Introduction
Allergic asthma is a common inflammatory disease,
which affects ~300 million people worldwide (1). Allergic asthma results from exposure
to allergens which may include pollens, house dust, animal dander,
inhalants, foods and air pollutants (2). Patients with allergic asthma exhibit
clinical features including wheezing, breathlessness, dyspnea and
coughing (3). These symptoms
result from airway inflammation, which is associated with the
recruitment of inflammatory cells into the airway, mucus
overproduction and airway hyper-responsiveness (2). Airway inflammation is a complex
response that is associated with numerous factors including T
helper (Th) type 2 cytokines, proinflammatory proteins, chemokines
and growth factors (1). The
importance of these mediators in the development of allergic asthma
has been demonstrated in previous studies (2–4). Among these
mediators, inducible nitric oxide synthase (iNOS) has been shown to
have a pivotal role in the production of nitric oxide (NO), which
acts as a powerful aggravator of allergic asthma (5). Asthmatic conditions are associated
with an overexpression of iNOS in the airways, and significantly
elevated NO production (5).
Previous studies have demonstrated that NO activates
proinflammatory signaling and Th2 responses in allergic asthma
(6). Furthermore, the suppression
of iNOS expression in the airways has been shown to reduce
asthmatic responses in numerous asthma models (7).
Thuja orientalis (TO) is an evergreen tree,
from which extracts are used in Korean traditional medicine. It is
used to treat numerous diseases, including gout, rheumatism,
diarrhea and chronic tracheitis, as demonstrated by numerous in
vivo and in vitro studies (8–11). Previous studies have
demonstrated that TO possesses antioxidant, anticancer, and
anti-inflammatory properties (12–14). However, there are currently
no studies on the effects of TO on asthmatic responses that include
airway hyperresponsiveness (AHR), inflammation, and mucus
hypersecretion.
The aims of the present study were to examine the
effects of TO on airway inflammation in an ovalbumin (OVA)-induced
allergic asthma murine model, by measuring Th2 cytokines, AHR,
chemokines, immunoglobulin (Ig) E, and histologically analyzing
lung tissue. To further investigate the possible anti-inflammatory
mechanisms of TO, the expression levels of proinflammatory
proteins, including iNOS, cyclooxy-genase (COX)-2 and matrix
metalloproteinase (MMP)-9, were determined in RAW264.7 cells and
the allergic asthma murine model.
Materials and methods
Cell culture and viability
RAW264.7 murine macrophage cells were purchased from
the American Type Culture Collection (Manassas, VA, USA). The cells
were incubated at 37°C in 5% CO2 in Dulbecco's modified
Eagle's media (DMEM; Gibco-BRL, Carlsbad, CA, USA), supplemented
with 100 U/ml penicillin, 100 µg/ml streptomycin and 5.5%
fetal bovine serum (Gibco-BRL). The cells were also treated with
0.05% dimethyl sulfoxide as a vehicle control. The cells were
seeded in 96-well plates at a density of 5×104
cells/well and incubated in serum-free medium in the presence of
different concentrations of TO. Following a 24 h incubation, the
cellular viability was determined using an MTT assay. TO was
obtained from the Plant Extract Bank at the Korea Research
Institute of Bioscience and Biotechnology (PB1411.1; Daejeon, South
Korea). All experiments were performed in triplicate. TO
concentrations were used that have been shown to be nontoxic in
other biological systems, based on MTT results (Fig. 1A).
Measurement of NO production
The cells (2.5×105 cells/ml) were seeded
in 96-well plates in phenol red-free DMEM, and treated with
different concentrations (10, 20, 30, and 40 µg/ml) of TO
for 1 h, followed by an incubation in the presence of
lipopolysaccharide (LPS; 1 µg/ml) for 24 h. Nitrite
accumulation in the culture medium was measured using Griess
reagent (Promega Corporation, Madison, WI, USA). The absorbance was
measured at 535 nm, using a microplate reader (Bio-Rad, Hercules,
CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from the cells using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA). A total of 2 µg of RNA was reverse transcribed, to
synthesize single-stranded cDNA, using the commercially available
kit (Qiagen, Valencia, CA, USA). The cDNA was then subjected to
PCR. The primer sequences (Bioneer, Daejeon, Korea) used were as
follows: tumor necrosis factor (TNF)-α, sense, 5′-GTG GAA CTG GCA
GAA GAG GC-3′, antisense, 5′-AGA CAG AAG AGC GTG GTG GC-3′; COX-2,
sense, 5′-CAA GTC TTT GGT CTG GTG CCT G-3′, antisense, 5′-GTC TGC
TGG TTT GGA ATA GTT GC-3′; iNOS, sense, 5′-CAA GAG TTT GAC CAG AGG
ACC-3′, antisense, 5′-TGG AAC CAC TCG TAC TTG GGA-3′; interleukin
(IL)-6, sense, 5′-GAG GAT ACC ACT CCC AAC AGA CC-3′, antisense,
5′-AAG TGC ATC ATC GTT GTT CAT ACA-3′; MMP-9, sense, 5′-AAG CAC ATG
CAG AAT GAG TAC CG-3′, antisense, 5′-GTG GGA CAG CTT CTG GTC
GAT-3′; and β-actin, sense, 5′-CGC TCA TTG CCG ATA GTG AT-3′; and
antisense 5′-TGT TTG AGA CCT TCA ACA CC-3′. The PCR products were
fractionated and analyzed qualitatively by 1.5% agarose gel
electrophoresis, stained with 5 µg/ml ethidium bromide for
visualization.
Gelatin zymography
The cells (2.5×105 cells/ml) were seeded
into 96-well plates in phenol red-free DMEM and treated with
different concentrations (10, 20, 30 and 40 µg/ml) of TO for
1 h, followed by an incubation in the presence of LPS for 24 h. The
cell supernatants were collected and were loaded for gelatin
zymography. SDS−PAGE zymography was performed according to the
methods of Heussen and Dowdle (15), to determine gelatinase activities.
