Introduction
Tendons are dense connective tissues, which are
responsible for transferring forces generated by muscles to the
opposite side of the joint, and supporting normal movement and
stability (1,2). Tendons are commonly subjected to
injury, due to various causes (3).
A clinical issue associated with tendon repair is the formation of
adhesions between a tendon and the surrounding synovial sheath,
which may seriously affect the recovery of tendon function.
Therefore, tendon adhesion is an important clinical issue (4). The treatment methods used to reduce
adhesion in injured flexor tendons include the use of
anti-inflammatory agents (5),
hyaluronan (6), electric fields
(7) and ultrasound (8). The use of biomaterials for the
prevention of adhesion has recently been widely studied in the
clinical setting. For example, biocompatible phospholipid polymer
MPC and hyaluronan have been applied for tendon repair (9,10).
However, the underlying cellular and molecular mechanisms of
biomaterial treatment of tendon repair remain to be elucidated in
order to improve therapeutic methods.
Chitosan, which is derived by partial deacetylation
of chitin from crustacean shells, is a polysaccharide copolymer
that consists of glucosamine [β-(1–4)-linked 2-amino-2-deoxy-D-glucose] and
N-acetylglucosamine (2-a cetamido-2-deoxy-D-glucose) (11,12).
Due to its biological sensitivity and security, chitosan has been
implemented in antimicrobial and antitumor treatment, immune
modification, and has been applied in tissue engineering as a
bio-scaffold to allow skin or bone cell growth (13). Previous studies have demonstrated
the therapeutic action of chitosan on tendon repair, including
inhibition of fibroblast growth (14), improved adhesive capacity (15), and cell proliferation and collagen
production (16).
Previous studies have demonstrated the regulatory
role of chitosan on tendon adhesion; however, the underlying
mechanism remains to be fully elucidated. Sirtuin (SIRT)1 is an
NAD+-dependent histone deacetylase for numerous histone
and non-histone substrates, which participate in various
physiological functions, including cell proliferation, apoptosis
and inflammation (17). SIRT1 has
previously been reported to be essential for the inhibition of
apoptosis and inflammatory responses in human tenocytes (18), thus suggesting that SIRT1 may be an
effective therapeutic target for adhesion prevention. However,
information regarding the role of SIRT1 signaling in tendon repair
is considered to be insufficient, particularly in vivo.
In the present study, a rabbit flexor tendon injury
model and human tenocytes were used to evaluate the effects of
chitosan on tendon adhesion, and investigate the underlying
mechanism. In the present study, a rabbit flexor tendon injury
model and human tenocytes were used to evaluate the effects of
chitosan on tendon adhesion. Whether SIRT1 and its downstream
signaling are involved in the anti-adhesion action of chitosan were
also investigated.
Materials and methods
Animals and surgery
A total of 30 mature male New Zealand white rabbits
(age, 6 months; weight, 1.84±0.89 kg), purchased from the Animal
Breeding Center (Zhejiang University, Hanhzhou, China), were
randomly divided into three equal groups: Group 1, saline
treatment; group 2, chitosan treatment (Sigma-Aldrich, St. Louis,
MO, USA); and group 3, chitosan + nicotinamide treatment
(nicotinamide is an inhibitor of SIRT1; Sigma-Aldrich). The left
hind limbs of the rabbits in each group were designated as the
injured tendon, and the right hind limbs were designated as the
normal flexor tendons for each group. The rabbits were maintained
in individual standard rabbit cages, with access to a standard
rabbit diet and water ad libitum. The study was approved by
the ethics committee of The First Affiliated Hospital of Medical
School of Zhejiang University (Hangzhou, China).
