Introduction
Flavonoids from natural biological sources,
including tea and fruit, and have attracted considerable attention
for their applicability in the prevention and treatment of stroke
(1). Epidemiological evidence
supported the therapeutic potential of these compounds in a variety
of pre-clinical models of ischemic brain injury (2). Quercetin is a flavonoid and an
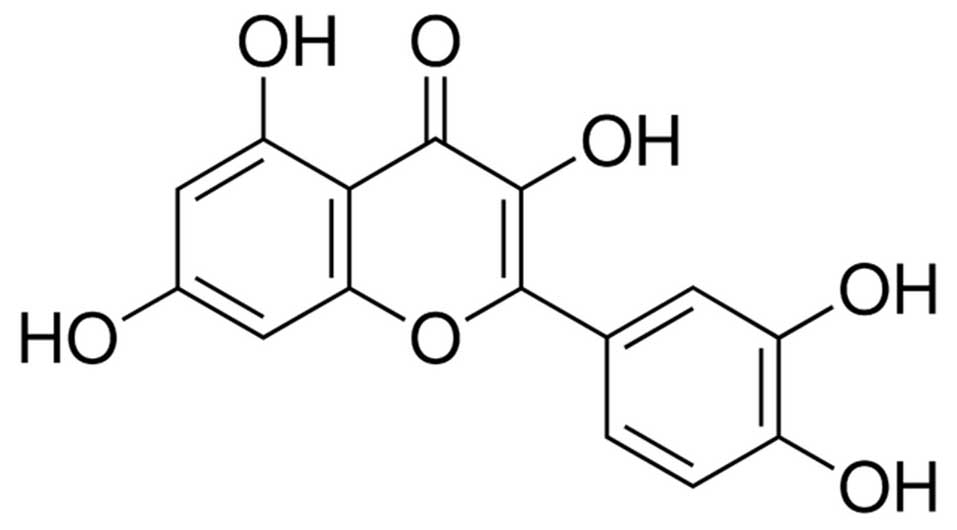
important polyphenolic antioxidant (Fig. 1) (3). It was reported that quercetin was
able to maintain blood-brain barrier integrity by scavenging active
oxygen (4). It was suggested that
oral treatment with quercetin nanocapsules may exert a protective
effect against oxidative damage and prevent pyramidal neurons in
the hippocampus from ischemia/reperfusion (I//R) (5). However, the exact mechanism of the
neuroprotective effect of quercetin against global brain I/R injury
has remained elusive.
Apoptosis is one of the processes leading to cell
death after cerebral ischemia (6).
In response to mitochondrial oxidative stress, the mitochondrial
outer membrane becomes permeabilized (7), which leads to the translocation of
B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax) from the
cytoplasm to the mitochondria, eventually causing the release of
cytochrome c (8). Oxidative
stress is the result of a redox imbalance between the generation of
reactive oxygen species (ROS) and the compensatory response from
the endogenous anti-oxidant network. ROS are now known to act as
secondary messengers during I/R (9), and it was reported that dietary
flavonoids such as quercetin can block ROS generation (10). Furthermore, a study showed that
quercetin can reduce the activation of apoptosis in focal cerebral
ischemia in rat brain tissue and the mechanism may involve the
brain-derived neurotrophic factor/tropomyosin receptor kinase
B/phosphoinositide 3 kinase (PI3K)/Akt signaling pathway (11). Akt is a serine/threonine protein
kinase, and the primary role of Akt signaling is to tansduct growth
factor-mediated cell survival and block apoptosis, which is
activated by phosphorylation. Pro-apoptotic factors, including
Bcl-2-associated death promoter (Bad) and caspase-9 and caspase-3
are modulated by Akt (12). The
neuroprotective effect of Akt in cerebral ischemia has been
extensively studied (13,14). Temporary Akt phosphorylation of
serine 473 was shown to occur after focal cerebral ischemia and
phosphorylated Akt-positive cells exhibited decreased levels of DNA
damage, indicating that the PI3K/Akt pathway is involved in
neuronal cell survival in I/R injury (15).
In view of these findings, the present study
investigated whether oral administration of quercetin can reduce
motor performance deficits and neuronal cell loss in the CA1
hippocampus using a rat model of global cerebral I/R injury.
Materials and methods
Animals
All experiments were performed on a total of 284 6–8
week-old male Sprague Dawley (SD) rats (280–320 g; Xi'an
Experimental Animal Center of the Medical College, Xi'an Jiaotong
University, Xi'an, China), housed at constant temperature (21±1°C)
and a 12-h light/dark cycle (6 am to 6 pm). Food and water were
available ad libitum. All animal handling as well as
surgical and sacrification procedures were conducted in compliance
with the guidelines of the Institutional Animal Care and Use
Committee of Xi'an Jiaotong University (XJTU), which oversees
conformity with national laws (Animal Welfare Act) and
accreditation policies (Association for Assessment and
Accreditation of Laboratory Animal Care). All experimental
protocols in the present study were approved by the The Laboratory
Animal Care and Use Committee of Xi'an Jiaotong University (Xi'an,
China) Animals were transported to an approved animal unit 12–24 h
prior to the experiment to reduce stress responses on the day of
surgery.
Quercetin treatment
Quercetin (≥95%) was purchased from Sigma-Aldrich
(St. Louis, MO, USA). Quercetin powder was dissolved in distilled
water, and was stored at a final concentration of 1 g/ml. The
solution was administered by oral gavage to the SD rats. Rats in
the sham + vehicle group were given an equivalent volume of vehicle
(distilled water, 10 ml/kg, orally (p.o.); once daily). Volumes of
drug solution required for the administration of 5, 10 or 20
mg/kg/day quercetin equated to 3 ml for a 300-g rat, which is an
acceptable volume for oral gavage.
Rat model of global brain I/R
The global cerebral ischemia reperfusion model was
established by a four-vessel occlusion (4-VO) ligation method as
previously described (16). Rats
were randomly assigned to four groups: Sham + vehicle group, I/R +
vehicle group, I/R + 5 mg/kg/day quercetin group and I/R + 10
mg/kg/day quercetin group. Prior to surgery, the animals were
housed overnight with free access to tap water. Global cerebral
ischemia was induced using the 4-VO method with slight modification
(17). Briefly, on the first day,
rats were anesthetized with chloral hydrate (35 mg/100 g weight;
Puzhen Bio Tech Ltd., Shanghai, China) and both vertebral arteries
were electrocauterized permanently through the alar foramen of the
first cervical vertebra. On the next day, under chloral hydrate
anesthesia, bilateral carotid artery occlusion was performed by
atraumatic micro-vessel clamps for 15 min to induce ischemia prior
to the release of the carotid artery clamps and onset of the
reperfusion. The neck incision was then closed with 3-0 aseptic
non-absorbable silk sutures in a simple interrupted pattern.
Sham-operated animals were treated similarly to those in the
ischemic group, but neither the vertebral arteries nor the common
carotid arteries were occluded. The rectal temperature was
maintained at 36–37°C for all animals throughout the experiment.
