Introduction
Cadmium (Cd) is an occupationally and
environmentally important toxic metal with no known biological
function (1). It is well known
that Cd affects neurological and behavioral impairment, including
memory deficits (2), alteration of
social contact (3), olfactory
dysfunction (4), synaptic function
and neurotransmission (5,6). Therefore, Cd is considered to be a
possible etiological factor in neurodegenerative diseases. However,
the exact mechanism by which Cd elicits its neurotoxic effects is
not well elucidated.
Impaired mitochondrial function, oxidative stress,
accumulation of protein aggregates and autophagic stress are common
in many pathologies, including neurodegenerative diseases (7). Oxidative stress is a disturbance of
the cellular redox balance in favor of the pro-oxidants, and leads
to disruption of cellular macromolecules (for example, degradation
of proteins, cross-links in DNA and membrane fatty acid
peroxidation). However, elevated reactive oxygen species (ROS)
concentrations also influence signal transduction. At the cellular
level, Cd induces oxidative stress in numerous organisms, which
results in physiological damage to different organs (8,9).
Neurons in the brain are vulnerable to oxidative damage due to
their high metabolic activity, low antioxidant capacity and
non-replicative nature (10).
Excessive quantities of Cd-induced ROS can modify proteins, lipids
and DNA, and alter their functions, which contributes to the
activation of mTOR signaling and leads to neuronal apoptosis
(11). These findings indicate
that ROS are important regulators in Cd neurotoxicity.
Autophagy is a catabolic process that responds to
starvation or other stress conditions whereby cell constituents
(damaged organelles, unfolded proteins and intracellular pathogens)
are engulfed within autophagosomes and eventually degraded by
lysosomal enzymes to sustain cellular homeostasis (12,13).
However, a massive and persistent autophagy may result in cell
death, termed autophagic cell death (14). Autophagy is largely considered to
be non-selective, where preferential autophagy of damaged or excess
organelles (including the endoplasmic reticulum and mitochondria)
occurs. However, there is accumulating evidence for selective
autophagic processes in response to ROS (10,15).
Our previous study demonstrated that exposure of PC-12 cells to Cd
stimulates autophagy, which serves as a temporary survival pathway
in cells under stress by delaying the occurrence of apoptosis
(16). The aim of the present
study was to determine the association between oxidative stress and
Cd-induced autophagy in PC-12 cells.
Materials and methods
Materials
RPMI-1640 medium, fetal bovine serum (FBS) and horse
serum were purchased from Life Technologies (Grand Island, NY,
USA). Cd(CH3COO)2·3H2O (Cd),
N-acetyl-L-cysteine (NAC), 2-,7-dichlorofluorescein diacetate
(DCFH-DA), polyclonal rabbit anti-LC3B (cat. no. L7543),
monodansylcadaverine (MDC), chloroquine (CQ) and poly-L-lysine were
purchased from Sigma-Aldrich (St. Louis, MO, USA). JC-1, a
bicinchoninic acid (BCA) protein assay kit was provided by Beyotime
Institute of Biotechnology (Haimen, Jiangsu, China) and propidium
iodide (PI) was purchased from BD Pharmingen (San Diego, CA, USA).
Monoclonal rabbit anti-β-actin (cat. no. 4970), horseradish
peroxidase (HRP)- and fluorescein isothiocyanate (FITC)-conjugated
goat anti-rabbit IgG (cat. nos. 7074 and 4412, respectively) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The enhanced chemiluminescence (ECL) solution (SuperSignal
Chemiluminescent HRP substrate) was obtained from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA) and lactate dehydrogenase (LDH)
was purchased from Beckman Coulter, Inc. (Brea, CA, USA).
Cell cultures
The rat pheochromocytoma cell line, PC-12 was
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). The PC-12 cell line was cultured in
an antibiotic-free RPMI-1640 medium (temperature, 37°C; atmosphere,
5% CO2), which was supplemented with 10%
heat-inactivated horse serum and 5% FBS.
Immunofluorescence
The PC-12 cells (2.0×105 cells/ml) were
cultivated on cover slips that were pre-coated with poly-L-lysine
and treated with 20 μmol/l Cd for 4 h. After washing in
phosphate-buffered saline (PBS), the cells were fixed with 4%
paraformaldehyde in PBS for 30 min at 4°C, permeabilized with 0.5%
Triton X-100 (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) and blocked with 5% bovine serum albumin (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) for 20 min at
room temperature. The cells were incubated with anti-LC3B rabbit
antibody in a blocking solution for 2 h, washed in blocking
solution and stained with FITC-conjugated anti-mouse IgG for 1 h.
The cell nuclei were subsequently stained with DAPI. Following
washing, the samples (~300 cells) were observed under a
fluorescence microscope (Leica DMI3000 B; Leica Microsystmes,
Wetzlar, Germany).
Labeling of autolysosome with MDC
PC-12 cells (2.5×105 cells/ml) were grown
on cover slips, pretreated with or without 5 μmol/l CQ for
30 min, followed by treatment with or without 20 μmol/l Cd
for a further 4 h. After incubation, cells were washed in PBS and
fixed for 30 min using a fresh solution of 4% paraformaldehyde.
Following staining with 50 μmol/l MDC, the cells were
immediately analyzed by fluorescence microscopy (Leica DMI3000
B).
