Introduction
Acute myeloid leukemia (AML) is an aggressive
hematopoietic malignancy and primarily treated by chemotherapy.
Although various chemotherapeutic agents have been developed for
AML treatment, they affect normal cells, which causes unpleasant
side effects, including hepatotoxicity, cardiotoxicity,
hematotoxicity and infection, and which restricts their dosage and
clinical efficacy. Moreover, resistance to conventional
chemotherapeutic agents is a common reason for unsuccessful
chemotherapy. Recent studies have suggested natural products as
potent chemotherapeutic drugs for AML to improve the therapeutic
efficacy and lower the side effects (1–3). For
instance, β-elemene, an active component of the medicinal herb
Curcuma wenyujin, induced apoptosis in human leukemia HL-60,
NB4, K562 and HP100-1 cells through downregulation of cellular
FLICE-like inhibitory protein and generation of reactive oxygen
species (4).
In recent years, a series of pentacyclic
triterpenoid compounds, including ursolic acid (5), oleanolic acid (6) and betulinic acid (7), were reported to exhibit anti-tumor
activity, and therefore, triterpene acids are thought to have great
potential as novel drugs for the treatment of malignant tumors.
Asiatic acid (AA) is a pentacyclic triterpenoid derived from the
medicinal plant Centella asiatica (family, Apiaceae).
A wide range of beneficial effects of AA have been reported in
hepatofibrosis (8), diabetes
(9), ultraviolet (UV)
radiation-induced photo aging and cerebral ischemia (10), and wound healing (11). Furthermore, AA has been reported to
be cytotoxic to several solid tumor cell lines, including malignant
glioma (12), human hepatoma
(13), colon cancer (14), melanoma (15) and gastric cancer (16). Moreover, AA has attracted attention
for its multiple protective effects against drug-induced
hepatotoxicity, neurotoxicity and ulcers. Due to its potent
anti-inflammatory, anti-cancer and chemo-protective activities, AA
has been suggested to be a promising chemopreventive and
therapeutic agent. However, to the best of our knowledge, the
effect of AA on hematological malignant cells has not been
investigated to date. In the present study, the effect of AA on
HL-60 cells was investigated using MTT cell proliferation assays
and assessment of the apoptotic rate using flow cytometric analysis
and confocal microscopy. Furthermore, the mechanism of the
anti-cancer effect of AA was investigated by assessing changes in
levels of B-cell lymphoma 2 (Bcl-2) family proteins and proteins
involved in the mitogen-activated protein kinase (MAPK) signaling
pathway using western blot analysis. The present study provided a
molecular basis for the clinical application of AA in patients with
acute leukemia.
Materials and methods
Materials
AA (C30H48O5;
molecular weight, 488.7 g/mol), dimethyl sulfoxide (DMSO), Hoechst
33258 and MTT were purchased from Sigma-Aldrich (St Louis, MO,
USA). The Annexin V-fluorescein isothiocyanate (FITC)/propidium
iodide (PI) reagent kit was purchased from Nanjing Key-Gen Biotech
Co, Ltd (Nanjing, China). The bicinchoninic acid (BCA) Protein
Assay kit, chemiluminescence reagent kit and polyvinylidene
difluoride (PVDF) membranes were purchased from Pierce
Biotechnology, Inc, USA. The rabbit polyclonal anti-extracellular
signal-regulated kinase (ERK)1/2 (cat. no. 9102), rabbit monoclonal
anti-phosphorylated (p-)ERK1/2 (cat. no. 4377), rabbit polyclonal
anti-c-Jun N-terminal kinase (JNK) (cat. no. 9252), rabbit
polyclonal anti-P-JNK (cat. no. 9251), rabbit polyclonal anti-p38
(cat. no. 9212), rabbit monoclonal anti-P-p38 (cat. no. 9215),
rabbit polyclonal anti-Bcl-2 (cat. no. 2872), rabbit polyclonal
anti-survivin (cat. no. 2803), rabbit polyclonal anti-myeloid cell
leukemia 1 (Mcl-1) (cat. no. 4572), and mouse monoclonal
anti-β-actin (cat. no. 3700) antibodies were provided by Cell
Signaling Technology, Inc, (Danvers, MA, USA). Horseradish
peroxidase-conjugated goat anti-rabbit (cat. no. 111-035-003) and
donkey anti-mouse (cat. no. 715-035-150) immunoglobulin G secondary
antibodies were purchased from Jackson Immuno Research
Laboratories, Inc (West Grove, PA, USA). Z-DEVD-FMK was purchased
from Medical and Biological Laboratories Co, Ltd, (Nagoya, Japan).
Fetal bovine serum (FBS) was obtained from Hangzhou Sijiqing
Biological Engineering Materials Co, Ltd (Hangzhou, China).
