Introduction
Puromycin aminonucleoside (PAN) is widely used as a
model of nephrotic syndrome and focal segmental glomerulosclerosis.
Dysfunction in the slit diaphragm caused by PAN is associated with
the development of massive proteinuria. Although glomerular injury
is the primary effect of PAN, renal handling of the excess filtered
proteins may contribute to tubulointerstitial lesions. The excess
of protein delivered to the proximal tubule results in
inflammation, tubular epithelial mesenchymal transition (EMT) and
interstitial fibrosis (1,2).
Previous studies have demonstrated a significant
renoprotective effect of the active form of vitamin D, or
calcitriol (1,25-dihydroxycholecalciferol), in kidney diseases of
various etiologies (3). Calcitriol
activity is mediated through the vitamin D receptor (VDR), a member
of the nuclear receptor superfamily (4). Administration of paricalcitol, a
vitamin D analogue, reduced glomerulosclerosis and proteinuria,
which prevented podocyte injury in a model of adriamycin-induced
nephropathy (5). In addition,
paricalcitol protected the kidneys against renal damage in
obstructive nephropathy (6),
possibly due to its ability to preserve tubular epithelial
integrity via EMT prevention. Calcitriol regulates two major
pathways involved in a number of pathological processes, the renin
angiotensin system (RAS) (7) and
the nuclear factor (NF)-κB pathway (8). Calcitriol has been well established
as a negative regulator of the RAS by suppressing the prorenin gene
(9). In addition, NF-κB, a major
mediator of the immune response, is involved in regulating
inflammatory cytokines and chemo-kines, including monocyte
chemotactic protein 1, plasminogen activator inhibitor-1 and
tumor-necrosis factor (TNF)-α, which are important in renal damage
by inducing inflammation and fibrogenesis (10). Calcitriol may interfere with NF-κB
signaling by inducing the formation of a complex between the VDR
and the p65 subunit of NF-κB, preventing the complex from binding
to DNA (8).
In the present study, the effect of calcitriol in
PAN-induced nephrotic syndrome in rats was investigated. It was
also determined whether calcitriol is beneficial for minimizing
renal damage in a pre-established model of proteinuria.
Materials and methods
The experimental protocol was approved by the
Ethical Committee of the Federal University of São Paulo (CEP 0741,
UNIFESP, São Paulo, Brazil). The study used 12-week-old male Wistar
rats (150–200 g) supplied by the animal facility of the Federal
University of São Paulo. The rats were housed in cages with ad
libitum access to standard rat chow and tap water, in a
temperature-controlled environment (23°C) with a 12 h light/dark
cycle. One week prior to PAN administration, the right kidney was
removed under anesthesia with 40 mg/kg ketamine and 20 mg/kg
xylazine (Syntec, Hortolândia, Brazil). Nephrosis was induced using
a single intraperitoneal injection of PAN (100 mg/kg body weight;
Sigma-Aldrich, St. Louis, MO, USA). The animals were divided into
three groups: Control (CTL; n=5), PAN treatment (PAN; n=5) and PAN
combined with calcitriol treatment (PAN + calcitriol; n,5).
Calcitriol (Abbott, Milan, Italy) treatment started eight weeks
after PAN administration when proteinuria was established.
Calcitriol was administered by subcutaneous injection (0.5 mg/kg
bodyweight) five times a week for four weeks. All animals were
sacrificed 12 weeks after the onset of PAN administration.
Periodically, retro-orbital blood samples were
obtained from the animals under ketamine and xylazine anesthesia.
Additionally, 24-h urine samples were collected in metabolic cages
(Tecniplast, Buguggiate, Italy). A colorimetric assay was used to
measure concentrations of creatinine (Creatinine kit; Labtest
Diagnóstica, Lagoa Santa, Brazil), calcium (Arsenazo III kit;
Labtest Diagnóstica) and inorganic phosphate (Inorganic Phosphorous
kit; Beckman Coulter, Miami, FL, USA). Urine protein was measured
using a colo-rimetric assay (Sensiprot; Labtest Diagnóstica). At
completion of the experimental protocol, the animals were
anesthetized with ketamine and xylazine, blood was collected from
the abdominal aorta and the remaining kidney was excised. Animals
were sacrificed via anesthetic overdose (160 mg/kg ketamine and 80
mg/kg xylazine; Syntec). For the mRNA and protein expression
analyses, the kidney samples were immediately frozen in liquid
nitrogen and kept at −80°C until use. For the histochemical and
immunohistochemical analyses, the kidney samples were fixed in
tamponated formaldehyde (Merck KGaA, Darmstadt, Germany) and
following several washes in ethanol (Merck KGaA) and xylene
(Labsynth, Diadema, Brazil), the samples were embedded in paraffin
wax (Labsynth).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was purified from the whole kidney using
the phenol and guanidine isothiocyanate-cesium chloride method with
TRIzol® (Gibco-BRL, Gaithersburg, MD, USA), according to
the manufacturer’s instructions. Total RNA (2 μg) was
treated with DNase (RQ1 RNase-free DNase; Promega, Madison, WI,
USA) to avoid genomic DNA contamination and reverse-transcribed
into cDNA by adding a mixture containing 0.5 mg/ml of oligo(dT)
(Invitrogen Life Technologies, Carlsbad, CA, USA), 10 mM
DL-dithiothreitol (Invitrogen Life Technologies), 0.5 mM
deoxynucleoside triphosphates (Invitrogen Life Technologies) and
200 units of reverse transcriptase enzyme (SuperScript RT II;
Invitrogen Life Technologies). The mRNA expression levels were
estimated using RT-qPCR (7500 PCR system; Applied Biosystems,
Carlsbad, CA, USA) using specific primers for each molecule as
follows (forward and reverse, respectively): TGF-β1
(5′-GCTGTGCAGGTGTTGAGCC-3′ and 5′-TCAGTCCCAAACGTCGAGGT-3′),
interleukin (IL)-6 (5′-TGTATGAACAGCGATGATGCAC-3′ and
5′-GGTTATATCCAGTTTGGAAGCATCC-3′), IL-10
(5′-ATTGAACCACCCGGCATCTAC-3′ and 5′-GGTTTTCCAAGGAGTTGCTCC-3′). The
relative expression of the target genes was normalized to the
housekeeping gene β-actin (5′-CCTCTATGCCAACACAGTGC-3′ and
5′-ACATCTGCTGGAAGGTGGAC-3′). All primers were synthesized by
Integrated DNA Technologies (Coralville, IA, USA). PCR product
accumulation was monitored using SYBR Green I intercalating dye
(Applied Biosystems, Warrington, UK), which exhibits increased
fluorescence upon binding with double-stranded DNA.
