Introduction
AP25 is an anti-angiogenic and anti-tumor peptide
with molecular targets, including integrin α5β1 and αvβ3 (1). AP25 inhibited the growth of the
MGC-803, HeLa, HCT116, A549, MDA-MB-435 and MDA-MB-231 cell lines
in vitro with half maximal inhibitory concentration values
of 8–24 μM. AP25 also significantly inhibited the growth of
B16F10 in C57Bl/6 mice and the growth of HCT116 human colon
carcinoma cells in severe combined immunodeficient mice (1). This in vivo inhibitory effect
is attributed to the inhibition of tumor cell growth and inhibition
of angiogenesis (1). The molecular
mechanisms by which AP25 inhibits the growth of MGC-803 cells were
investigated in the present study.
Cell cycle progression in mammalian cells is
strictly regulated by integrin-mediated adhesion to the
extracellular matrix and by binding of growth factors to their
receptors (2). This regulation is
mediated by G1 phase cyclin-dependent kinases (CDKs), which are
downstream of the signaling pathways under the integrated control
of integrins and growth factors (3). The regulation of the G1 phase by CDKs
is now fairly well understood (4).
Of the major components in the G1 phase CDK system (cyclins D and
E, CDK4, CDK2 and their inhibitors), integrin/receptor tyrosine
kinase (RTK) signaling has been most closely associated with the
induction of cyclin D1 (5). The
induction of cyclin D1 mRNA has most frequently been attributed to
the activation of extracellular signal-regulated kinases (ERKs)
(5). In the majority of cases, the
activation of the Ras/Raf/mitogen-activated protein kinase
kinase/ERK cascade induces cyclin D1 gene expression. Conversely,
inhibition of the Ras pathway inhibits cyclin D1 gene expression.
By contrast, phosphoinositide 3-kinase (PI3K) is required for the
expression and stability of cyclin D1 in certain cell lines
(6,7). Furthermore, previous studies have
indicated that activation of Rac by integrins is able to increase
cyclin D1 levels in endothelial cells (8) and overexpression of activated Rac
also stimulated cyclin D1 gene expression in fibroblasts (6). In addition to the aforementioned
signal transduction pathways, c-Jun N-terminal kinases (JNKs) and
p38 mitogen-activated protein kinases also are important downstream
of the integrins (9,10).
RNA interference is a process in which
double-stranded RNA triggers the degradation of a homologous mRNA.
Lentivirus-mediated RNA interference may be used to inhibit the
expression of homologous genes in different mammalian cells, in
vitro and in vivo. It is an important tool in gene
function analysis and gene therapy (11). Ras is activated in cancer cells by
the mutation of ras alleles (12) and also by perturbations in RTK
signaling (13). The three ras
isoforms, H-, N- and K-ras, are ubiquitously expressed in mammalian
cells and are able to interact with the same set of effector
molecules (14). A previous study
confirmed that K-ras recruited Raf-1 to the plasma membrane and
activated Raf-1 more efficiently than H-ras, the latter of which
was a more potent activator of other signaling pathways, such as
the PI3K pathway (14).
Furthermore, K-ras mutations occur in 50% of colon cancer cases and
90% of pancreatic cancer cases, whereas N- and H-ras mutations are
extremely uncommon (14).
Therefore, RNA interference specific for K-ras was performed in
order to weaken the signal transduction via the Ras/Raf/MEK/ERK
pathway, while simultaneously retaining the basic functions of the
remaining ras proteins (N- and H-ras), which are important for the
maintenance of growth and the survival rate of the infected
cells.
In the present study, RNA interference of K-ras was
performed to investigate the role of the main signaling pathway
downstream of the integrins and the RTKs. Chemical inhibitors of
the key enzymes in various signaling pathways downstream of
integrins were also included to investigate how integrin engagement
resulted in decreased expression levels of cyclin D1.
Materials and methods
Cell culture, antibodies and
reagents
Peptide AP25 was synthesized by GL Biochem Ltd.
(Shanghai, China) with a purity of >95%. The MGC-803 gastric
carcinoma cell line as well as 293 cells were purchased from the
American Type Culture Collection (Shanghai, China) and maintained
in Dulbecco's modified Eagle's medium with 10% fetal bovine serum
(FBS; Invitrogen Life Technologies, Grand Island, NY, USA). The
chemical inhibitors of key enzymes in various signaling pathways
used in the present study included the Src-selective kinase
inhibitor PP1 (cat no. 567809; Merck, Darmstadt, Germany), the PI3K
inhibitor wortmannin (cat no. BML-ST415-0001; Biomol, Plymouth
Meeting, PA, USA) and the JNK-specific inhibitor SP600125 (cat no.
EI-305-0010; Biomol).
Generation of K-ras-specific
lentivirus
K-ras-specific lentivirus was prepared as previously
described (15). The synthetic
transcription short hairpin (sh)RNA template sequences were as
follows: forward, 5′-AAC GGT CAT CCA GTG TTG TCA TGC TTT CAAGAG AAG
CAT GAC AAC ACT GGA TGA CCT TTT TTC-3′ and reverse, 5′-TCG AGA AAA
AAG GTC ATC CAG TGT TGT CAT GCT TCT CTT GAA AGC ATG ACA ACA CTG GAT
GAC CGTT-3′. The sequences were provided by Shanghai Gene Pharma
Co., Ltd. (Shanghai, China). The above synthetic and annealed
duplexes were sub-cloned between the XhoI and HpaI
sites of the plasmid PLentilox3.7 (Invitrogen Life Technologies) to
form the expression plasmid PLentilox3.7-shRNA, which was used to
transform Escherichia coli DH5α. The bacteria were spread on
a lysogeny broth plate containing ampicillin (Hyclone, Logan, UT,
USA). The positive colonies were selected and plasmids were
identified by enzyme digestion and sequencing as described
previously (15).