Cell culture
The leukemia cell line HL-60 was obtained from
American Type Culture Collection (Rockville, MD, USA). Human
peripheral blood mononuclear cells (PBMCs) from healthy volunteers
(five males and five females between 20 and 50 years old) were
obtained by Ficoll-Hypaque (Lonza Ltd., Wakersville, MD, USA)
density gradient centrifugation at 1,000 × g, according to the
manufacturer’s instructions. All cells were cultured in RPMI 1640
medium (Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin solution (Invitrogen Life Technologies) in
a 5% CO2 humidified atmosphere at 37°C. Written informed
consent for blood utilization was obtained from all volunteers. The
procedures were approved by the ethics committee of Tongji Medical
College, Huazhong University of Science and Technology (Hubei,
China).
Cell proliferation assay
The effects of AA on the proliferation of HL-60
cells and PBMCs were detected using an MTT assay. Briefly, the
cells were maintained in RPMI-1640 medium (Thermo Fisher
Scientific, Waltham, MA, USA) until reaching the mid-logarithmic
growth phase and then seeded in 96-well plates with or without AA
at various concentrations (10, 20, 30, 40, 50 and 60 μmol/l)
at a density of 2×105 cells per well. Following
incubation for a set period of time, 20 μl MTT (5 mg/ml) was
added and the cells were incubated for another 3 h at 37°C. The
supernatant was discarded of and 150 μl DMSO was added. The
plate was gently agitated until the blue formazan crystals were
fully dissolved. The absorbance (A) was measured at 490 nm using a
microplate reader (Infinite F50; Tecan Spectra, Switzerland), and
the cell proliferation inhibition ratio (%) was calculated using
the following formula: [1-(Aexperimental
sample/Acontrol sample)]×100.
Annexin V-FITC/PI double-labeled flow
cytometry
The apoptotic rate was measured in HL-60 cells
treated with AA at different concentrations alone or in combination
with Z-DEVD-FMK. Four-color flow cytometry (FCM) was applied to
detect the expression of Annexin V-FITC and the exclusion of PI.
The cells positive for Annexin V-FITC and negative for PI
represented the early apoptotic cells, whereas the cells positive
for both markers represented the late apoptotic cells. The total
apoptotic rate was the sum of the number of early and late
apoptotic cells. Briefly, HL-60 cells were collected after the
treatment using eppendorf tubes, washed twice with
phosphate-buffered saline (PBS; Sigma-Aldrich) and resuspended in
500 μl binding buffer. A total of 5 μl Annexin V-FITC
was added and the samples were maintained at room temperature for
10 min. Next, 5 μl PI was added and the cells were incubated
for another 10 min in the dark. The fluorescence intensity was
detected using a flow cytometer (Becton-Dickinson, Franklin Lakes,
NJ, USA).
Hoechst 33258 staining
HL-60 cells were treated with 40 μmol/l AA
for 24 h and the nuclear fragmentation was visualized using Hoechst
33258 staining. Briefly, 1×105 cells were seeded in
12-well plates and incubated with AA. After 24 h, the cells were
collected, washed twice with PBS and fixed in 4% paraformaldehyde
(Sigma-Aldrich, St. Louis, MO, USA) for 10 min at room temperature
before being deposited on polylysine-coated slides. After 30 min,
the adherent cells were permeabilized by incubation with 0.1%
Triton X-100 (Sigma-Aldrich) for 5 min at 4°C. The cells were then
incubated with Hoechst 33258 for 30 min at room temperature, rinsed
with PBS and mounted on coverslips using glycerol (Sigma-Aldrich).
Finally, cells were visualized using an Olympus BH-2 fluorescence
microscope (Olympus, Tokyo, Japan).
Western blot analysis
All of the HL-60 cells treated with AA at various
concentrations for 24 h were collected. The cells were washed twice
with PBS and completely lysed in a lysis buffer containing protease
inhibitors (Pierce, Thermo Scientific, Waltham, MA, USA). The
extracts were centrifuged at 12,000 × g for 15 min at 4°C, and the
clear supernatants containing the total protein were isolated. The
protein concentration was quantified using the BCA assay (Pierce,
Thermo Scientific). Equal amounts of protein (40 μg per
lane) were separated by 10–12% SDS-polyacrylamide gel
electrophoresis, and the proteins were then transferred to PVDF
membranes. The membranes were blocked in 5% non-fat milk
(Sigma-Aldrich) for 2 h at room temperature and then probed with
the specific primary antibodies (1:500) at 4°C overnight, and
corresponding secondary antibody (1:1,000) at room temperature for
1 h. The specific protein bands were visualized using an Enhanced
Chemiluminescence Detection system (GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA).
Statistical analysis
Each experiment was repeated three times. Values are
expressed as the mean ± standard deviation and analyzed using SPSS
13.0 statistical software for Windows (SPSS, Inc., Chicago, IL,
USA). The comparisons between each group were analyzed using a
one-way analysis of variance and the Student-Newman-Keuls (SNK)
test. P<0.05 was considered to indicate a statistically
significant difference between values.
