Introduction
During the past decade, a large number of studies
have shown that Rho-kinase (ROCK) is one of the major kinases
involved in cell movement. It participates in the RhoA-ROCK
signaling pathway and regulates smooth muscle cell contraction by
regulating the Ca2+ sensitization mechanism (1–4).
The levels of myosin light chain (MLC)
phosphorylation, which is dually regulated by
Ca2+/calmodulin (CaM)-dependent myosin light chain
kinase (MLCK) and Ca2+-independent myosin light chain
phosphatase (MLCP), is an important factor affecting the extent of
smooth muscle contraction (5). The
regulation of MLC phosphorylation by MLCK causes smooth muscle
contraction, whereas the inhibition of MLCP can enhance the extent
of MLC phosphorylation and smooth muscle contraction and increase
Ca2+ sensitivity, a phenomenon known as Ca2+
sensitization (6,7).
In the RhoA-ROCK signaling pathway, several agonists
activate RhoA through a series of mechanisms, leading to ROCK
activation and the regulation of Ca2+ sensitization,
which enhance gallbladder smooth muscle contraction (8,9).
Studies have shown that ROCK inhibitors, including Y-27632 and
fasudil, can inhibit smooth muscle contraction (10,11),
which prompted us to study the mechanisms by which they affect the
RhoA-ROCK pathway and Ca2+ sensitization.
In the present study, changes in ROCK expression in
gallbladder smooth muscle cells were assessed and the role of ROCK
protein in the regulation of gallbladder contractility during the
lithogenic process was investigated using a guinea pig model of
gallstone formation. The findings of the present study provided
experimental evidence on animals, which contributes to the
elucidation of the etiology of gallstone formation and may aid in
its prevention.
Materials and methods
Experimental animals
30 Male guinea pigs weighing 250–300 g were obtained
from the Experimental Animal Center of Renmin Hospital of Wuhan
University (Wuhan, China). The animals were randomly divided into
the control group, the gallstone model group and the fasudil
interference group.
Animal models
Gallstone development was induced in the guinea pigs
in accordance with previously described methods (12). The control group was fed a normal
diet, whereas the model group and fasudil group were fed a 2%
cholesterol-enriched diet for 60 days. From the 20th day onward,
the fasudil group were treated with the known ROCK inhibitor,
fasudil (5 mg/kg; Chase Sun Pharmaceutical Co., Ltd., Tianjin,
China), by intraperitoneal injection (0.2 ml fasudil per 100 g body
weight diluted in normal saline) twice a day. Animals in the
control group and model group were injected intraperitoneally with
the same volume of sterile saline twice daily.
Specimen collection
Fasting gallbladder volume (FV) and
fasting gallbladder bile volume (FB)
Five guinea pigs from each group were randomly
selected on days 30 and 60, respectively. After a 12-h fast, the
guinea-pigs were anesthetized by intraperitoneal injection of 6%
chloral hydrate (350 mg/kg; Hubei Hechang Chemical Co., Ltd.,
Wuhan, China). The longest length and two perpendicular diameters
[width (W), height (H)] of the gallbladder were measured under
natural conditions. The cystic duct was blocked with a vascular
clamp and the volume of bile drawn from the gallbladder was
recorded (FB). The gallbladder bile was stored for determination of
total cholesterol (TC) and triglyceride (TG) contents.
Collection of gallbladder wall
tissue
After removing the gallbladder, the gallbladder
lumen was cut longitudinally to determine whether it contained
biliary sludge or gallstones. The gallbladder was washed using cold
saline, and the residual tissue in the gallbladder wall was
stripped off. A number of gallbladder tissue samples were clipped
from the gallbladder body, rinsed three times with
phosphate-buffered saline (Wuhan Boster Biological Engineering Co.,
Ltd., Wuhan, China), fixed with 10% formaldehyde solution (Hubei Ju
Sheng Technology Co., Ltd., Hubei, China), dehydrated and embedded
in paraffin (Wuhan Boster Biological Engineering Co., Ltd.) for
immunohistochemical analysis. Further tissue samples were
snap-frozen in liquid nitrogen and stored at −80°C.
Detection of methods
FV and FB
The longest length and two perpendicular diameters
(W, H) of the guinea-pig gallbladder were measured under natural
conditions. These results were used to calculate FV using Dodd’s
ellipsoid formula, V=0.52xLxWxH (ml). A vascular clamp was used to
block the cystic duct, and the total volume of the bile drawn from
the gallbladders was recorded (FB; ml).
TC and TG
TC and TG contents were determined using a TC and TG
kit (Sigma-Aldrich, St. Louis, MO, USA) on an Bayer Advia 2400
automatic biochemical analyzer; Siement, Germany) using the oxidase
and enzyme colorimetric methods, respectively (performed by the
Department of Laboratory, Renmin Hospital of Wuhan University,
Wuhan, China).