The successful establishment of the global cerebral I/R model was
verified by identifying certain symptoms, including mydriasis,
tachypnea, pale eyeballs and lip cyanosis.
Neurological score assessment
An independent observer serially evaluated the
neurological functional outcome after graded injury using the Neuro
Deficit Score (NDS) system as described previously (18). Neurologic evaluation was performed
once daily at the same time (5:00 p.m.) by the same investigator,
who was unaware of the group assignment at 12, 24 and 48 h after
I/R injury. The NDS ranges from 80 (best) to 0 (brain dead) and it
includes a sub-score of general behavioral deficits: Consciousness
as normal, stuporous or unresponsive and arousal with eye opening
and respiration as normal, abnormal (hypo- or hyperventilation) or
absent. Brainstem function sub-scores are assessed with: i)
Olfaction as response to smell of food; ii) vision, as head
movement to light; presence of iii) pupillary light reflex; iv)
corneal reflex; v) startle reflex; response to vi) whisker
stimulation and vii) swallowing liquids or solids were also tested.
Sub-score in motor assessment included strength testing as normal,
abnormal (either stiff or weak) and absence of movement. All
randomized animals were evaluated at baseline to ensure a normal
neurologic score.
Morris water maze (MWM)
The Morris water maze assay was executed as
previously described (19,20). The MWM consists of a black circular
pool (180 cm diameter, 45 cm high) filled with water (30 cm depth)
at 22±1°C and is virtually divided into four equivalent quadrants:
North-east (NE), north-west (NW), south-east (SE) and south-west
(SW). A 2-cm submerged escape platform (12 cm diameter) was placed
in the middle of one of the quadrants equidistant from the sidewall
and the center of the pool. The pool was surrounded by several
distal visual cues. Animal behavior was monitored by a video camera
and quantified using Ethovision v1.90 (Noldus, Wageningen, The
Netherlands). Prior to starting the MWM acquisition phase, animals
received a cued learning training to instruct the animals on the
procedures required to learn the MWM task. For cued learning, the
pool is the same as that in the MWM version except that the
platform is flagged above the water by ~12 cm and curtains are
closed around the maze to avoid access to distal visual cues. To
ensure that rats are using the flagged cue to locate the platform,
the location of the goal and the starting point were both moved to
new positions during each trial. Four trials per day were performed
until the rat reached the platform in two consecutive trials with a
latency of <20 sec. After completing cued learning, rats were
trained following the standard MWM learning protocol consisting of
four consecutive trials during three days, starting randomly from
one of the four quadrants each time. A trial began by placing the
rat into the water facing the wall of the pool. The rat was guided
to the platform if it failed to escape within 90 sec and was
allowed to stay on the platform for 20 sec maximum prior to
returning to its cage for a new trial (inter-trial interval, 20
sec). On day four, a probe trial was performed, in which the escape
platform was removed from the pool and the rat was allowed to swim
for 60 sec. The trial began with the rat in the quadrant opposite
the usual platform location. The time spent in each quadrant was
recorded. In order to discriminate against the chance level, the
percentage of time spent in the target quadrant over the total time
spent in the target and the opposite quadrant (chance level at 50%)
was determined. The acquisition learning phase lasting 3 days
provided a measure of spatial learning and reference memory, while
the probe trial at day four measured spatial memory and retrieval
capabilities.
Pathological analysis
Pathological Analysis was performed after 24 h of
I/R injury. Hematoxylin and eosin (H&E; Puzhen Bio Tech Ltd.)
staining was performed according to standard protocols. Briefly,
brain tissues were fixed in 4% (w/v) paraformaldehyde and embedded
in paraffin and then cut into 4-µm sections. The slices
underwent hematoxylin and eosin staining, respectively, for 20 and
2 min following de-waxing. After H&E staining, images were
captured using a microscope (Nikon Eclipse 80i; Nikon, Tokyo,
Japan) to assess morphological differences among the experimental
groups.
Apoptosis in brain tissues due to I/R injury was
examined by terminal dUTP nick end labeling (TUNEL) analysis. Brain
tissues were resected, fixed in paraformaldehyde for 24 h, imbedded
in paraffin and 5-µm sections were prepared. The TUNEL assay
was performed using an apoptag peroxidase in situ apoptosis
detection kit (Chemicon International, Temecula, CA, USA). Briefly,
the sections were digested using proteinase K and the endogenous
peroxidase activity was blocked using 3% hydrogen peroxide in
phosphate-buffered saline (PBS). The sections were then placed in
equilibration buffer and incubated with working strength of TdT
enzyme in a humidifying chamber at 37°C for 1 h. The reaction was
terminated with a stop/wash buffer provided with the kit. The
apoptotic nuclei were identified by detection of direct immuno
peroxidase staining of digioxigenin-labeled DNA in the test
sections.
Assessment of hippocampal cell viability
and apoptosis
At 24 h following I/R injury, 24 rats (n=6/group)
were intraperitoneally anesthetized and sacrificed by cervical
dislocation to obtain the brain. The hippocampus was isolated and
placed in a petri dish containing cold PBS (0–4°C) on ice. The
cerebral cortex (0.5×0.5 cm) between the optic chiasma and
mamillary body was isolated and stored on ice in a centrifuge tube
containing PBS (0–4°C). Single-cell suspensions were prepared
within 30 min and the cell density was adjusted to
1×106/ml. A total of 100 µl cell suspension was
placed in a 5-ml test tube and mixed with 5 µl Annexin
V/fluorescein isothiocyanate and 10 µl propidium iodide (20
µl/ml). The mixture was incubated in the dark at room
temperature for 15 min. 400 µl PBS was added to the tube and
cells were analyzed by flow cytometry (FACSCalibur; BD Biosciences,
Franklin Lakes, NJ, USA).
Measurement of ROS
To assess ROS formation, 2′,7′-dichlorofluorescein
diacetate (DCFH-DA; Sigma-Aldrich) was used as previously descried
(21). Briefly, the hippocampus
was isolated, homogenized using a manual glass homogenizer, and
incubated with DCFH-DA (100 µM) at 37°C for 30 min. Cooling
of the reaction mixture in ice terminated the reaction. The
formation of the oxidized fluorescent derivative (DCF) was
monitored at excitation and emission wavelengths of 488 and 525 nm,
respectively, using a fluorescence spectrophotometer (SP-Max
3500FL; Shanghai Flash Spectrum Bio Tech Co., Ltd., Shanghai,
China). The free radical content was quantified using a DCF
standard curve and results were expressed as nanomoles of DCF
formed per milligram of protein.
Western blot analysis
At 24 h following I/R injury, rats were
intraperitoneally anesthetized and sacrificed by cervical
dislocation to obtain the brain. Brain tissues (comprising the
cortex and striatum) were collected and stored at −80°C. Tissues
were harvested and resuspended in lysis buffer, washed with
ice-cold PBS and lysed in extraction buffer, containing 40 mmol/l
Tris-HCl (pH 7.5), 150 mmol/l KCl, 1 mmol/l EDTA, 1% Triton X-100,
100 mmol/l NaVO3, 1 mmol/l PMSF, supplemented with the
protease inhibitor cocktail (Roche, Basel, Switzerland). The
protein (50 µg) was separated on 10% SDS-PAGE gels and
transferred onto nitrocellulose membranes. The membranes were
blocked with 5% non-fat milk in Tris-buffered saline (TBS) at 37°C.