Results
AA inhibits the proliferation of HL-60
cells
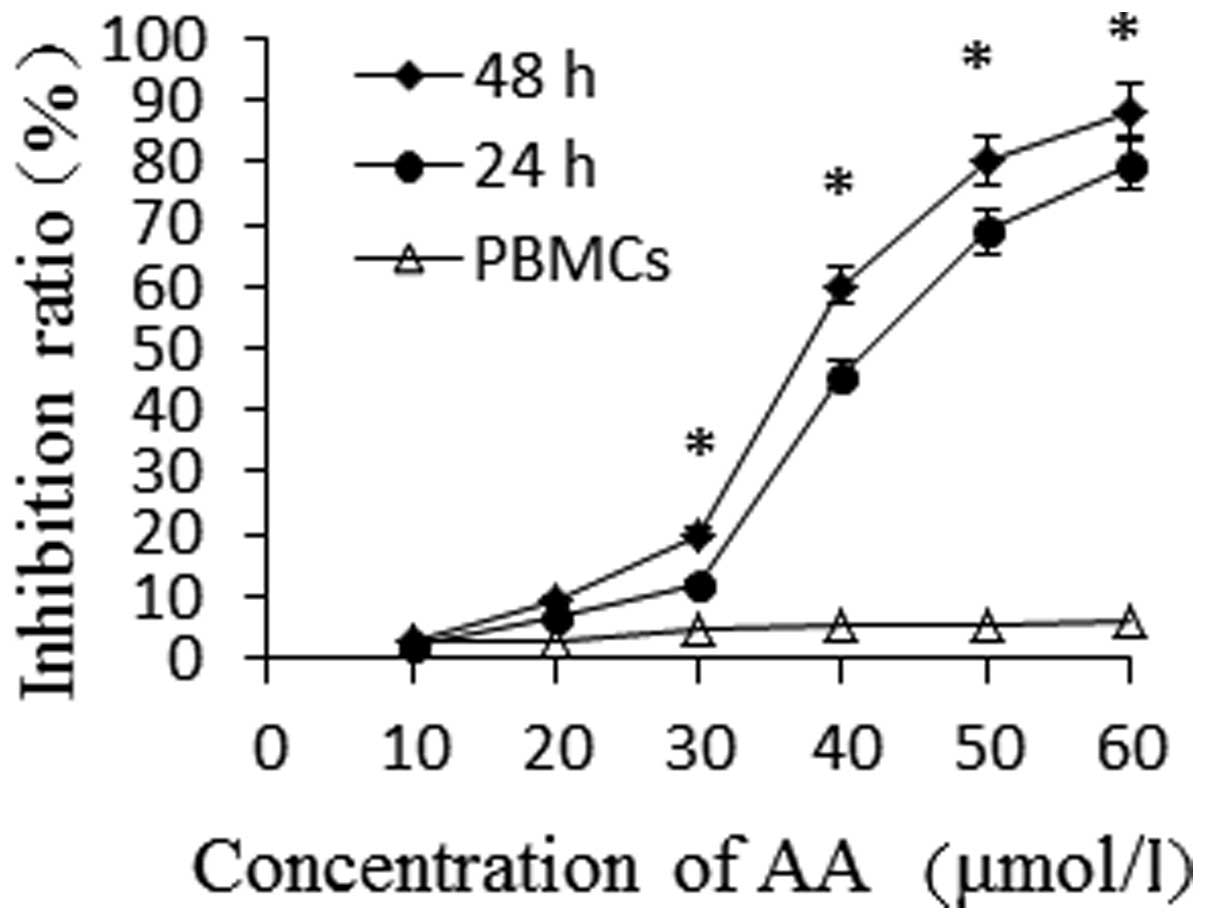
An MTT assay was used to detect the cytotoxicity of
different concentrations of AA (0, 10, 20, 30, 40, 50 and 60
μmol/l) on HL-60 cells for 24 and 48 h and PBMCs for 24 h.
As shown in Fig. 1, the growth of
HL-60 cells was found to be inhibited by AA in a time- and
dose-dependent manner. The proliferation inhibition rate of HL-60
cells significantly increased following incubation with AA at
various concentrations for 24 h (P<0.05), while AA showed low
toxicity to PBMCs. When the same concentration of AA was used, the
proliferative rate of HL-60 cells was shown to be inhibited in a
time-dependent manner (P<0.05). The IC50-values of AA
for HL-60 cells at 24 and 48 h were 46.67 μmol/l and 36.42
μmol/l, respectively.
