Introduction
Radiotherapy is a common therapeutic strategy for
cancer. However, its effectiveness is usually limited by the
inherent sensitivity of normal tissue to ionizing radiation, which
contributes to the generation of a large number of oxidizing free
radicals causing irreversible cell apoptosis (1). Therefore, it is necessary to develop
novel strategies to prevent radiation-induced apoptosis of normal
cells.
Cistanche, a genus of parasitic plants mainly
distributed in arid lands and desert, have been commonly used as a
traditional Chinese herbal medicine for treating various disorders,
including renal deficiency, morbid leucorrhea, chronic infection
and hematopoietic disorders (2).
Extensive studies have been conducted to investigate the biological
and physiochemical properties of Cistanche, which revealed
that it exhibited neuroprotective, anti-inflammatory, antioxidant
and antiaging effects (3). The
stems of Cistanche salsa, a parasitic plant native to the
northwest China with phenylethanoid glycosides (PhGs) as the major
active components, have been considered as an important traditional
Chinese herbal medicine for treating renal dysfunction and
neurasthenia (4). Acteoside, one
type of PhG derived from Cistanche showed antioxidative,
hepatoprotective, antiviral, antimetastasis, and anti-inflammatory
properties (5–7). However, few studies have been
performed to investigate its protective effects against
radiation-induced damage.
The present study aimed to investigate the
inhibitory effects of acteoside on radiation-induced apoptosis in
human skin fibroblasts and the underlying mechanism
Materials and methods
Materials
Human skin fibroblasts (HSFs) were purchased from
Fuxiang Biotechnology Co., Ltd. (Shanghai, China). Dulbecco’s
modified Eagle’s medium (DMEM) was obtained from Sangon Biotech
Co., Ltd. (Shanghai, China). Fetal bovine serum (FBS) was provided
by Sangon Biotech (Shanghai, China). Antibodies against
procaspase-3 (cat. no. sc-7148), Bcl-2 (cat. no. sc-783), Bax (cat.
no. sc-493), JNK (cat. no. sc-7345), p-JNK (cat. no. sc-6254), ERK
(cat. no. sc-94), p-ERK (cat. no. sc-7383) and β-actin (cat. no.
sc-47778) were purchased from Santa Cruz Biotechnology Inc. (Santa
Cruz, CA, USA).
Extraction and identification of
acteoside
Cistanche salsa (C.A. Mey.) G. Beck (10 kg)
was extracted with ethanol as previously described (8). Then the mixture was concentrated, and
the residue was suspended in water, followed by extraction with
ethyl acetate and n-butyl alcohol. The n-butyl alcohol fraction was
isolated by an SP825 chromatograph system (Sigma-Aldrich, St.
Louis, MO, USA). Acteoside (825 mg) was isolated by further
separation over Sephadex LH-20 (Sigma-Aldrich, St. Louis, MO, USA).
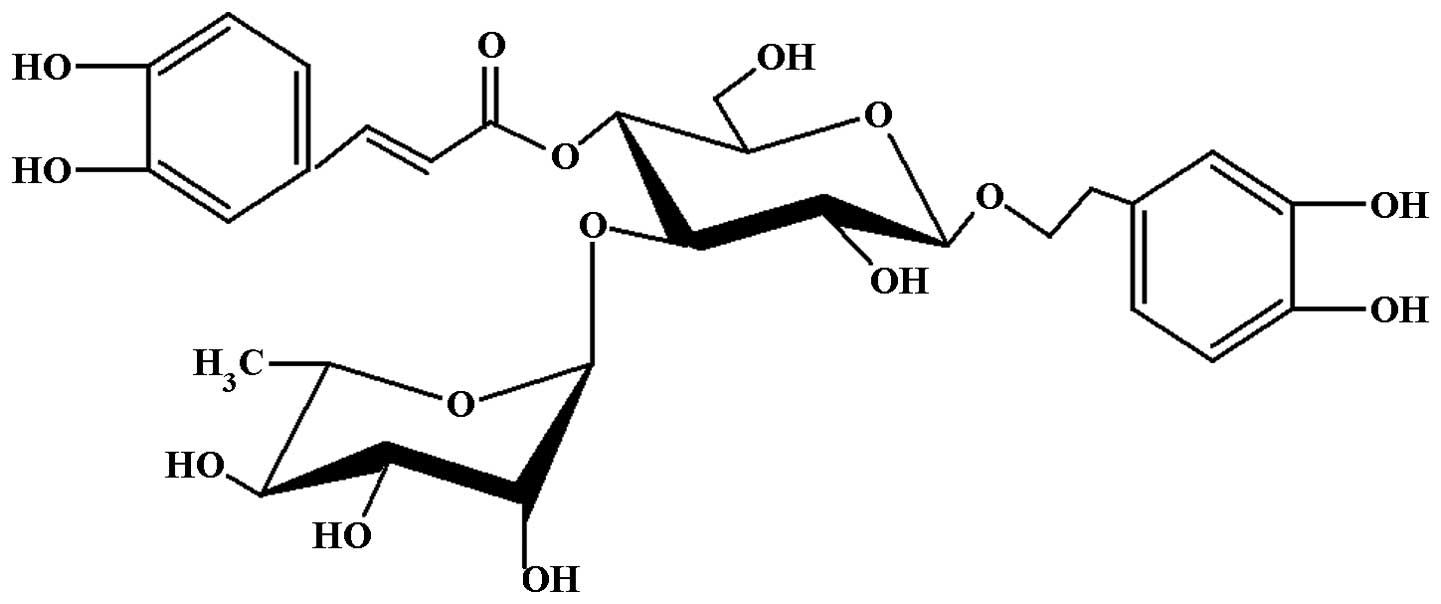
The structure of acteoside (Fig.