Confluent 293 cells were cultured in a six-well
culture plate to 70–80% confluency. The recombinant lentivirus was
generated from 293 cells co-transfected with calcium phosphate
precipitation of plasmids: i) PLentilox-shRNA, and ii) pCMV∆R8.91,
a plasmid expressing the human immunodeficiency virus-1 gag/pol,
tat and rev genes required for efficient lentivirus production, and
iii) a plasmid expressing the vesicular stomatitis virus envelope
glycoprotein (G), pLR-VSV-G (Invitrogen Life Technologies,
Carlsbad, CA, USA), at 15:15:7.5 μg/plate. Cells transfected
with empty PLentilox3.7 were used as the control. After 72 h, the
viral supernatant was collected and concentrated 500-fold by
ultra-high speed centrifugation at 35,000 x g, at 4°C for 2 h. The
viral titer was determined to be 1×107 transducing
units/ml. The viral stocks were stored frozen at −80°C until
usage.
Infection of MGC-803 cells with
K-ras-specific lentivirus
A total of 0.05 ml (3×106 cells/ml)
MGC-803 cells were seeded into each well of a six-well plate and
cultured to 50% confluence. Viral infection was initiated by
addition of 0.9 ml viral stock solution, 0.1 ml complete culture
medium and 6 μl polybrene (Invitrogen Life Technologies,
Grand Island, NY, USA). After 6 h, the viral solution was removed
and 2 ml fresh medium with 10% FBS was added. After 48 h, the cells
were observed under a fluorescence microscope (Olympus DP72;
Olympus, Tokyo, Japan). The efficiency of infection was determined
by the percentage of cells exhibiting green fluorescent protein
expression. The MGC-803 cells successfully infected with
K-ras-specific or control virus were termed MGC-803 shRNA or
MGC-803 NC.
Preparation of protein samples
MGC-803, MGC-803 shRNA and MGC-803 NC cells were
washed with ice-cold phosphate-buffered saline (PBS) three times. A
total of 300 μl ice-cold cell lysis buffer
(radioimmunoprecipitation assay buffer; Hyclone) with 1%
phenylmethylsulfonyl fluoride and 1% phosphatase inhibitor was
added to each 24-cm2 culture flask. The cells were
scraped off the flask quickly with a cell scraper. The cell lysate
was collected and preserved on ice for 20 min to complete lysis.
The supernatant was collected by centrifugation at 8,000 x g for 10
min at 4°C for protein concentration analysis using a bicinchoninic
acid assay kit from Tiangen Biotech Co., Ltd. (Beijing, China).
Western blot analysis
A total of 100 μg total protein in the whole
cell lysate from MGC-803, MGC-803 shRNA and MGC-803 NC cells were
subjected to a 12% SDS-PAGE, followed by blotting with specific
antibodies. The protein bands were visualized with an enhanced
chemiluminescence plus (ECL+) substrate (Tanon Science and
Technology Co., Ltd., Shanghai, China). The primary antibodies
consisted of a mouse anti-K-ras monoclonal antibody (OP-24;
Calbiochem, Darmstadt, Germany; 1:100), a rabbit anti-p-ERK
polyclonal antibody (9101; Cell Signaling Technology, Danvers, MA,
USA; 1:500), a rabbit anti-ERK polyclonal antibody (9102; Cell
Signaling Technology; 1:500), a rabbit anti-cyclin D-1 monoclonal
antibody (2922; Cell Signaling Technology; 1:1,000) and a mouse
anti-β-actin monoclonal antibody (TA-09; ZSBG-Bio; Beijing, China;
1:1,000). The membranes were incubated with the primary antibodies
overnight at 4°C. Gray value analysis was performed with ImageJ
software version 1.46 (National Institutes of Health, Bethesda, MD,
USA) and the results were expressed as a comparison of the gray
value of a protein band with that of the corresponding β-actin
band.
Cell cycle analysis
MGC-803 cells were growth-arrested by contact
inhibition for 24 h. A total of 200 μl (6×106
cells/ml) cells were added to each well of a six-well plate in the
presence of 0, 3.2 or 50 μM AP25. After 12 h, the cells were
harvested and fixed in ice-cold ethanol. The fixed cells were
dehydrated at 4°C for 30 min in PBS and were stained with propidium
iodine (5 μg/ml; Takara Bio, Inc., Otsu, Japan). The cells
were then analyzed using a BD FACSCalibur flow cytometer (BD
Biosciences, Mountain View, CA, USA).
Cell proliferation assays
A total of 100 μl (2×104 cells/ml)
MGC-803 or MGC-803 shRNA cells were seeded into each well of a
96-well plate. After 12 h, AP25 or chemical inhibitors at 15
μM PP1, 10 μM SP600125 and 30 μM wortmannin
dissolved in complete cell medium were added. For each
concentration there were five repeat wells. After 48 h, cell
proliferation was detected with an MTT assay at a detection
wavelength of 570 nm and reference wavelength of 630 nm. The
proliferation inhibitory effect of AP25 was calculated as
(Anegative control − Adrug)/Anegative
control × 100%.
Statistical analysis
All values are expressed as the mean ± standard
deviation. All statistical analyses were performed using the
statistical software package SPSS 17.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference and P<0.01 was considered to indicate a
highly significant difference between values.