ROCK expression
ROCK expression in the gallbladder smooth muscle
tissues was detected using immunohistochemical methods.
Immunohistochemistry was conducted according to the manufacturer’s
instructions of the SP-9000 kit (Boster Biological Technology Co,
Ltd., Wuhan, China). The evaluation standard was as follows: All
cells were counted in 10 randomly selected high-power fields and
semi-quantitative results were evaluated on the basis of the degree
of staining and the percentage of stained cells. The paraffin
sections were de-waxed using xylene (Shanghai Senfi Chemical Co.,
Ltd., Shanghai, China) for antigen retrieval by heat-induced
retrieval using citrate buffer (Shanghai Gunsuo Biological
Technology Co., Ltd., Shanghai, China) and following elimination of
the endogenous peroxidase activity, the sections were blocked with
goat serum. Next, the unlabeled rabbit anti-mouse polyclonal
antibody (cat. no. BA1701-1; Wuhan Boster Biological Engineering
Co., Ltd.) primary antibody was added and incubated for 1–2 h. The
biotin-labeled goat anti-rabbit polyclonal secondary antibody (cat.
no. BA1003; Wuhan Boster Biological Engineering Co., Ltd.) was then
added and incubated at 37°C for 15 min, followed by horseradish
peroxidase-labeled streptavidin for 15 min. The diaminobenzidine
chromogenic kit was used to stain the tissue sections, which was
followed by re-staining with hematoxylin. The sections were
observed under a light microscope (Olympus CX31-LV320; Olympus Co.,
Ltd., Beijing, China), with the positive cells displaying