Briefly, zymogram gels, consisting of 10% polyacrylamide gel
containing SDS and 1% gelatin, were used as the MMP substrate. The
gels were washed in 2.5% Triton X-100 for 1 h to remove the SDS,
followed by an incubation at 37°C for 16 h in developing buffer (1
M Tris-HCl, pH 7.5 with CaCl2). The gels were
subsequently stained with 25% methanol/8% acetic acid, containing
Coomassie Brilliant Blue (Amresco, Solon, OH, USA). Gelatinase
activity was visualized as white bands on a blue background, this
represented the areas of proteolysis.
Mouse model of OVA-induced allergic
asthma
Female BALB/c specific pathogen-free mice (six weeks
old) were purchased from Koatech Co. (Pyeongtaek, South Korea).
Experimentation began after a two week quarantine and
acclimatization period. All experimental procedures were approved
by the Institutional Animal Care and Use Committee of the Korea
Research Institute of Bioscience and Biotechnology. The mice were
sensitized on days 0 and 14, by an intraperitoneal (i.p) injection
of 20 µg OVA (Sigma-Aldrich, St. Louis, MO, USA) emulsified
with 2 mg aluminum hydroxide in 200 µl phosphate-buffered
saline (PBS) buffer (pH 7.4). On days 21, 22, and 23, the mice
received airway challenges of OVA (1% (w/v)) for 1 h using an
ultrasonic nebulizer (NE-U12; Omron Corp., Tokyo, Japan). TO and
montelukast, which was used as a positive control, were orally
administered to the mice at a dose of 30 mg/kg body weight, one
hour prior to OVA challenge. Airway responsiveness was indirectly
assessed 24 h after the final challenge, using single-chamber,
whole body plethysmography (Allmedicus Co. Ltd., Seoul, South
Korea). The mice were sacrificed 48 h after the final challenge, by
i.p injection of pentobarbital (50 mg/kg; Hanlim Pharm. Co. Ltd.,
Seoul, South Korea), and tracheostomies were performed. To obtain
bronchoalveolar lavage fluid (BALF), ice-cold PBS (0.5 ml) was
infused into the lung three times, and the fluid was withdrawn each
time by tracheal cannulation (total volume 1.5 ml). Total
inflammatory cell numbers were determined by counting the cells in
≤5 squares of a hemocytometer, following the exclusion of dead
cells, with Trypan blue staining. Differential BALF cell counts
were performed using Diff-Quik® staining reagent (IMEB
Inc., San Marcos, CA, USA), according to the manufacturer's
instructions.
Measurements of cytokine and chemokine
levels in the BALF and IgE in the serum
The levels of IL-4, IL-5, and IL-13 in the BALF were
measured using ELISA kits (R&D SystemS, Minneapolis, MN, USA)
according to the manufacturer's instructions. The levels of total
IgE and OVA-specific IgE in the serum were also measured by ELISA.
Microtiter plates were coated with anti-IgE antibodies (anti-mouse
IgE; 10 g/ml; Serotec, Oxford, UK), in PBS-Tween® 20,
and incubated with BALF or a plasma sample. The plates were then
washed four times with wash solution (PBS, containing 0.05% Tween
20 (Biosesang, GyeongGi-Do, Korea), and 200 µl of
o-Phenylene-diamine dihydrochloride (Sigma-Aldrich) was added to
each well. The plates were incubated for 10 min in the dark, and
the absorbance was measured at 450 nm.
Western blot analysis
The murine lung tissue was homogenized (1/10 w/v)
using a homogenizer with Tissue Lysis/Extraction reagent
(Sigma-Aldrich), containing a protease inhibitor cocktail
(Sigma-Aldrich). Protein concentrations were determined using a
protein assay reagent (Bio-Rad), according to the manufacturer's
instructions. Equal amounts of total cellular protein (30
µg) were separated by 12% SDS-PAGE and then transferred to
polyvinylidene fluoride membranes (Millipore, Darmstadt, Germany).
The membranes were incubated with blocking solution (5% skim milk;
Becton Dickinson, Franklin Lakes, NJ, USA), followed by an
overnight incubation at 4°C with the appropriate primary antibody.
The following primary antibodies and dilutions were used:
anti-β-actin (1:2,000 dilution; Cell Signaling Technology Inc.,
Danvers, MA, USA), anti-iNOS (1:1,000 dilution; Abcam, Cambridge,
MA, USA) and anti-MMP-9 (1:1,000 dilution; Cell Signaling
Technology Inc.). The blots were washed three times with
Tris-buffered saline containing Tween® 20 (TBST),
followed by an incubation with a 1:10,000 dilution of horseradish
peroxidase-conjugated secondary antibody (Jackson ImmunoResearch
Laboratories Inc., Westgrove, PA, USA) for 30 min at room
temperature. The blots were washed three additional times with
TBST, and visualized using an enhanced chemiluminescence kit
(Thermo Fisher Scientific, Waltham, MA, USA). Densitometic values
for each protein were measured using a Chemi-Doc system
(Bio-Rad).
Histology
After the BALF samples had been obtained, the lung
tissues were fixed in 4% (v/v) paraformaldehyde. The tissues were
embedded in paraffin, sectioned at 4 µm, and stained with
hematoxylin & eosin solution (Sigma-Aldrich) and Periodic
acid-Schiff (IMEB Inc) in order to estimate inflammation and mucus
production, respectively.