Transmission electron microscopy
Treated cells were washed and fixed for 30 min in
2.5% glutaraldehyde. The samples were treated with 1.5% osmium
tetroxide, dehydrated with alcohol and embedded in epoxy resins.
The ultrathin sections were contrasted with uranyl acetate and lead
citrate for electron microscopy. Electron micrographs were obtained
using a Philips CM120 transmission electron microscope (Philips,
Eindhoven, The Netherlands).
Western blot analysis
PC-12 cells (3.0×105) were preincubated
with 100 μmol/l NAC for 1 h, and were then incubated with 20
μmol/l Cd for a further 4 h. Following treatment, the cells
were washed twice with cold PBS, extracted into RIPA lysis buffer
on ice for 30 min, sonicated for 10 sec and centrifuged at 12,000 ×
g for 10 min at 4°C. The protein content was determined using a BCA
protein assay kit. Equal quantities of protein samples (60
μg) were uploaded and separated by 15% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and transferred onto
nitrocellulose membranes. The membranes were blocked in 5% nonfat
milk in Tris-buffered saline with 0.1% Tween-20 at room temperature
for 2 h, and incubated with the indicated primary antibodies
(anti-LC3B, 1:1,000; anti-β-actin, 1:2,000) overnight at 4°C and
with HRP-conjugated secondary antibodies at room temperature for
1.5 h. The signal was detected using ECL reagents. The band
intensity was determined using the gel image analysis system, Image
Lab version 2.0.1 (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
and was normalized against β-actin.
ROS determination
Following treatment, the cells were collected,
incubated with 20 μmol/l DCFH-DA at 37°C for 20 min in the
dark, and washed twice with PBS. The cells were analyzed using a
FACSAria flow cytometer (BD Biosciences, San Jose, CA, USA).
Mitochondrial membrane potential (ΔΨm)
assay
Changes in the ΔΨm were measured by flow cytometry
using JC-1 dye. Following treatment, cells were selected and
incubated in a JC-1 staining solution for 20 min at 37°C. The cells
were subsequently rinsed twice with the JC-1 staining buffer. The
fluorescence intensity of the mitochondrial JC-1 monomers and
aggregates was detected using flow cytometry. The ΔΨm of the PC-12
cells was calculated as the ratio of red (aggregates) to green
(monomers) fluorescence.
PI staining to assess DNA
fragmentation
The PI staining assay and flow cytometry were used
to evaluate the extent of cell death. Apoptotic cells are
characterized by a hypoploid DNA fluorescence pattern (17). Briefly, following treatment, cells
were collected and suspended in 100 μl binding buffer
containing 5 μl PI dye solution. Following incubation in the
dark at 25°C for 25 min, the cells were analyzed using a FACSAria
flow cytometer.
LDH activity assay
Following treatment, the supernatant of the cell
culture was harvested. The PC-12 cells were rinsed with PBS and
lysed with 1% Triton X-100 at 37°C for 30 min. The supernatants and
cell lysates were prepared according to the manufacturer's
instructions for the LDH assay and examined using an automatic
biochemical analyzer (AU480; Beckman Coulter, Inc.). The LDH result
is expressed as the proportion of leakage with respect to the total
level of LDH.
Statistical analysis
Results are presented as the mean ± standard
deviation (SD). Significance was assessed by one-way analysis of
variance following appropriate transformation to normalized data
and equalized variance where required. Statistical analysis was
performed using IBM SPSS statistics 19.0 (IBM SPSS, Armonk, NY,
USA) and P<0.05 was considered to indicate a statistically
significant difference.
Results
Activation of autophagy
The distribution of endogenous LC3-II in cells prior
to and following Cd treatment for 4 h was monitored by indirect
immunofluorescence staining to examine whether autophagy was
induced by Cd treatment. As demonstrated in Fig. 1A, a greater number of dots were
apparent in Cd-treated neurons when compared with the control
cells. In addition, the treated cells were stained with MDC, a
fluorescent dye that selectively recognizes autolysosomes (13). The Cd-treated cells reveal a
diffuse punctate pattern, which indicates the activation of
autophagy, while the control cells demonstrate faint fluorescence
(Fig. 1B and C; P<0.01).
Following treatment with CQ, which disrupts the function of
lysosomes and inhibits autophagy at a late stage, Cd co-treatment
resulted in the accumulation of large MDC-positive vesicles. To
further demonstrate that autophagy is induced by Cd, transmission
electron microscopy was performed on untreated or Cd-treated cells.
As depicted in Fig. 1D, Cd-treated
cells exhibited cup-shaped membranous structures, and initial and
degradative autophagosome structures that were not present in the
control cells. Furthermore, western blot analysis revealed that the
levels of LC3-II protein markedly increased following Cd treatment
(Fig. 2A). These results indicate
that Cd treatment induces autophagy in PC-12 cells.