1) was identified by spectroscopic techniques (HPLC and NMR)
(9). The purity of acteoside was
≥99% based on the HPLC analysis with a wavelength of 333 nm.
Cell culture
HSF cell line was cultured in Dulbecco’s modified
Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/ml
penicillin and 100 µg/ml streptomycin (Sigma-Aldrich) at
37°C in a humidified atmosphere of 5% CO2-95% air. The
cells were harvested and prepared for further analysis once
exponential growth was achieved.
Experimental design
The cells were divided into: i) Control group, which
was subjected to no radiation; ii) radiation group, in which the
cells were only subjected to radiation; iii) experimental group, in
which the confluent cells were preincubated with 50 µg/ml
acteoside for 2 h followed by radiation; and (iii) positive control
group, in which the cells were preincubated with 50 µg/ml
paeoniflorin followed by radiation. For the radiation, HSF cells
preincubated with acteoside or paeoniflorin were exposed to X-ray
beams with a dose-rate of 3 Gy/min generated from a Varian 2300 C/D
medical linear accelerator (Varian Medical Systems Inc., Palo Alto,
CA, USA). The radiation field was 25×25 cm at the isocenter in the
plane perpendicular to the beam. The total dose for the radiation
was 16.0 Gy.
MTT assay
Cell survival after radiation was determined using
an MTT assay as previously described (10). In brief, 2×104 cells
were plated in 200 µl culture medium in 96-well plates and
incubated for 48 h. At the indicated time points (2, 24, 48 and 72
h after radiation), a total of 20 µl MTT (Sigma-Aldrich)
solution in phosphate-buffered saline was added to each well. After
4 h of incubation, the supernatant was removed and 150 µl
dimethyl-sulfoxide was added to each well to terminate the
reaction. The absorbance at 570 nm was determined using a
Biokinetics plate reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Annexin V assay for apoptosis
Cellular apoptosis was determined using an Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit
(Invitrogen Life Technologies, Grand Island, NY, USA). In brief,
1×106 cells were harvested and washed with PBS. Then the
cells were resuspended in 500 µl binding buffer.
Subsequently, the cells were incubated in 5 µl Annexin
V-FITC and 10 µl prodium iodide (PI) solution for 5 min at
room temperature in the dark. Finally, FITC fluorescence was
analyzed by Expose ADC software (Beckman-Coulter, San Diego, CA,
US).
Cell cycle analysis
Cell cycle analysis was performed using a FACScan
flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose,
CA, USA). The cells were then washed with PBS (pH 7.4) and fixed
with 70% ice-cold ethanol at 4°C overnight. After that, the cells
were stained with PI solution (20 µg/ml) for 15 min at room
temperature. The percentage of cells in G1, S and G2/M phase of the
cell cycle was determined according to a previous study (11). Data acquisition and analysis were
controlled by CellQuest version 5.2 software (BD Biosciences, San
Diego, CA, USA).
Determination of ROS level
Generation of intracellular ROS was assessed as
described by Cathcart et al (12). Briefly, after radiation, HSF
obtained from the groups were incubated with 10 µM DCFH-DA
(Sigma-Aldrich) for 30 min. Subsequently, ROS production was
measured using a flow cytometer (Beckman-Coulter, San Diego, CA,
USA). The intensity of dichlorofluorescein (DCF) fluorescence was
measured with an excitation wavelength and an emission wavelength
of 485 and 530 nm, respectively.
Western blot analysis
Western blot analysis was performed as previously
described (13). In brief, the
cells were treated with 0.5 ml radioimmunoprecipitation assay
(RIPA) lysis buffer (Beijing ComWin Biotech Co., Ltd., Beijing,
China), followed by centrifugation at 16,363 × g at 4°C for 15 min.
The obtained proteins (100 µg) were separated by
electrophoresis on a 10% SDS-PAGE gel and transferred to a Hybond-P
polyvinyldifluoride (PVDF) membrane (EMD Millipore, Bedford, MA,
USA). Then the membrane was blocked with 5% (w/v) non-fat dry milk
and incubated with primary antibodies against procaspase-3 (1:200),
Bcl-2 (1:200), Bax (1:200), JNK (1:200), p-JNK (1:200), ERK
(1:200), p-ERK (1:200), and β-actin (1:3,000, Fude Biological
Technology, Hangzhou, China) overnight at 4°C. Then the mixture was
incubated with horseradish peroxidase-conjugated goat anti-mouse
IgG (1:5,000; Fude Biological Technology; cat. no. FD-GAM007) for 1
h at room temperature. After washing with PBS, the bound primary
antibody was visualized with the Enhanced Chemiluminescence system
from Amersham (Piscataway, NJ, USA) and exposed to film. The same
membrane was probed for β-actin (Boster Corporation, Wuhan, China),
a loading control. The relative density of proteins to β-actin was
analyzed with the AlphaEaseFC software (Genetic Technologies, Inc.
Miami, FL. USA).
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). SPSS 16.0 software (SPSS Inc., Chicago, IL, USA)
was used for data analysis. Analysis of variance was conducted to
compare the inter-group difference. P<0.05 was considered to
indicate a statistically significant difference.