Western blot analysis
The kidney fragments were homogenized using a
Polytron homogenizer (Kinematica, Lucerne, Switzerland) in ice-cold
buffer [50 mM TRIS (Sigma-Aldrich), 150 mM NaCl (Labsynth), 1.0%
nonidet-P-40 (Bio-Rad Laboratories, Inc., Hercules, CA, USA), 0.5%
sodium deoxycholate (Sigma-Aldrich), 0.1% SDS, (pH 8.0;
Sigma-Aldrich)] containing protease inhibitors (AEBSF, aprotinin,
bestatin, E-64, leupeptin, pepstatin A) (Protease Inhibitor
Cocktail; Sigma-Aldrich). Total protein was quantified using a
modified Lowry method (Bio-Rad DC protein assay reagent; Bio-Rad
Laboratories, Inc.). Protein samples (50 μg) were separated
according to size by 12% SDS-PAGE and electroblotted onto
nitrocellulose membranes (GE Healthcare Life Sciences, Little
Chalfont, UK). The membrane blots were probed with primary
antibodies overnight at 4°C and with horseradish peroxidase
(HRP)-conjugated secondary antibodies for 1 h at 4°C. The primary
antibodies were obtained from the following sources: mouse
monoclonal anti-GAPDH (cat. no. AM4300; 1:4,000; Ambion, Austin,
TX, USA), mouse monoclonal anti-TGF-β1 (cat. no. T0438; 1:500;
Sigma-Aldrich), mouse polyclonal anti-p47 phox (cat. no. 07–500;
1:1,000; Millipore, Billerica, MA, USA), rabbit polyclonal
anti-CuZn superoxide dismutase (SOD) (cat. no. 07–403; 1:1,000;
Millipore), rabbit polyclonal anti-renin (cat. no. sc-22752; 1:200;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), rabbit
monoclonal anti-Smad3p (cat. no. ab52903; 1:500; Abcam, Cambridge,
UK) and rabbit polyclonal anti-p65 NF-κB (cat. no. 06–418; 1:1,000;
Millipore). The goat anti-rabbit (cat. no. NA934V; 1:20,000) and
rabbit anti-mouse (cat. no. A9044; 1:60,000) HRP-conjugated
secondary antibodies were purchased from GE Healthcare Life
Sciences and Sigma-Aldrich, respectively. The protein bands were
visualized using the Immobilon Western HRP substrate (Millipore).
The obtained bands were quantified using the Luminescent Image
Analyzer-LAS 4000 and Image Gauge V3.1 software (Fuji Photo Film
Co, Tokyo, Japan).
Light microscopy studies
The paraffin-embedded fragments were cut into
5-μm sections using a rotary microtome (Leica, Herlev,
Denmark). The tissue slides were deparaffinized in xylene three
times (5 min each), and gradually rehydrated through a series of
graded ethanol (100% twice for 5 min, 95% for 5 min, 70% for 5 min
and 50% for 5 min). Histological sections were stained using
picrosirius red staining kit (1% Sirius red in saturated picric
acid; EasyPath, Indaiatuba, Brazil) for 24 h, or hematoxylin and
eosin (Labsynth) and examined under light microscopy (Nikon Eclipse
2000 equipped with Nikon DS-Fi2; Nikon Corporation, Tokyo, Japan).
The fibrotic area stained with picrosirius solution was quantified
using Corel Photo-Paint 12 (CorelDRAW version 12; Corel
Corporation, Ottawa, ON, Canada) and UTHSCSA - ImageTool software
(version 3.0; University of Texas Health Science Center, San
Antonio, TX, USA).
Immunohistochemistry
The kidney slices were deparaffinized and
rehydrated, as described above. To expose the antigens, the kidney
sections were boiled in a target retrieval solution [citrate buffer
(pH 6.0) for fibroblast-specific protein 1 (FSP1), TRIS buffer (pH
9.0) for α-smooth muscle actin (α-SMA) and ED-1] for 30 min.
Endogenous peroxidase activity was blocked with 3%
H2O2 (Labsynth) for 10 min at room
temperature. Nonspecific binding was prevented by incubating the
sections with a protein blocker (Dako, Carpinteria, CA, USA). The
sections were incubated overnight at 4°C with primary antibodies:
α-SMA (cat. no. A2547; 1:500; Sigma-Aldrich), FSP1 (cat. no. A5114;
1:400; Dako) or CD68/ED-1 (cat. no. MCA341R; 1:100; Serotec,
Oxford, UK). Following washing with Tris-buffered saline (TBS) [50
mM TRIS, 150 mM NaCl], the sections were incubated with a
horseradish peroxidase-conjugated polymer (Dako) for 30 min at room
temperature. The slides were rinsed with TBS and the sites of
antibody-antigen binding were visualized with 3,3′-diaminobenzidine
(Dako). The sections were lightly counterstained with hematoxylin.
The analyses were performed using light microscopy (Eclipse 2000
camera Nikon DS-Fi2) and the stained proteins were quantified using
Corel Photo-Paint 12 (CorelDRAW version 12) and UTHSCSA-ImageTool
software (version 3.0).
Statistical analysis
Results are expressed as the mean ± standard error.