AA induces apoptosis in HL-60 cells
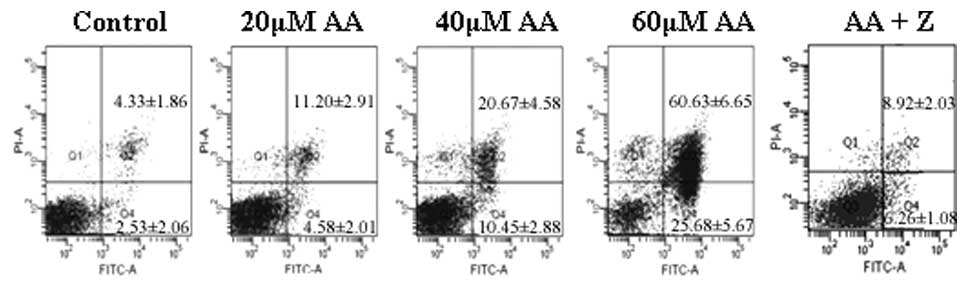
Annexin V-FITC/PI double staining and flow
cytometric analysis were utilized to assess the apoptotic rate of
HL-60 cells treated with AA, as shown in Fig. 2. The early apoptotic rates of HL-60
cells treated with 0, 20, 40 and 60 μmol/l AA for 24 h were
4.58±2.01, 10.45±2.88 and 25.68±5.67%, respectively, which were
significantly higher than that of the control group (2.53±2.06%).
The late apoptotic rates were 11.20±2.91, 20.67±4.58 and
60.63±6.65%, respectively, while that of the control group was
significantly lower (4.33±1.86%). To further identify whether
AA-induced apoptosis in HL-60 cells is caspase-dependent, caspase
inhibitor of Z-VAD-FMK was used in combination with 60
μmol/l AA. Z-DEVD-FMK was found to significantly decrease
the apoptotic rate of AA-treated HL-60 cells (15.72±3.82%,
P<0.05).
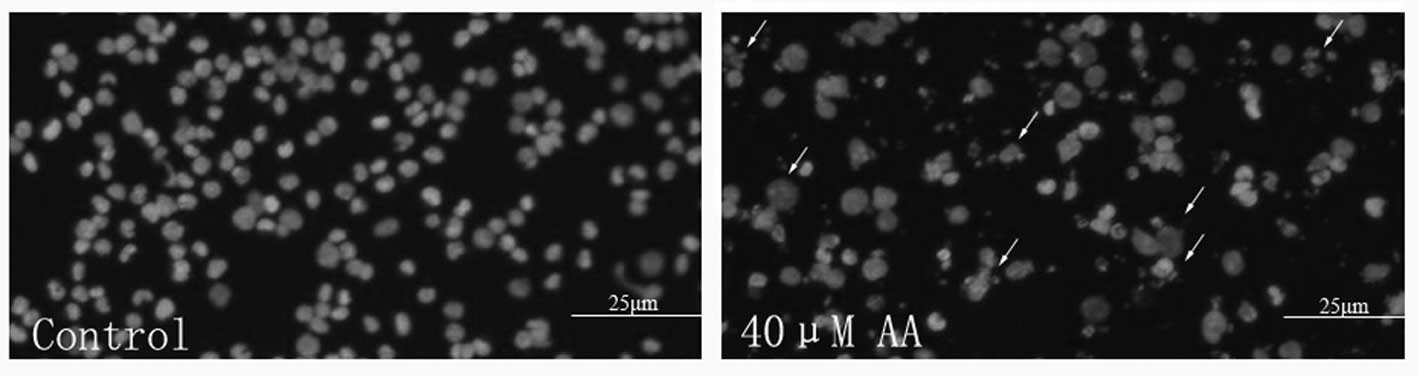
HL-60 cells treated with AA for 24 h were also
stained with Hoechst 33258 to visualize the nuclear changes. HL-60
cell nuclei were regular in shape in the control group, whereas
apparent apoptotic bodies were observed among HL-60 cells after
treatment with 40 μmol/l AA. The nuclei of these apoptotic