Statistical analyses
The data are expressed as the means ± standard error
of the mean. Statistical significance was determined using analyses
of variance, followed by multiple comparison tests with Dunnet's
adjustment A P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of TO on NO production in
LPS-stimulated RAW264.7 cells
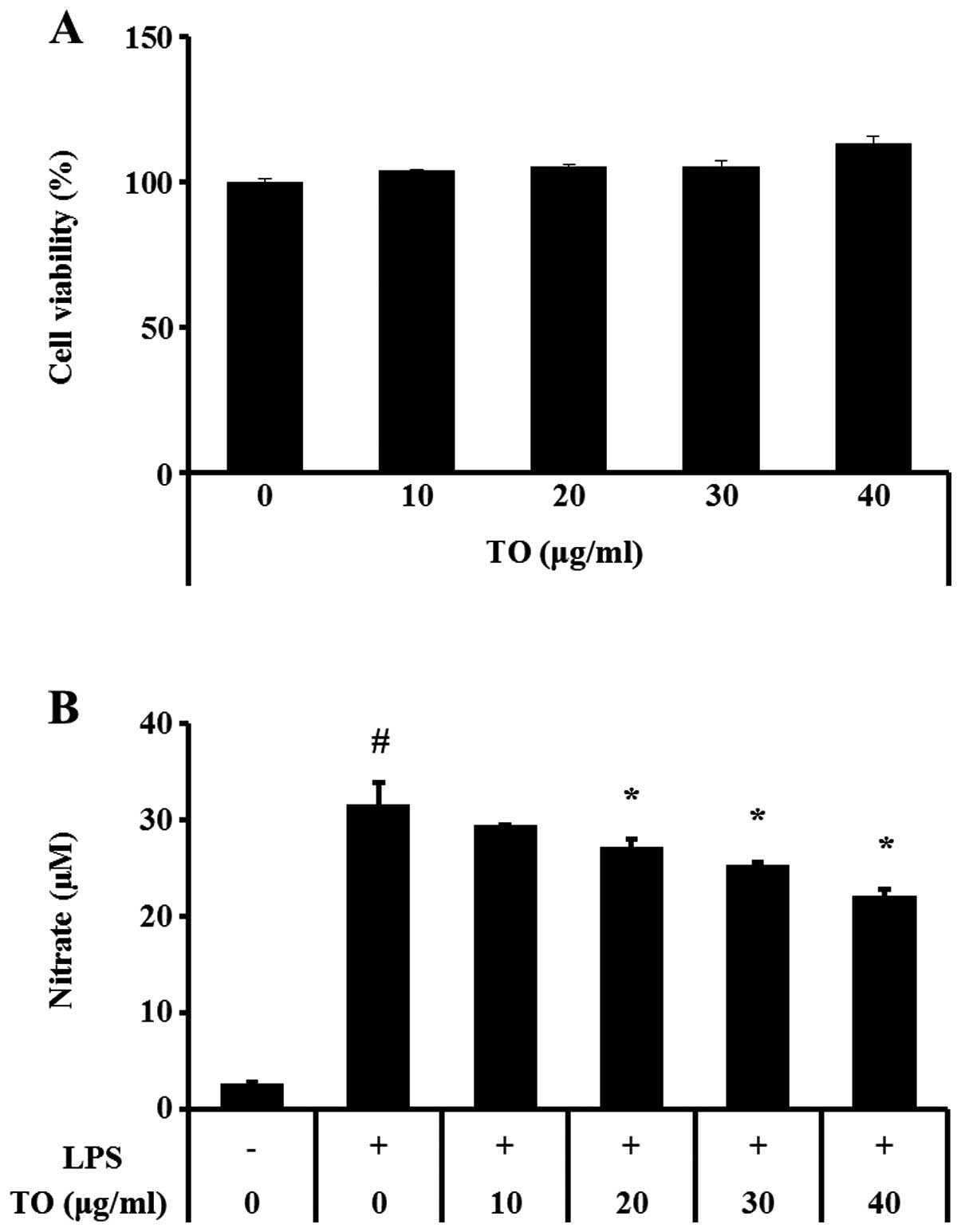
Treatment with TO did not induce toxicity in
RAW264.7 murine macrophage cells at concentrations ≤40 µg/ml
(Fig. 1A). NO production was
significantly increased in the LPS-stimulated RAW264.7 cells, as
compared with the control cells. Conversely, the TO-treated cells
exhibited concentration-dependent reductions in NO production, as
compared with the LPS-stimulated RAW264.7 cells (Fig. 1B).
Effects of TO on the mRNA expressions
levels of proinflammatory mediators in LPS-stimulated RAW264.7
cells
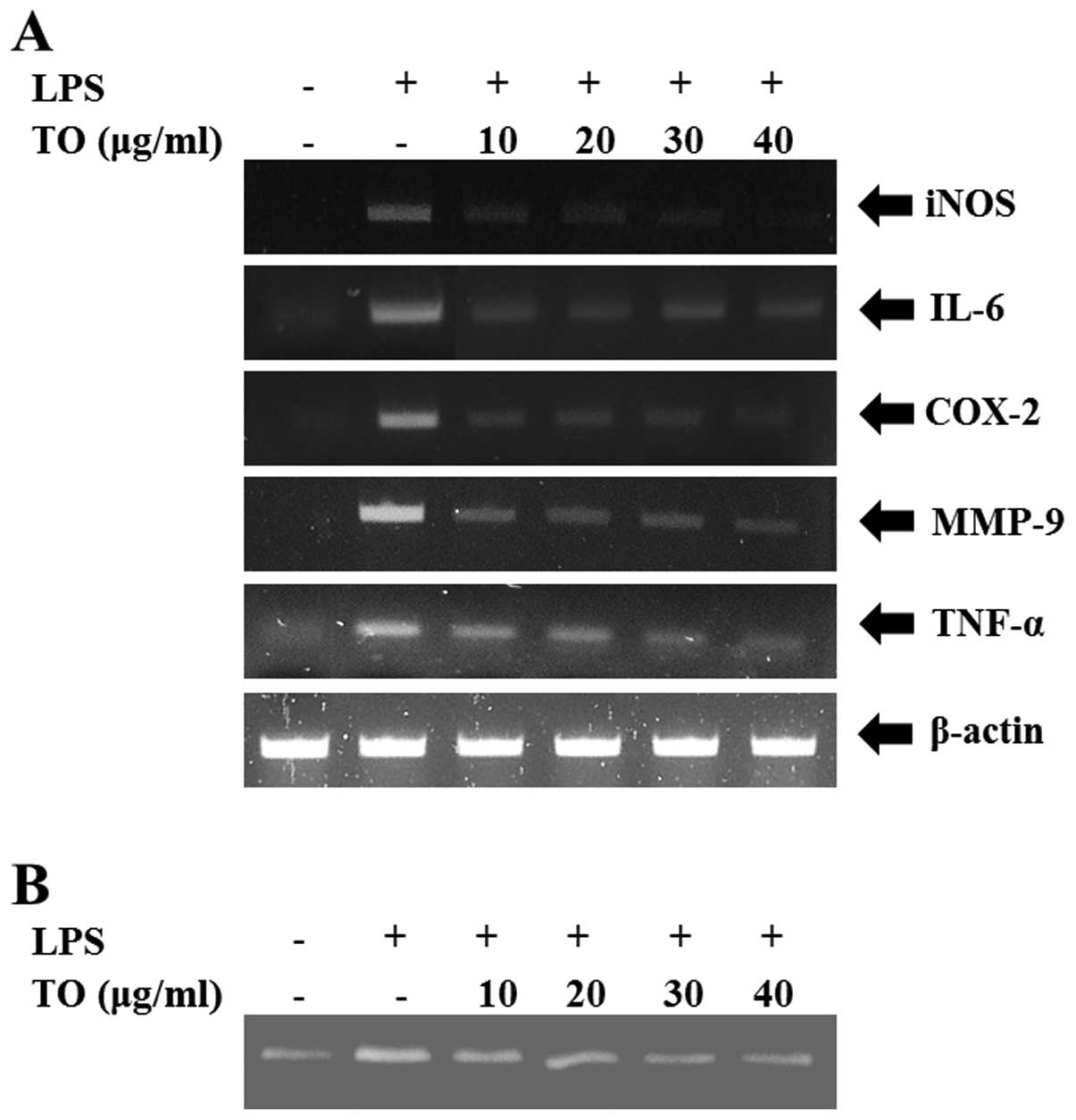
The LPS-stimulated RAW264.7 cells exhibited