Rabbit anti-Bcl-2 polyclonal antibody (1:1,000; Cell Signaling
Technology, Danvers, MA, USA; cat no. 2876), rabbit anti-Bcl-xL
polyclonal antibody (1:1,000; Cell Signaling Technology, cat no.
2762), rabbit anti-Bad monoclonal antibody (1:1,000; Cell Signaling
Technology, Danvers, MA, USA, cat no. 9293), rabbit phospho (p-)Bad
(Ser136) monoclonal antibody (1:1,000; Cell Signaling Technology,
Danvers, MA, USA, cat no. 4366), rabbit anti-survivin polyclonal
antibody (1:1,000; Cell Signaling Technology, Danvers, MA, USA, cat
no. 2803), rabbit anti-Akt polyclonal antibody (1:1,000; Cell
Signaling Technology, Danvers, MA, USA, cat no. 9272), rabbit
anti-p-Akt (Ser473) monoclonal antibody (1:1,000; Cell Signaling
Technology, Danvers, MA, USA, cat no. 4060), and rabbit
anti-β-actin monoclonal antibody (1:1,000; Cell Signaling
Technology, Danvers, MA, USA, cat no. 4967) were diluted in freshly
prepared PBS containing 3% skimmed milk powder, and blots were
incubated in primary antibodies at 4°C overnight. Horseradish
peroxidase-linked goat anti-rabbit IgG antibody (1:5,000; Beijing
Biosynthesis Biotechnology Co., Ltd. Beijing, Cihna; cat. no.
bs-0295G-HRP) was used as a secondary antibody in TBS containing 5%
non-fat milk and the blots were incubated for 3 h at room
temperature. Antigen-antibody complexes were detected using an
enhanced chemiluminescence kit (ECL Plus; GE Healthcare Life
Sciences, Chalfont, UK), and bands were analyzed using ImageJ
version 1.42q software (National Institutes of Health, Bethesda,
MD, USA).
Statistical analyses
Unless otherwise indicated, values are expressed as
the mean ± standard error of the mean. Data were analyzed using
SPSS 20.0 software for Windows (International Business Machines,
Armonk, NY, USA). Differences between groups were analyzed using a
one-way analysis of variance, and when significant, t tests
were employed for post hoc comparisons. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Quercetin treatment increases survival of
rats following I/R
Prior to specific studies on the protective effect
of quercetin on neuronal cells following I/R, an experiment was
performed to confirm an appropriate concentration of quercetin for
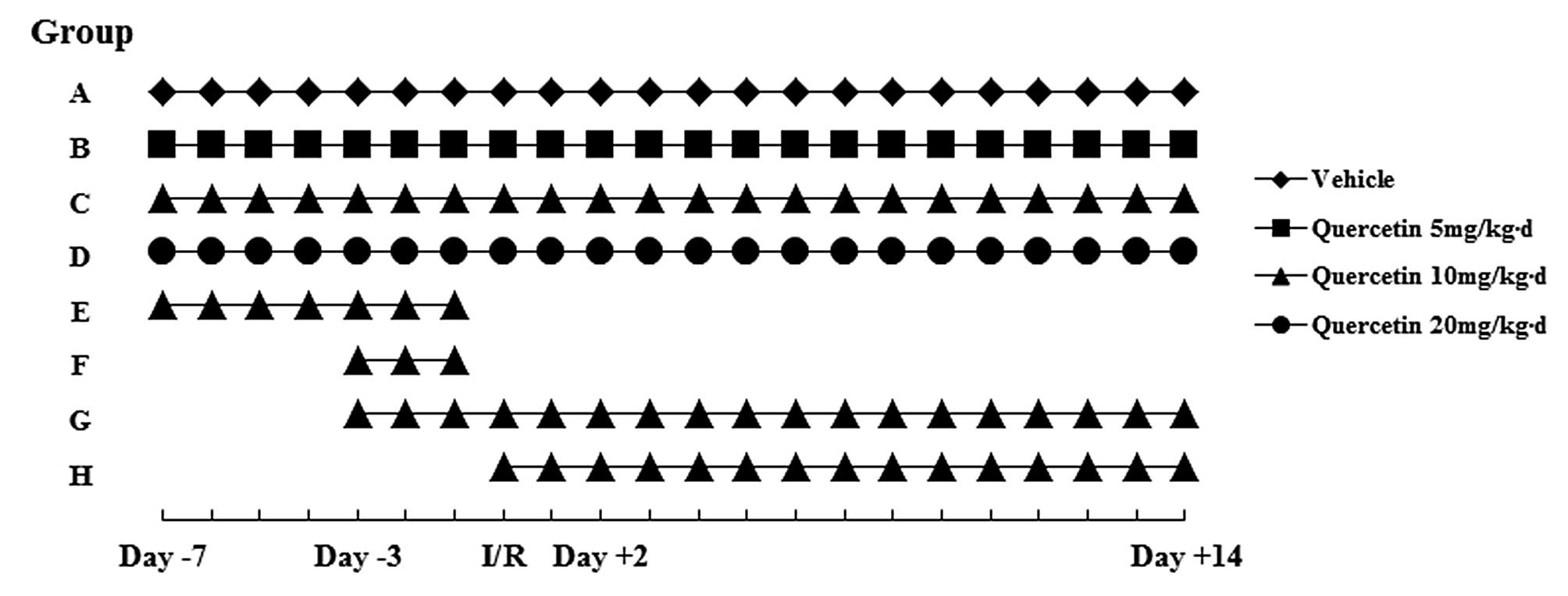
the treatment of cerebral I/R injury. The experimental design is
illustrated in Fig. 2. Rats were
divided into four groups: A, I/R + vehicle; B, I/R + quercetin 5
mg/kg/day; C, I/R + quercetin 10 mg/kg/day; and D, I/R + quercetin
20 mg/kg/day. All rats were given individual treatment from 7 days
prior to I/R to 14 days after I/R. As shown in Fig 3A, quercetin treatment significantly
decreased I/R-induced death. Survival rates in groups B, C and D
were significantly higher than that in group A; however, there was
no significant difference between groups C and D. In the sham group
treated with quercetin (20 mg/kg/day for 21 days), none of the
animals died (results not shown). The second experiment was
executed to identify the appropriate timing of quercetin treatment
to achieve a reduction of I/R injury. All rats were treated with
quercetin (10 mg/kg/day) for different periods; rats were divided
into four groups: E, from day -7 until I/R; F, from day -3 until
I/R; G, from day -3 to day +14; H, from I/R to day+14 (Fig. 2). The survival rates in groups C
and G were higher than those in all other groups with the same
concentration of quercetin treatment. No significant difference was
detected between group C and group G, suggesting that treatment
time may not influence the motility of rats after I/R. Therefore,
rats were treated with 5 or 10 mg/kg/day quercetin from day -3
until the end of the experiments.
