365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jo YS, Huong DT, Bae K, Lee MK and Kim YH:
Monoamine oxidase inhibitory coumarin from Zanthoxylum
schinifolium. Planta Med. 68:84–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paik SY, Koh KH, Beak SM, Paek SH and Kim
JA: The essential oils from Zanthoxylum schinifolium pericarp
induce apoptosis of HepG2 human hepatoma cells through increased
production of reactive oxygen species. Biol Pharm Bull. 28:802–807.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsai IL, Lin WY, Teng CM, Ishikawa T,
Doong SL, Huang MW, Chen YC and Chen IS: Coumarins and antiplatelet
constituents from the root bark of Zanthoxylum schinifolium. Planta
Med. 66:618–623. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen IS, Lin YC, Tsai IL, Teng CM, Ko FN,
Ishikawa T and Ishii H: Coumarins and anti-platelet aggregation
constituents from Zanthoxylum schinifolium. Phytochemistry.
39:1091–1097. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao LH, Lee YJ, Kang DG, Kim JS and Lee
HS: Effect of Zanthoxylum schinifolium on TNF-alpha-induced
vascular inflammation in human umbilical vein endothelial cells.
Vascul Pharmacol. 50:200–207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li W, Sun YN, Yan XT, Yang SY, Kim EJ,
Kang HK and Kim YH: Coumarins and lignans from Zanthoxylum
schinifolium and their anticancer activities. J Agric Food Chem.
61:10730–10740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jun DY, Kim JS, Park HS, Han CR, Fang Z,
Woo MH, Rhee IK and Kim YH: Apoptogenic activity of auraptene of
Zanthoxylum schinifolium toward human acute leukemia Jurkat T cells
is associated with ER stress-mediated caspase-8 activation that
stimulates mitochondria-dependent or -independent caspase cascade.
Carcinogenesis. 28:1303–1313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim MY, Kim DH and Do MS:
B-cell-activating factor is a regulator of adipokines and a
possible mediator between adipocytes and macrophages. Exp Mol Med.
45:e42013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rhyu J, Kim MS, You MK, Bang MA and Kim
HA: Pear pomace water extract inhibits adipogenesis and induces
apoptosis in 3T3-L1 adipocytes. Nutr Res Pract. 8:33–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000.PubMed/NCBI
|
|
32
|
Ntambi JM and Young-Cheul K: Adipocyte
differentiation and gene expression. J Nutr. 130:3122S–3126S.
2000.
|
|
33
|
Tong Q and Hotamisligil GS: Molecular
mechanisms of adipocyte differentiation. Rev Endocr Metab Disord.
2:349–355. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cornelius P, MacDougald OA and Lane MD:
Regulation of adipocyte development. Annu Rev Nutr. 14:99–129.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kohn AD, Summers SA, Birnbaum MJ and Roth
RA: Expression of a constitutively active Akt Ser/Thr kinase in
3T3-L1 adipocytes stimulates glucose uptake and glucose transporter
4 translocation. J Biol Chem. 271:31372–31378. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Uto-Kondo H, Ohmori R, Kiyose C, Kishimoto
Y, Saito H, Igarashi O and Kondo K: Tocotrienol suppresses
adipocyte differentiation and Akt phosphorylation in 3T3-L1
preadipocytes. J Nutr. 139:51–57. 2009. View Article : Google Scholar
|
|
37
|
Zhang HH, Huang J, Düvel K, Boback B, Wu
S, Squillace RM, Wu CL and Manning BD: Insulin stimulates
adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One.
4:e61892009. View Article : Google Scholar : PubMed/NCBI
|