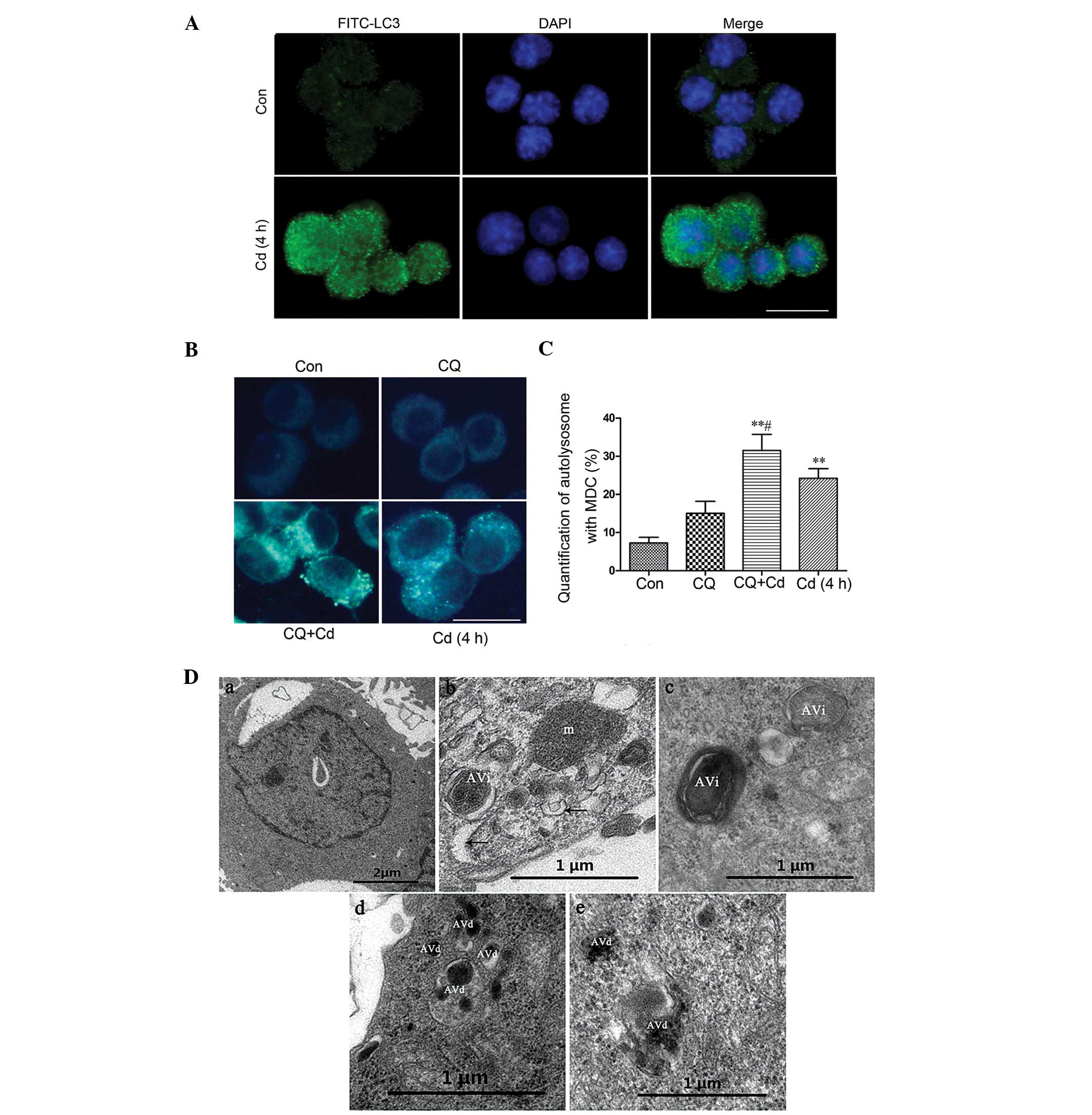 | Figure 1Activation of autophagy. (A) PC-12
cells were treated with 20 μmol/l Cd for 4 h and stained by
indirect immunofluorescence. (B) PC-12 cells were pretreated with 5
μmol/l CQ for 30 min, followed by treatment with 20
μmol/l Cd for another 4 h. MDC staining analysis was
performed by fluorescence microscopy. (C) Quantitation of ~300
cells containing autolysosomes, reported as the mean ± standard
deviation; **P<0.01, compared with the control and
#P<0.05 compared with the Cd 4 h group (bar = 10
μm). (D) Transmission electron microscopy analysis. (a)
Untreated control cells demonstrating normal distribution of
organelles; (b–e) autophagic compartments at various stages.