increased relative mRNA expression levels of iNOS, COX2, IL-6,
TNF-α and MMP-9. However, TO-treated cells exhibited a significant
reduction in the relative mRNA expression levels of these
proinflammatory mediators, as compared with the LPS-stimulated
cells (Fig. 2A). Furthermore,
MMP-9 activity was increased in the LPS-stimulated RAW264.7 cells,
whereas reductions were observed in the TO-treated cells (Fig. 2B).
Effects of TO on AHR in
OVA-sensitized/challenged mice
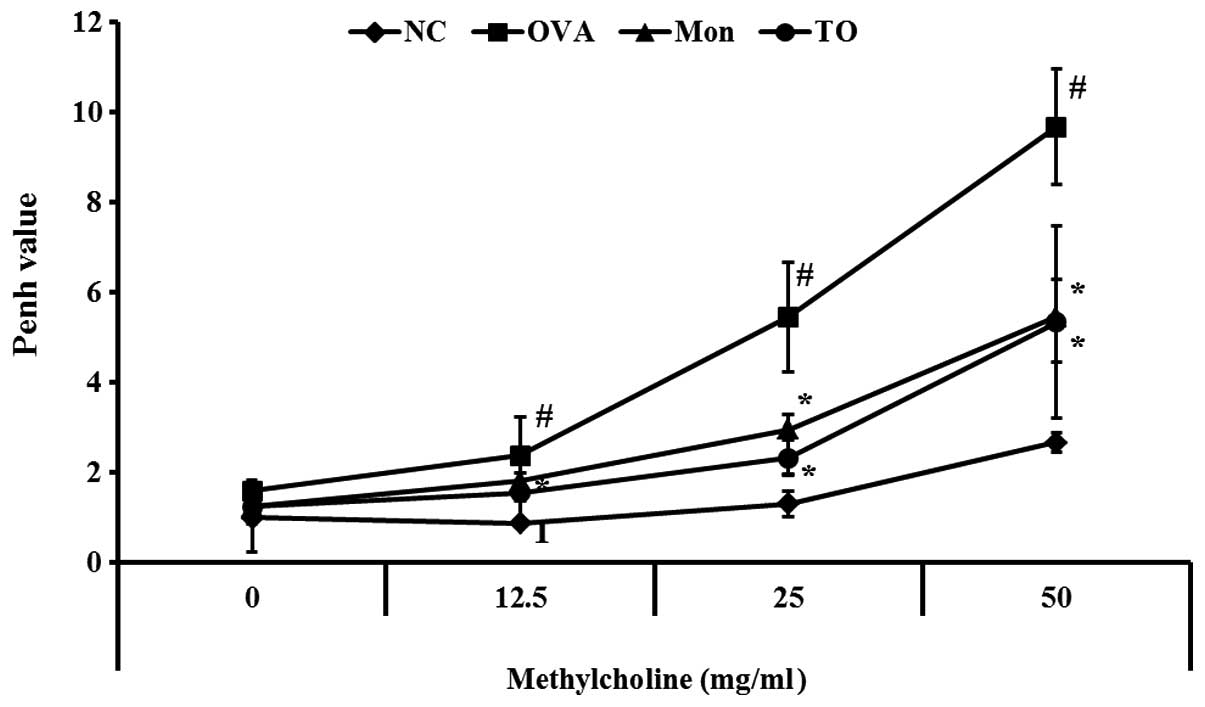
AHR was significantly increased in the
OVA-sensitized/challenged mice, as demonstrated by an increased
concentration of methylcholine, as compared with the normal
controls. The AHR, induced by the OVA challenge, was reduced in the
positive control montelukast-treated mice. The TO-treated mice also
exhibited significant reductions in AHR, as compared with the
OVA-sensitized/challenged mice (Fig.
3).