brown-yellow staining. The degree of cell staining was scored as
follows: 0, no or negligible staining; 1, pale yellow staining; 2,
brown-yellow staining; 3, brown staining.
All immunohistochemical tissue sections were
analyzed by a computer image analysis program under identical
conditions, and the average optical density in the image was
regarded as the positive rate of ROCK expression (13).
Statistical analysis
Data were analyzed by SPSS 16.0 statistical and
processing software (SPSS, Inc, Chicago, IL, USA). Values are
expressed as the mean ± standard error and were analyzed by one-way
analysis of variance, whereas the counts are expressed as
percentages and were analyzed using the χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Changes of the FV and FB and gallstone
formation
In the model group, biliary sludge and gallstone
formation was observed in one guinea pig on day 30 and in three
animals on day 60, while the two and six counterparts in the
fasudil group were affected, respectively.
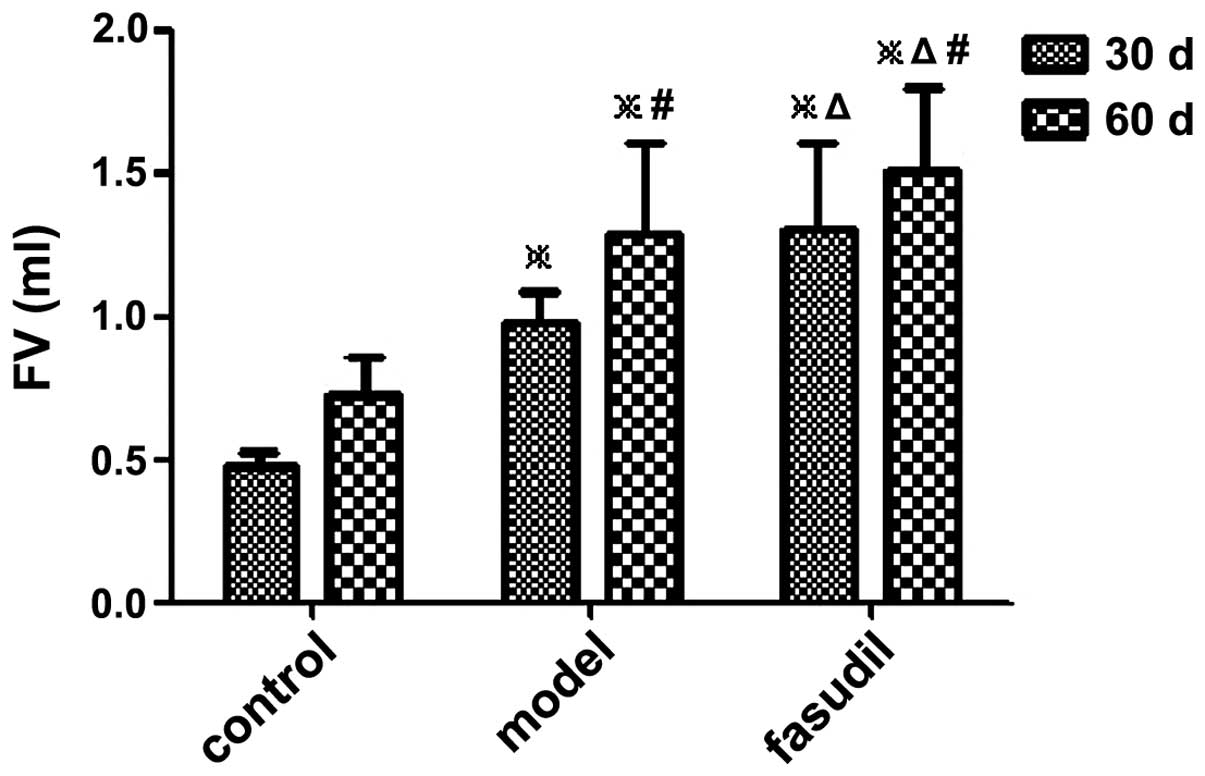
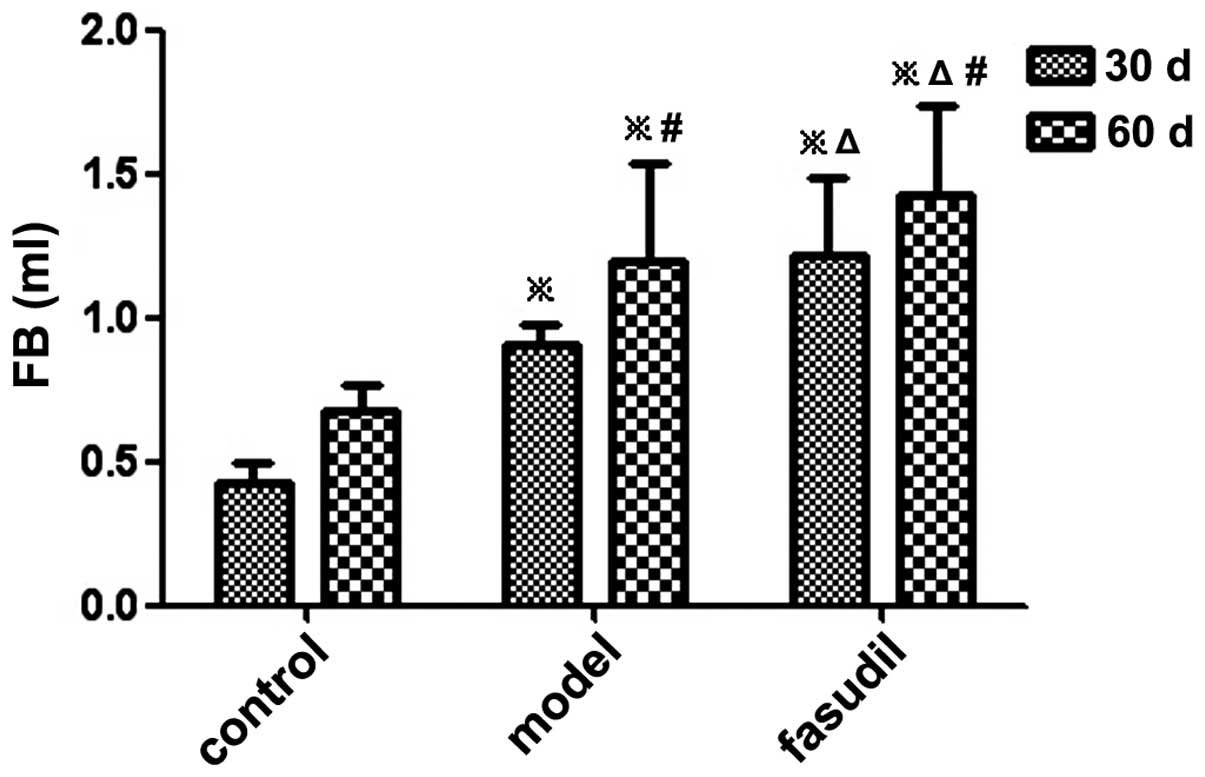
The FV in the fasudil group was larger than that in
the model group at the same time-point. There was a significant
difference in the FV and FB between the model group and the fasudil
group, and differences between the three groups were also
significant (P<0.05) (Figs. 1
and 2; Table I).
 | Table IComparison of gallstone formation
rate, FV and FB. |
Table I
Comparison of gallstone formation
rate, FV and FB.