Cup-shaped membranous structures in the cytoplasm are indicated by
the arrows. m, mitochondria; AVi, initial autophagosome; AVd,
degradative autophagosome. FITC-LC3, fluorescein
isothiocyanate-light chain 3; Con, control; Cd, cadmium; CQ,
chloroquine; MDC monodansylcadaverine. |
Involvement of ROS in Cd-triggered
autophagy
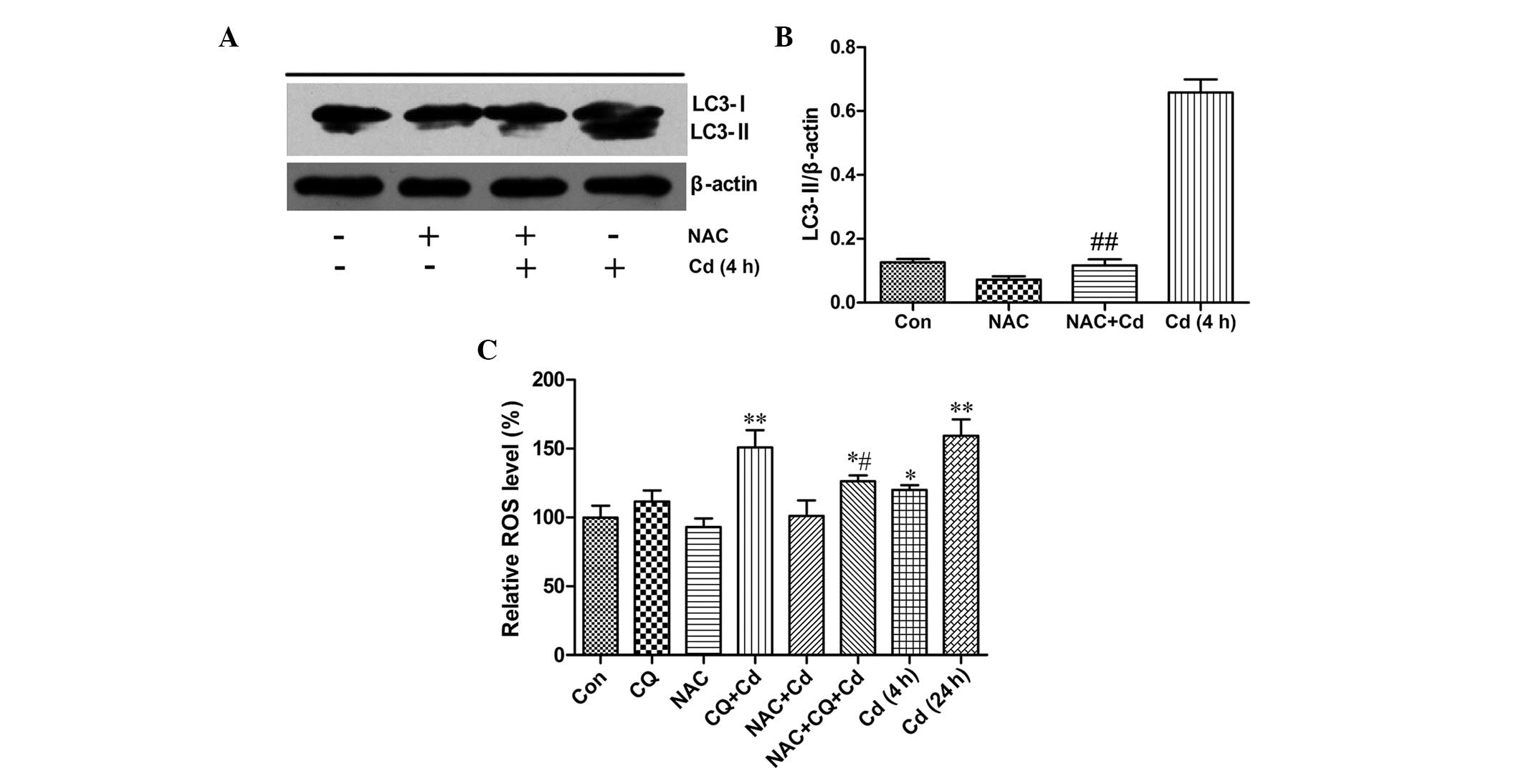
To identify whether the ROS scavenger, NAC exerts an
effect on Cd-induced autophagy, western blot analysis was
conducted. As Fig. 2A and B
demonstrate, compared with the Cd treatment group, the antioxidant,
NAC significantly inhibited the LC3-II protein level (P<0.01).
The result indicates that Cd-mediated autophagy may be blocked by
NAC and ROS production may be involved in inducing autophagy.
To further identify the above-mentioned hypothesis,
ROS levels were assessed using flow cytometry. As shown in Fig. 2C, treatment of PC-12 cells with Cd
for 4 h and 24 h increased intracellular ROS levels significantly
(P<0.05 and P<0.01, respectively) compared with the control.
Co-treatment with 20 μmol/l Cd and CQ for 4 h also
significantly increased ROS production when compared with the
control group (P<0.01). However, NAC reduced the ROS
concentrations that were induced by Cd and CQ co-treatment
(P<0.05). The result revealed that Cd-mediated autophagy may be
dependent on its ability to generate ROS.
Roles of ROS and autophagy in Cd-induced
cytotoxicity
In the case of treatment with 20 μmol/l Cd,
there was a significant decrease in ΔΨm following treatment for 4 h
(P<0.05) and 24 h (P<0.01), a 4-h co-treatment of Cd and CQ
also significantly decreased ΔΨm (P<0.01; Fig. 3A). Although the difference was not
significant, it appeared that NAC did not prevent, but promoted
this event induced by Cd and CQ treatment in PC-12 cells. The
findings indicate that ROS production protects PC-12 cells from
inhibiting the decline of ΔΨm by inducing autophagy.