cells were condensed, and the nuclear envelopes appeared lytic
(Fig. 3).
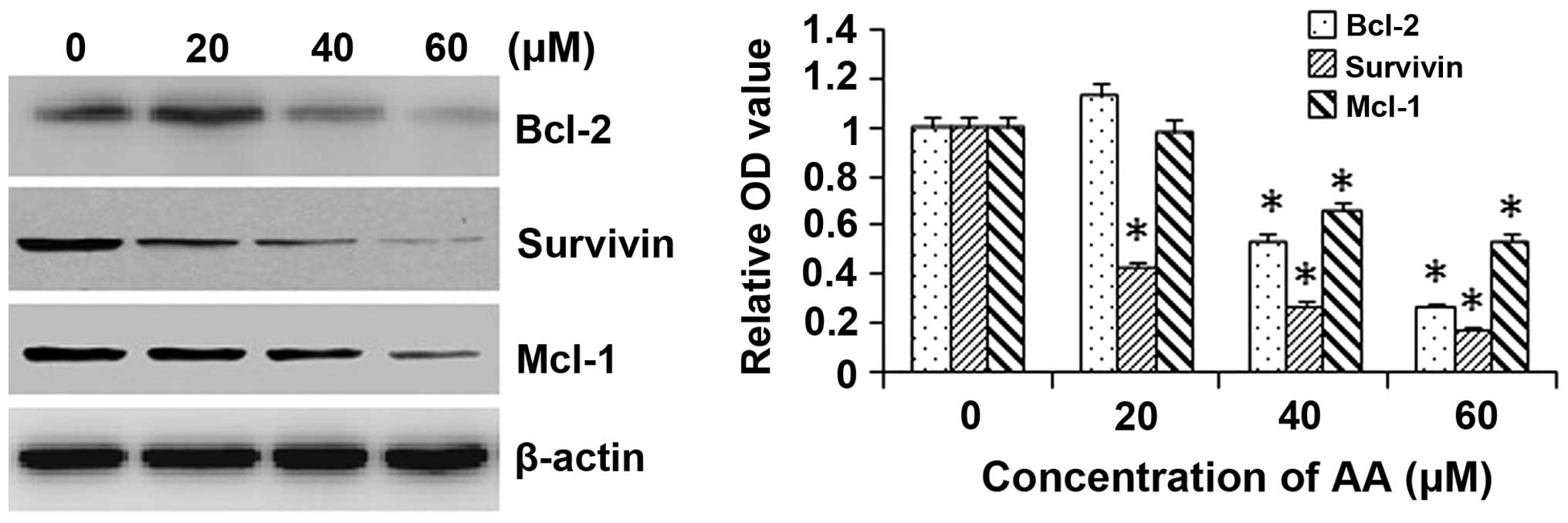
AA decreases the expression of
apoptosis-associated proteins in HL-60 cells
To investigate the mitochondrial apoptotic events
involved in AA-induced apoptosis, changes in the levels of
anti-apoptotic proteins Bcl-2, survivin and Mcl-1 were assessed by
western blot analysis. AA treatment attenuated the expression
levels of the anti-apoptotic proteins Bcl-2, survivin and Mcl-1 in
a concentration-dependent manner in HL-60 cells (Fig. 4). These results suggested that
Bcl-2, survivin and Mcl-1 have essential roles in AA-mediated
induction of apoptosis.
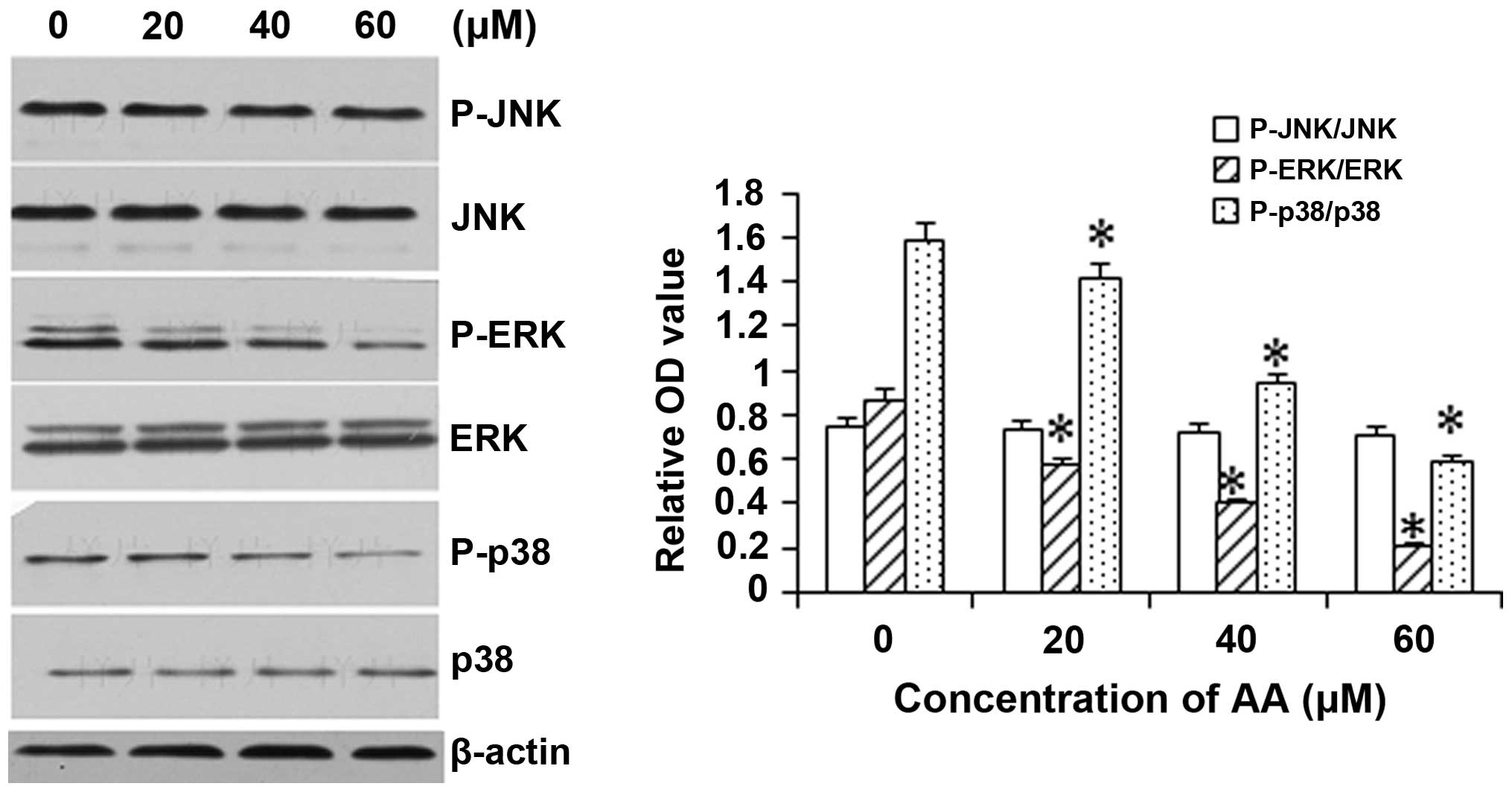
AA inhibits the activation of ERK and p38
but not JNK
To assess the involvement of the MAPK pathway in the
mechanism underlying the cytotoxicity of AA, the levels and
activation (phosphorylation) of JNK, p38 and ERK1/2 were assessed
in AA-treated HL-60 cells. As shown in Fig. 5, exposure of HL-60 cells to 20, 40
and 60 μM AA resulted in a significant inhibition of
phosphorylation of p38 and ERK1/2 in a dose-dependant manner, while
basal activation of p38 and ERK was not altered. However, AA
treatment did not JNK levels (including the phosphorylated and
unphosphorylated forms).