Results
K-ras interference in MGC-803 cells by
lentiviral infection
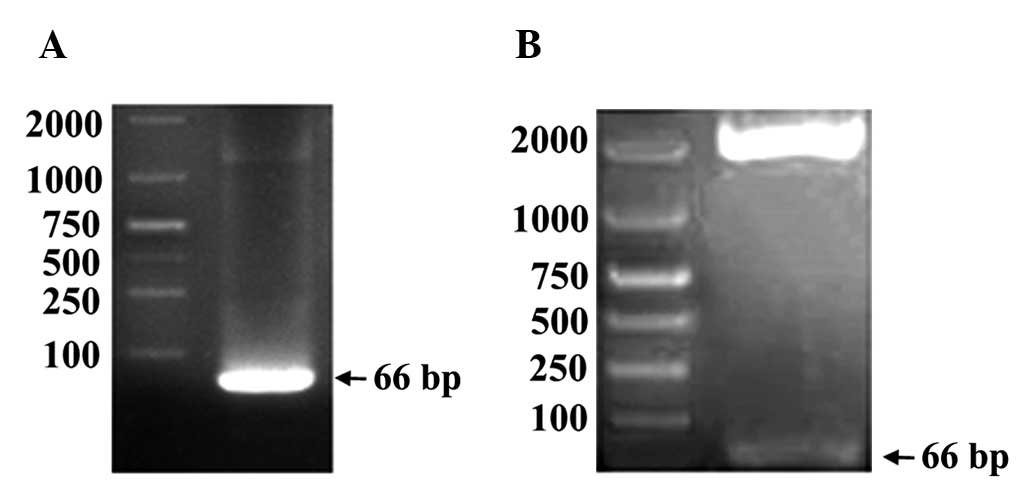
K-ras-specific lentiviruses were generated and
verified using gel electrophoresis (Fig. 1). Successful infection of MGC-803
cells was performed. Under a fluorescence microscope, >90%
MGC-803 shRNA cells (Fig. 2A) and
MGC-803 NC cells (Fig. 2B)
exhibited marked fluorescence, indicating a successful infection
and target gene transcription. Using western blot analysis, K-ras
protein expression in MGC-803 shRNA cells was significantly
decreased, whereas that in the MGC-803 NC cells exhibited no
substantial change (Fig. 2C and
E). Furthermore, the expression of p-ERK, the important kinase
downstream of ras, and cyclin D1 decreased significantly in MGC-803
shRNA cells, whereas that in MGC-803 NC cells remained almost
identical to that in the intact cells (Fig. 2D and E). These results indicated
that K-ras interference reduced the levels of signaling of the
Ras/Raf/MEK/ERK pathway in MGC-803 cells and the level of
expression of cyclin D1 was also decreased.
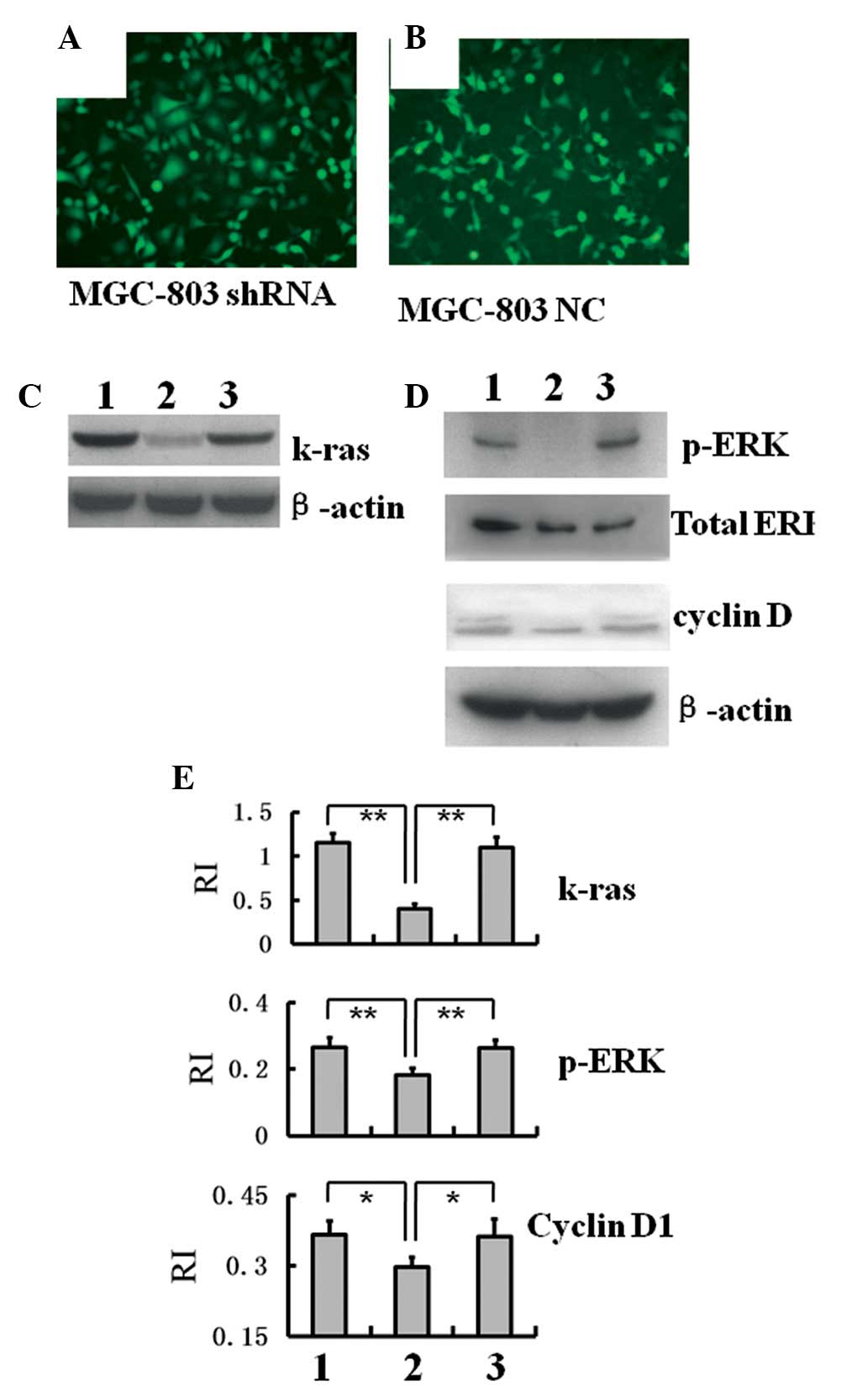 | Figure 2Decreased expression levels of K-ras,
p-ERK and cyclin D1 in MGC-803 shRNA cells. (A) Fluorescence
microscopy image of MGC-803 cells infected with K-ras-specific
lentiviruses (MGC-803 shRNA; magnification, ×200). (B) Fluorescence
microscopy image of MGC-803 cells infected with control
lentiviruses (MGC-803 NC; magnification, ×200). Western blot
analysis of (C) K-ras and (D) p-ERK and cyclin D1 expression levels
in MGC-803, MGC-803 shRNA and MGC-803 NC cells. (E) Gray value
analysis of the protein bands in C and D to compare the relative
protein expression levels of K-ras, p-ERK and cyclin D1
(**P<0.01). RI was obtained by comparison of the gray
value of a protein band with that of the corresponding β-actin
band. Groups: 1, intact MGC-803; 2, MGC-803 shRNA; 3, MGC-803 NC.