During surgery, the rabbits were administered an
intramuscular injection of 2% xylazine (1 mg/kg; Sigma-Aldrich) as
premedication, and 10% Ketamin-HCl (60 mg/kg; Sigma-Aldrich) for
anesthesia. The thigh of the rabbit was bound using an elastic
bandage as a tourniquet, and a 2-cm longitudinal incision was made
in the plantar skin between the proximal and distal interphalangeal
joints of the toes. The flexor digitorum profundus tendons were
transected using a blade, through the incision in the tendon
sheath. Finally, the injured tendons were surgically repaired using
6-0 sutures (Hanhzhou AiPu Medical Instrument, Co., Ltd., Hangzhou,
China) and the skin covering the lesion was sealed with a simple
continuous pattern, using non-absorbable 5-0 silk sutures (Hanhzhou
AiPu Medical Instrument, Co., Ltd). A rectangular window was
created in the cast at the site of injury (plantar surface of the
hind limb) for injection of the reagents. At 6 h post-surgery, 0.2
ml saline, 0.2 ml chitosan, or 0.2 ml chitosan + 5 mM/kg
nicotinamide was injected into the hind limb, through the
rectangular window, once per day for six weeks.
Adhesion and mechanical assessment
At 6 weeks post-surgery, the rabbits (n=10/group)
were sacrificed via 10 ml air via the ear intravenously and the
flexor tendons were harvested and stored at −20°C for subsequent
morphological and histological analyses. Gross and histological
evaluation of tendon adhesion was initially performed. The standard
degree of adhesion was determined, as mentioned previously
(12,19). Gross evaluation for adhesion degree
was determined as follows: None (no adhesions), filmy (separable
from the surrounding tissue), mild (not separable from the
surrounding tissue), moderate (35–60% of the injured area) and
severe (>60% of the injured area). Evaluation was performed by
an investigator in a blinded-manner. Histological evaluation for
adhesion formation was determined as follows: None (no adhesions),
mild (<33% of the tendon), moderate (33–66% of the tendon
surface) and severe (>66% of the tendon surface). The average
grade from the grades of 10 slides was calculated. In addition, the
flexor tendons were fixed on biomechanical instruments, in order to
assess mechanical strain. Pretension was set at 10 N and the
maximum tensile breakage of the tendons was recorded at a 20 mm/min
drawing speed.
Cell lines, culture conditions and
treatment
Human tenocytes were derived from five healthy toe
tendon explants, which were collected fresh from surgery at the
First Affiliated Hospital of Zhenjiang University. In brief, the
tendons were stripped aponeurosis under a microscope (DM5500 B;
Leica Microsystems, Wetzlar, Germany) and were washed with Hank's
solution. The tendon was then subjected to digestion with 0.25%
trypsin and 0.1% collagenase for 20 min at of 37°C. The digested
tendons were collected, washed with Hank's solution and cut into
1–2 mm3 fragments. The fragments were exposed to 0.25%
trypsin and 0.1% collagenase for 1 h at 37°C in order for second
digestion. The digested mixture was filtered and centrifuged. The
sediment was washed and made into a cell suspension. The cells were
counted and inoculated at 5×105/ml. Finally, the cells
were cultured in phenol red-free Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% charcoal stripped fetal bovine serum
prior to the subsequent experimental protocols The cells were
pretreated with chitosan (5, 10 or 50 µg/ml) for 30 min, and
were then split into two groups, those that were activated by
interleukin (IL)-1β (10 ng/ml), and those that were not. High
purity chitosan and IL-Iβ were purchased from Sigma-Aldrich,
dissolved in normal saline and added to the culture medium
according to the indicated concentrations. All reagents were
obtained from Invitrogen Life Technologies, (Carlsbad, CA, USA).
All subsequent experiments were conducted 1 h after IL-1β
supplementation, unless otherwise stated.
MTT viability assay
The effects of chitosan and IL-1β on cell viability
were detected using an MTT assay (Sigma-Aldrich), which is based on
the uptake of 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium
bromide. The tenocytes (2×103 cells/well) were cultured
in 96-well plates 24 h prior to treatment with chitosan. Following
treatment with the reagents, the MTT solution was added to each
well and incubated for 2 h at 37°C. SDS buffer (10%) supplemented
with 0.01 M HCl was then added to the cells and left for 12 h.
Subsequently, the absorbance at 570 nm was measured using a
spectrophotometer (Perkin Elmer LS-55; PerkinElmer Inc., Waltham,
MA, USA). Independent experiments were performed in triplicate.