| Group | n | Rate of gallstone
formation (%)
| FV (cm3)
| FB (ml)
|
|---|
| 30 days | 60 days | 30 days | 60 days | 30 days | 60 days |
|---|
| Control | 10 | 0 | 0 | 0.48±0.05 | 0.73±0.13 | 0.43±0.07 | 0.68±0.09 |
| Model | 10 | 10a | 30a,c | 0.98±0.11a | 1.29±0.32a,c | 0.91±0.07a | 1.20±0.34a,c |
| Fasudil | 10 | 20a,b | 60a,b,c | 1.31±0.30a,b | 1.51±0.29a,b,c | 1.22±0.27a,b | 1.43±0.31a,b,c |
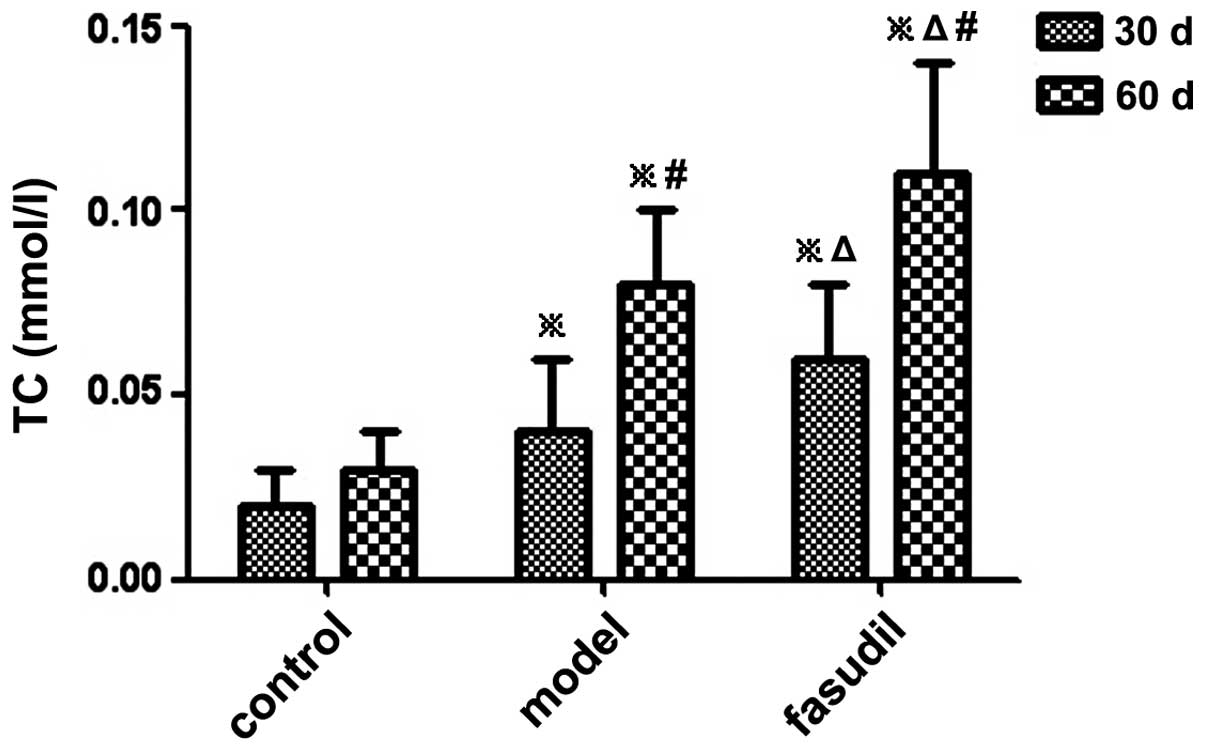
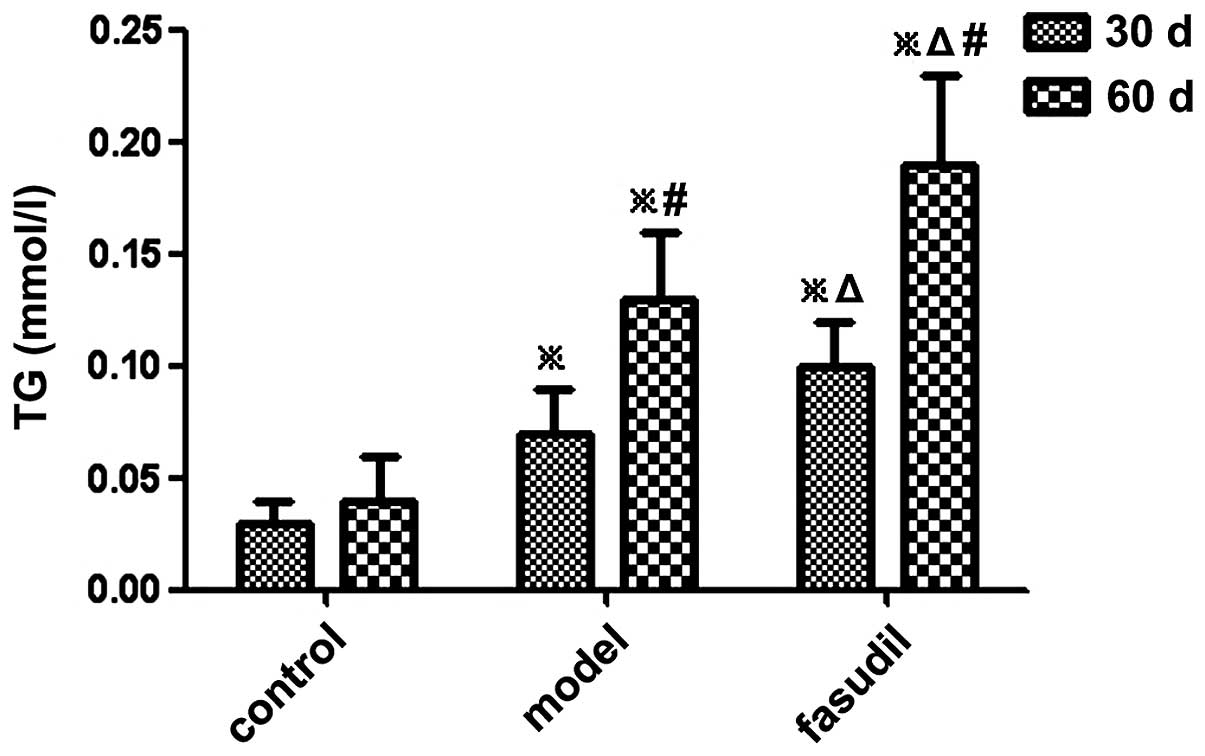
Changes in TC and TG contents in
gallbladder bile
The levels of TC and TG in the gallbladder bile of
guinea pigs were significantly increased in the model group and the
fasudil group compared with those in the control group (P<0.05)
(Figs. 3 and 4; Table
II).