Results
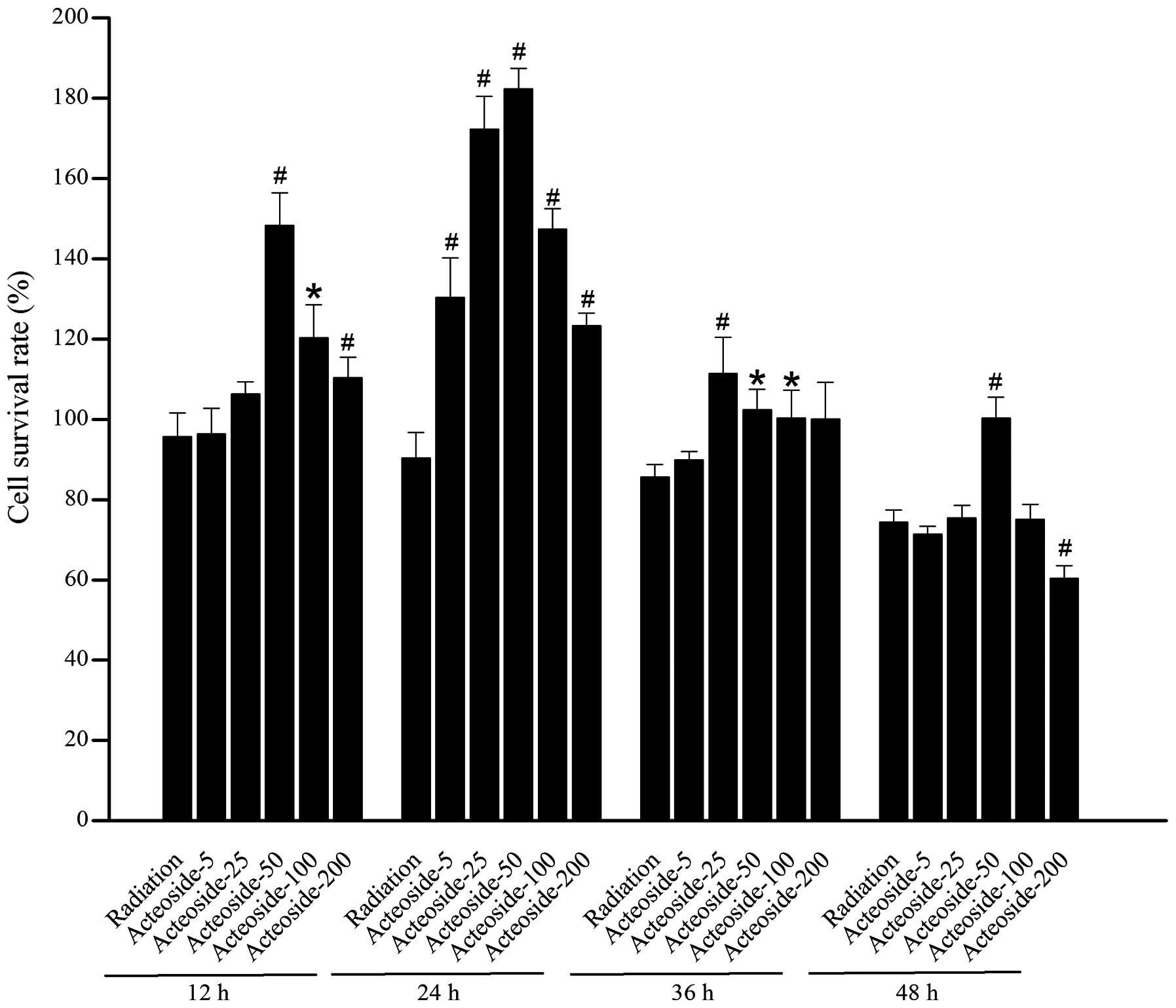
Effect of acteoside on the viability of
HSF
A marked decrease was noted in the cellular
viability of cells exposed to X-ray beams compared with the normal
control at 48 (P<0.05) and 72 h (P<0.05), respectively
(Table I). For the cells
pretreated using various concentrations of acteoside, higher cell
survival rates were obtained than that of radiation group (Fig. 2).
 | Table IProliferation activity indicated by
OD570 value of human skin fibroblasts. |
Table I
Proliferation activity indicated by
OD570 value of human skin fibroblasts.