Effects of TO on BALF inflammatory cell
counts from OVA-sensitized/challenged mice
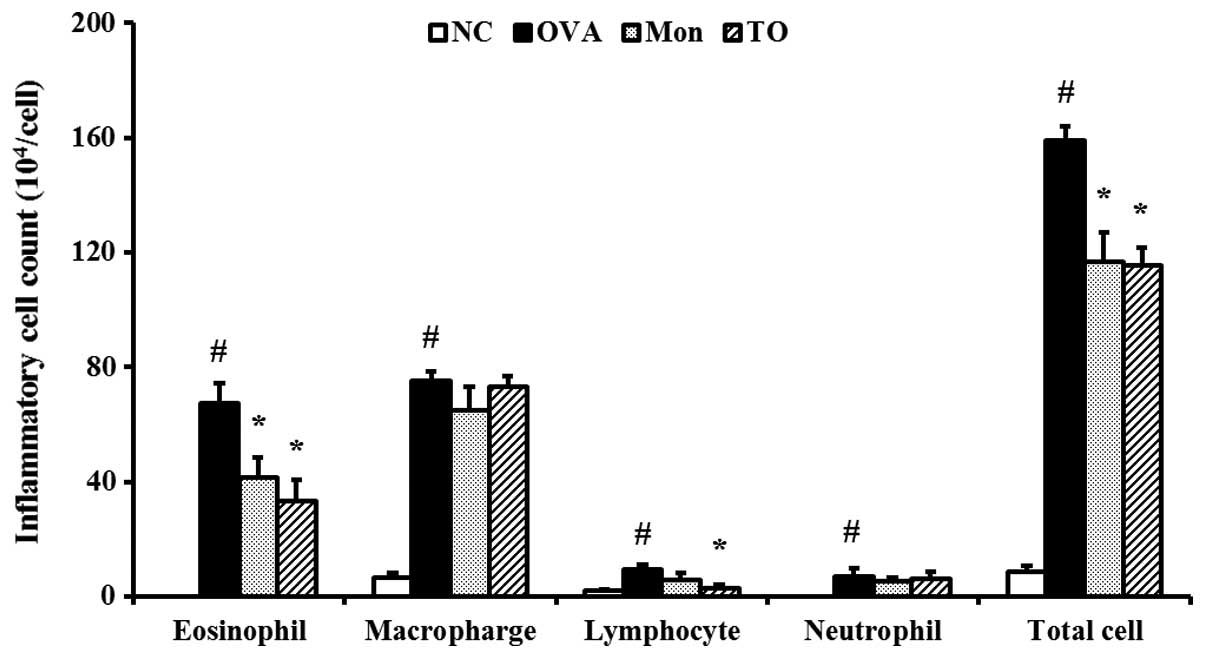
The OVA-sensitized/challenged mice exhibited a
significant increase in the number of inflammatory cells present in
the BALF, relative to the normal control mice (Fig. 4). However, the TO-treated mice
exhibited significant reductions in the number of inflammatory
cells, particularly eosinophils, in the BALF, as compared with the
OVA-sensitized/challenged mice.
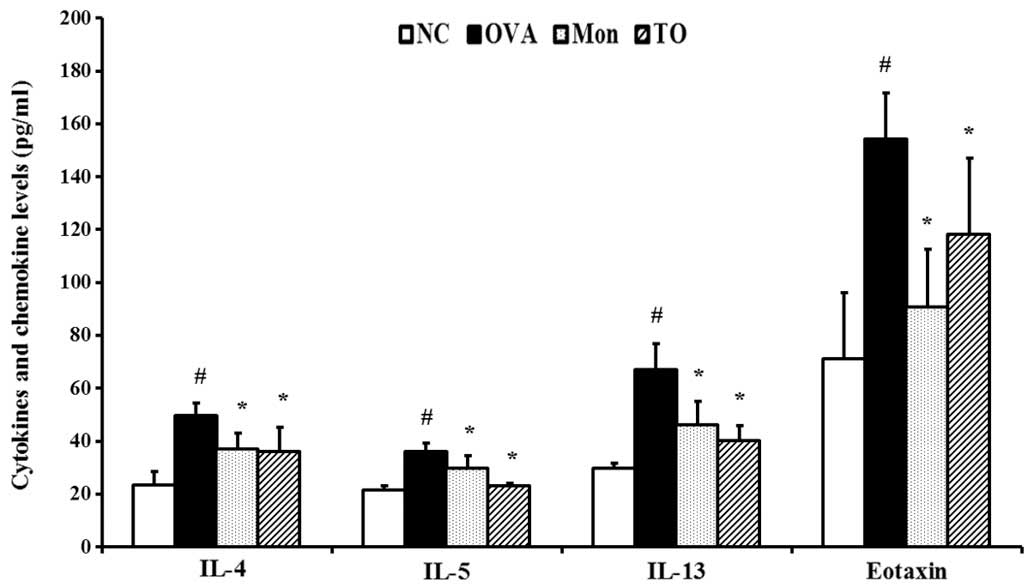
Effects of TO on IL-4, IL-5, IL-13, and
eotaxin in the BALF of OVA-sensitized/challenged mice
The OVA-sensitized/challenged mice showed marked
elevations of Th2 cytokines, including IL-4, IL-5, and IL-13, in
the BALF as compared with the normal controls. The elevations of
Th2 cytokines induced by an OVA-challenge in the TO-treated mice
were significantly reduced (Fig.
5). The production of eotaxin was also significantly increased
in the OVA-sensitized/challenged mice; however, eotaxin production
in the TO-treated mice was markedly reduced, as compared with the
OVA-sensitized/challenged mice.