Discussion
In previous studies, numerous natural products,
including pentacyclic triterpenoids, have been discovered to be
potent anti-leukemic agents for AML therapy (5–7). The
present study was the first, to the best of our knowledge, to
report the cytotoxic activity of AA on the human acute leukemia
cell line HL-60. HL-60 cells treated with AA underwent apoptosis in
a dose- and time-dependent manner. It was demonstrated that AA
attenuated the expression levels of Bcl-2, survivin and Mcl-1, and
also the phosphorylation of ERK and p38 in a time-dependent
manner.
The Bcl-2 family of proteins consists of
pro-apoptotic effector proteins, including Bcl-2 associated X
protein and Bcl-2 homologous antagonist killer, as well as
anti-apoptotic proteins, including Bcl-2, Bcl extra large (Bcl-xL)
and Mcl-1. Bcl-2 proteins exert anti-apoptotic effects through
regulating the permeabilization of the mitochondrial outer
membrane, a key step in apoptosis. Their complex network of
interactions in the cytosol and mitochondria determines the fate of
the cells (17). Cancer cells
often violate key cellular checkpoints that would normally drive
the cells to die by programmed cell death. As a result, they
require to overcome the apoptotic stress either by reducing the
expression of pro-apoptotic factors or, more frequently, by
upregulating anti-apoptotic molecules, including Bcl-2, BcL-XL and
MCL-1 (18,19). Bcl-2 and MCL-1 are critical for the
development and maintenance of hematologic malignancies (20,21).
Constitutively high levels of Bcl-2 and Mcl-1 have been associated
with a more aggressive malignant phenotype and/or drug resistance
to various classes of chemotherapeutic agents in cancer (22,23).
This anti-apoptotic subfamily of proteins is currently considered a
major target in the development of novel methods to improve
treatment outcomes for leukemia patients. In the previous decade,
several drugs directed at inhibiting Bcl-2 have been tested in the
clinic, with several of them showing promising effects,
particularly in lymphoid malignancies (24). Retroviruses encoding BimSL62A/F69A,
which selectively bind Mcl-1, were able to selectively diminish
survival of cells in two samples of clinical AML (21). In the present study, AA was
demonstrated to decrease the anti-apoptotic activity in HL-60 cells
through downregulating Bcl-2 and Mcl-1 expression, in consistency
with the previously reported effect of AA on Bcl-2 expression in
colon cancer, melanoma and breast cancer cells (15,25,26).
Survivin (BIRC5) is a member of the family of
inhibitors of apoptosis proteins (IAPs) and has been implicated in
the control of cell survival as well as regulation of mitosis in
cancer, including solid tumors and hematological malignancies
(27–29). Upon activation of pro-apoptotic
cell signaling, survivin is released from the mitochondria and
inhibits caspases-3 and -9. This function requires association with
hepatitis B X-interacting protein and/or with X-linked IAP and is
inhibited by SMAC-DIABLO (27,28).
The regulation of survivin expression and function is complex and
occurs at various levels, including transcription, differential
splicing, protein degradation and intracellular sequestration via
different ligands (28).
Overexpression of suvivin is correlated with advanced disease,
accelerated time of recurrence, reduced survival and resistance to
therapy (30). AML patients with
overexpression of survivin showed an unfavorable response to
chemotherapy in 81.2% of the patients and shorter median survival
time (30 days) compared to that of patients with normal expression
(29). Thus, targeting survivin
with small-molecule inhibitors by their anti-sense approaches or
natural IAP antagonist mimetics may be an attractive strategy of
anti-leukemia treatment. Such agents can either directly induce
apoptosis of tumor cells or sensitize them to other cytotoxic
agents, hence overcoming drug resistance (31,32).