| Incubation time
(h) | OD570
|
|---|
| Control group | Radiated group | Acteoside group |
|---|
| 2 | 0.288±0.025 | 0.280±0.023 | 0.421±0.058b |
| 24 | 0.306±0.020 | 0.287±0.025 | 0.547±0.072b |
| 48 | 0.437±0.037 | 0.358±0.020a | 0.445±0.079c |
| 72 | 0.576±0.075 | 0.425±0.076a | 0.585±0.073 |
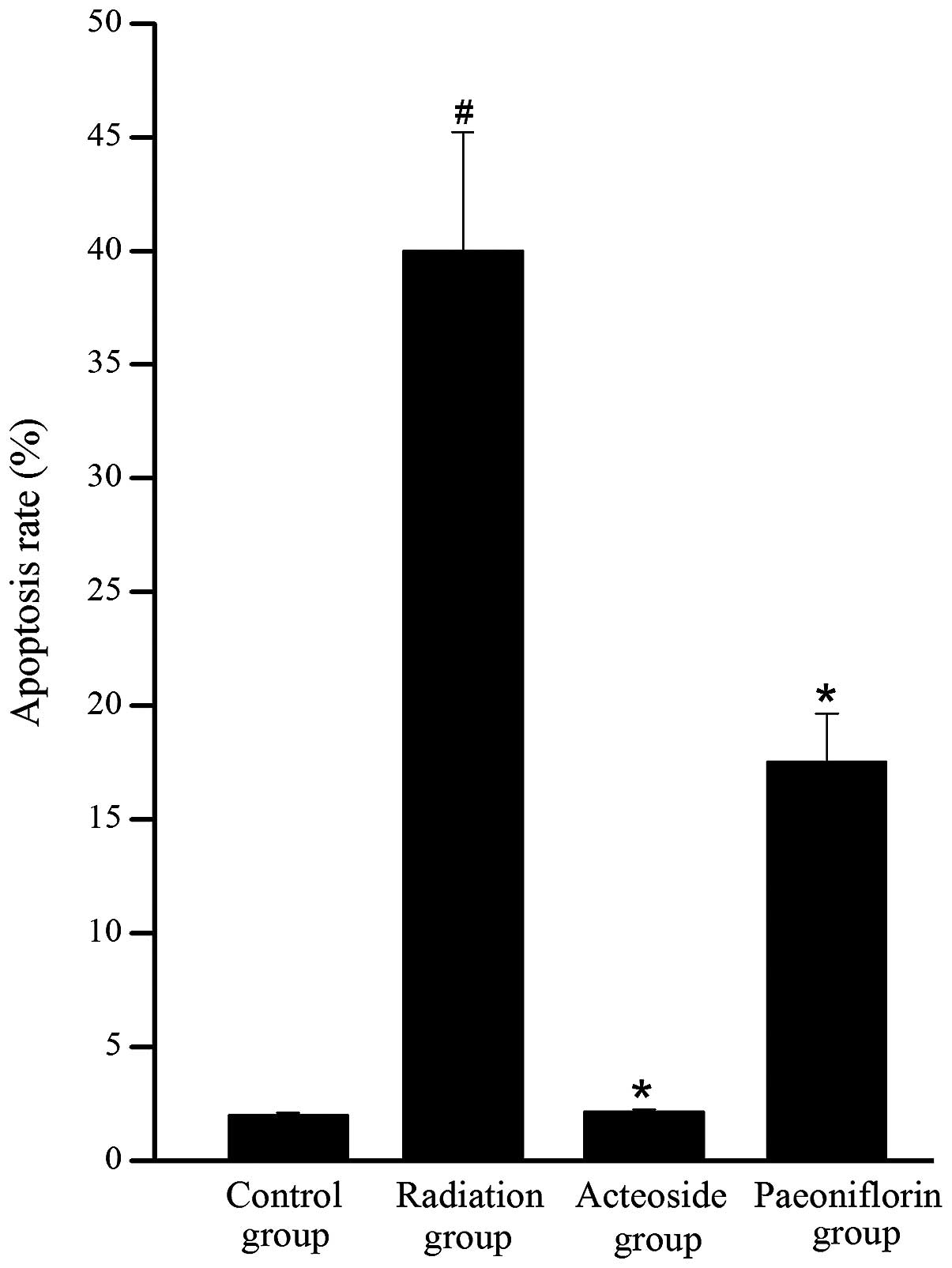
Effect of acteoside on cell
apoptosis
To investigate whether the reduction in cell
viability was due to apoptosis, cytometric analysis was performed
using a flow cytometer. As shown in Fig. 3, a significant increase was
observed in the apoptosis of cells subjected to radiation compared
with the control group (P<0.05). Conversely, the apoptosis rates
in the acteoside and paeoniflorin groups were significantly reduced
compared with that of the radiation group (P<0.05).
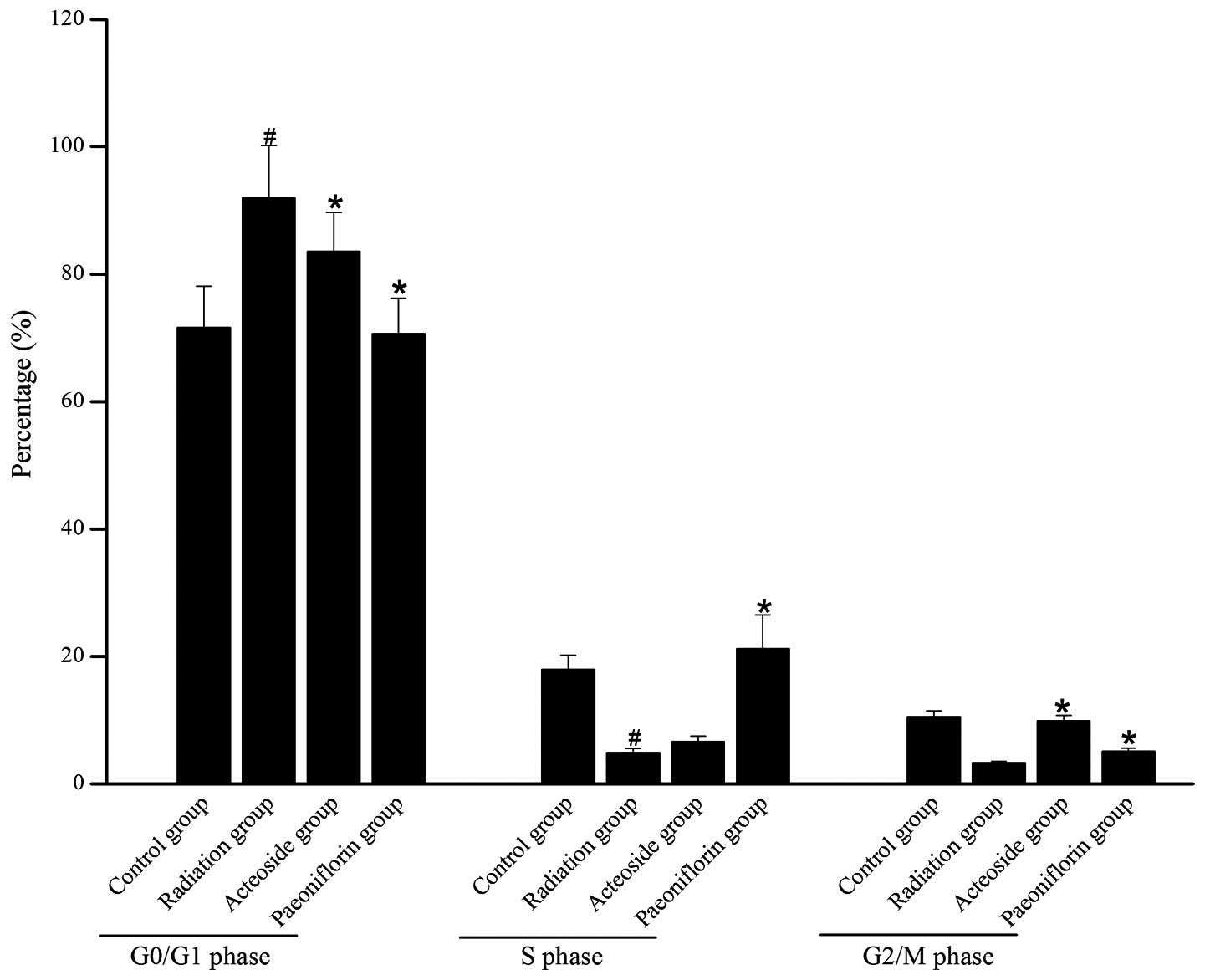