Western blot analysis
The rabbit flexor tendons or human tenocytes were
lysed using ice-cold lysis buffer containing: 50 mmol/l Tris-HCl
(pH 7.4); 1% NP-40; 150 mmol/l NaCl; 1 mmol/l EDTA; 1 mmol/l
phenylmethylsulfonyl fluoride; and complete proteinase inhibitor
mixture (one tablet/10 ml; Roche Molecular Biochemicals,
Indianapolis, IN, USA). A Bicinchoninic Acid Protein Assay kit
(Beyotime Institute of Biotechnology, Haimen, China) was used to
quantify the protein concentration within the lysate and western
blot analysis was subsequently performed. Proteins (40 µg)
were separated by 10% SDS-PAGE and transferred to a nitrocellulose
membrane (EMD Millipore, Bilerica, MA, USA). The membrane was then
probed with mouse anti-goat polyclonal antibody against SIRT1 (cat.
no. sc-19857; Santa Cruz Biotechnology, Inc., Dallas, TX, CA),
rabbit polyclonal antibody against acetylated p65 (cat. no. 3045s;
Cell Signaling Technology, Inc., Beverly, MA, USA), rabbit
polyclonal antibody against acetylated p53 (cat. no. 2570s; Cell
Signaling Technology, Inc) and rabbit polyclonal antibody against
β-actin (cat. no. A2066; Sigma-Aldrich), followed by incubation
with anti-goat (cat. no. ab157532) or anti-rabbit (cat. no.
ab191866) secondary antibodies (Abcam, Cambridge, MA, USA). All
antibodies were used at concentrations and dilutions as recommended
by the manufacturer's instructions (dilutions ranged between 1:100
and 1:10,000 for western blot analysis). The blots were visualized
by enhanced chemiluminescence (ECL) using ECL reagent (Pierce
Biotechnology, Inc., Rockford, IL, USA) and images were captured on
X-ray films (ChampGel 6000; Beijing Sage Creation Science, Co.,
Ltd., Beijing, China).
Apoptosis analysis
Detection and quantification of apoptosis was
performed using flow cytometry. After treatment with the reagents,
the tenocytes were cultured with binding buffer, Annexin V-Enhanced
Green Fluorescent Protein and prop-idium iodide (Sigma-Aldrich).
The mixture was then incubated at room temperature for 15 min in
the dark, followed by flow cytometry (FACScan; BD Biosciences,
Franklin Lakes, NJ, USA). The percentage of apoptotic cells was
quantified using CellQuest software version 5.1 (BD
Biosciences).
Inhibition of SIRT1 by RNA
interference
Double-stranded oligonucleotides 5′-GAT CCC GTT GGA
TGA TAT GAC ACT GTT CAA GAG ACA GTG TCA TAT CAT CCA ACT TTT TTG GAA
A-3′ (SIRT1 target sequence underlined) were cloned into the
pSuperiorRetroPuro vector (Oligoengine, Seattle, WA, USA). The
plasmid was packaged into a retrovirus by transfection of the
amphotropic packaging cell line LA (Cell Biolabs, Inc. USA). A
virus expressing a scrambled small interfering (si)RNA
(5′-GATCCCGCCGTCGTCGATAAGCAATATTTGATATC
CGATATTGCTTATCGACGACGGCTTTTTTA-3′; pSuperi-orRetroPuro vector)
served as a control. Human tenocytes were infected with the SIRT1
siRNA retrovirus and selected using 0.5 g/ml puromycin
(Sigma-Aldrich) for 10 days. Cells were harvested for the
subsequent apoptotic assay and western blot analysis.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
statistical analysis software (SPSS Inc., Chicago, IL, USA). Data
was analyzed using one-way analysis of variance. Statistical
analyses of cell viability and apoptosis were conducted using
Student's t-test. All data were expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Adhesion formation
Following surgery, the flexor tendons were harvested
from the rabbits, in order to determine the state of adhesion.