Thereofore, the present study evaluated the effect of AA on
survivin in HL-60 cells. The results showed that, in addition to
bcl-2 protein, survivin is likely to be involved in the mechanism
of AA-induced apoptosis.
MAPKs, a family of serine/threonine kinases, are
mediators of intracellular signals in response to various stimuli.
JNK, p38 and ERK1/2 are the three main members of three different
MAPK pathways that can be activated by growth factors, DNA damage,
cytokines, oxidant stresses, UV light, anti-cancer drugs and
osmotic shock (33,34). These signaling pathways regulate a
variety of cellular activities, including proliferation,
differentiation, survival and death. Deviation from the strict
control of MAPK signaling pathways has been implicated in the
development of numerous human diseases, including Alzheimer’s
disease (AD), Parkinson’s disease (PD), amyotrophic lateral
sclerosis (ALS) and various types of cancer (35). The present study showed that AA
significantly inhibited phosphorylation of p38 and ERK without
affecting the JNK pathway. According to previous studies, the role
of these three MAPK pathways in cancer has remained controversial,
as MAPK pathways have been shown to mediate pro-apoptotic as well
as anti-apoptotic signals in different systems, apparently
depending on the stimulus and cell type involved. For instance, the
apoptosis of K562 cells induced by icaritin was accompanied by the
inhibition of activation of p-ERK and p-P38, while activation of
p-ERK and p-P38 were shown to be critical mediators in AA-induced
cell growth inhibition of human breast cancer cell lines (26,36).
JNK and p38 are activated by cellular stress and have been
associated with apoptosis (37).
However, certain studies have indicated that the JNK is required
for interleukin-3-mediated cell survival and that p38 is associated
with the development of chemoresistance by activating nuclear
factor kappa B (38,39).
In conclusion, the results of the present study
suggested that AA induced apoptosis through inhibiting Bcl-2,
survivin and MAPK signaling pathways in HL-60 human acute leukemia
cells. These results strongly suggested that AA may be a valuable
agent for molecular targeted therapy of human acute leukemia.
Acknowledgments
The authors would like to acknowledge Dr Fei Zhao
(Institute of Hematology, Union Hospital, Tongji Medical College,
Huazhong University of Science and Technology, Wuhan, China) for
analyzing the data.
References
|
1
|
Ben-Ayre E, Attias S, Tadmor T and Schiff
E: Herbs in hemato-oncological care: An evidence-based review of
data on efficacy, safety, and drug interactions. Leuk Lymphoma.
51:1414–1423. 2010. View Article : Google Scholar
|
|
2
|
Wang N, Li DY, Niu HY, et al:
2-hydroxy-3-methylanthraquinone from Hedyotis diffusa Willd induces
apoptosis in human leukemic U937 cells through modulation of MAPK
pathways. Arch Pharm Res. 36:752–758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Q, Huai L, Zhang C, et al: Icaritin
induces AML cell apoptosis via the MAPK/ERK and PI3K/AKT signal
pathways. Int J Hematol. 97:617–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu Z, Wang R, Xu L, Xie S, Dong J and Jing
Y: β-Elemene piperazine derivatives induce apoptosis in human
leukemia cells through down-regulation of c-FLIP and generation of
ROS. PLoS One. 6:e158432011. View Article : Google Scholar
|
|
5
|
Shin SW and Park JW: Ursolic acid
sensitizes prostate cancer cells to TRAIL-mediated apoptosis.
Biochim Biophys Acta. 1833:723–730. 2013. View Article : Google Scholar
|
|
6
|
Liu L, Fu J, Li T, et al: NG, a novel
PABA/NO-based oleanolic acid derivative, induces human hepatoma
cell apoptosis via a ROS/MAPK-dependent mitochondrial pathway. Eur
J Pharmacol. 691:61–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang P, Li Q, Li K, et al: Betulinic acid
exerts immunoregulation and anti-tumor effect on cervical carcinoma
(U14) tumor-bearing mice. Pharmazie. 67:733–739. 2012.PubMed/NCBI
|
|
8
|
Tang LX, He RH, Yang G, et al: Asiatic
acid inhibits liver fibrosis by blocking TGF-beta/Smad signaling in
vivo and in vitro. PLoS One. 7:e313502012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J, He T, Lu Q, Shang J, Sun H and
Zhang L: Asiatic acid preserves beta cell mass and mitigates
hyperglycemia in streptozocin-induced diabetic rats. Diabetes Metab
Res Rev. 26:448–454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee KY, Bae ON, Serfozo K, et al: Asiatic
acid attenuates infarct volume, mitochondrial dysfunction and
matrix metalloproteinase-9 induction after focal cerebral ischemia.