shRNA, short hairpin RNA; p-ERK, phosphorylated extracellular
signal-regulated kinase; RI, relative intensity; NC, negative
control. |
AP25 inhibits MGC-803 cell growth by
decreasing p-ERK and cyclin D1 levels
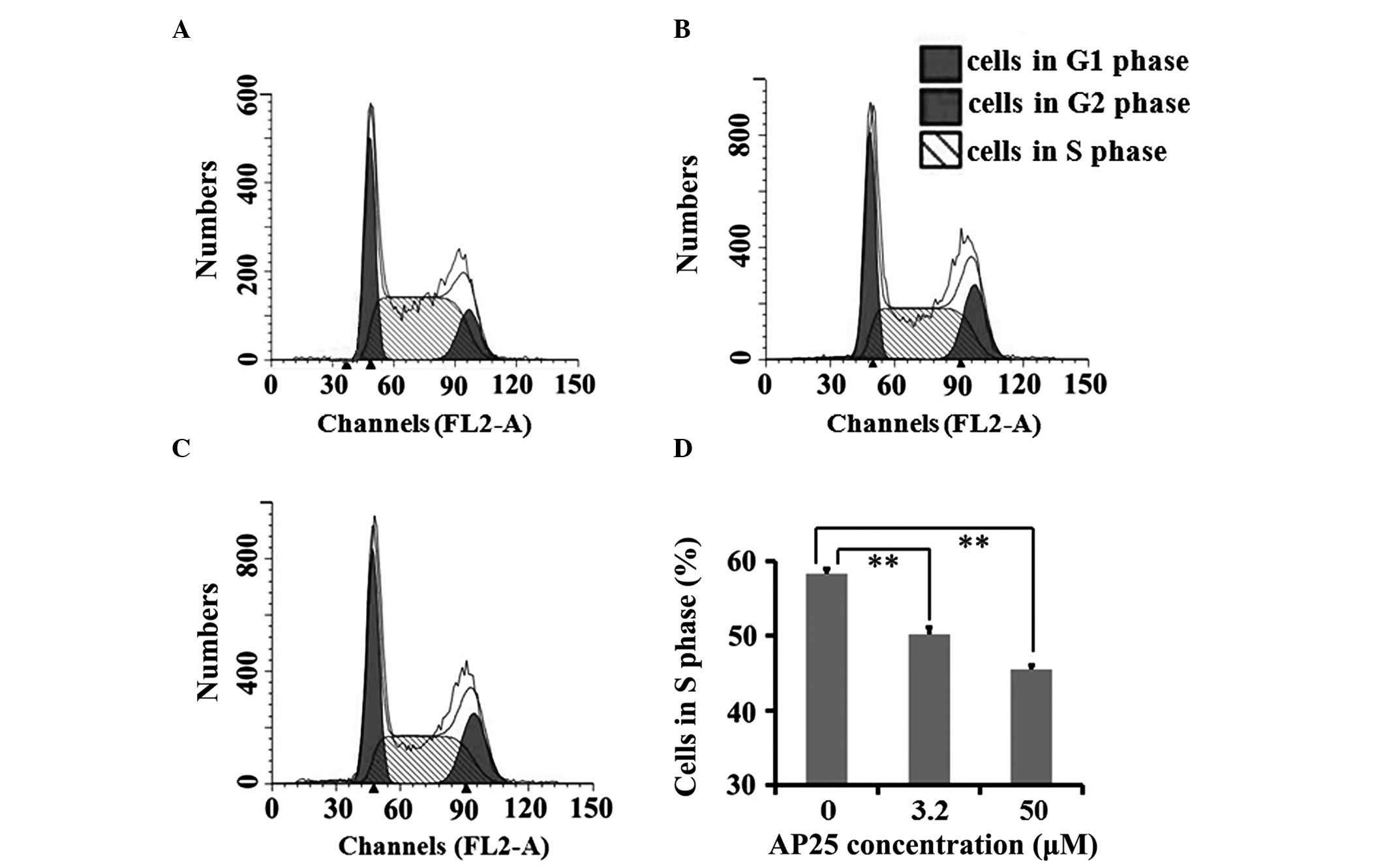
Cell cycle analysis was performed on intact MGC-803
cells and as shown in Fig. 3, AP25
treatment significantly decreased the percentage of intact MGC-803
cells in S phase after a 12 h incubation in complete medium.