Effect of acteoside on the cell
cycle
Fig. 4 shows the
distribution of cells in different phases of the cell cycles in
each group. Compared with the control group, the majority of the
cells subjected to X-ray radiation were arrested at the G0/G1
phase, and few cells were arrested at S phase or G2/M phase. For
the irradiated cells pre-incubated with acteoside, the number of
cells blocked at G0/G1 phase was reduced compared with that of the
radiation group (P<0.05).
Effect of acteoside on cell production of
ROS
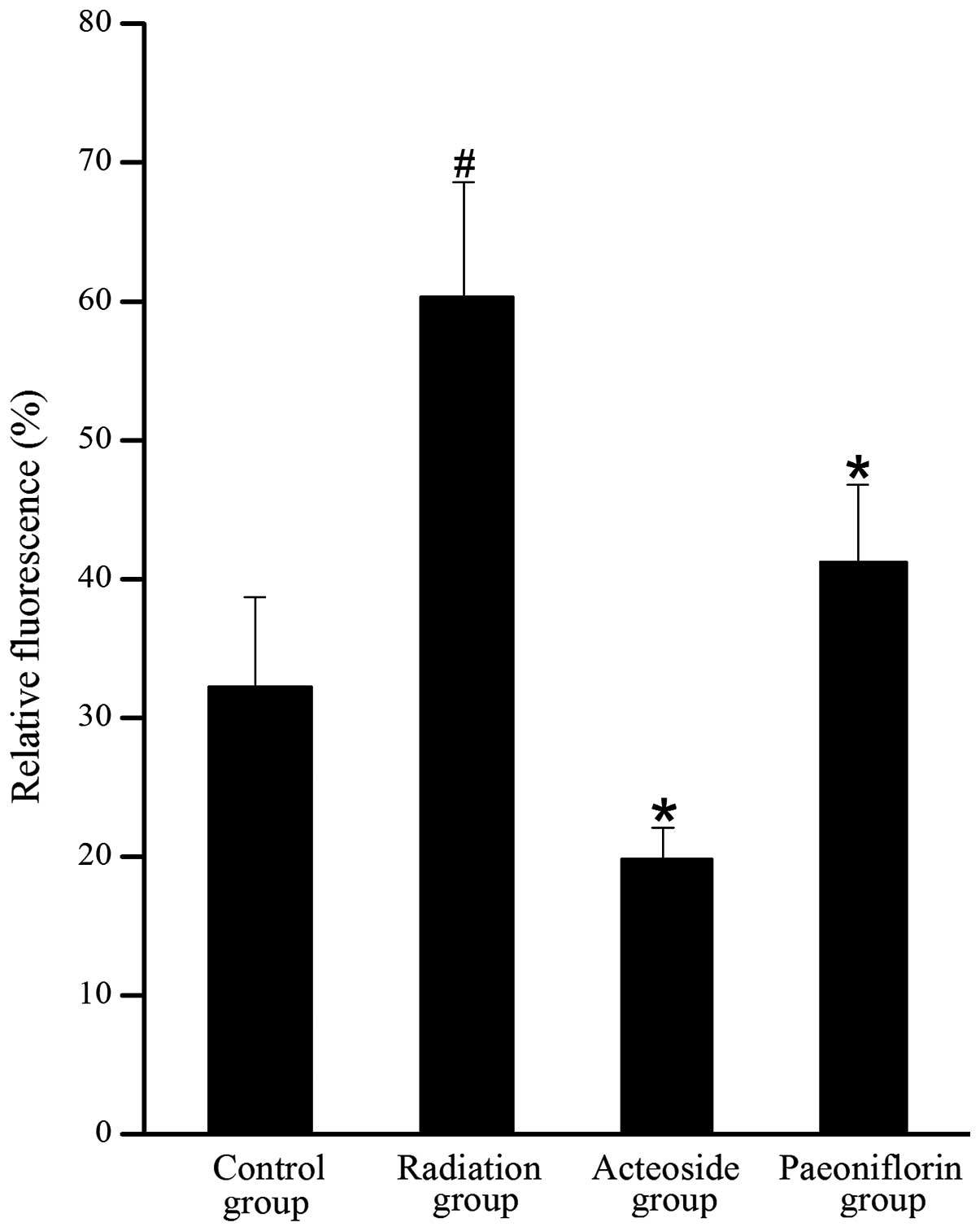
As shown in Fig. 5,
the level of DCFH-DA in the cells treated with X-ray was 1.8-fold
higher than that of the control group (P<0.01). Compared with
the radiation group, the generation of ROS was completely abrogated
by pre-incubation with acteoside or paeoniflorin (P<0.05).