 | Table IIComparison of TC and TG in bile. |
Table II
Comparison of TC and TG in bile.
| Group | n | TC (mmol/l)
| TG (mmol/l)
|
|---|
| 30 days | 60 days | 30 days | 60 days |
|---|
| Control | 10 | 0.02±0.01 | 0.03±0.01 | 0.03±0.01 | 0.04±0.02 |
| Model | 10 | 0.04±0.02a | 0.08±0.02a,c | 0.07±0.02a | 0.13±0.03a,c |
| Fasudil | 10 | 0.06±0.02a,b | 0.11±0.03a,b,c | 0.10±0.02a,b | 0.19±0.04a,b,c |
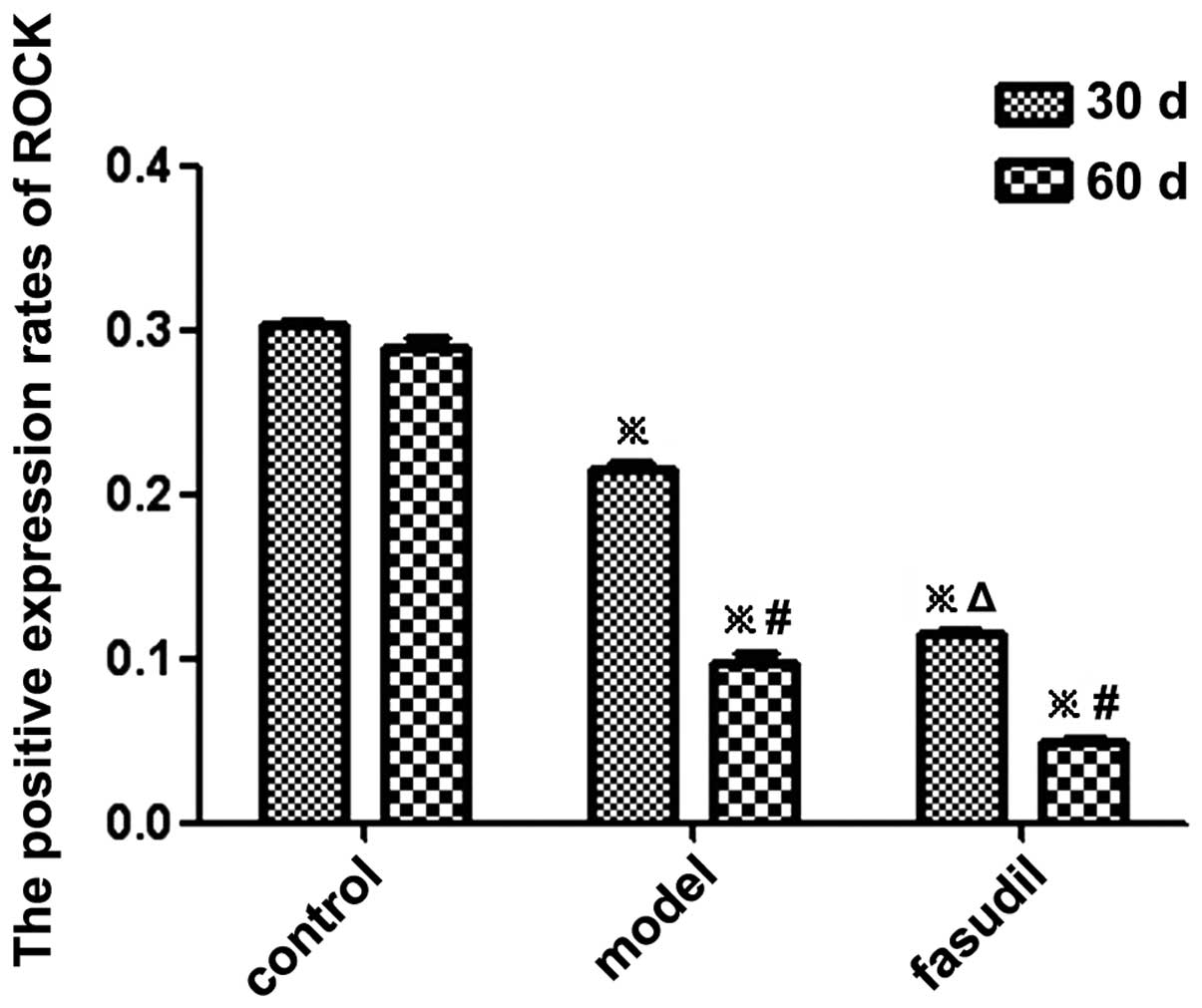
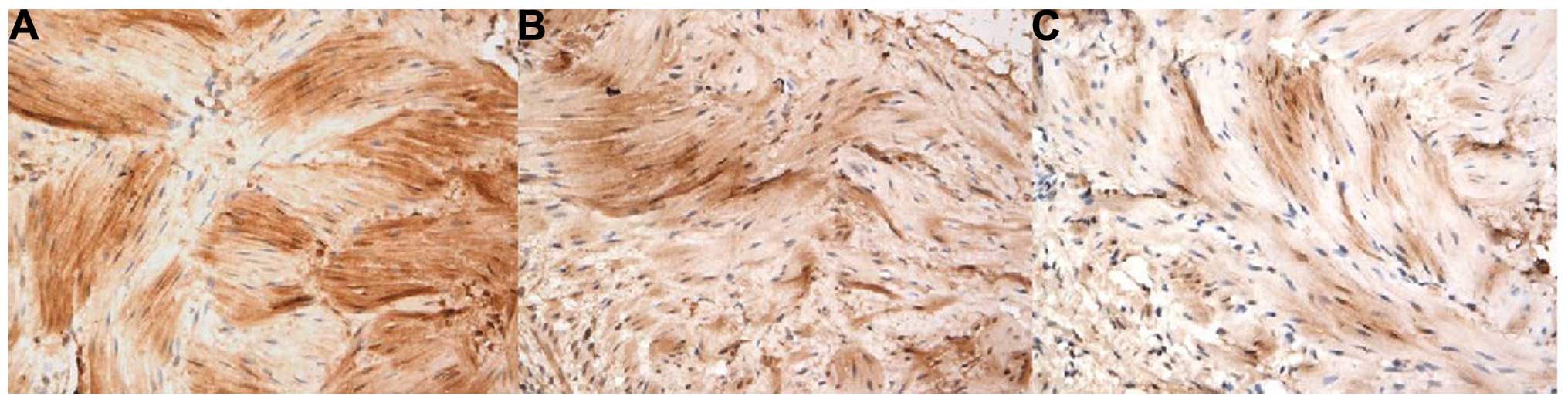
ROCK expression in gallbladder smooth
muscles
The presence of yellow granules indicated positive
expression of ROCK in gallbladder smooth muscle cells, and the
optical density value, which was obtained using computer image
analysis software (Image-Pro Plus 5.0; Media Cybernetics, Inc.,
Rockville, MD, USA), was used to quantify the expression rate of
ROCK. It was observed that the positive expression rate of ROCK in
the model group and the fasudil group was significantly lower than
that in the control group (P<0.05) (Figs. 5 and 6; Table
III).
 | Table IIIExpression of ROCK in gallbladder
smooth muscle. |
Table III
Expression of ROCK in gallbladder
smooth muscle.
| Group | n | ROCK (OD)
|
|---|
| 30 days | 60 days |
|---|
| Control | 10 | 0.3025±0.0025 | 0.2889±0.0056 |
| Model | 10 |
0.2145±0.0051a |
0.0981±0.0046a,c |
| Fasudil | 10 |
0.1153±0.0022a,b |
0.0494±0.0023a,b,c |
Discussion
Cholelithiasis is a common medical condition
worldwide, whose mechanism has not been completely elucidated to
date. Its incidence rate is 10–20% in western countries (2). The incidence of gallstones is
increasing along with the improvement of the standard of living
conditions and due to rare use of ultrasound and other forms of
medical examination, it increased from 2–7% (3) to 8–10% (4) today. >80% of cholelithiasis cases
are gallstone-associated conditions (14). Researchers have attached great
importance to the pathological significance of gallbladder in
cholesterol-associated gallstone formation in the previous studies
(15–17). Studies suggested that gallstone
formation was not totally dependent on the physical and chemical
changes of bile. The gallbladder, and particularly its dysfunctions
regarding its contraction, have a key role in
cholesterol-associated gallstone formation (18). In 1983, Doty found that the
emptying function of the gallbladder decreased significantly during
the formation of cholesterol monohydrate crystals (CMCs) (19). Further evidence provided by flash
radiography and ultrasound analysis indicated that the emptying and
refilling of the gallbladder with bile and bile metabolism were
clearly inhibited in patients with cholecystolithiasis (20).