The data were analyzed by SigmaStat 2.0 software (Systat Software
Inc., San Jose, CA, USA), using one-way analysis of variance
followed by Tukey’s test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of calcitriol on proteinuria and
serum markers
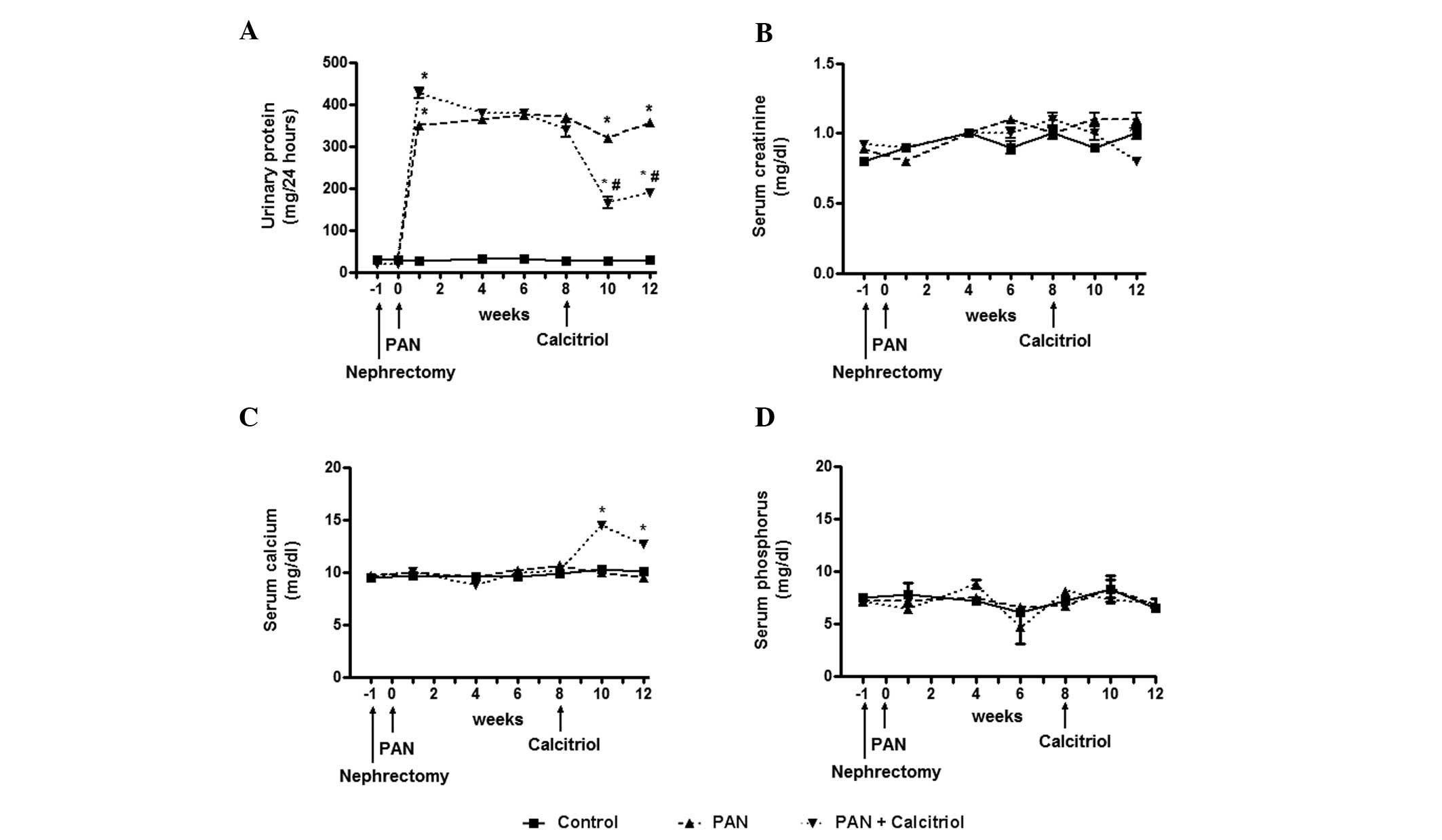
As expected, one week after puromycin injection,
intense proteinuria developed, which was significantly reduced by
calcitriol treatment (Fig. 1A).
Despite massive proteinuria, a change in the plasma creatinine
concentration was not detectable (Fig.
1B). Treatment with calcitriol increased the serum calcium
concentration at 10 weeks (Fig.
1C), which subsequently decreased at 12 weeks. There was no
significant change in plasma phosphorus concentration (Fig. 1D).
Calcitriol ameliorates renal damage and
interstitial fibrosis
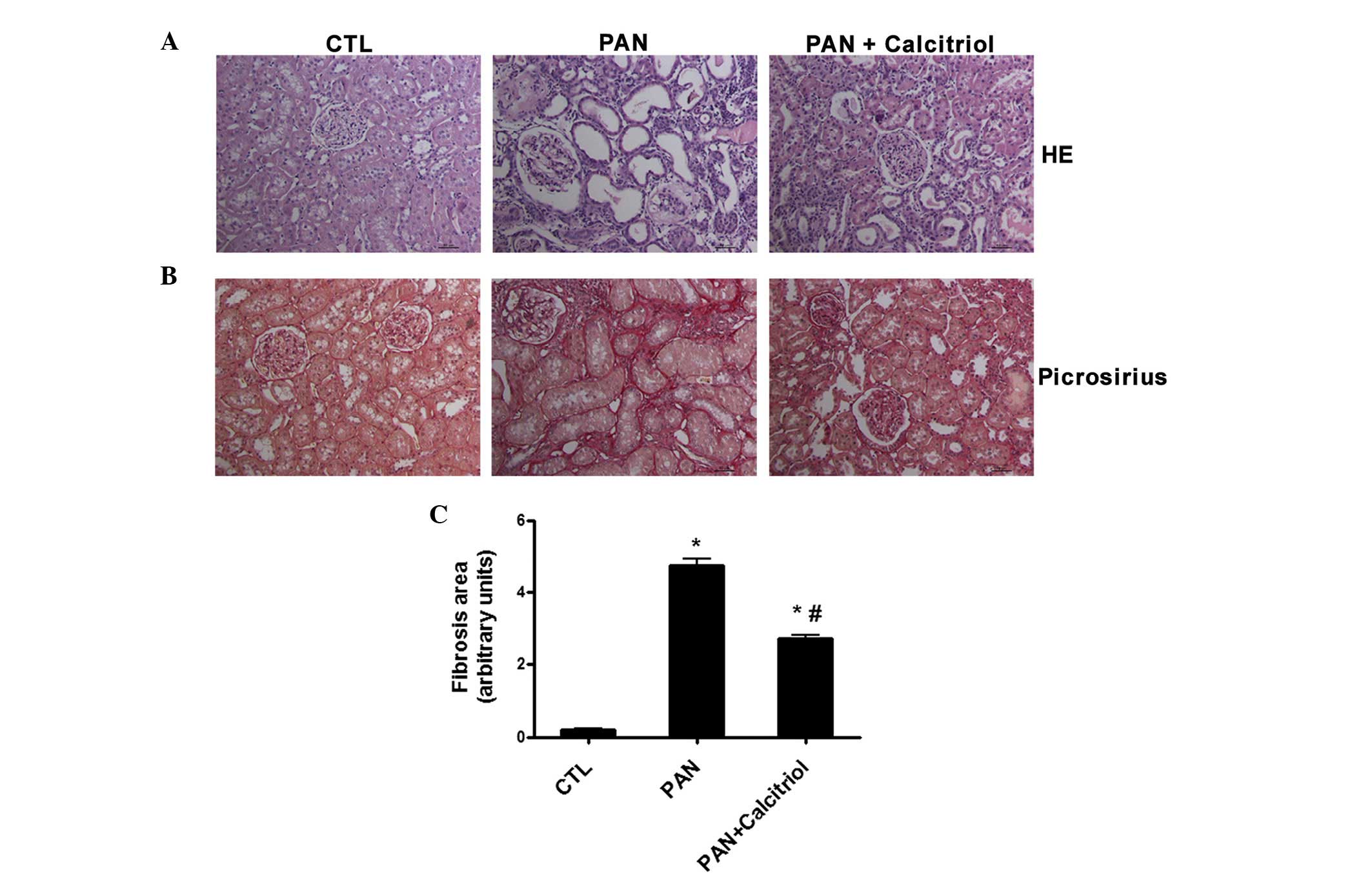
Kidney histology using H&E staining (Fig. 2A) revealed severe renal damage 12
weeks after puromycin administration, char-acterized by