Motility is a critical indicator to evaluate
neuroprotective effects following I/R injury (22). To assess the neuroprotective effect
of quercetin, motility was assessed using two experiments in the
present study.
Oral administration of quercetin
attenuates I/R-induced performance deficits
To evaluate the functional neuroprotective effect of
quercetin in global cerebral I/R injury, neurological evaluation
was performed at 12, 24 and 48 h after cerebral I/R injury.
NDS was significantly decreased at 12, 24 and 48 h
in I/R rats compared with those that underwent quercetin treatment
and I/R (Fig. 4A). Compared to the
sham + vehicle group, 15 min cerebral I/R caused a significant
decrease in NDS (78.8±2.7 vs. 17.6±5.4; 79.4±1.3 vs. 29.0±10.8; and
79.4±1.3 vs. 32.6±7.8 at 12, 24 and 48 h, respectively; P<0.05).
Treatment with 5 and 10 mg/kg/day quercetin significantly increased
the NDS caused by I/R (33.2±8.1 and 43.4±12.1 vs. 17.6±5.4;
41.6±4.9 and 51.8±7.8 vs. 29.0±10.8; 49.4±7.2 and 66.2±6.2 vs.
32.6±7.8 at 12, 24 and 48 h, respectively; P<0.05).
Spatial learning and long-term memory is
differently affected in I/R + quercetin-treated and untreated I/R
rats
In the MWM test, differences between groups
regarding cognitive abilities which are known to depend on the
hippocampal function were assessed using the MWM task. In the cued
learning task, animals in the sham + vehicle group reached the set
goal on the first training day, while quercetin-treated and
untreated I/R animals required 3 and 4 days of training,
respectively. Seven days following cerebral I/R, animals performed
the standard MWM acquisition task, which measures spatial learning.
The escape latency was measured 8, 9 and 10 days after ischemia
(Fig. 4B). Animals in the sham +
vehicle group performed better than I/R animals (14.5±2.7, 13.1±1.9
and 12.8±2.1 sec in the sham + vehicle group vs. 31.0±8.4, 28.7±5.2
and 26.6±5.5 sec in the I/R group on day 8, 9 and 10,
respectively). Significant differences were revealed in escape
latency between the sham + vehicle and I/R groups (P<0.05). This
result confirmed that I/R elicited significant deficits in spatial
learning. At days 8, 9 and 10, the escape latency was differently
affected in I/R + quercetin-treated and I/R animals (26.2±5.7,
22.8±5.0 and 19.5±3.6 sec in the 5 mg/kg/day group and 18.8±4.3,
17.8±3.9 and 15.1±2.4 sec in the 10 mg/kg/day group). Quercetin
dose-dependently decreased the escape latency, and the two doses (5
and 10 mg/kg/day) caused a statistically significant effect in the
escape latency at days 8, 9 and 10 after I/R (P<0.01 vs.
ischemic animals). Significant group differences were revealed
during the acquisition phase trial between I/R + quercetin-treated
groups (5 and 10 mg/kg/day) and the I/R group. These results
revealed that Quercetin-treated rats showed an attenuated spatial
learning deficit.
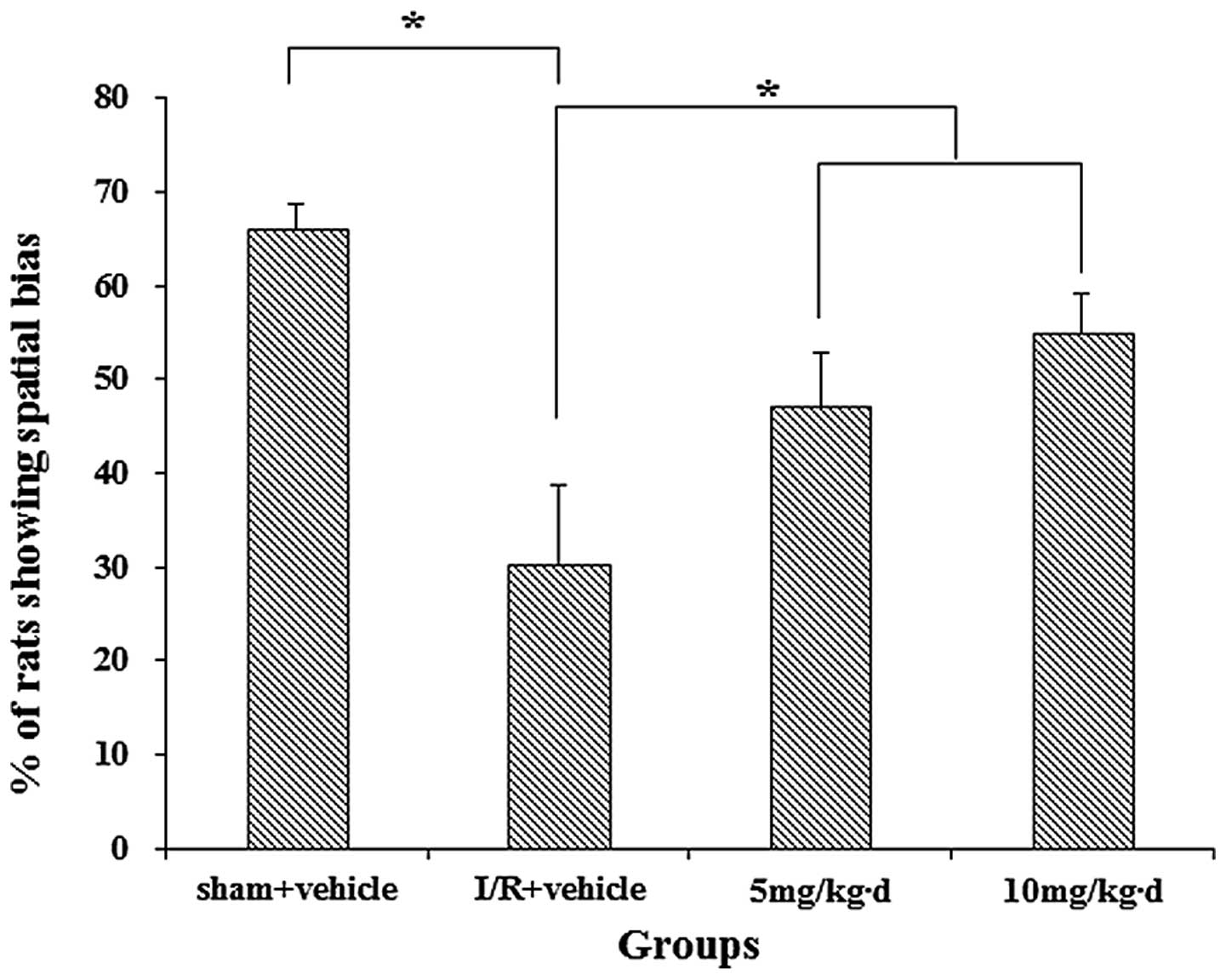
In the probe MWM trial, similar patterns were
observed in terms of the percentages of animals spending more time
in the target quadrant than in any other quadrant (Fig. 5), referred to as spatial bias.