The present study investigated changes in the
expression of ROCK, an important regulatory enzyme in the pathway
of gallbladder smooth muscle contraction regulation, and further
explored its role in gallbladder smooth muscles contraction
function and gallstone formation. The results showed that the FV
and FB of guinea pigs were significantly increased after receiving
a high-cholesterol diet for up to 60 days. The contents of TC and
TG in bile were elevated with increasing time of receiving the
high-cholesterol diet. One guinea pig was observed to have
gallstones on day 30, and three animals had gallstones on day 60,
while no gallstones were observed in the control group. This
suggested that the increase of the cholesterol concentration in the
gallbladder bile may have an important role in gallstone formation.
Cholesterol may impair the contraction of the gallbladder smooth
muscles, favoring gallstone formation. The same phenomenon was
found in fasudil group, in which eight guinea pigs presented with
gallstone formation.
Furthermore, ROCK expression in gallbladder smooth
muscles was markedly reduced in the model group and the fasudil
group. The reduction in ROCK levels may be one of the main reasons
for the inhibition of the gallbladder contraction function in
guinea pigs following cholesterol intake or fasudil treatment.
The primary mechanism by which ROCK regulates smooth
muscle cell contraction is the phosphorylation and
dephosphorylation of MLC. MLC is phosphorylated by CaM-dependent
MLCK and dephosphorylated by Ca2+-independent MLCP.
Therefore, the intracellular elevation of Ca2+ levels
results in MLCK activation and subsequent phosphorylation of MLC,
finally leading to the contraction of smooth muscle cells (21,22).
However, MLC phosphorylation and the tension of smooth muscles do
not rely on increased intracellular levels of Ca2+.
Certain agonists, including phenylephrine, acetylcholine, U-44619
and endothelin (3–5), can combine with G protein-coupled
receptors, inducing the contraction of smooth muscle cells through
enhancing intracellular Ca2+ levels and Ca2+
sensitivity (23,24). The inhibition of MLCP may increase
Ca2+ sensitivity in smooth muscle cells and further
increase the extent of MLC phosphorylation and smooth muscle
tension at a constant Ca2+ concentration, which in turn
causes myofilament contraction. When ROCK expression is inhibited
or reduced, MLC phosphorylation is decreased, whereas the MLCP
activity is enhanced.
In the present study, the expression of ROCK was
shown to be reduced alongside gallbladder stone formation following
feeding on a high-cholesterol diet or treatment with fasudil. The
underlying mechanism is likely to be the inhibitory effect of ROCK
on MLCP. The decreased expression of ROCK enhanced the activation
of MLCP, decreased the phosphorylation of MLC and weakened the
contraction of gallbladder smooth muscle cells. All of the above
mechanisms promote cholestasis and gallstone formation. The results
of the present study indicated that ROCK expression has an
important role in gallbladder contraction function and may be
involved in gallstone formation.
In the fasudil treatment group, the expression of
ROCK in gallbladder smooth muscles at the same time-point was
decreased more markedly than that in the model group, while the
rate of gallstone formation was identical in the two groups.
This implied that fasudil is able to significantly
inhibit ROCK expression in gallbladder smooth muscles and impair
their contraction, therefore inhibiting their emptying function of
the gallbladder and favoring gallstone formation as a result. High
cholesterol diets as well as fasudil were able to significantly
inhibit ROCK expression, leading to the promotion of gallstone
formation. Therefore, enhancement of the expression of ROCK in
gallbladder smooth muscles may be a novel strategy for the
prevention and treatment for cholelithiasis.
Acknowledgments
The authors would like to thank the Central
Laboratory of Renmin Hospital of Wuhan University for excellent
technical assistance.
References
|
1
|
Somlyo AP and Somlyo AV: Ca2+
sensitivity of smooth muscle and non-muscle myosin II: Modulated by
G proteins, kinases and myosin phosphatase. Physiol Rev.
83:1325–1358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seasholtz TM, Majumdar M and Brown JH: Rho
as a mediator of G protein-coupled receptor signaling. Mol
Pharmacol. 55:949–956. 1999.PubMed/NCBI
|
|
3
|
Mack CP, Somlyo AV, Hautmann M, Somlyo AP
and Owens GK: Smooth muscle differentiation marker gene expression
is regulated by RhoA-mediated actin polymerization. J Biol Chem.