Expression of apoptosis-associated
proteins
To investigate the effects of acteoside on the
expression of apoptosis-associated proteins, the expression of
procaspase-3, Bax, Bcl-2, p-ERK, ERK, p-JNK and JNK, was
determined. For the cells subjected to radiation, expression of
procaspase-3 was downregulated compared with that of the control
group (P<0.01, Fig. 6).
However, compared with the radiation group, expression of
procaspase-3 showed marked increase in the cells preincubated with
acteoside or paeoniflorin (P<0.01). Compared with the radiation
group, cells treated using acteoside or paeoniflorin showed
significant increase in the radiation group (P<0.05). The
expression of Bax was significantly upregulated in cells subjected
to radiation compared with that of the control group (P<0.01).
Nevertheless, for the cells preincubated with acteoside or
paeoniflorin, a significant decrease was noted in the expression of
Bax compared with the radiation group (P<0.05). In addition, the
radiation group showed a significant decrease in the expression of
Bcl-2 compared with the control group (P<0.01), and this
phenomenon was reversed in the acteoside and paeoniflorin groups.
The phosphorylation levels of ERK and JNK were significantly
increased after radiation compared with that of the control group
(P<0.01). However, the enhanced expression of phosphorylated ERK
and JNK was totally reversed by prior treatment with acteoside or
paeoniflorin at 50 µg/ml.
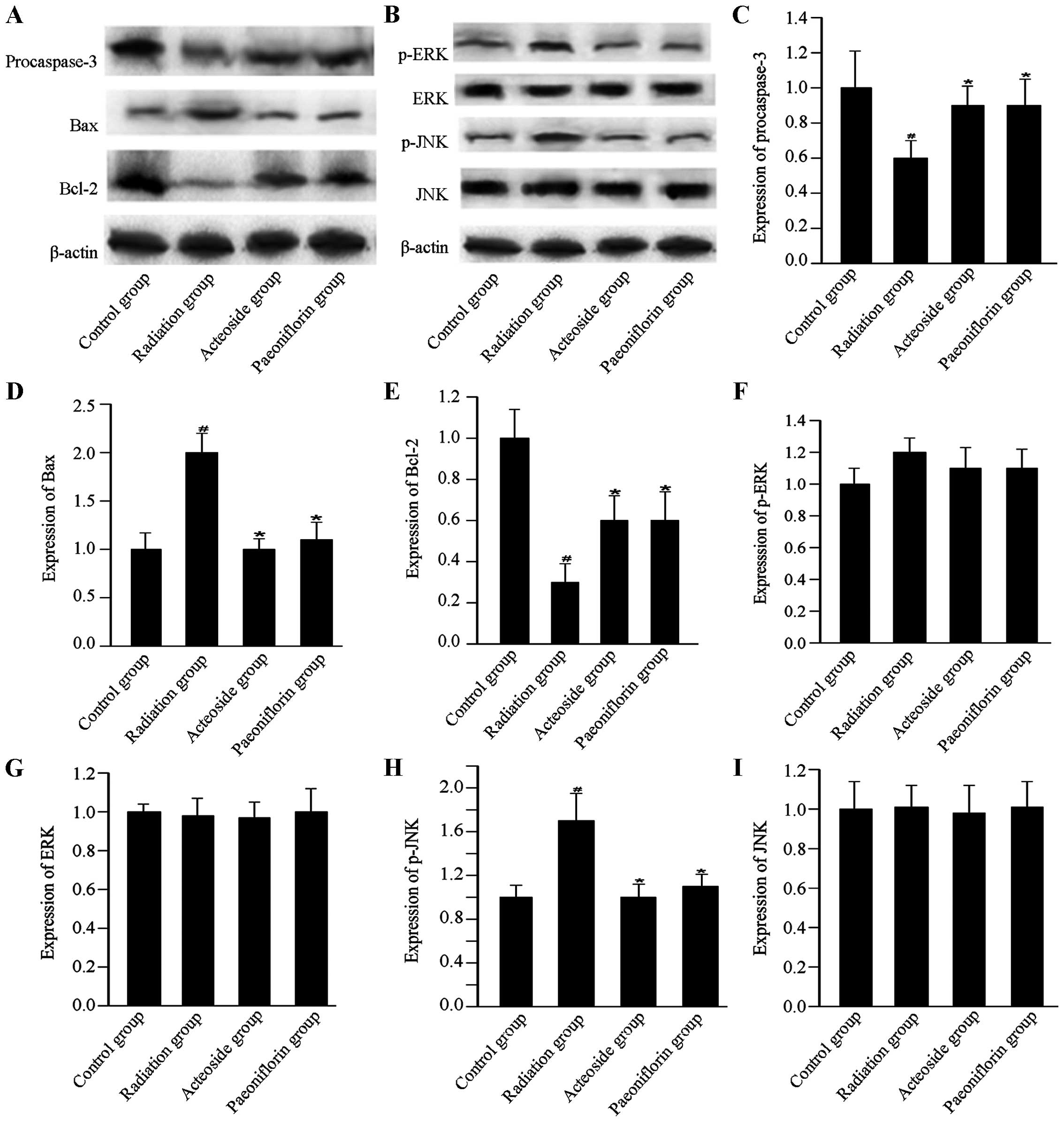 | Figure 6Effect of acteoside on the expression
of apoptosis-related proteins using western blot analysis. (A)
Levels of apoptosis-targeted proteins, including procaspase-3, Bax
and Bcl-2 in the control group, radiation group, acteoside group
and paeoniflorin group. (B) Levels of apoptosis-targeted proteins,
including p-ERK, ERK, p-JNK and JNK were examined using western
blot. The quantification of (C) procaspase-3, (D) Bax, (E) Bcl-2,
(F) p-ERK, (G) ERK, (H) p-JNK, and (I) JNK was normalized to
β-actin. #P<0.01 vs. control; *P<0.01
vs. the irradiation group. |
Discussion
C. salsa has been widely used in the
treatment of renal dysfunction and malnutrition (14). This study focused on the protective
effects of acteoside, an active component extracted from C.
salsa, on the radiation-induced apoptosis in vitro. The
results demonstrated that acteoside could attenuate the apoptosis
induced by X-ray beams through the removal of ROS, and involved in
the modulation of the mitogen-activated protein kinase (MAPK)
signaling pathway.
In previous studies, paeoniflorin has been reported
to show various pharmacological activities, including inhibiting
apoptosis (15,16). In this study, paeoniflorin was set
as a positive control, based on which to investigate the effects of
acteoside on radiation-induced injury. On this basis, the cell
survival rates of irradiated cells preincubated with paeoniflorin
and acteoside (with a concentration of 5, 25, 50, 100 and 200
µg/ml, respectively) were compared. The results showed that
acteoside could attenuate the cellular apoptosis of cells that