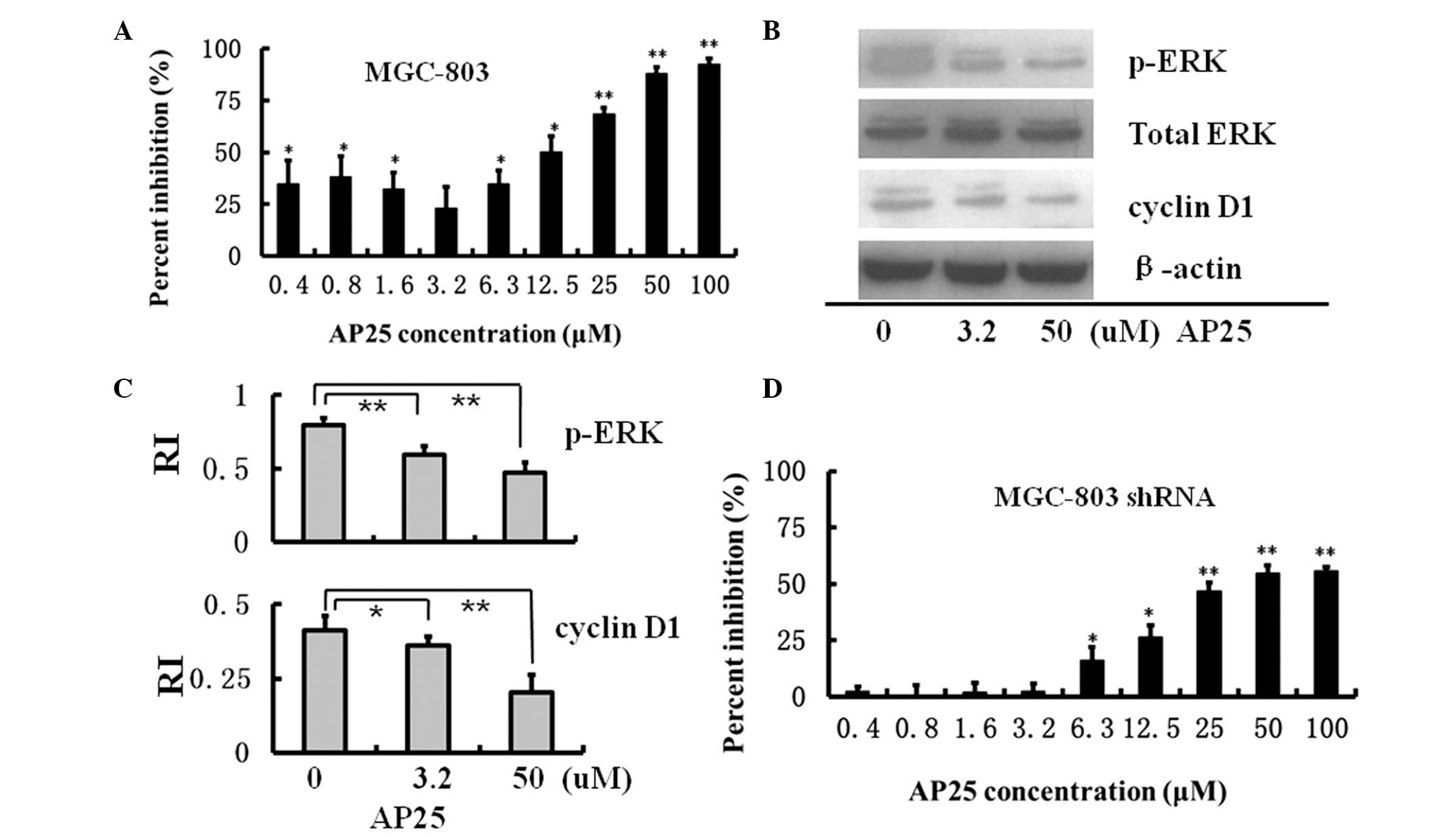
Using MTT assays, AP25 treatment was revealed to
inhibit intact MGC-803 cell growth at low (0.4 to 3.2 μM)
and high concentrations (6.3 to 100 μM). At a concentration
of 100 μM, AP25 treatment almost completely inhibited
MGC-803 cell growth after a 48-h incubation (Fig. 4A).
AP25 contains a sequence termed ES-2, comprising
amino acids 60–70 of endostatin (1). ES-2 covers one of the two active
domains of endostatin to interact with integrin α5β1 and induce the
inhibitory effect of endostatin on angiogenesis (16). It has been observed that endostatin
causes G1 arrest of endothelial cells (17) and inhibits human umbilical vein
endothelial cell (HUVEC) growth by decreasing the expression levels
of cyclin D1 within the cells (18). Importantly, endostatin was unable
to arrest cyclin D1-overexpressing endothelial cells, suggesting
that cyclin D1 is a critical target for the action of endostatin
(18). It was also demonstrated
that through interactions with free extracellular ligands,
integrins interact with growth factor receptors by interfering with
the Ras/Raf/ERK/cyclin D1 pathway, which is an important signal
transduction pathway downstream of growth factor receptors
(3). The expression levels of
cyclin D1 and p-ERK within intact MGC-803 cells were evaluated
following AP25 treatment. As shown in Fig. 4B and C, AP25 decreased the
expression levels of cyclin D1 and p-ERK in a dose-dependent manner
in intact MGC-803 cells. Together with the data in Fig. 3, it was revealed that AP25
inhibited intact MGC-803 cell growth and decreased cyclin D1
expression via the Ras/Raf/MEK/ERK pathway.