Effects of TO on total IgE and
OVA-specific IgE in the serum of OVA-sensitized/challenged
mice
Total IgE was markedly elevated in the
OVA-sensitized/challenged mice, as compared with the normal
controls (Table 1). However, the
TO-treated mice exhibited a significant reduction in the total
serum IgE levels, as compared with the OVA-sensitized/challenged
mice. These findings were consistent with the OVA-specific IgE
serum results. The OVA-sensitized/challenged mice exhibited
significantly increased OVA-specific IgE levels in the serum,
whereas the TO-treated mice exhibited marked reductions, as
compared with the OVA-sensitized/challenged mice.
 | Table ISerum levels of total immunoglobulin
(Ig)-E and ovalbumin (OVA)-specific IgE in serum. |
Table I
Serum levels of total immunoglobulin
(Ig)-E and ovalbumin (OVA)-specific IgE in serum.
| Groups | Total IgE
(ng/ml) | OVA-specific
IgE
(ng/ml) |
|---|
| NC | 64.0±11.39 | – |
| OVA | 1023.1±122.43# | 196.3±27.2# |
| Mon | 258.6±100.37* | 67.2±23.0* |
| TO | 453.9±110.53* | 110.5±34.1* |
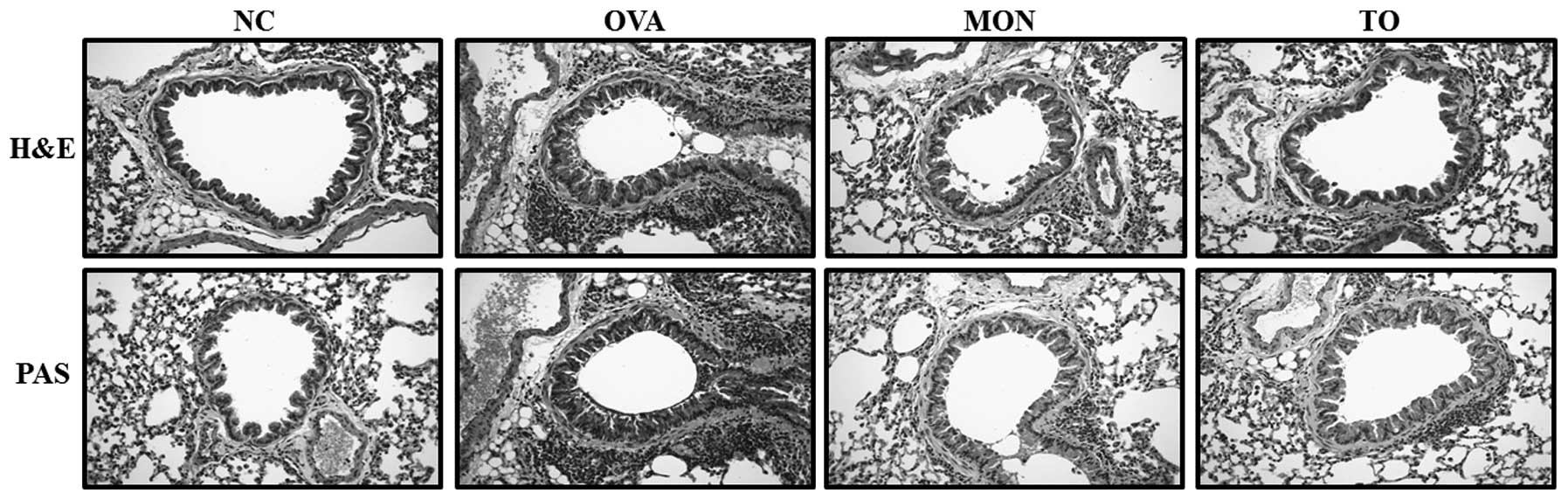
Effects of TO on inflammatory responses
and mucus produc- tion in the lung tissue of OVA-challenged
mice
Lung sections from the OVA-sensitized/challenged
mice were examined for inflammatory cell infiltration into the
peribronchial and perivascular lesions. The TO-treated mice
exhibited reductions in airway inflammation, as compared with the
OVA-sensitized/challenged mice (Fig.
6). Mucus production was also increased in the bronchial
airways of the OVA-sensitized/challenged mice. Conversely, the
TO-treated mice exhibited reductions in mucus production (Fig. 6).
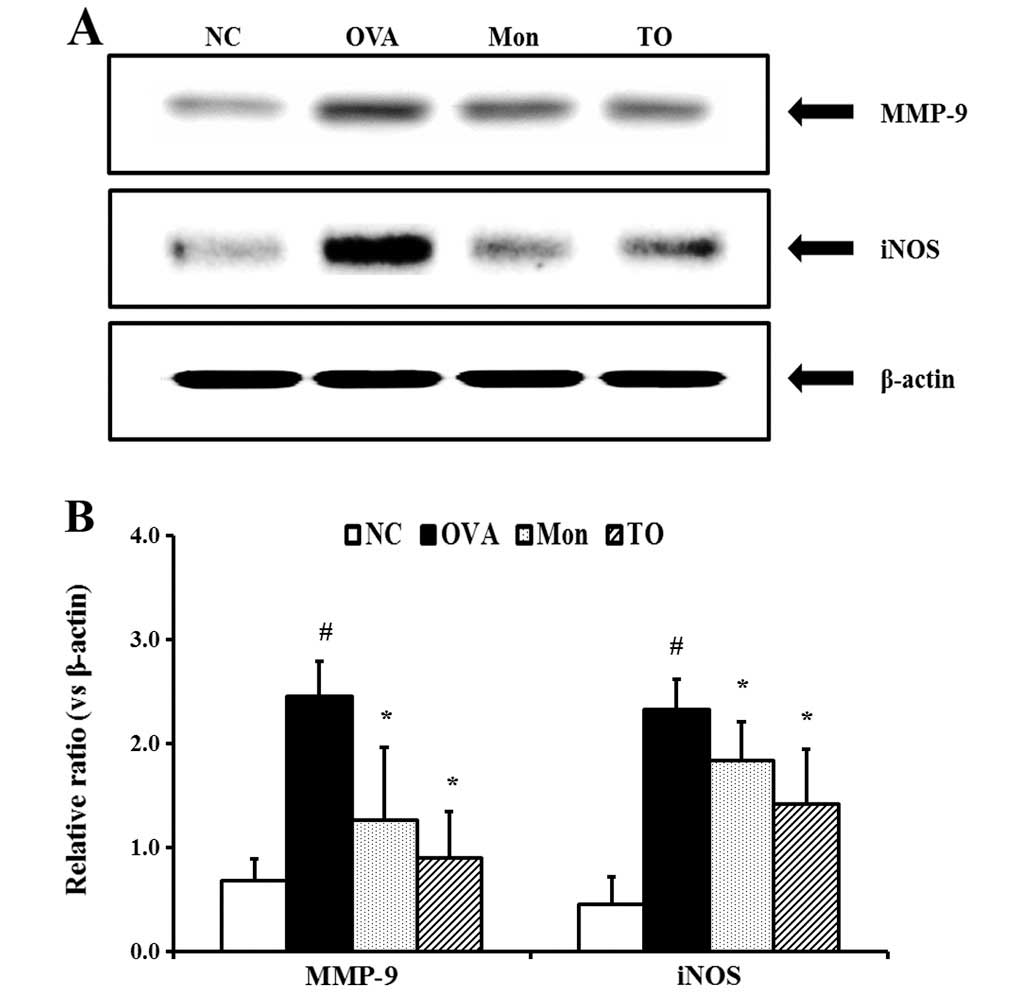
Effects of TO on the expression of iNOS
and MMP-9 in the lung tissues of the OVA-sensitized/challenged
mice
The relative protein expression levels of iNOS in
the lung tissue was significantly increased in the
OVA-sensitized/challenged mice, as compared with the normal
controls (Fig. 7A and B). However,
the TO-treated mice exhibited marked decreases in the protein
expression of iNOS, as compared with the OVA-sensitized/challenged
mice. The relative protein expression levels of MMP-9 in the lung
tissue were similar to that of iNOS. The OVA-sensitized/challenged
mice exhibited significantly increased MMP-9 protein expression in
the lung tissue, as compared with the normal controls, whereas the
TO-treated mice exhibited a marked decrease in the MMP-9 protein
expression, relative to the OVA-sensitized/challenged mice.
Discussion
In the present study, the effects of TO on the
development of asthma were evaluated using a murine model of
OVA-induced asthma, and RAW264.7 murine macrophage cells. The
TO-treated mice exhibited significant reductions in inflammatory
cell numbers, Th2 cytokines and eotaxin in the BALF, serum IgE and
AHR, as compared with the OVA sensitized/challenged mice. The
OVA-challenge induced airway inflammation and mucus hypersecretion,
which was shown to be suppressed in the lung tissues of the
TO-treated mice; iNOS and MMP-9 expression levels were also
reduced. In the LPS-stimulated RAW264.7 cells, TO treatment induced
a significant reduction in NO production, and decreased the
relative mRNA expression levels of iNOS, MMP-9, TNF-α, COX-2 and
IL-6.
Eosinophilic airway inflammation is an important
feature of allergen-induced asthma. Eosinophils contain various