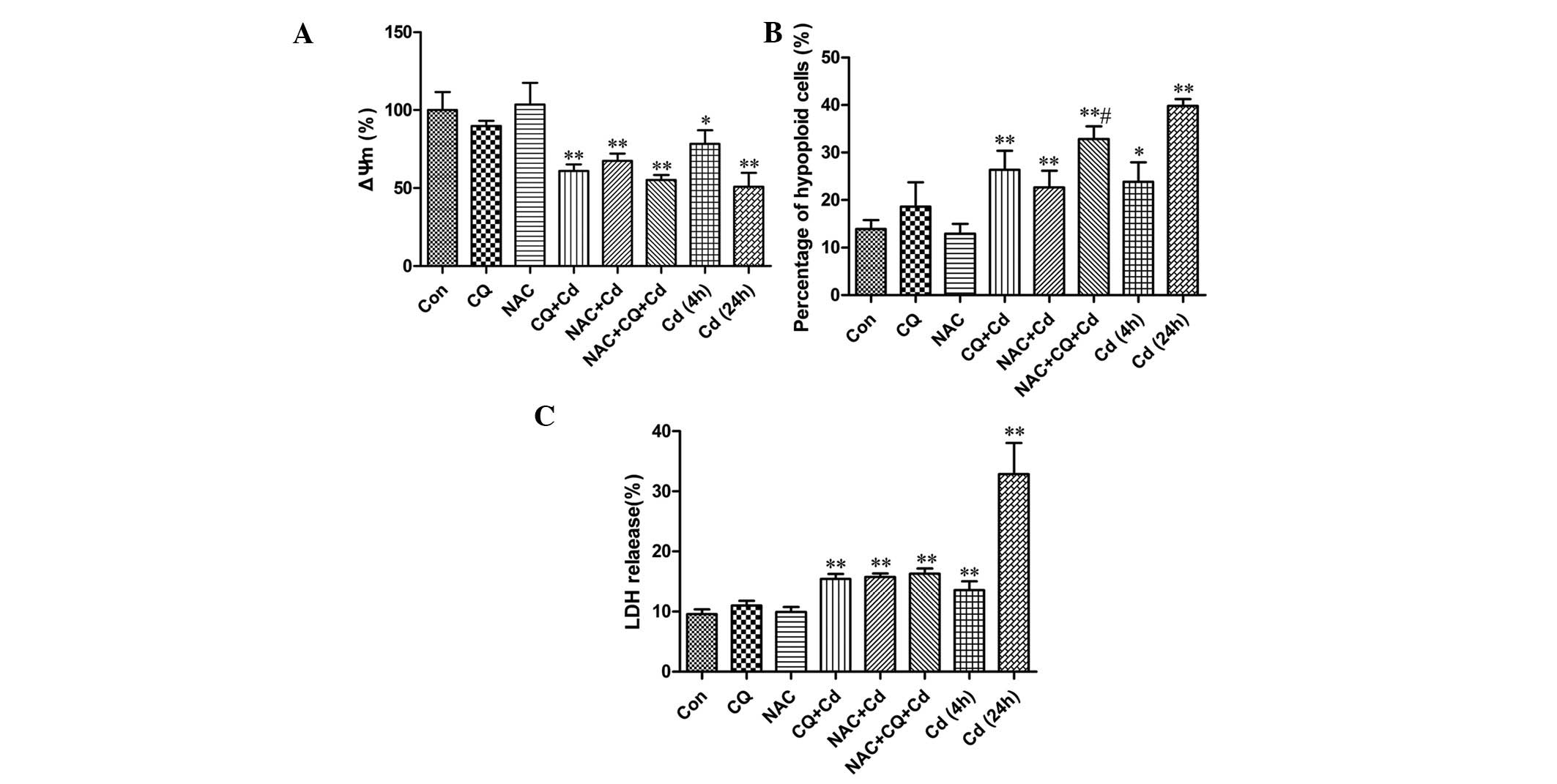 | Figure 3Roles of ROS and autophagy in
Cd-induced cytotoxicity. PC-12 cells were preincubated with 5
μmol/l CQ for 30 min and/or 100 μmol/l NAC for 1 h,
then incubated with 20 μmol/l Cd for another 4 h. Or PC-12
cells were treated with 20 μmol/l Cd for 24 h. (A) Flow
cytometry was used to examine changes in the ΔΨm, (B) hypoploid
apoptotic cell numbers were determined by propidium iodide staining
and (C) the LDH assay was detected using a cell viability assay kit
(n=3). *P<0.05, **P<0.01 compared with
the control, and #P<0.05 compared with the
co-treatment group of Cd and CQ. ΔΨm, mitochondrial membrane
potential; Con, control; CQ, chloroquine; NAC, N-acetyl-L-cysteine;
Cd, cadmium; LDH, lactate dehydrogenase; ROS, reactive oxygen
species. |
To examine the role of ROS and autophagy associated
with Cd-induced cytotoxicity, a PI staining assay was performed.
The results demonstrated that Cd treatment increased the quantity
of hypoploid apoptotic cells following 4 h (P<0.05) and 24 h
(P<0.01) of treatment when compared with the control.
Furthermore, CQ augmented the proportion of apoptotic cells
following a 4-h treatment with Cd (P<0.01). In addition, PC-12
cells that were co-treated with NAC, Cd and CQ further accelerated
the apoptosis rate when compared with the CQ and Cd co-treatment
group (P<0.05; Fig. 3B).
Collectively, these results indicate that ROS generation inhibits
apoptosis by inducing autophagy in PC-12 cells.