Stroke. 43:1632–1638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Somboonwong J, Kankaisre M, Tantisira B
and Tantisira MH: Wound healing activities of different extracts of
Centella asiatica in incision and burn wound models: an
experimental animal study. BMC Complement Altern Med. 12:1032012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kavitha CV, Agarwal C, Agarwal R and Deep
G: Asiatic acid inhibits pro-angiogenic effects of VEGF and human
gliomas in endothelial cell culture models. PLoS One. 6:e227452011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee YS, Jin DQ, Kwon EJ, et al: Asiatic
acid, a triterpene, induces apoptosis through intracellular Ca2+
release and enhanced expression of p53 in HepG2 human hepatoma
cells. Cancer Lett. 186:83–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang XL, Yang XY, Jung HJ, et al: Asiatic
acid induces colon cancer cell growth inhibition and apoptosis
through mitochondrial death cascade. Biol Pharm Bull. 32:1399–1405.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park BC, Bosire KO, Lee ES, Lee YS and Kim
JA: Asiatic acid induces apoptosis in SK-MEL-2 human melanoma
cells. Cancer Lett. 218:81–90. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida M, Fuchigami M, Nagao T, et al:
Antiproliferative constituents from Umbelliferae plants VII Active
triterpenes and rosmarinic acid from Centella asiatica. Biol Pharm
Bull. 28:173–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reed JC: Bcl-2-family proteins and
hematologic malignancies: history and future prospects. Blood.
111:3322–3330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ni Chonghaile T, Sarosiek KA, Vo TT, et
al: Pretreatment mitochondrial priming correlates with clinical
response to cytotoxic chemotherapy. Science. 334:1129–1133. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reed JC and Pellecchia M: Apoptosis-based
therapies for hematologic malignancies. Blood. 106:408–418. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Glaser SP, Lee EF, Trounson E, et al:
Anti-apoptotic Mcl-1 is essential for the development and sustained
growth of acute myeloid leukemia. Genes Dev. 26:120–125. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perego P, Righetti SC, Supino R, et al:
Role of apoptosis and apoptosis-related proteins in the
cisplatin-resistant phenotype of human tumor cell lines. Apoptosis.
2:540–548. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Campbell KJ, Bath ML, Turner ML, et al:
Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of
hematopoietic stem/progenitor cells and enhances drug resistance.
Blood. 116:3197–3207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robinson BW, Behling KC, Gupta M, et al:
Abundant anti-apoptotic BCL-2 is a molecular target in leukaemias
with t (4;11) translocation. Br J Haematol. 141:827–839. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bunpo P, Kataoka K, Arimochi H, et al:
Inhibitory effects of asiatic acid and CPT-11 on growth of HT-29
cells. J Med Invest. 52:65–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu YL, Kuo PL, Lin LT and Lin CC: Asiatic
acid, a triterpene, induces apoptosis and cell cycle arrest through
activation of extracellular signal-regulated kinase and p38
mitogen-activated protein kinase pathways in human breast cancer
cells. J Pharmacol Exp Ther. 313:333–344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tamm I, Wang Y, Sausville E, et al:
IAP-family protein survivin inhibits caspase activity and apoptosis
induced by Fas (CD95), Bax, caspases and anticancer drugs. Cancer
Res. 58:5315–5320. 1998.PubMed/NCBI
|
|
28
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ibrahim AM, Mansour IM, Wilson MM, Mokhtar
DA, Helal AM and Al Wakeel HM: Study of survivin and X-linked
inhibitor of apoptosis protein (XIAP) genes in acute myeloid
leukemia (AML). Lab Hematol. 18:1–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adida C, Berrebi D, Peuchmaur M,
Reyes-Mugica M and Altieri DC: Anti-apoptosis gene, survivin and
prognosis of neuroblastoma. Lancet. 351:882–883. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smolewski P and Robak T: Inhibitors of
apoptosis proteins (IAPs) as potential molecular targets for
therapy of hematological malignancies. Curr Mol Med. 11:633–649.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu Z, Li E, Liu Y, et al: Bufalin induces
the apoptosis of acute promyelocytic leukemia cells via the
downregulation of survivin expression. Acta Haematol. 128:144–150.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olson JM and Hallahan AR: p38 MAP kinase:
a convergence point in cancer therapy. Trends Mol Med. 10:125–129.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu JF, Li ZJ, Zhang GS, et al: Icaritin
shows potent anti-leukemia activity on chronic myeloid leukemia in
vitro and in vivo by regulating MAPK/ERK/JNK and JAK2/STAT3/AKT
signalings. PLoS One. 6:e237202011. View Article : Google Scholar
|
|
37
|
Huh JE, Kang KS, Chae C, Kim HM, Ahn KS
and Kim SH: Roles of p38 and JNK mitogen-activated protein kinase
pathways during cantharidin-induced apoptosis in U937 cells.
Biochem Pharmacol. 67:1811–1818. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hendrickx N, Volanti C, Moens U, et al:
Up-regulation of cyclooxygenase-2 and apoptosis resistance by p38
MAPK in hypericin-mediated photodynamic therapy of human cancer
cells. J Biol Chem. 278:52231–52239. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu C, Minemoto Y, Zhang J, et al: JNK
suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2
family protein BAD. Mol Cell. 13:329–340. 2004. View Article : Google Scholar : PubMed/NCBI
|