underwent radiation treatment. To investigate the potential
mechanism underlying this effect, the abrogation of intracellular
reactive oxygen species, the regulation of Bcl-2 family members,
the activation of caspases, as well as the modulation of MAPK
signaling pathways were determined.
Overproduction of ROS has been considered as the
major cause of oxidative stress, which results in apoptosis, aging,
tissue inflammation and degeneration (17). The scavenging activity of ROS has
been considered to contribute to the apoptosis-inhibiting effects
in vivo and in vitro (18). In the present study, HSF
preincubated with acteoside showed a significant decrease in the
generation of intracellular ROS. Therefore, it was hypothesized
that acteoside-induced cell protection may be correlated with the
scavenging effects on oxygen radicals. In addition, the assumption
is strongly supported by the results that acteoside could
effectively block the radiation-induced expression of
apoptosis-related proteins.
The susceptibility of cells to death signals is
dependent, in part, on the ratio between pro-apoptotic and
anti-apoptotic Bax/Bcl-2 proteins (19). Bcl-2 acts to prevent the release of
cytochrome c and caspase activation, while Bax has the
opposite function, which in turn promotes the release of cytochrome
c into the cytosol from mitochondria and activates caspase 3
(20,21). For the cells preincubated with
acteoside prior to radiation, the ratio of Bax/Bcl-2 decreased
compared with the cells that underwent radiation. This indicated
that acteoside was pivotal in apoptosis through inhibiting the
X-ray mediated Bax/Bcl-2 imbalance. Additionally, the decreased
expression of procaspase-3 induced by X-ray radiation was reversed
by pretreatment with acteoside. These results indicated that
acteoside could, at least in part, inhibit X-ray-induced apoptosis
in HSF cells.
The MAPK signaling pathway is important in the
transmission of extracellular signals to the nucleus. To the best
of our knowledge, there are three classes of MAPKs in mammals, JNK,
ERK and p38 MAPK. Generally, the activation of ERK contributed to
cell proliferation, while activation of JNK and/or p38MAPK is
important in the regulation of cell death (22). To date, the mechanism of how
acteoside is involved in the inhibition of cellular apoptosis is
still not well defined. In a previous study, Pu et al
(23) reported that acteoside
extracted from C. salsa could inhibit the cellular apoptosis
induced by 1-methyl-4-phenylpyridinium ion in cerebellar granule
neurons by inhibiting caspase activation. However, the role of
acteoside in the regulation of the MAPK signaling pathway is still
not well defined. In our study, the expression of p-JNK was
upregulated in the acteoside group compared with the radiation
group. Addtionally, the increased expression of p-ERK may
contribute to the cellular proliferation. All these facts may
contribute to the protective effects of acteoside on the inhibition
of radiation induced apoptosis. The results strongly suggested that
acteoside treatment could attenuate or counteract the radiation
damage in HSF cells.