For Cd injured PC-12 cells, the LDH leakage rate
(Fig. 3C) demonstrated a
significant difference when compared with the control group
(9.58±0.76%) after cells had been treated with Cd for 4 h
(13.56±1.45%; P<0.01). Furthermore, the LDH leakage rate
markedly increased to 32.87±4.47% following a 24-h Cd treatment
(P<0.01). CQ increased the release of LDH following treatment
with 20 μmol/l Cd for 4 h (15.41±0.84%; P<0.01). However,
NAC did not protect PC-12 cells against injury that was caused by
CQ and Cd co-treatment (16.05±0.79%) (Fig. 3C), indicating that the protective
effect of ROS is irrelevant to reducing necrosis.
Discussion
The bioaccumulation of Cd has been demonstrated as
toxic to humans and environmental health. Cd induces damaging and
repair processes in which the cellular redox status is critical
(18). Thus, understanding the
interface between stress adaptation and cell death/autophagy is
important for understanding redox biology and the disease
pathogenesis that is caused by Cd.
Cd-induced autophagy has been reported in in
vitro and in vivo models (19–21).
A previous study has shown that Cd-induced autophagic death is
dependent on ROS activation in skin epidermal cells (22). However, the function and mechanism
of autophagy remains unknown. The results of the present study
indicate that Cd-induced ROS increase is closely associated with
protective autophagy in PC-12 cells. Inhibition of autophagy, by
the addition of CQ, resulted in a further decreased capacity to
reduce mitochondrial content and an increased sensitivity to Cd,
which supports the hypothesis that autophagy is a cellular
protective mechanism response to Cd treatment. Notably, NAC reduced
intracellular ROS levels and decreased Cd-induced autophagy, but
enhanced the mitochondrial damage and apoptotic cell death that was
induced by the inhibition of autophagy. Previous studies
demonstrated that high levels of intracellular glutathione inhibit
mitophagy in yeast (23) and
increased superoxide anion production stimulates mitophagy
(24), which supports the findings
of the present study that increased ROS, induced by Cd, acts as a
signaling molecule to activate mitophagy.
Generation of ROS is hypothesized to be the basis
for Cd-induced toxicity. In certain instances, ROS formation
induced by Cd depletes endogenous redox scavengers, inhibits
antioxidant enzymes and the mitochondrial electron transport chain,
causes mitochondrial damage, and triggers apoptosis and necrosis
(8,25,26).
However, Cd-induced ROS formation also activates various
antioxidative components as a result of a disturbed redox balance
and a consecutively induced signal transduction cascade (18). The result of the present study
indicated that an appropriate quantity of ROS is not harmful, but
essential in order to induce autophagy for cell survival. The
initial ROS response to Cd induction is an initiation of autophagy
to enable self-clearance. Autophagy may act as a cellular
antioxidation defense mechanism, although its function is not
inherently antioxidative. Notably, in the present study when
autophagy was inhibited and the PC-12 cells were exposed to Cd at a
late stage (24 h), ROS accumulation was observed in the PC-12
cells; however, apoptosis and necrosis also became evident at this
point. These results indicate that a massive and persistent ROS is
likely to be accompanied by an induction of mitochondrial
dysfunction leading to cell death, which is consistent with the
hypothesis of neuronal cell death as a consequence of increased
oxidative stress (11). The
underlying reason for these variances remains unknown, however
might be associated with differences in the capacity to activate
autophagy, the quantity of ROS and the time exposed to Cd.
Therefore, oxidative stress is hypothesized to be essential for
cell survival to trigger autophagy at the initial stage. As an
increasing quantity of Cd enters into cells, the overwhelming
oxidative stress may be harmful to those cells. Further studies are
required to investigate the ROS threshold in cell survival and
death.
The crosstalk between autophagy, redox signaling and
mitochondrial dysfunction is not well understood (10). Mitochondria are generators of and
targets for ROS, and oxidative stress is indivisibly linked to
mitochondrial dysfunction (27).
Mitochondrial turnover is dependent on autophagy, which results in
membrane potential variances, ROS production (28) and occasionally cell death (29). Under certain stress conditions, one
possible explanation is that the accumulation of toxic proteins and
the decrease in mitochondrial function leads to further
overwhelming oxidative stress when the autophagic process is
disrupted (30–32). By contrast, redox signaling or
mitochondrial dysfunction may also influence autophagy. The results
of the present study conform to these two hypotheses. Although ROS
production has been identified as demonstrating a positive role in
Cd-induced autophagy, it remains unclear as to which species of ROS
activate the autophagy in Cd-treated PC-12 cells. Furthermore, the
present findings do not exclude the possibility that mitochondrial
dysfunction is also involved in the induction of autophagy, as ROS
production may result from either the mitochondria themselves or
the plasma membrane oxidases (33). Further investigation is required to
resolve these queries.