interstitial expansion and an increase in tubular lumen, possibly
due to impaired tubular reabsorption. These alterations were
minimized by calcitriol treatment (Fig. 2A). In addition, the weak collagen
deposition detected in the tubulointerstitium and glomeruli in the
nephrectomized control rats was markedly increased in the kidneys
of the PAN-treated animals (Fig.
2B). Calcitriol administration was associated with
significantly less collagen staining compared with that in the
untreated proteinuric animals based on quantification of the
picrosirius-positive areas (collagen deposition; Fig. 2C).
Calcitriol attenuates fibroblast
activation
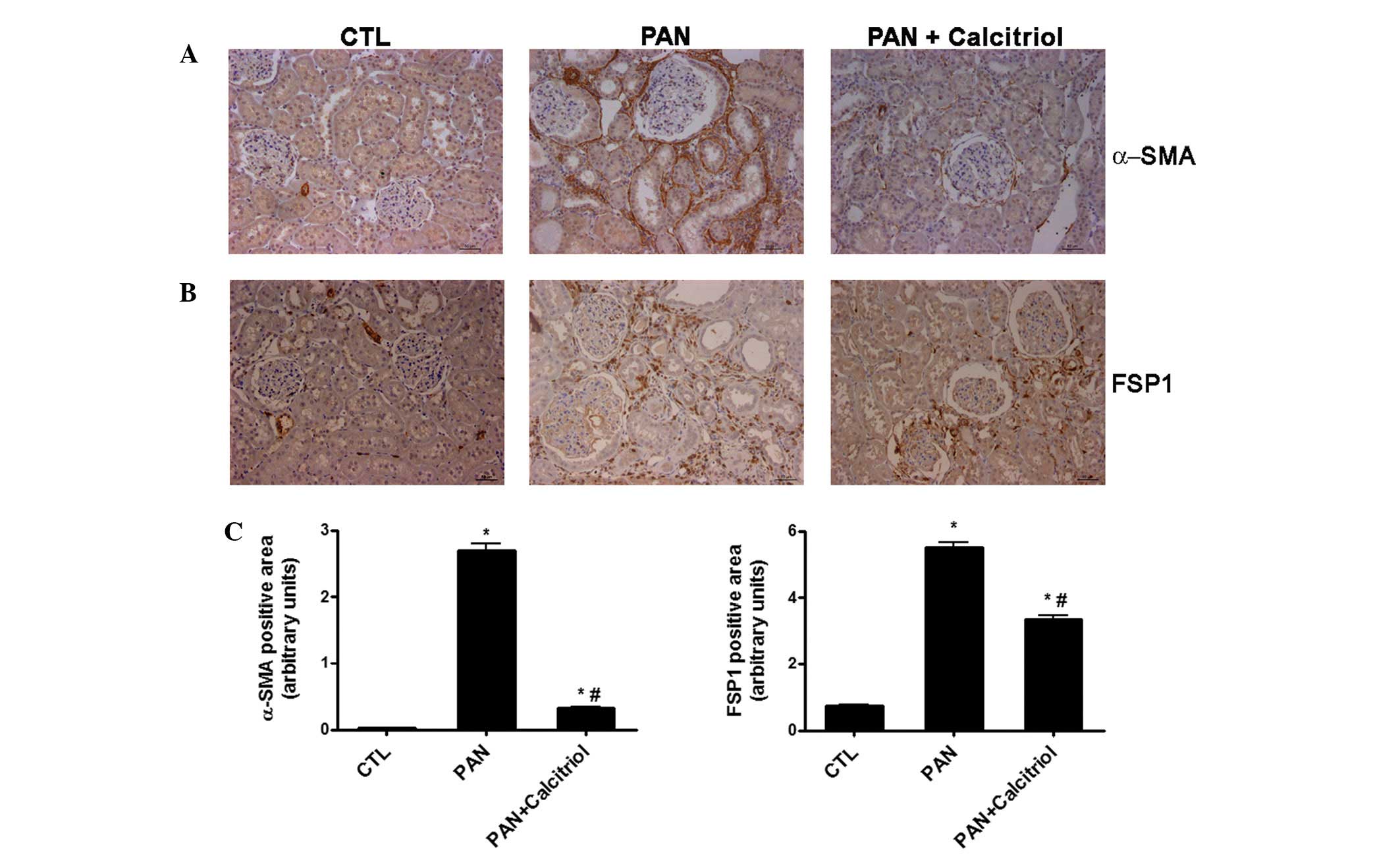
The expression levels of the fibroblast markers
α-SMA and FSP1 were examined. There was an increase in the
expression of the two fibroblast markers in the proteinuric animals
(Fig. 3A and B). However, α-SMA
was detected in the periglomerular region and interstitial space,
while FSP1 staining was identified mainly in the interstitium.