Based on the gross (10) and
histological evaluations of adhesion (Table I), the numbers of rabbits
exhibiting severe and moderate levels of adhesion werelowest in the
chitosan-treated rabbits (group 2; 0 severe and 2 moderate; n=10),
when compared with the levels in the normal saline-treated rabbits
(group 1; 6 severe and 3 moderate; n=10), and the effect of
chitosan was reversed following nicotinamide treatment (group 3; 5
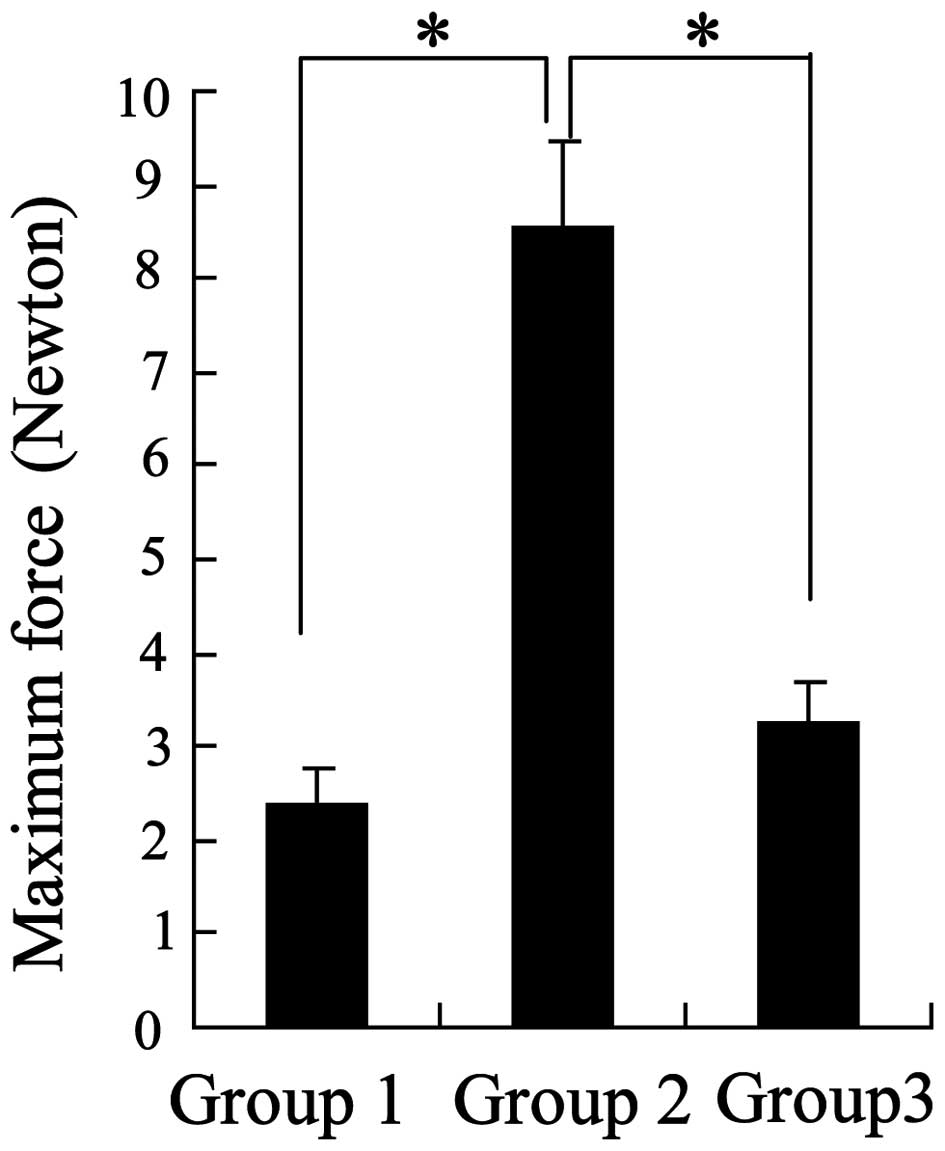
severe and 3 moderate; n=10). Furthermore, analysis of the maximum
force generated by the tendon anastomoses demonstrated that the
greatest forces were observed in the chitosan group (Fig. 1). These results indicated that
chitosan may possess anti-adhesive bioactivity.
 | Table IGross and histological evaluation of
tendon adhesion. |
Table I
Gross and histological evaluation of
tendon adhesion.