MTT assays were also performed on MGC-803 shRNA
cells. As shown in Fig. 4D, at low
concentrations (0.4 to 3.2 μM), AP25 exhibited no inhibitory
effect on MGC-803 shRNA cell growth. As K-ras interference in
MGC-803 shRNA cells decreased the expression levels of p-ERK and
cyclin D1, it is possible that AP25 was not able to further
suppress cell signaling in the Ras/Raf/ERK/cyclin D1 pathway to
further inhibit the growth of MGC-803 shRNA cells. However, at high
concentrations (25 to 100 μM), AP25 exerted a marked
inhibition of MGC-803 shRNA cell growth, which indicated that at
high concentrations, AP25 used alternative signaling pathways to
inhibit MGC-803 shRNA cell growth.
Src, JNK and PI3K are key enzymes in the
signaling pathways activated by 25 μM AP25
To further investigate which signaling pathways
mediated the inhibitory effect of AP25 at high concentrations,
chemical inhibitors of Src (PP1), JNK (SP600125), and PI3K
(wortmannin) were included in the MTT assay and the western blot
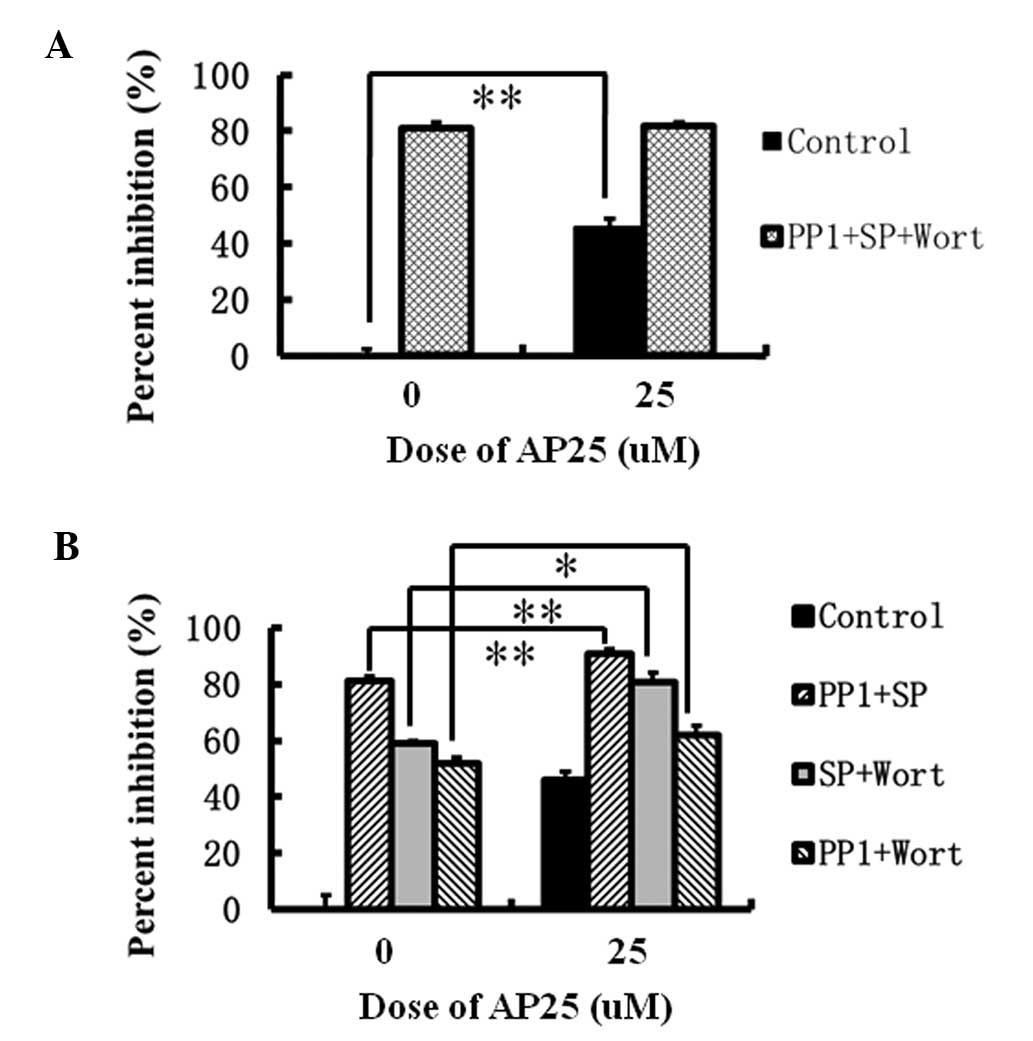
analysis. As shown in Fig. 5A, in
the MTT assay, 25 μM AP25 exhibited a 45% inhibition of
MGC-803 shRNA cell growth. Quantities of 15 μM PP1, 10
μM SP600125 and 30 μM wortmannin together caused an
80% inhibition of MGC-803 shRNA cell growth, whereas the additional
presence of 25 μM AP25 did not further increase the
combinatorial inhibition, indicating that the signaling pathways
activated by AP25 at high concentrations are within the scope of
the Src-, JNK- and PI3K-associated pathways, in addition to the
Ras/Raf/ERK pathway. Furthermore, the combinatorial inhibition of
MGC-803 shRNA cell growth by two of the three enzyme inhibitors in
the presence or absence of 25 μM AP25 were compared. A
significant difference was identified in the combinatorial
inhibition by PP1 and SP600125 in the presence (90%) or absence
(80%) of 25 μM AP25 (P<0.01) (Fig. 5B), indicating that an alternative
signaling pathway existed downstream of that activated by 25
μM AP25 in MGC-803 shRNA cells in which PI3K is important.