Calcitriol treatment reduced α-SMA and FSP1 expression, as shown in
the semiquantitative analysis (Fig.
3C).
Renoprotective mechanisms of
calcitriol
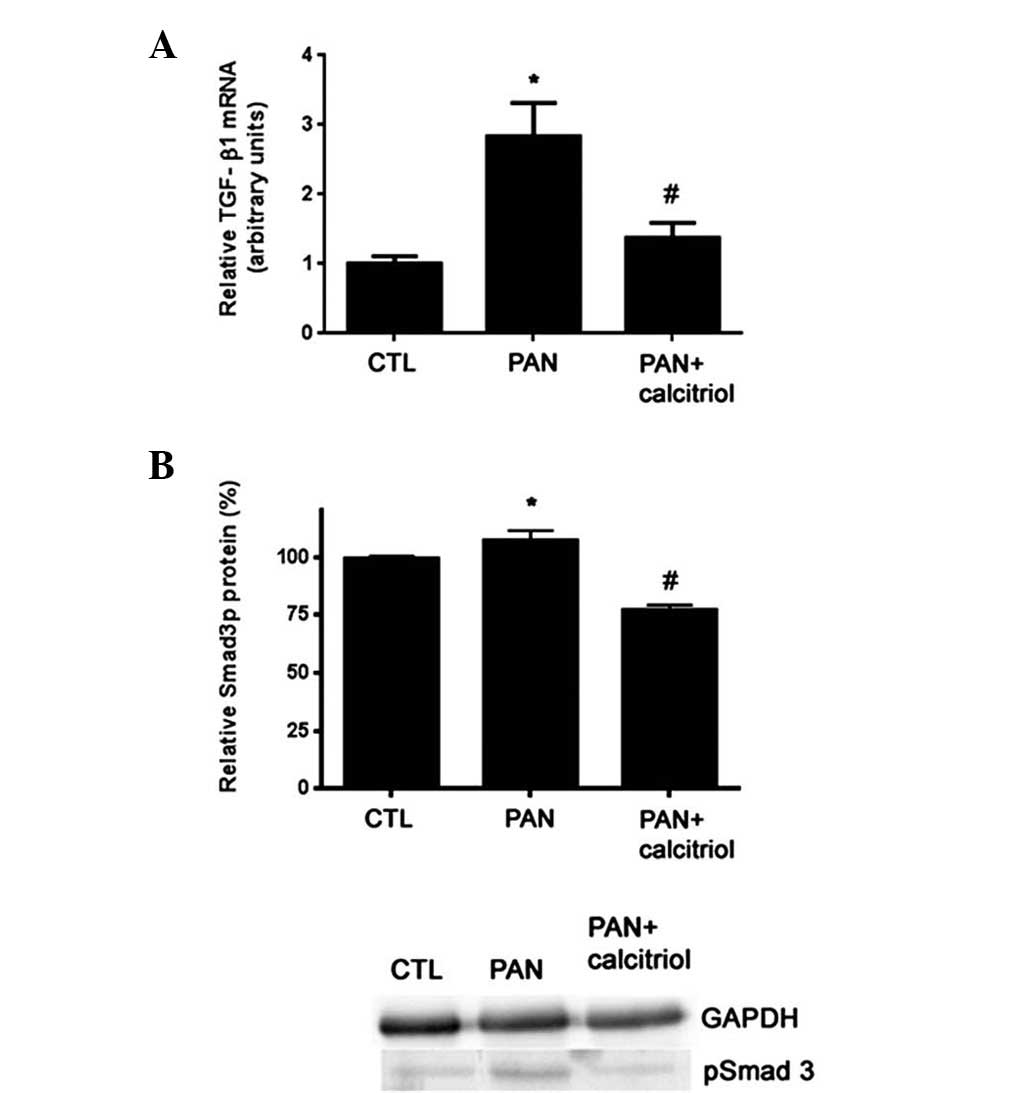
The levels of TGF-β1 and the signaling molecule
Smad3 were analyzed using RT-PCR and western blotting,
respectively. Compared with the control rats, the proteinuric
animals exhibited increased TGF-β1 mRNA expression and
phosphorylated Smad3 (pSmad3) (Fig.