Significant differences in spatial bias were identified between the
sham + vehicle group and rats subjected to I/R (P<0.05). This
may be due to an I/R-induced impairment in spatial memory
retention. By contrast, quer-cetin-treated animals more effectively
discriminated the target quadrant, and the fraction of rats showing
spatial bias after quercetin treatment was significantly higher
than that in the vehicle-treated I/R group. Compared with untreated
animals, quercetin-treated animals subjected to I/R spent more time
in the target quadrant than in any other quadrant (P<0.05). The
fact that the performance of quercetin-treated rats improved in the
probe trials may support that quercetin improves long-term spatial
memory retention.
Quercetin reduces neuronal cell apoptosis
caused by I/R injury
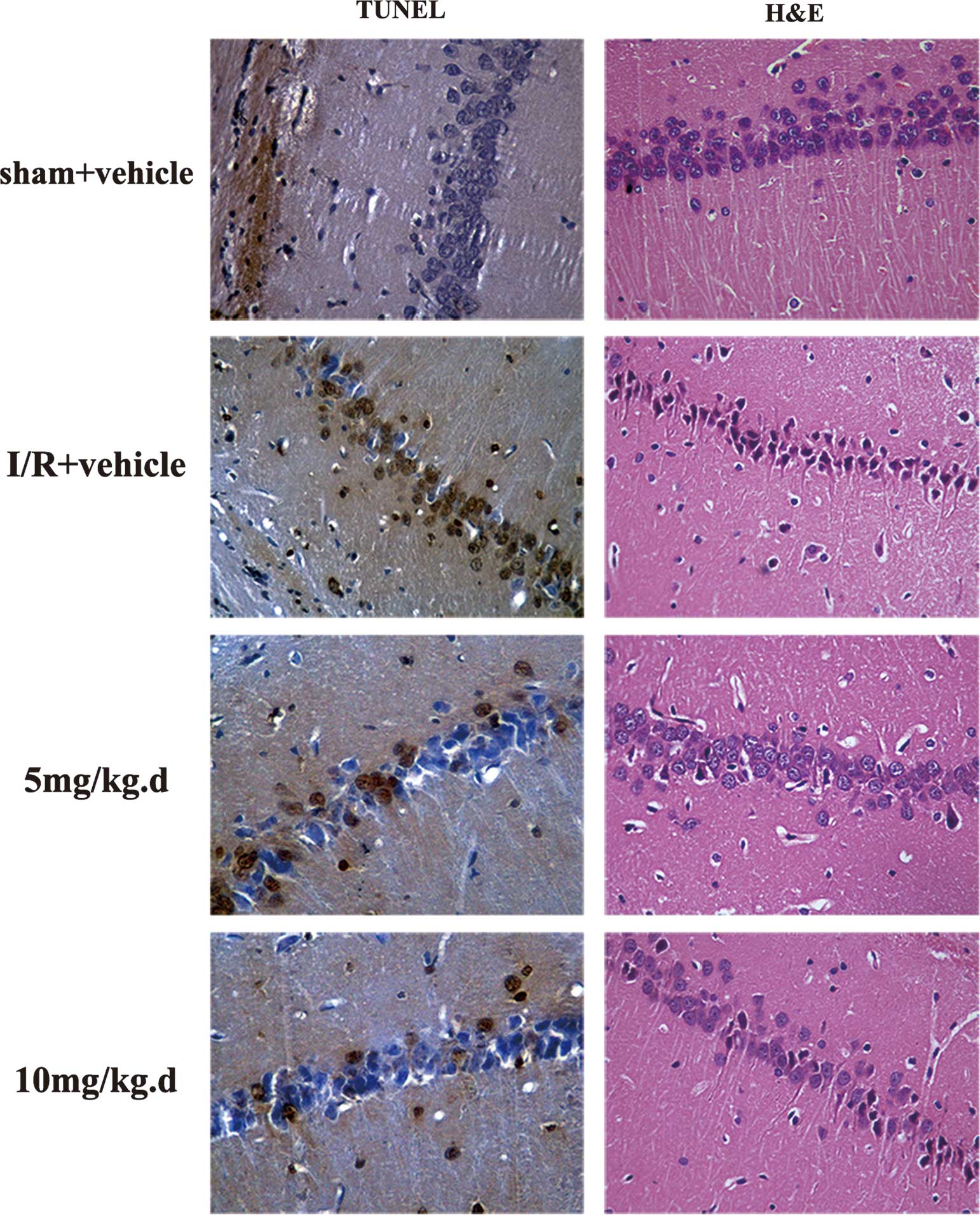
To detect the effect of quercetin on histological
alteration after cerebral I/R injury in the hippocampus,
dose-response associations regarding the neuroprotective effects of
pre-treatment with quercetin on the CA1 hippocampus of rats
subjected to I/R were examined. Four groups of rats were orally
administered once daily with vehicle or quercetin (5 or 10
mg/kg/day). All animals were subjected to 15 min ischemia 24 h
after the last administration of water or quercetin. Neuronal cell
loss in the hippocampus was assessed 24 h after I/R by counting the
number of apoptotic cells in brain sections from these structures
using the TUNEL assay (Fig. 6) and
FACS (Fig. 7). Quercetin treatment
caused a dose-dependent increase in neuronal cell survival in the
dorsal hippocampus. As shown in Fig.
6, the TUNEL-positive cells were significantly decreased in the
quercetin-treated group. This neuroprotective effect was also
illustrated by H&E staining (Fig.
6). According to the FACS assay, the ratios of apoptotic cells
were 3.57±0.38% for the sham + vehicle treatment group, 35.56±5.39%
for the I/R + vehicle treatment group, 19.28±3.64% for the I/R + 5
mg/kg/day quercetin treatment group and 9.41±1.16% for the I/R + 10
mg/kg/day quercetin treatment group (Fig. 7). Increasing the dose of quercetin
to 40 mg/kg/day did not produce any further improvement of neuronal
cell survival (data not shown).
Quercetin-treatment decreases ROS
formation
The fluorogenic compound DCFH-DA is one of the most
prominent markers to reflect the overall oxidative status in cells.
Within the cell, esterases cleave the acetate groups on DCFH-DA,
thus trapping the reduced probe (DCFH) intracellularly. ROS in the
cells lead to the oxidation of DCFH, yielding the fluorescent
product DCF. As shown in Fig. 8,
rats that had undergone transient global cerebral ischemia for 15
min followed by 1 h of reperfusion exhibited a significantly
increase in ROS production in the hippocampus (P<0.01) as
compared with levels in vehicle-treated sham animals. By contrast,
the I/R induced ROS overproduction following 12 and 24 h of
reperfusion was significantly decreased by quercetin in the
hippocampus (P<0.01; Fig. 8).
No alterations in DCFH levels were observed in sham animals.