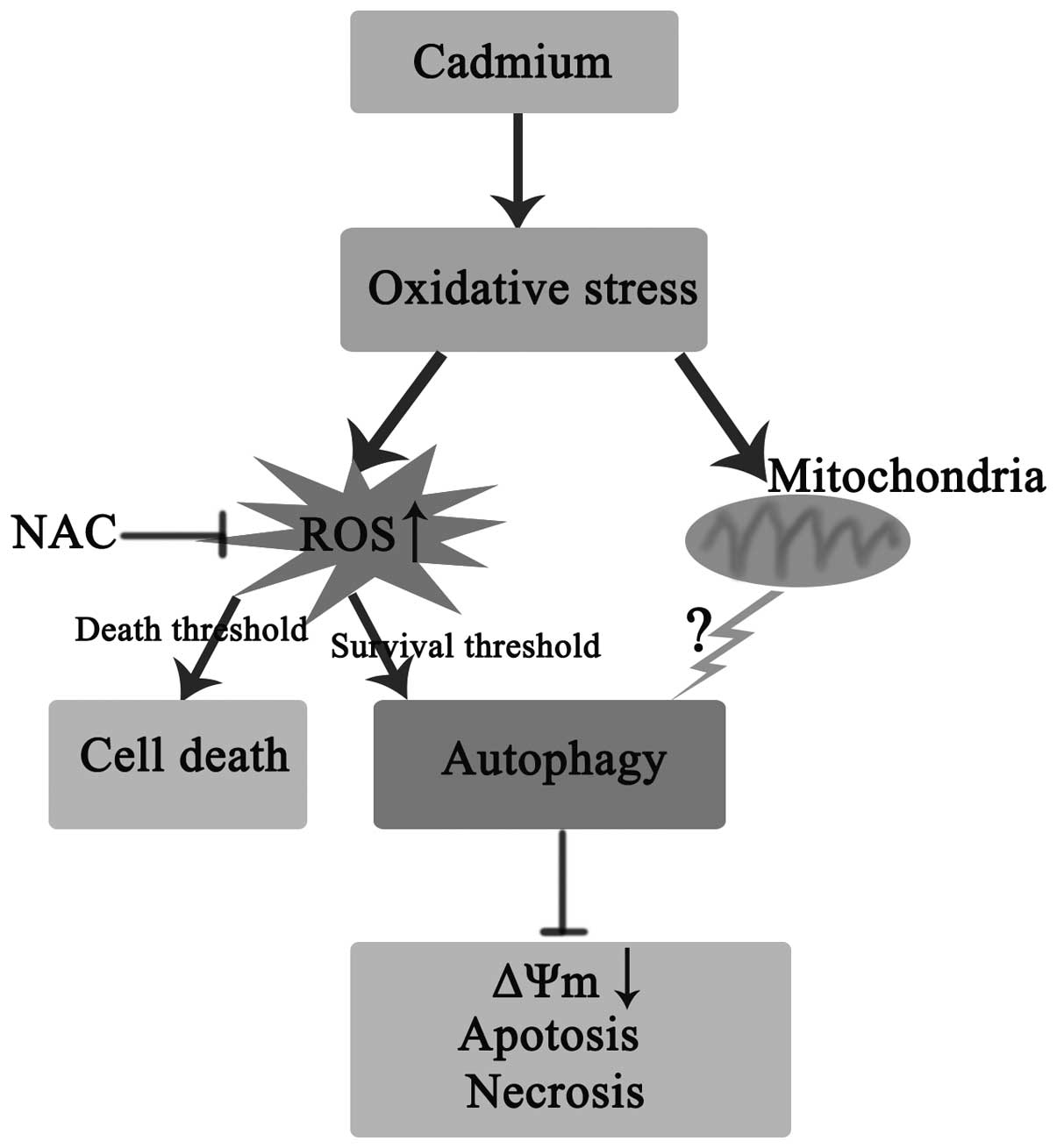
In conclusion, autophagy has been demonstrated as
significant in PC-12 cell survival following Cd treatment. At the
early stages of Cd exposure, ROS function as signaling molecules to
trigger autophagy as a survival mechanism in Cd-induced oxidative
stress. However, excessive ROS production may lead to cell death
(Fig. 4). The present study
provides an insight for future investigation of Cd-induced
neurotoxicity.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 31302058, 31101866
and 31172373), a Project Funded by the Priority Academic Program
Development of Jiangsu Higher Education Institutions (PAPD) and the
Joint Foundation of Guizhou Province (grant no. LKB [2013]02). The
authors would like to thank Dr Maozhi Hu for technical assistance
in flow cytometry analysis.
References
|
1
|
Templeton DM and Liu Y: Multiple roles of
cadmium in cell death and survival. Chem Biol Interact.
188:267–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lukawski K, Nieradko B and
Sieklucka-Dziuba M: Effects of cadmium on memory processes in mice
exposed to transient cerebral oligemia. Neurotoxicol Teratol.
27:575–584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Curtis JT, Hood AN, Chen Y, Cobb GP and
Wallace DR: Chronic metals ingestion by prairie voles produces
sex-specific deficits in social behavior: an animal model of
autism. Behav Brain Res. 213:42–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mascagni P, Consonni D, Bregante G,
Chiappino G and Toffoletto F: Olfactory function in workers exposed
to moderate airborne cadmium levels. Neurotoxicology. 24:717–724.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernández-Pérez B, Caride A, Cabaleiro T
and Lafuente A: Cadmium effects on 24h changes in glutamate,
aspartate, glutamine, GABA and taurine content of rat striatum. J
Trace Elem Med Biol. 24:212–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minami A, Takeda A, Nishibaba D, Takefuta
S and Oku N: Cadmium toxicity in synaptic neurotransmission in the
brain. Brain Res. 894:336–339. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schapira AH and Gegg M: Mitochondrial
contribution to Parkinson's disease pathogenesis. Parkinsons Dis.