4). Calcitriol treatment significantly reduced TGF-β1 and
pSmad3 expression. Regarding the function of RAS in this model, it
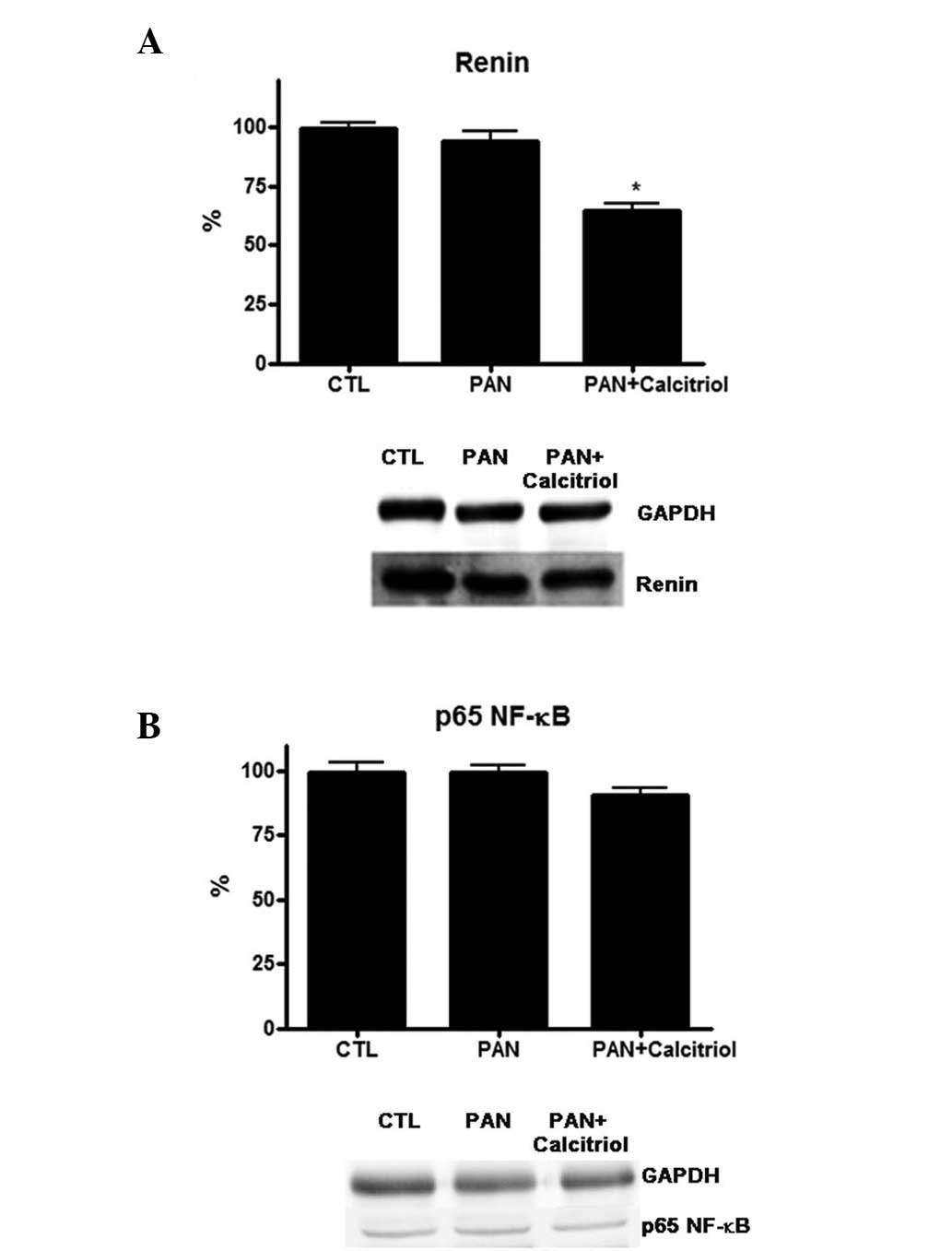
was observed that PAN did not alter renin expression, but
calcitriol treatment significantly reduced renin levels (Fig. 5A). Calcitriol was able to regulate
the NF-κB pathway; however, no detectable alterations were observed
for the NF-κB signaling protein p65 in the PAN and calcitriol
groups (Fig. 5B).
Calcitriol reduces renal
inflammation
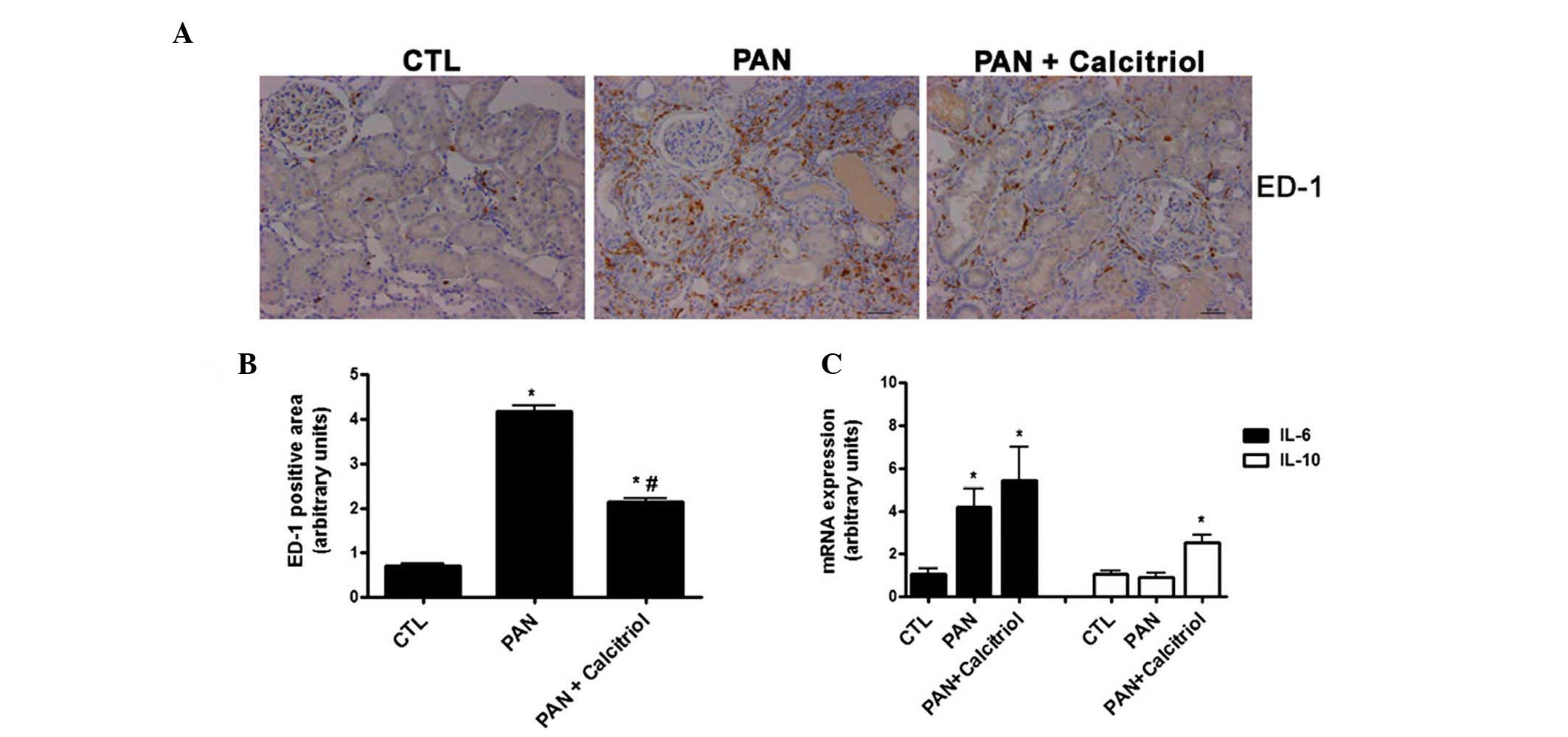
There was increased macrophage infiltration in the
kidneys in the PAN group based on increased ED-1 staining (Fig. 6A and B). Calcitriol reduced the
presence of macrophages, indicating a possible decrease in
inflammation. There was increased gene expression of the
proinflammatory cytokine IL-6 in the PAN group (Fig. 6C). Although calcitriol treatment
did not change IL-6 expression, calcitriol significantly increased
expression of the anti-inflammatory cytokine IL-10.