Similarly, the significant difference in the combinatorial
inhibition of MGC-803 shRNA growth by SP600125 and wortmannin in
the presence (80%) and absence (60%) of 25 μM AP25
(P<0.01) (Fig. 5B) indicated
that the Src-associated signaling existed downstream of the pathway
initiated by 25 μM AP25. The significant difference
identified between the combinatorial inhibition by PP1 and
wortmannin in the presence (65%) and absence (55%) of 25 μM
AP25 (P<0.05) confirmed that the signaling pathway with JNK as
an important factor also existed downstream of that initiated by 25
μM AP25 (Fig. 5B).
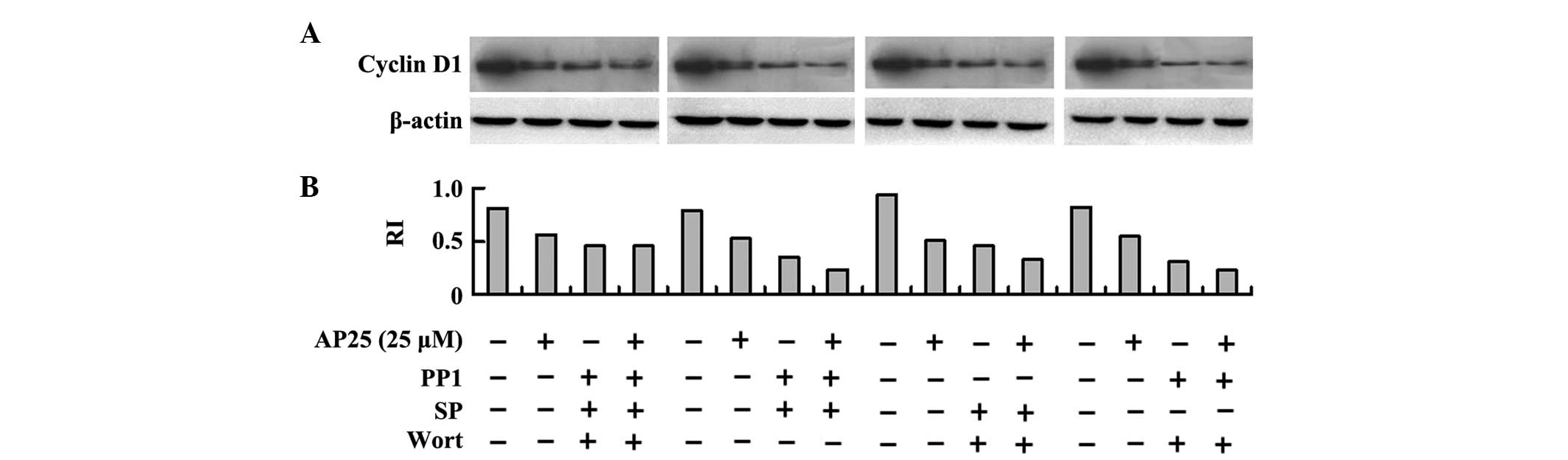
Western blot analysis of cyclin D1 expression levels
within MGC-803 shRNA cells confirmed the results of the MTT assay.
In Fig. 6A and B, treatment with
25 μM AP25 decreased the expression levels of cyclin D1. The
combinatorial inhibition of Src, JNK and PI3K also decreased the
expression levels of cyclin D1, whereas the combinatorial
inhibition by the three inhibitors in the presence of 25 μM
AP25 did not further decrease the expression levels of cyclin D1.
By contrast, co-treatment with 25 μM AP25 further decreased
cyclin D1 expression in addition to the combinatorial effect of PP1
and SP100625, the combinatorial effect of SP100625 and wortmannin
and the combinatorial effect of PP1 and wortmannin.
Discussion
Anti-angiogenic reagents often have differential
effects at low or high concentrations, such as the anti-angiogenic
peptide HM-3, which is formed by binding of the RGD sequence to the
C-terminus of ES-2. HM-3 inhibits HUVEC migration at 5 μM,
whereas at 38 μM, it promotes HUVEC migration (19). Similarly, AP25 inhibited HUVEC
migration at 0.2 μM, whereas at 12.8 μM, it promoted
HUVEC migration. A possible reason for this may be that at higher
concentrations, HM-3 and AP25 activate alternative signaling
pathways and the crosstalk of the various signaling pathways
results in a reversal of the cellular behavior. According to the
experimental results of the present study, AP25 at low
concentrations inhibited MGC-803 cell growth mainly through the
Ras/Raf/ERK/cyclin D1 pathway, whereas at high concentrations, AP25
activated alternative signaling pathways. As AP25 exhibited an
inhibitory effect on MGC-803 cell growth, key signaling pathways
and key enzymes may be confirmed only in a negative way, such as
blocking the Ras/Raf/MEK/ERK pathway by K-ras interference, which,
at low concentrations, reduced the inhibitory effect of AP25 on
MGC-803 shRNA cell growth. In addition, key signaling pathways and
key enzymes were confirmed only after examining the effects of high
doses of AP25; following the omission of one out of three enzyme
inhibitors, high concentrations of AP25 further increased the
combinatorial inhibitory effect of the other chemical
inhibitors.