Quercetin modulates the expression of
anti-apoptotic proteins following I/R in a fashion that is
characteristic for neuroprotection
To reveal the effect of quercetin on
apoptosis-associated proteins in brain tissues after I/R injury,
the expression of Bcl-2, Bcl-xL, survivin and cleaved caspase-3 was
assessed in the hippocampus 24 h following I/R (Fig. 9). I/R decreased the expression
levels of Bcl-2 and Bcl-xL as compared with those in the sham +
vehicle group, while it increased the levels of cleaved caspase-3.
Administration of quercetin prior to I/R (quercetin-I/R) was able
to significantly increase the expression of Bcl-2, Bcl-xL and
survivin, as well as restore levels of cleaved caspase-3 (Fig. 9).
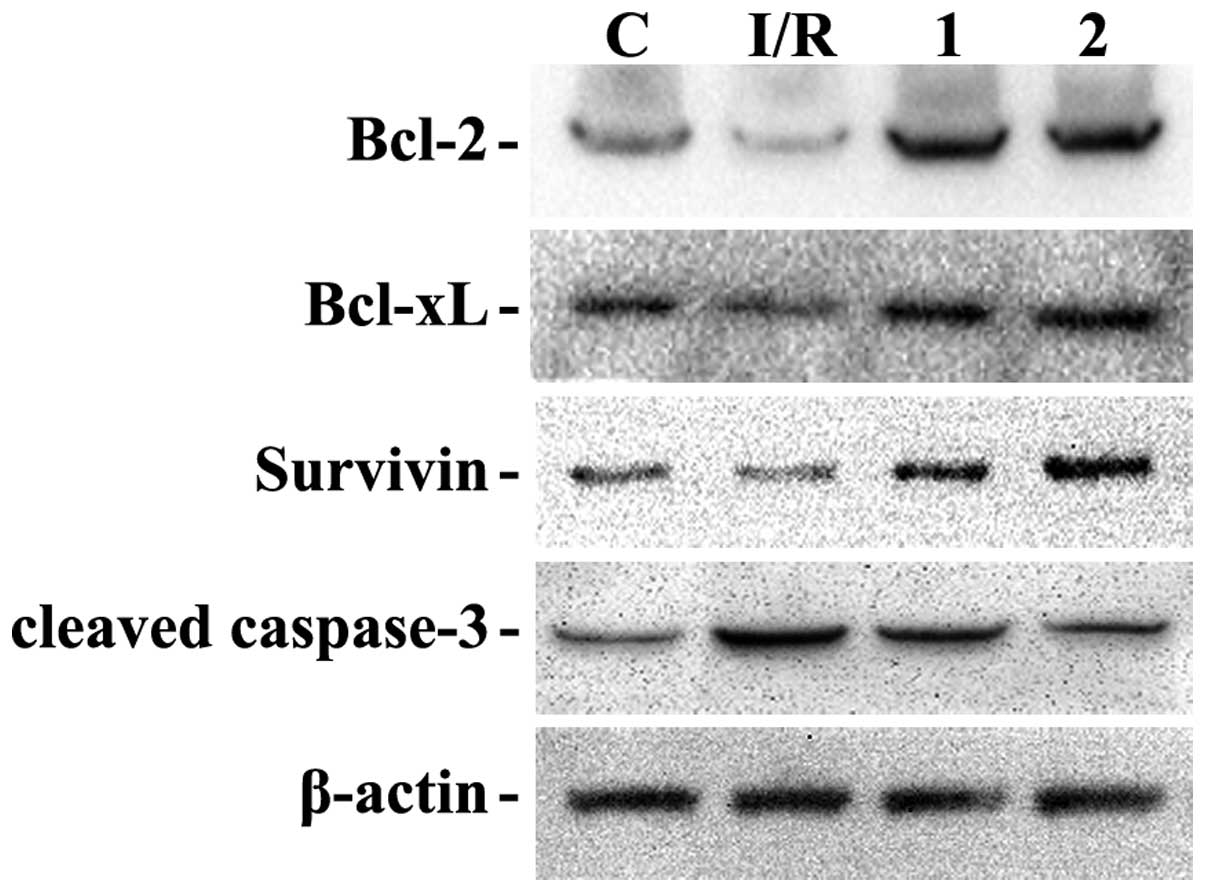 | Figure 9QC modulates the expression of
anti-apoptotic genes following I/R in a fashion characteristic for
neuroprotection. C, sham + vehicle group; I/R, I/R group; 1, IR + 5
mg/kg/d QC treatment; 2, IR + 10 mg/kg/d QC treatment. 24 h after
I/R, QC treatment significantly upregulated Bcl-2, Bcl-xL and
survivin, while it downregulated cleaved caspase-3, which was
enhanced by I/R in the hippocampus compared with the sham + vehicle
group. I/R, ischemia/reperfusion; QC, quercetin; Bcl-2, B-cell
lymphoma 2; xL, extra large. |
Effects of quercetin on Akt signaling in
brain after cerebral I/R injury
In the final experiment, the effects of quercetin on
Akt signaling as well as its mainly apoptosis-associated substrate,
Bad, were assessed. As shown in Fig.
10, at 24 h after I/R, the phosphorylation of Akt as well as
Bad levels were significantly upregulated in the hippocampi of
animals in the quercetin-treated I/R group compared with those in
the vehicle-treated I/R group.
Discussion
The neuroprotective effects of quercetin on the
central nervous system have been previously reported, and most of
these studies were based on focal cerebral ischemia models
(11,23,24).
The advantage of the focal ischemia model is that the injury can be
evaluated, which cannot be performed in the global brain ischemia
model. However, global brain ischemia occurs in a certain
percentage of patients with heart dysfunction, i.e. myocardial
infarction, who usually contract reperfusion injury-induced brain
damage. Therefore, global brain ischemia is another prevalent brain
I/R injury in the clinic. Previous studies have demonstrated that
cerebral I/R injury causes neurological deficits and neuronal cell
apoptosis has an important role in the evolution of I/R injury in
the brain (25,26). In the present study, treatment with
quercetin was shown to significantly improve neurological function
at 12, 24 and 48 h after cerebral ischemia according to NDS. These
results were in accordance with those of previous studies (11,23);
however, to the best of our knowledge, the present study was the
first to report that quercetin protects certain neuronal cells from
global cerebral I/R injury.
Behavioral parameters are useful measures of
functional deficits following experimental global cerebral ischemia
and the degree of sensorimotor dysfunction as an important
indicator of the severity of injury. The motor function was found
to be disturbed by neurological evaluation. The present study
proved that the motor function was impaired after ischemic insults
in rats and was significantly ameliorated by treatment with
quercetin. These findings are in accordance with earlier studies
where motor deficits were attenuated by treatment with
anti-oxidants (4,11,23).