Effect of calcitriol on oxidative
stress
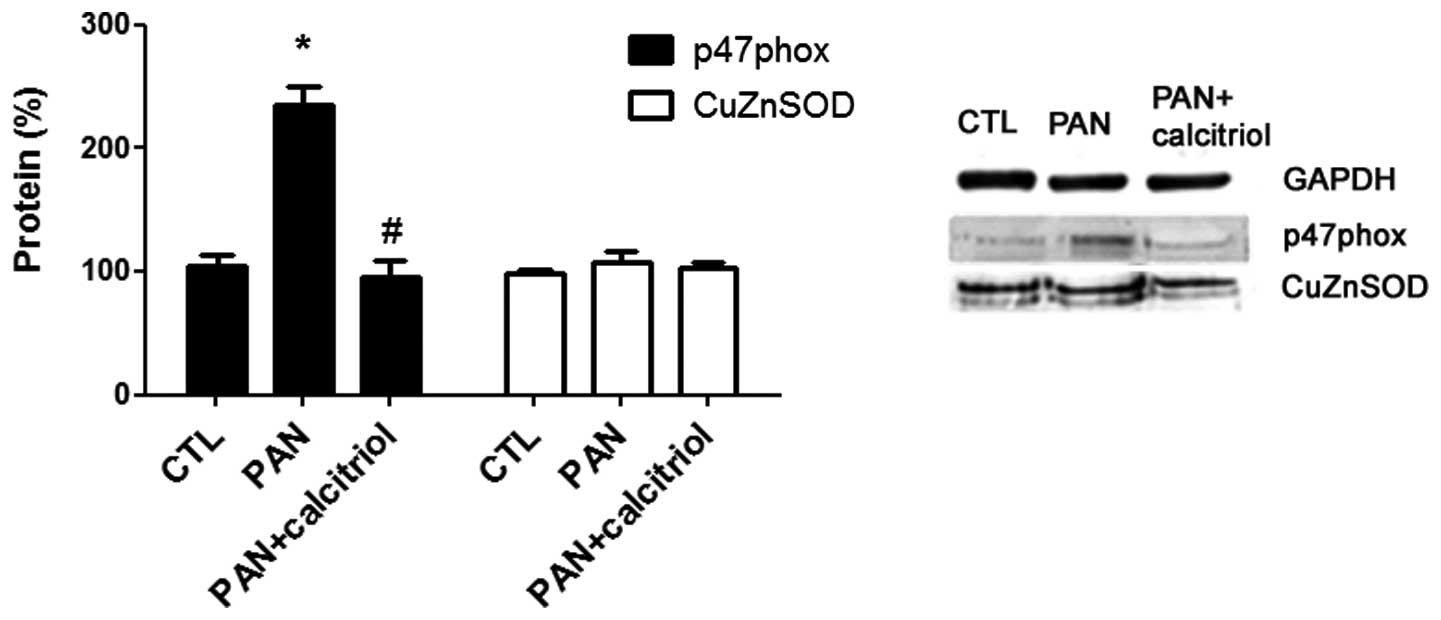
The mechanism of oxidative stress in the
pathophysiology of puromycin nephropathy was assessed through
analyzing the expression of two enzymes involved in this mechanism,
p47 phox, a subunit of nicotinamide adenine dinucleotide phosphate
(NADPH) oxidase and CuZnSOD, an antioxidant enzyme. There was a
significant increase in p47 phox expression in the PAN group and
calcitriol-treatment reduced this increase to near the control
group level (Fig. 7). No
significant differences in CuZnSOD expression were identified.
Discussion
PAN-induced nephropathy is characterized by podocyte
injury, resulting in glomerulosclerosis, tubular damage and
interstitial fibrosis. PAN nephropathy was reproduced in a rat
model in the present study and was characterized by nephrotic level
proteinuria with no detectable change in serum creatinine.
Calcitriol administered eight weeks after PAN-induced renal injury
significantly reduced proteinuria. Although podocyte function and
morphology was not evaluated in the present study, a previous study
demonstrated that the vitamin D analogue paricalcitol may prevent
podocyte lesions in adriamycin nephropathy (5) and the reno-protective effect of
paricalcitol was considered to be primarily due to the prevention
of podocyte injury.
The protein overload in the tubules resulted in
epithelial cell damage with functional and structural changes,
including EMT (11). In the
present study, there was a significant change in tubular structure
with lumen dilation, indicating impaired reabsorptive capacity of
the tubular epithelial cells. Although PAN induced an increase in
the EMT markers FSP1 and α-SMA, these markers were predominately
identified in interstitial cells but not in tubular cells. This
finding suggested that EMT was not the main mechanism of fibrosis
in this model, although the involvement of EMT in interstitial
fibrosis cannot be fully ruled out. Calcitriol treatment was able
to minimize the overexpression of FSP1 and α-SMA induced by PAN,
suggesting that the beneficial effects of calcitriol
supplementation on fibrogenesis were mediated, at least in part, by
reduced fibroblast activation, although the origin of fibroblasts,
either resident and/or infiltrating, was not determined in the
present study.
Calcitriol was able to improve renal morphology and
reduce the fibrotic area with less collagen deposition. TGF-β1, one
of the most relevant profibrotic factors in the kidney, was
increased by PAN as well as its signaling pathway, represented by
pSmad3, which was activated by puromycin. Calcitriol completely
inhibited this pro-fibrotic mechanism, suggesting that the actions
of vitamin D in renal fibrosis may involve a downregulation of the
TGF-β1/Smad3 axis.