As previously reported, the Ras/Raf/MEK/ERK pathway
is the main pathway regulating cell cycle progression via
regulation of cyclin D1 expression (5). This was confirmed in the present
study, which showed that AP25 caused cell cycle arrest in untreated
and treated MGC-803 cells and decreased p-ERK and cyclin D1
expression levels. K-ras interference was successfully induced and
it was confirmed that the Ras/Raf/MEK/ERK pathway was the main
signal transduction pathway at low concentrations of AP25 (0.4 to
3.2 μM) to inhibit MGC-803 cell growth. However, at 25
μM, alternative pathways were activated, indicating that
only after K-ras interference and Src, JNK and PI3K inhibition by
chemical inhibitors, 25 μM AP25 no longer inhibited MGC-803
shRNA cell growth. It is noteworthy that AP25 is a freely moving
ligand for integrins. The conventional role of integrins is to
promote cell growth and the survival rate when interacting with
immobilized extracellular matrix glycoproteins. However, when
interacting with freely moving extracellular ligands, such as AP25,
they accumulate intracellular signaling molecules and generate
signal transduction pathways to inhibit cell growth (20). The target signaling pathways
selected in the present study were those that were well
investigated and downstream of integrins interacting with
immobilized ligands (21). It was
observed that when interacting with freely moving ligands,
integrins generated intracellular signaling with a similar set of
key molecules, but with a different effect. The proximal mechanisms
of integrin signaling remain to be elucidated and require further
investigation to explain how promoting effect of integrin agonists
on cell growth switches to an inhibitory effect with a similar set
of intracellular key molecules. This may improve the current
understanding of the mechanism by which integrin antagonists
exhibit their anti-tumor effects in clinical trials.
Acknowledgments
The present study was supported by 863
High-Technology Development Planning (grant no. SQ2011SF11B02030),
the National Natural Science Foundation of China (grant no.
81301902), the National Science and Technology Major Projects of
New Drugs (grant nos. 2012ZX09103301-004 and 2014ZX09508007) and
the International Projects of Scientific Cooperation and
Communication (grant no. 2012DFG32000) in China.
References
|
1
|
Yin R, Zheng H, Xi T and Xu HM: Effect of
RGD-4C position is more important than disulfide bonds on
antiangiogenic activity of RGD-4C modified endostatin derived
synthetic polypeptide. Bioconjug Chem. 21:1142–1147. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stoker M, O'Neill C, Berryman S and Waxman
V: Anchorage and growth regulation in normal and virus-transformed
cells. Int J Cancer. 3:683–693. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwartz MA and Assoian RK: Integrins and
cell proliferation: regulation of cyclin-dependent kinases via
cytoplasmic signaling pathways. J Cell Sci. 114:2553–2560.
2001.PubMed/NCBI
|
|
4
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1 phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roovers K and Assoian RK: Integrating the
MAP kinase signal into the G1 phase cell cycle machinery.
Bioessays. 22:818–826. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gille H and Downward J: Multiple ras
effector pathways contribute to G(1) cell cycle progression. J Biol
Chem. 274:22033–22040. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takuwa N, Fukui Y and Takuwa Y: Cyclin D1
expression mediated by phosphatidylinositol 3-kinase through
mTOR-p70(S6K)-independent signaling in growth factor-stimulated NIH
3T3 fibroblasts. Mol Cell Biol. 19:1346–1358. 1999.PubMed/NCBI
|
|
8
|
Huang S, Chen CS and Ingber DE: Control of
cyclin D1, p27(Kip1), and cell cycle progression in human capillary
endothelial cells by cell shape and cytoskeletal tension. Mol Biol
Cell. 9:3179–3193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verma G, Bhatia H and Datta M: Gene
expression profiling and pathway analysis identify the integrin
signaling pathway to be altered by IL-1β in human pancreatic cancer
cells: role of JNK. Cancer Lett. 320:86–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niederlechner S, Baird C and Wischmeyer
PE: P38MAP kinase, but not phosphoinositol-3 kinase, signal
downstream of glutamine-mediated fibronectin-integrin signaling
after intestinal injury. Nutr J. 12:882013. View Article : Google Scholar :
|
|
11
|
Lois C, Refaeli Y, Qin XF and Van Parijs
L: Retroviruses as tools to study the immune system. Curr Opin
Immunol. 13:496–504. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bos JL: ras oncogenes in human cancer: a
review. Cancer Res. 49:4682–4689. 1989.PubMed/NCBI
|
|
13
|
Shields JM, Pruitt K, McFall A, Shaub A
and Der CJ: Understanding Ras: 'it ain't over 'til it's over'.
Trends Cell Biol. 10:147–154. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan J, Roy S, Apolloni A, Lane A and
Hancock JF: Ras isoforms vary in their ability to activate Raf-1
and phosphoinositide 3-kinase. J Biol Chem. 273:24052–24056. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng L, Li G, Xi L, et al: Hepatitis B
virus inhibition in mice by lentiviral vector mediated short
hairpin RNA. BMC Gastroenterol. 9:732009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Olsson AK, Johansson I, Akerud H, et al:
The minimal active domain of endostatin is a heparin-binding motif
that mediates inhibition of tumor vascularization. Cancer Res.
64:9012–9017. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dhanabal M, Volk R, Ramchandran R, Simons
M and Sukhatme VP: Cloning, expression, and in vitro activity of
human endostatin. Biochem Biophys Res Commun. 258:345–352. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanai J, Dhanabal M, Karumanchi SA, et al:
Endostatin causes G1 arrest of endothelial cells through inhibition
of cyclin D1. J Biol Chem. 277:16464–16469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu H, Pan L, Ren Y, et al: RGD-modified
angiogenesis inhibitor HM-3 dose: dual function during cancer
treatment. Bioconjug Chem. 22:1386–1393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garmy-Susini B and Varner JA: Roles of
integrins in tumor angiogenesis and lymphangiogenesis. Lymphat Res
Biol. 6:155–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cox D, Brennan M and Moran N: Integrins as
therapeutic targets: Lessons and opportunities. Nat Rev Drug
Discov. 9:804–820. 2010. View
Article : Google Scholar : PubMed/NCBI
|