In conclusion, this study demonstrates that
acteoside could protect the human skin fibroblasts from X-ray
induced apoptosis by scavenging the intracellular ROS, decreasing
Bax/Bcl-2 ratio and downregulating the activity of procas-pase-3,
as well as modulating the MAPK signaling pathways.
Acknowledgments
This study is supported by the National Nature
Science Foundation of China (grant no. 81060333), the National
Nature Science Foundation of China (grant no. 81360670) and
theApplication and Development Program Foundation from Technology
Board of Urumqi (grant no. Y111310032).
References
|
1
|
Mothersill C and Seymour C:
Radiation-induced bystander effects: are they good, bad or both?
Med Confl Surviv. 21:101–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang Y and Tu PF: Analysis of chemical
constituents in Cistanche species. J Chromatogr A. 1216:1970–1979.
2009. View Article : Google Scholar
|
|
3
|
Xuan GD and Liu CQ: Research on the effect
of phenylethanoid glycosides (PEG) of the Cistanche deserticola on
anti-aging in aged mice induced by D-galactose. Zhong Yao Cai.
31:1385–1388. 2008.In Chinese.
|
|
4
|
Geng X, Song L, Pu X and Tu P:
Neuroprotective effects of phenylethanoid glycosides from
Cistanches salsa against
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced
dopaminergic toxicity in C57 mice. Biol Pharm Bull. 27:797–801.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koo KA, Kim SH, Oh TH and Kim YC:
Acteoside and its aglycones protect primary cultures of rat
cortical cells from glutamate-induced excitotoxicity. Life Sci.
79:709–716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He ZD, Lau KM, Xu HX, Li PC, Pui-Hay and
But P: Antioxidant activity of phenylethanoid glycosides from
Brandisia hancei. J Ethnopharmacol. 71:483–486. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diaz AM, Abad MJ, Fernández L, Silván AM,
De Santos J and Bermejo P: Phenylpropanoid glycosides from
Scrophularia scorodonia: in vitro anti-inflammatory activity. Life
Sci. 74:2515–2526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lei L, Yang F, Zhang T, Tu P, Wu L and Ito
Y: Preparative isolation and purification of acteoside and
2′-acetyl acteoside from Cistanches salsa (C.A. Mey) G Beck by
high-speed counter-current chromatography. J Chromatogr A.
912:181–185. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Gan L, Li GQ, Deng L, Zhang X and
Deng Y: Pharmacokinetics of plantamajoside and acetoside from
Plantago asiatica in rats by liquid chromatography-mass
spectrometry. J Pharm Biomed Anal. 89:251–256. 2014. View Article : Google Scholar
|
|
10
|
Demidenko ZN, Vivo C, Halicka HD, et al:
Pharmacological induction of Hsp70 protects apoptosis-prone cells
from doxorubicin: comparison with caspase-inhibitor- and
cycle-arrest-mediated cytoprotection. Cell Death Differ.
13:1434–1441. 2006. View Article : Google Scholar
|
|
11
|
Chiu SJ, Lee MY, Chen HW, Chou WG and Lin
LY: Germanium oxide inhibits the transition from G2 to M phase of
CHO cells. Chem Biol Interact. 141:211–228. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cathcart R, Schwiers E and Ames BN:
Detection of picomole levels of hydroperoxides using a fluorescent
dichlorofluorescein assay. Anal Biochem. 134:111–116. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurien BT and Scofield RH: Western
blotting. Methods. 38:283–293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chinese Pharmacopoeial Commission:
Pharmacopoeia of the People’s Republic of China. 1. People’s
Medical Publishing House; Beijing, China: pp. 1262010
|
|
15
|
Hu S, Sun W, Wei W, et al: Involvement of
the prostaglandin E receptor EP2 in paeoniflorin-induced human
hepatoma cell apoptosis. Anticancer Drugs. 24:140–149. 2013.
View Article : Google Scholar
|
|
16
|
Tsuboi H, Hossain K, Akhand AA, et al:
Paeoniflorin induces apoptosis of lymphocytes through a
redox-linked mechanism. J Cell Biochem. 93:162–172. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valko M, Rhodes CJ, Moncol J, Izakovic M
and Mazur M: Free radicals, metals and antioxidants in oxidative
stress-induced cancer. Chem Biol Interact. 160:1–40. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
An Z, Qi Y, Huang D, et al: EGCG inhibits
Cd(2+)-induced apoptosis through scavenging ROS rather than
chelating Cd(2+) in HL-7702 cells. Toxicol Mech Methods.
24:259–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang HL, Chen CS, Chang WH, et al: Growth
inhibition and induction of apoptosis in MCF-7 breast cancer cells
by Antrodia camphorata. Cancer Lett. 231:215–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: a
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ryan KM, Phillips AC and Vousden KH:
Regulation and function of the p53 tumor suppressor protein. Curr
Opin Cell Biol. 13:332–337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Junttila MR, Li SP and Westermarck J:
Phosphatase-mediated crosstalk between MAPK signaling pathways in
the regulation of cell survival. FASEB J. 22:954–965. 2008.
View Article : Google Scholar
|
|
23
|
Pu X, Song Z, Li Y, Tu P and Li H:
Acteoside from Cistanche salsa inhibits apoptosis by
1-methyl-4-phenylpyridinium ion in cerebellar granule neurons.
Planta Med. 69:65–66. 2003. View Article : Google Scholar : PubMed/NCBI
|