Chronic inflammation is an important mechanism in
tissue injury and fibrogenesis (12). PAN induced increased macrophage
infiltration with increased expression of the pro-inflammatory
cytokine IL-6. Calcitriol was shown to have a potent
anti-inflammatory effect in different experimental models of kidney
disease (13,14). Additionally, clinical studies have
demonstrated that calcitriol treatment suppressed IL-6 and TNF-α
expression in patients with chronic kidney disease (15). In the present study, calcitriol
reduced the presence of ED-1-positive cells, indicating less
macrophage infiltration; however, calcitriol did not reduce IL-6
mRNA expression, but of note, calcitriol upregulated the
anti-inflammatory cytokine IL-10, suggesting an indirect effect of
calcitriol in PAN-induced inflammation.
Reactive oxygen species formed by oxidative stress
are important mediators of renal disease induced by puro-mycin
(16). The results of the present
study demonstrated the presence of PAN-induced oxidative stress
represented by a significant increase in the expression of p47phox,
an NADPH oxidase subunit, with no change in SOD expression,
resulting in an imbalance between prooxidant and antioxidant
mechanisms. Treatment with calcitriol decreased p47phox expression
and thus reduced oxidative stress. Finch et al (17) reported that paricalcitol
ameliorated oxidative stress by increasing CuZnSOD expression in
uremic rats. In addition, treatment with an antioxidant attenuated
renal interstitial fibrosis following ureteral obstruction
(18), indicating a function for
the redox state in fibrosis progression. In the present study, the
effect of calcitriol in reducing oxidative stress was mediated, at
least in part, via TGF-β downregulation.
There is evidence of puromycin-induced RAS
activation in nephropathy (19);
however, intrarenal renin expression did not change in the present
model. Calcitriol has been well accepted to suppress prorenin gene
expression and reduce RAS activity and although PAN does not have
an effect on renin expression, calcitriol induced a 30% decrease in
renin protein expression, but the impact of this reduction on the
renoprotective effect of calcitriol in the present study remains
elusive.
In conclusion, although the functional and
histological parameters did not completely return to control
levels, calcitriol treatment significantly ameliorated the
progression of puromycin-induced renal fibrosis. In addition,
calcitriol was effective in decreasing the accumulation of
extracellular matrix, reduced inflammation and downregulated the
TGF-β1 pathway. Therefore, calcitriol supplementation may be a
strategy to reduce renal damage in proteinuric kidney disease.
Acknowledgments
This study was supported by grants from the
Coordenação de Aperfeiçoamento de Nível Superior, Conselho Nacional
de Desenvolvimento Científico e Tecnológico, Fundação Oswaldo Ramos
and Fundação de Amparo à Pesquisa do Estado de São Paulo.
References
|
1
|
Birn H and Christensen EI: Renal albumin
absorption in physiology and pathology. Kidney Int. 69:440–449.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeisberg M and Neilson EG: Mechanisms of
tubulointerstitial fibrosis. J Am Soc Nephrol. 21:1819–1834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li YC: Renoprotective effects of vitamin D
analogs. Kidney Int. 78:134–139. 2010. View Article : Google Scholar
|
|
4
|
Haussler MR, Whitfield GK, Haussler CA, et
al: The nuclear vitamin D receptor: biological and molecular
regulatory properties revealed. J Bone Miner Res. 13:325–349. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He W, Kang YS, Dai C and Liu Y: Blockade
of Wnt/beta-catenin signaling by paricalcitol ameliorates
proteinuria and kidney injury. J Am Soc Nephrol. 22:90–103. 2011.
View Article : Google Scholar :
|
|
6
|
Tan X, Li Y and Liu Y: Paricalcitol
attenuates renal interstitial fibrosis in obstructive nephropathy.
J Am Soc Nephrol. 17:3382–3393. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Freundlich M, Quiroz Y, Zhang Z, et al:
Suppression of renin-angiotensin gene expression in the kidney by
paricalcitol. Kidney Int. 74:1394–1402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan X, Wen X and Liu Y: Paricalcitol
inhibits renal inflammation by promoting vitamin D
receptor-mediated sequestration of NF-kappaB signaling. J Am Soc
Nephrol. 19:1741–1752. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan W, Pan W, Kong J, et al:
1,25-dihydroxyvitamin D3 suppresses renin gene transcription by
blocking the activity of the cyclic AMP response element in the
renin gene promoter. J Biol Chem. 282:29821–29830. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guijarro C and Egido J: Transcription
factor-kappa B (NF-kappa B) and renal disease. Kidney Int.
59:415–424. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ibrini J, Fadel S, Chana RS, et al:
Albumin-induced epithelial mesenchymal transformation. Nephron Exp
Nephrol. 120:e91–e102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee SB and Kalluri R: Mechanistic
connection between inflammation and fibrosis. Kidney Int Suppl.
119:S22–S26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Panichi V, Migliori M, Taccola D, et al:
Effects of 1,25(OH)2D3 in experimental mesangial proliferative
nephritis in rats. Kidney Int. 60:87–95. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schleithoff SS, Zittermann A, Tenderich G,
Berthold HK, Stehle P and Koerfer R: Vitamin D supplementation
improves cytokine profiles in patients with congestive heart
failure: a double-blind, randomized, placebo-controlled trial. Am J
Clin Nutr. 83:754–759. 2006.PubMed/NCBI
|
|
15
|
Alborzi P, Patel NA, Peterson C, et al:
Paricalcitol reduces albuminuria and inflammation in chronic kidney
disease: a randomized double-blind pilot trial. Hypertension.
52:249–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diamond JR, Bonventre JV and Karnovsky MJ:
A role for oxygen free radicals in aminonucleoside nephrosis.
Kidney Int. 29:478–483. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Finch JL, Suarez EB, Husain K, et al:
Effect of combining an ACE inhibitor and a VDR activator on
glomerulosclerosis, proteinuria and renal oxidative stress in
uremic rats. Am J Physiol Renal Physiol. 302:F141–F149. 2012.
View Article : Google Scholar
|
|
18
|
Akin M, Demirbilek S, Ay S, et al:
Attenuation of ureteral obstruction-induced renal injury by
polyenylphosphatidylcholine. Int J Urol. 14:350–356. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yayama K, Konishi K, Ohta A, et al:
Elevation of plasma angiotensinogen in rats with experimentally
induced nephrosis. Nephron. 63:89–93. 1993. View Article : Google Scholar : PubMed/NCBI
|