Hippocampus-dependent cognitive impairments were
quantified using the MWM. It was found that compared with
vehicle-treated animals, quercetin-treated rats more effectively
learned and remembered tasks in the MWM. By contrast, I/R animals
performed poorly at the acquisition phase of the task and failed
memory retention tests. In the acquisition phase of the MWM task,
quercetin-treated animals performed better than their I/R peers,
which spent more time to locate the hidden platform, while only
animals in sham + vehicle group showed successful preference for
the target quadrant in the probe trial and animals that received
I/R only were impaired the most among all groups. Analysis of
escape latency (quantified as the time required to locate the
hidden platform) and spatial bias (defined as the percentage of
time spent in the target area with regard to the time spent in all
quadrants), which represent spatial learning and memory retention,
respectively, clearly showed that animals subjected to I/R injury
alone were the most seriously affected, while quercetin-treated
animals performed better regarding preference for platform location
and the target quadrant. The results showed that quercetin had a
significant positive effect in terms of spatial learning and
long-term memory retention. Therefore, the present study confirmed
that quercetin treatment effectively reversed cognitive impairment
following I/R injury. The findings suggested that further studies
on quercetin are warranted to determine its usefulness as a
potential agent for the prevention of I/R injuries.
The behavioral impact is closely linked to the
degree of neuronal dysfunction. Functional deficits are a common
neurological sequel in animal models of cerebral ischemia. The
present study demonstrated that treatment with quercetin has a
far-reaching protective effect against the consequences of ischemic
insults. A rat model of global cerebral I/R injury was used for the
behavioral and biochemical tests, which is widely used to study the
neuroprotective effect of quercetin, because it represents the
biochemical and pathological features of stroke in humans,
including oxidative stress, mitochondrial dysfunction and apoptosis
(27). In response to the
oxidative load in mitochondria, the outer membrane of mitochondria
becomes permeabilized (7),
resulting in the translocation of Bax from the cytosol to the
mitochondria and the release of cytochrome c. Since delayed
neuronal degeneration is mostly due to apoptosis, the present study
further investigated the number of apoptotic cells by TUNEL
staining. The results showed less TUNEL-positive cells in the
quercetin treatment group compared with that in the vehicle-treated
I/R rats. Previous studies reported decreased protein levels of
Bcl-2 and increased protein levels of Bax and cleaved-caspase-3 in
MCAO rats following I/R (28,29).
The present study demonstrated that quercetin treatment was
associated with pronounced upregulation of Bcl-2, Bcl-xL and
survivin, as well as downregulation of cleaved-caspase-3. These
results provided further evidence that quercetin is able to reduce
apoptosis following ischemia through inhibiting the
mitochondria-dependent caspase-mediated apoptotic pathway.
ROS generation in endothelial cells during
hypoxia/reoxygenation is well documented (30,31).
Excess ROS from endogenous sources can account for autocrine and
paracrine cellular damage during reperfusion (32,33).
The results of the present study showed that ROS production in the
brain increased after 1 h of reperfusion. Decreased ROS generation
following treatment with quercetin indicated that the
neuroprotection conferred by quercetin was due to its anti-oxidant
effect of attenuating oxidative stress.
Accumulating evidence suggested that the PI3K/Akt
signaling pathway has a crucial role in neuronal survival after
cerebral ischemia. Previous studies have reported that activation
of Akt signaling has neuroprotective effects against ischemic
neuronal injury (34,35). Several potential substrates for Akt
that are associated with cell survival after cerebral I/R injury.
To determine whether Akt activation is a major contributor to the
anti-apoptotic effect of quercetin following ischemia, the
phosphorylation of Akt after global cerebral I/R injury was
assessed using western blot analysis. Treatment with quercetin
significantly increased the expression of p-Akt compared with that
in the vehicle-treated group. The expression of p-Akt downstream
signaling proteins was further examined. Quercetin was shown to
upregulate the phosphorylation level of Bad, suggesting that Bad is
one of the downstream targets of quercetin-induced inhibition of
Akt. Previous studies have shown that blocking PI3K/Akt signaling
with the pharmacological inhibitor LY294002 significantly
attenuated the anti-apoptotic effect of quercetin (11). The results of the present study
suggested that the protective effect of quercetin against apoptosis
induced by ischemia is mediated by the PI3K/Akt pathway.
Quercetin alters the expression of anti-apoptotic
genes in response to I/R. The neuroprotective effects of flavonoids
have been linked to the activation of pro-survival signaling
mediated by the PI3/Akt and ERK pathways, which stimulate the
expression of the prototypical anti-apoptotic genes Bcl-2 and
X-linked inhibitor of apoptosis protein. I/R enhanced the
expression of Bcl-2 mRNA in the dorsal hippocampus, which was
completely reversed by quercetin (36). In situ hybridization
histochemical studies have localized the increases in Bcl-2 mRNA
following transient global cerebral ischemia to pyramidal neurons
in the hippocampus, which are exquisitely sensitive to ischemic
injury (37). Reversal of
I/R-induced increases in Bcl-2 gene expression by quercetin may
therefore reflect a reduction in N-methyl-D-aspartate
receptor-mediated cyclic adenosine monophosphate responsive element
binding protein signaling (38).
In conclusion, the present study demonstrated that
the oral administration of quercetin (5 and 10 mg/kg/day) prevented
motor performance deficits and markedly attenuated neuronal cell
loss in the dorsal hippocampus in a rat model of global cerebral
I/R injury. The neuroprotection of quercetin is associated with the
activation of PI3K/Akt signaling in response to ischemia-induced
apoptosis. Further investigation into the underlying mechanisms of
the anti-oxidant action of quercetin is required to determine
whether it can be an effective drug for the prevention of brain
injury following stroke.
Acknowledgments
This work was supported by grants from the Key
Science and Technology Program of Shaanxi Province [no. 2010k15-03
(1)].
References
|
1
|
Cherubini A, Ruggiero C, Morand C, et al:
Dietary antioxidants as potential pharmacological agents for
ischemic stroke. Curr Med Chem. 15:1236–1248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harwood M, Danielewska-Nikiel B,
Borzelleca JF, Flamm GW, Williams GM and Lines TC: A critical
review of the data related to the safety of quercetin and lack of
evidence of in vivo toxicity, including lack of
genotoxic/carcinogenic properties. Food Chem Toxicol. 45:2179–2205.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JC, Kim J, Park JK, Chung GH and Jang
YS: The antioxidant, rather than prooxidant, activities of
quercetin on normal cells: Quercetin protects mouse thymocytes from
glucose oxidase-mediated apoptosis. Exp Cell Res. 291:386–397.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lapi D, Vagnani S, Pignataro G, esposito
E, Paterni M and Colantuoni A: Protective effects of Quercetin on
Rat Pial micro-vascular changes during transient bilateral common
carotid artery occlusion and reperfusion. Front Physiol.
3:322012.
|
|
5
|
Ghosh A, Sarkar S, Mandal AK and Das N:
Neuroprotective role of nanoencapsulated quercetin in combating
ischemia-reperfusion induced neuronal damage in young and aged
rats. PLoS One. 8:e577352013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mattson MP, Duan W, Pedersen WA and
Culmsee C: Neurodegenerative disorders and ischemic brain diseases.
Apoptosis. 6:69–81. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kowaltowski AJ, Castilho RF and Vercesi
AE: Mitochondrial permeability transition and oxidative stress.
FEBS Lett. 495:12–15. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuwana T, Mackey MR, Perkins G, et al:
Bid, Bax and lipids cooperate to form supramolecular openings in
the outer mitochondrial membrane. Cell. 111:331–342. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lei C, Deng J, Wang B, et al: Reactive
oxygen species scavenger inhibits STAT3 activation after transient
focal cerebral ischemia-reperfusion injury in rats. Anesth Analg.