276:341–347. 2001. View Article : Google Scholar
|
|
4
|
Swärd K, Mita M, Wilson DP, Deng JT,
Susnjar M and Walsh MP: The role of RhoA and Rho-associated kinase
in vascular smooth muscle contraction. Curr Hypertens Rep. 5:66–72.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Emmert DA, Fee JA, Goeckeler ZM, Grojean
JM, Wakatsuki T, Elson EL, Herring BP, Gallagher PJ and Wysolmerski
RB: Rho-kinase-mediated Ca2+ independent contraction in
rat embryo fibroblasts. Am J Physiol Cell Physiol. 286:C8–C21.
2004. View Article : Google Scholar
|
|
6
|
Pfitzer G: Regulation of myosin
phosphorylation in smooth muscle. J Appl Physiol. 91:497–503.
2001.
|
|
7
|
Camello-Almaraz C, Macias B, Gomez-Pinilla
PJ, Alcon S, Martin-Cano FE, Baba A, Matsuda T, Camello PJ and Pozo
MJ: Developmental changes in Ca2+homeostasis and contractility in
gallbladder smooth muscle. Am J Physiol Cell Physiol.
296:C783–C791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amano M, Ito M, Kimura K, Fukata Y,
Chihara K, Nakano T, Matsuura Y and Kaibuchi K: Phosphorylation and
activation of myosin by Rho-associated kinase (Rho-kinase). J Biol
Chem. 271:20246–20249. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Otto B, Steusloff A, Just I, Aktories K
and Pfitzer G: Role of Rho proteins in carbachol-induced
contractions in intact and permeabilized guinea-pig intestinal
smooth muscle. J Physiol. 496:317–329. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nobe K and Paul RJ: Distinct pathways of
Ca (2+) sensitization in porcine coronary artery: effects of
Rho-related kinase and protein kinase C inhibition on force and
intracellular Ca (2+). Circ Res. 88:1283–1290. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iizuka K, Shimuza Y, Tsukagoshi H, Yoshii
A, Harada T, Dobashi K, Murozono T, Nakazawa T and Mori M:
Evaluation of Y-27632, a rho-kinase inhibitor, as a bronchodilator
in guinea pigs. Eur J Pharmacol. 406:273–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Everson GT: Gallbladder function in
gallstone disease. Gastroenterol Clin North Am. 20:85–110.
1991.PubMed/NCBI
|
|
13
|
Yang Jian-Ru: Average optical density in
quantitative medical image analysis. China JMIT. 4:322–323.
1999.
|
|
14
|
Tzovaras G and Rowlands BJ: Diagnosis and
treatment of sphincter of Oddi dysfunction. Br J Surg. 85:588–595.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Portincasa P, Di Ciaula A and van
Berge-Henegouwen GP: Smooth muscle function and dysfunction in
gallbladder disease. Curr Gastroenterol Rep. 6:151–162. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Erpecum KJ, Wang DQ, Moschetta A,
Ferri D, Svelto M, Portincasa P, Hendrickx JJ, Schipper M and
Calamita G: Gallbladder histopathology during murine gallstone
formation: relation to motility and concentrating function. J Lipid
Res. 47:32–41. 2006. View Article : Google Scholar
|
|
17
|
Lavoie B, Nausch B, Zane E, Leonard M,
Balemba O, Bartoo, Wilcox R, Nelson MT, Carey MC and Mawe G:
Disruption of gallbladder smoth muscle function is an early feature
in the development of cholesterol gallstone disease.
Neurogastroenterol Motil. 24:313–329. 2012. View Article : Google Scholar
|
|
18
|
Carey MC: Pathogenesis of gallstones. Am J
Surg. 165:410–419. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doty JE, Pitt HA, Kuchenbecker SL and
DenBesten L: Impaired gallbladder emptying before gallstone
formation in the prairie dog. Gastroenterology. 85:168–174.
1983.PubMed/NCBI
|
|
20
|
Jazrawi RP, Pazzip and Petroni ML:
Postgrandial gallbladder motor function: refilling and turnover of
bile in health and in cholelithiasis. Gastroenterology.
109:582–591. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sahan-Firat S, Tiftik RN, Nacak M and
Büyükafşar K: Rho kinase expression and its central role in ovine
gallbladder contractions elicited by a variety of excitatory
stimuli. Eur J Pharmacol. 528:169–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quinn T, Feighery R and Baird AW: Role of
Rho-kinase in guinea-pig gallbladder smooth muscle contraction. Eur
J Pharmacol. 534:210–217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uehata M, Ishizaki T, Satoh H, Ono T,
Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M
and Narumiya S: Calcium sensitization of smooth muscle mediated by
a Rho-associated protein kinase in hypertension. Nature.
389:990–994. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Büyükafşar K, Akça T, Nalan Tiftik R, et
al: Contribution of Rho-kinase in human gallbladder contractions.
Eur J Pharmacol. 540:162–167. 2006. View Article : Google Scholar
|