toxic stimuli including cytotoxic proteins, lipid mediators, free
radicals, and proinflammatory cytokines, which may cause asthmatic
responses including AHR, mucus hypersecretion, and airway
inflammation (16). The
accumulation and activation of eosinophils has previously been
shown to be associated with Th2 cytokines and chemokines (17,18).
Previous studies have demonstrated that Th2 cytokines, including
IL-4, IL-5 and IL-13, can cause eosinophil development, activation
and infiltration into the airways (19). Eotaxin is an eosinophil
chemoattractant which induces the recruitment of eosinophils into
asthmatic lesions (20). In the
present study, OVA-sensitized/challenged mice exhibited
significantly increased eosinophil BALF counts. However, the
TO-treated mice exhibited marked reductions as compared with the
OVA-sensitized/challenged mice; this finding was accompanied by
reductions in Th2 cytokines and eotaxin. These results indicate
that TO effectively suppressed eosinophilia induced by an OVA
challenge. Furthermore, the findings of the histological analyses
strongly supported the proposed effects of TO on asthma. In
asthmatic conditions, Th2 cytokines cause the development and
activation of eosinophils, which may aggravate asthmatic responses.
Experimental asthma animal models exhibit inflammatory cell
infiltration into peribronchail lesions, and mucus hypersecretion,
both of which were exhibited by the OVA-sensitized/challenged mice
in the present study. Conversely, the TO-treated mice exhibited
attenuated airway inflammation and mucus production, as compared
with the OVA-sensitized/challenged mice.
Proinflammatory mediators, including iNOS and MMP-9,
have crucial roles in the development of asthma. NO is produced
from L-arginine by iNOS, and acts as a strong activator of
inflammatory signaling (21).
Previous studies have shown that iNOS-derived NO aggravates airway
inflammation in allergic asthma (22). Conversely, the suppression of iNOS
attenuates asthmatic responses including airway constriction,
inflammation and remodeling processes (7). Furthermore, the overexpression of
iNOS results in an increase in MMP-9 which results in airway
remodeling, via the breakdown of the lung tissue extracellular
matrix (23). Previous studies
have demonstrated that MMP-9 is associated with the production of
numerous proinflammatory cytokines and growth factors, resulting in
the infiltration of inflammatory cells into the airway (24,25).
In the present study, TO-treated mice exhibited significant
reductions in relative MMP-9 and iNOS protein expression levels in
lung tissue, as compared with the OVA-sensitized/challenged mice.
These findings are consistent with the results of the in
vitro experiment. TO treatment significantly reduced the
production of NO, induced by LPS stimulation, in a
concentration-dependent manner. TO treatment also effectively
reduced the relative mRNA expression levels of MMP-9, iNOS, TNF-α
and IL-6, in the LPS-stimulated RAW264.7 cells. These results
indicate that the anti-asthmatic effects of TO are associated with
the suppression of iNOS and MMP-9.
In conclusion, TO was shown to be capable of
suppressing asthmatic responses, including eosinophilic airway
inflammation, AHR and mucus hypersecretion in the
OVA-sensitized/challenged mice; this may be through the inhibition
of iNOS and MMP-9. Therefore, the present study suggests that TO
may be used as a potential treatment for asthma.