113:153–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Priyadarsini RV and Nagini S: Quercetin
suppresses cytochrome P450 mediated ROS generation and NF kappaB
activation to inhibit the development of 7,12-dimethylbenz a
anthracene (DMBA) induced hamster buccal pouch carcinomas. Free
Radic Res. 46:41–49. 2012. View Article : Google Scholar
|
|
11
|
Yao RQ, Qi DS, Yu HL, Liu J, Yang LH and
Wu XX: Quercetin attenuates cell apoptosis in focal cerebral
ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling
pathway. Neurochem Res. 37:2777–2786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mayo LD and Donner DB: A
phosphatidylinositol 3-kinase/Akt pathway promotes translocation of
Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA.
98:11598–11603. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Janelidze S, Hu BR, Siesjo P and Siesjo
BK: Alterations of Akt1 (PKBalpha) and p70 (S6K) in transient focal
ischemia. Neurobiol Dis. 8:147–154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shibata M, Yamawaki T, Sasaki T, et al:
Upregulation of Akt phosphorylation at the early stage of middle
cerebral artery occlusion in mice. Brain Res. 942:1–10. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noshita N, Lewen A, Sugawara T and Chan
PH: Evidence of phosphorylation of Akt and neuronal survival after
transient focal cerebral ischemia in mice. J Cereb Blood Flow
Metab. 21:1442–1450. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue RL, He JX, Wang N, Yao FZ, Lv JR and
Wu G: Relationship between transmembrane signal transduction
pathway and DNA repair and the mechanism after global cerebral
ischemia-reperfusion in rats. Neurosci Bull. 25:115–121. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hadley G, Papadakis M and Buchan AM: A
method of inducing global cerebral ischemia. Methods Mol Biol.
1135:111–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Geocadin RG, Ghodadra R, Kimura T, et al:
A novel quantitative EEG injury measure of global cerebral
ischemia. Clin Neurophysiol. 111:1779–1787. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vorhees CV and Williams MT: Morris water
maze: procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006. View Article : Google Scholar
|
|
20
|
Stackman RW Jr, Lora JC and Williams SB:
Directional responding of C57BL/6J mice in the Morris water maze is
influenced by visual and vestibular cues and is dependent on the
anterior thalamic nuclei. J Neurosci. 32:10211–10225. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simao F, Matte A, Matte C, et al:
Resveratrol prevents oxidative stress and inhibition of
Na(+)K(+)-ATPase activity induced by transient global cerebral
ischemia in rats. J Nutr Biochem. 22:921–928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johansson SE, Larsen SS, Povlsen GK and
Edvinsson L: Early MEK1/2 inhibition after global cerebral ischemia
in rats reduces brain damage and improves outcome by preventing
delayed vasoconstrictor receptor upregulation. PLoS One.
9:e924172014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahmad A, Khan MM, Hoda MN, et al:
Quercetin protects against oxidative stress associated damages in a
rat model of transient focal cerebral ischemia and reperfusion.
Neurochem Res. 36:1360–1371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pandey AK, Hazari PP, Patnaik R and Mishra
AK: The role of ASIC1a in neuroprotection elicited by quercetin in
focal cerebral ischemia. Brain Res. 1383:289–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rizk NN, Rafols JA and Dunbar JC: Cerebral
ischemia-induced apoptosis and necrosis in normal and diabetic
rats: effects of insulin and C-peptide. Brain Res. 1096:204–212.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Solaroglu I, Tsubokawa T, Cahill J and
Zhang JH: Anti-apoptotic effect of granulocyte-colony stimulating
factor after focal cerebral ischemia in the rat. Neuroscience.
143:965–974. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yousuf S, Atif F, Ahmad M, et al:
Resveratrol exerts its neuroprotective effect by modulating
mitochondrial dysfunctions and associated cell death during
cerebral ischemia. Brain Res. 1250:242–253. 2009. View Article : Google Scholar
|
|
28
|
Wang R, Li G, Wang W and Li H: Effect of
compound preparation Tongqiao Jiannao capsules on neural cell
apoptosis and Bcl-2 and Bax protein levels in a rat model of brain
ischemia/reperfusion injury. Neural Regener Res. 3:871–874.
2008.
|
|
29
|
Babu PP, Yoshida Y, Su M, Segura M,
Kawamura S and Yasui N: Immunohistochemical expression of Bcl-2,
Bax and cytochrome c following focal cerebral ischemia and effect
of hypothermia in rat. Neurosci Lett. 291:196–200. 2000. View Article : Google Scholar
|
|
30
|
Wang T, Gu J, Wu PF, et al: Protection by
tetrahydroxystilbene glucoside against cerebral ischemia:
involvement of JNK, SIRT1 and NF-kappaB pathways and inhibition of
intracellular ROS/RNS generation. Free Radic Biol Med. 47:229–240.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu HW, Li HF, Wu XY, Zhao J and Guo J:
Reactive oxygen species mediate ERK activation through different
Raf-1-dependent signaling pathways following cerebral ischemia.
Neurosci Lett. 432:83–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Q, Sun AY, Simonyi A, et al: Ethanol
preconditioning protects against ischemia/reperfusion-induced brain
damage: Role of NADPH oxidase-derived ROS. Free Radic Biol Med.
43:1048–1060. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang JM, Xu HY, Zhang XJ, Li X, Zhang HB
and Ge PF: Role of mitochondrial function in the protective effects
of ischaemic postconditioning on ischaemia/reperfusion cerebral
damage. J Int Med Res. 41:618–627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Zhang ZG, Liu XS, Hozeska-Solgot
A and Chopp M: The PI3K/Akt pathway mediates the neuroprotective
effect of atorvastatin in extending thrombolytic therapy after
embolic stroke in the rat. Arterioscler Thromb Vasc Biol.
27:2470–2475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Endo H, Nito C, Kamada H, Nishi T and Chan
PH: Activation of the Akt/GSK3beta signaling pathway mediates
survival of vulnerable hippocampal neurons after transient global
cerebral ischemia in rats. J Cereb Blood Flow Metab. 26:1479–1489.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fang XX, Jiang XL, Han XH, Peng YP and Qiu
YH: Neuroprotection of interleukin-6 against NMDA-induced
neurotoxicity is mediated by JAK/STAT3, MAPK/ERK and PI3K/AKT
signaling pathways. Cell Mol Neurobiol. 33:241–251. 2013.
View Article : Google Scholar
|
|
37
|
Gu R, Liu M, Wang Y, Zhou Y and He H:
Angiopoietin-1 mRNA and Bcl-2 expression following estradiol
treatment in ovariectomized rats with focal cerebral
ischemia/reperfusion injury. Neural Regener Res. 4:780–785.
2009.
|
|
38
|
Kitagawa K: CREB and cAMP response
element-mediated gene expression in the ischemic brain. FEBS J.
274:3210–3217. 2007. View Article : Google Scholar : PubMed/NCBI
|