2011:1591602011.PubMed/NCBI
|
|
8
|
Thévenod F: Cadmium and cellular signaling
cascades: to be or not to be? Toxicol Appl Pharmacol. 238:221–239.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bertin G and Averbeck D: Cadmium: cellular
effects, modifications of biomolecules, modulation of DNA repair
and genotoxic consequences (a review). Biochimie. 88:1549–1559.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee J, Giordano S and Zhang J: Autophagy,
mitochondria and oxidative stress: cross-talk and redox signalling.
Biochem J. 441:523–540. 2012. View Article : Google Scholar :
|
|
11
|
Chen L, Xu B, Liu L, Luo Y, Zhou H, Chen
W, Shen T, Han X, Kontos CD and Huang S: Cadmium induction of
reactive oxygen species activates the mTOR pathway, leading to
neuronal cell death. Free Radic Biol Med. 50:624–632. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lavallard VJ, Meijer AJ, Codogno P and
Gual P: Autophagy, signaling and obesity. Pharmacol Res.
66:513–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy. Autophagy.
8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scarlatti F, Granata R, Meijer AJ and
Codogno P: Does autophagy have a license to kill mammalian cells?
Cell Death Differ. 16:12–20. 2009. View Article : Google Scholar
|
|
15
|
Scherz-Shouval R and Elazar Z: ROS,
mitochondria and the regulation of autophagy. Trends Cell Biol.
17:422–427. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, Zhu J, Zhang K, Jiang C, Wang Y,
Yuan Y, Bian J, Liu X, Gu J and Liu Z: Induction of cytoprotective
autophagy in PC-12 cells by cadmium. Biochem Biophys Res Commun.
438:186–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krysko DV, Vanden Berghe T, D'Herde K and
Vandenabeele P: Apoptosis and necrosis: detection, discrimination
and phagocytosis. Methods. 44:205–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cuypers A, Plusquin M, Remans T, Jozefczak
M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ,
et al: Cadmium stress: an oxidative challenge. Biometals.
23:927–940. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thévenod F and Lee WK: Cadmium and
cellular signaling cascades: interactions between cell death and
survival pathways. Arch Toxicol. 87:1743–1786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chiarelli R, Agnello M, Bosco L and
Roccheri MC: Sea urchin embryos exposed to cadmium as an
experimental model for studying the relationship between autophagy
and apoptosis. Mar Environ Res. 93:47–55. 2014. View Article : Google Scholar
|
|
21
|
Chargui A, Zekri S, Jacquillet G, Rubera
I, Ilie M, Belaid A, Duranton C, Tauc M, Hofman P, Poujeol P, et
al: Cadmium-induced autophagy in rat kidney: an early biomarker of
subtoxic exposure. Toxicol Sci. 121:31–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Son YO, Wang X, Hitron JA, Zhang Z, Cheng
S, Budhraja A, Ding S, Lee JC and Shi X: Cadmium induces autophagy
through ROS-dependent activation of the LKB1-AMPK signaling in skin
epidermal cells. Toxicol Appl Pharmacol. 255:287–296. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deffieu M, Bhatia-Kissová I, Salin B,
Galinier A, Manon S and Camougrand N: Glutathione participates in
the regulation of mitophagy in yeast. J Biol Chem. 284:14828–14837.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim EH and Choi KS: A critical role of
superoxide anion in selenite-induced mitophagic cell death.
Autophagy. 4:76–78. 2008. View Article : Google Scholar
|
|
25
|
Thévenod F: Catch me if you can! Novel
aspects of cadmium transport in mammalian cells. Biometals.
23:857–875. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
López E, Arce C, Oset-Gasque MJ, Cañadas S
and González MP: Cadmium induces reactive oxygen species generation
and lipid peroxidation in cortical neurons in culture. Free Radic
Biol Med. 40:940–951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murphy MP: How mitochondria produce
reactive oxygen species. Biochem J. 417:1–13. 2009. View Article : Google Scholar
|
|
28
|
Yen WL and Klionsky DJ: How to live long
and prosper: autophagy, mitochondria, and aging. Physiology
(Bethesda). 23:248–262. 2008. View Article : Google Scholar
|
|
29
|
Luo YH, Wu SB, Wei YH, Chen YC, Tsai MH,
Ho CC, Lin SY, Yang CS and Lin P: Cadmium-based quantum dot induced
autophagy formation for cell survival via oxidative stress. Chem
Res Toxicol. 26:662–673. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hashimoto M, Rockenstein E, Crews L and
Masliah E: Role of protein aggregation in mitochondrial dysfunction
and neurodegeneration in Alzheimer's and Parkinson's diseases.
Neuromolecular Med. 4:21–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gottlieb RA and Carreira RS: Autophagy in
health and disease. 5. Mitophagy as a way of life. Am J Physiol
Cell Physiol. 299:C203–C210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schneider L and Zhang J: Lysosomal
function in macromolecular homeostasis and bioenergetics in
Parkinson's disease. Mol Neurodegener. 5:142010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thannickal VJ and Fanburg BL: Reactive
oxygen species in cell signaling. Am J Physiol Lung Cell Mol
Physiol. 279:L1005–L1028. 2000.PubMed/NCBI
|