Acknowledgments
The present study was supported by grants from the
Korea Research Institute of Bioscience and Biotechnology Research
Initiative Program of the Republic of Korea (no. KGM1221521).
References
|
1
|
Lee MY, Seo CS, Lee JA, et al:
Anti-asthmatic effects of Angelica dahurica against
ovalbumin-induced airway inflammation via upregulation of heme
oxygenase-1. Food Chem Toxicol. 49:829–837. 2011. View Article : Google Scholar
|
|
2
|
Gueders MM, Bertholet P, Perin F, et al: A
novel formulation of inhaled doxycycline reduces allergen-induced
inflammation, hyperresponsiveness and remodeling by matrix
metalloproteinases and cytokines modulation in a mouse model of
asthma. Biochem Pharmacol. 75:514–526. 2008. View Article : Google Scholar
|
|
3
|
Holgate ST: The airway epithelium is
central to the pathogenesis of asthma. Allergol Int. 57:1–10. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou DY, Du Q, Li RR, Huang M, Zhang Q and
Wei GZ: Grape seed proanthocyanidin extract attenuates airway
inflammation and hyper-responsiveness in a murine model of asthma
by downregulating inducible nitric oxide synthase. Planta Med.
77:1575–1581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ricciardolo FL, Sterk PJ, Gaston B and
Folkerts G: Nitric oxide in health and disease of the respiratory
system. Physiol Rev. 84:731–765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Islam T, Breton C, Salam MT, et al: Role
of inducible nitric oxide synthase in asthma risk and lung function
growth during adolescence. Thorax. 65:139–145. 2010. View Article : Google Scholar
|
|
7
|
Prado CM, Leick-Maldonado EA, Yano L, et
al: Effects of nitric oxide synthases in chronic allergic airway
inflammation and remodeling. Am J Respir Cell Mol Biol. 35:457–465.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jung SH, Kim BJ, Lee EH and Osborne NN:
Isoquercitrin is the most effective antioxidant in the plant Thuja
orientalis and able to counteract oxidative-induced damage to a
transformed cell line (RGC-5 cells). Neurochem Int. 57:713–721.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee YJ, Hwang SM, Yoon JJ, et al:
Inhibitory effect of Thuja orientalis on TNF-α-induced vascular
inflammation. Phytother Res. 24:1489–1495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chae HS and Chin YW: Anti-allergic effects
of lambertianic acid from Thuja orientalis in mouse bone
marrow-derived mast cells. Immunopharmacol Immunotoxicol.
34:250–255. 2012. View Article : Google Scholar
|
|
11
|
Won JN, Lee SY, Song DS and Poo H:
Antiviral activity of the plant extracts from Thuja orientalis,
Aster spathulifolius, and Pinus thunbergii against influenza virus
A/PR/8/34. J Microbiol Biotechnol. 23:125–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu GH, Ryoo IJ, Kim YH, Choo SJ and Yoo
ID: Free radical scavenging and antielastase activities of
flavonoids from the fruits of Thuja orientalis. Arch Pharm Res.
32:275–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jung HW, Kang SY, Park KH, et al: Effect
of the semen extract of Thuja orientalis on inflammatory responses
in transient focal cerebral ischemia rat model and LPS-stimulated
BV-2 microglia. Am J Chin Med. 41:99–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim TH, Li H, Wu Q, Lee HJ and Ryu JH: A
new labdane diter-penoide with anti-inflammatory activity from
Thuja orientalis. J Ethnopharmacol. 146:760–767. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heussen C and Dowdel EB: Electrophoretic
analysis of plasminogen activators in polyacrylamide gels
containing sodium dodecyl sulfate and copolymerized substrate. Anal
Biochem. 102:196–202. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uhm TG, Kim BS and Chung IY: Eosinophil
development, regulation of eosinophil-specific genes, and role of
eosinophils in the pathogenesis of asthma. Allergy Asthma Immunol
Res. 4:68–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pope SM, Brandt EB, Mishra A, et al: IL-13
induces eosinophil recruitment into the lung by an IL-5- and
eotaxin-dependent mechanism. J Allergy Clin Immunol. 108:594–601.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pope SM, Zimmermann N, Stringer KF, Karow
ML and Rothenberg ME: The eotaxin chemokines and CCR3 are
fundamental regulators of allergen-induced pulmonary eosinophilia.
J Immuno. 175:5341–5350. 2005. View Article : Google Scholar
|
|
19
|
Mould AW, Ramsay AJ, Matthaei KI, Young
IG, Rothernberg ME and Foster PS: The effects of IL-5 and eotaxin
expression in the lung on eosinophil trafficking and degranulation
and the induction of bronchial hyperreactivity. J Immunol.
164:2142–2150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pease JE and Williams TJ: Chemokines and
their receptors in allergic disease. J Allergy Clin Immunol.
118:305–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hesslinger C, Strub A, Boer R, Ulrich WR,
Lehner MD and Braun C: Inhibition of inducible nitric oxide
synthase in respiratory diseases. Biochem Soc Trans. 37:886–891.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Starling CM, Prado CM, Leick-Maldonado EA,
et al: Inducible nitric oxide synthase inhibition attenuates lung
tissue responsiveness and remodeling in a model of chronic
pulmonary inflammation in guinea pigs. Respir Physiol Neurobiol.
165:185–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prado CM, Yano L, Rocha G, et al: Effects
of inducible nitric oxide synthase inhibition in bronchial vascular
remodeling-induced by chronic allergic pulmonary inflammation. Exp
Lung Res. 37:259–268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vermaelen KY, Cataldo D, Tournoy K, et al:
Matrix metallopro-teinase-9-mediated dendritic cell recruitment
into the airways is a critical step in a mouse model of asthma. J
Immunol. 171:1016–1022. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McMillan SJ, Kearley J, Campbell JD, et
al: Matrix metallopro-teinase-9 deficiency results in enhanced
allergen-induced airway inflammation. J Immunol. 172:2586–2594.
2004. View Article : Google Scholar : PubMed/NCBI
|