| Group | Gross evaluation of
tendon adhesion
| Histological degree
of tendon adhesion
|
|---|
| Severe | Moderate | Mild | Filmy | None | Severe | Moderate | Mild | None |
|---|
| 1 | 6 | 3 | 1 | – | – | 6 | 3 | 1 | – |
| 2 | – | 1 | 3 | 6 | – | – | 2 | 7 | 1 |
| 3 | 5 | 4 | 1 | – | – | 5 | 3 | 2 | – |
Expression levels of signaling
molecules
In addition to examination of the physiological
adhesion status, the flexor tendons were randomly selected and
disrupted using liquid nitrogen, in order to detect the expression
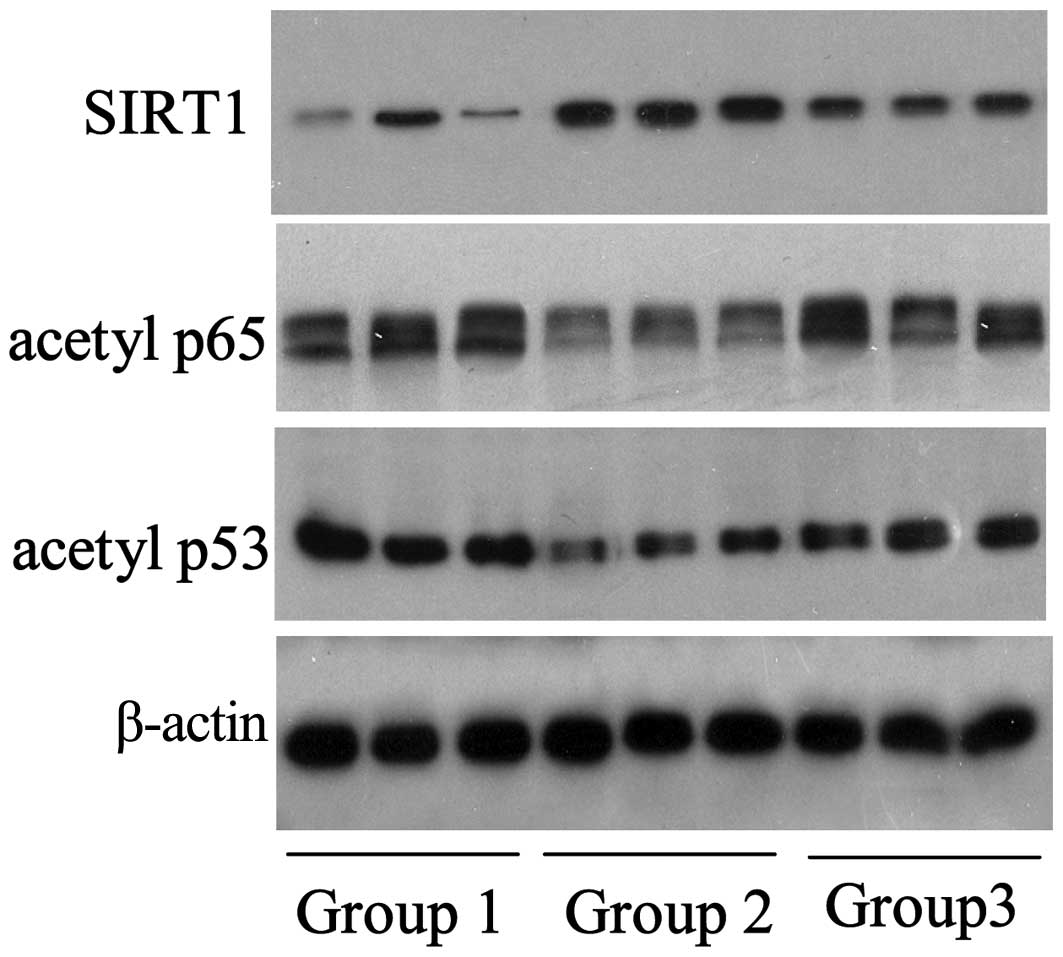
levels of specific signaling molecules. As shown in Fig. 2, the expression levels of SIRT1 in
the chitosan group (group 2; n=3/group) were elevated, when
compared with the control group; chitosan-induced SIRT1 protein
expression was observed to be inhibited by nicotinamide.
Conversely, the expression levels of the SIRT1-targeted proteins,
acetylated p65 and p53, decreased with the upregulation of SIRT1
and increased with the downregulation of SIRT1, which was induced
by nicotinamide.
Chitosan reverses IL-1β-induced
proliferation and apoptosis of human tenocytes
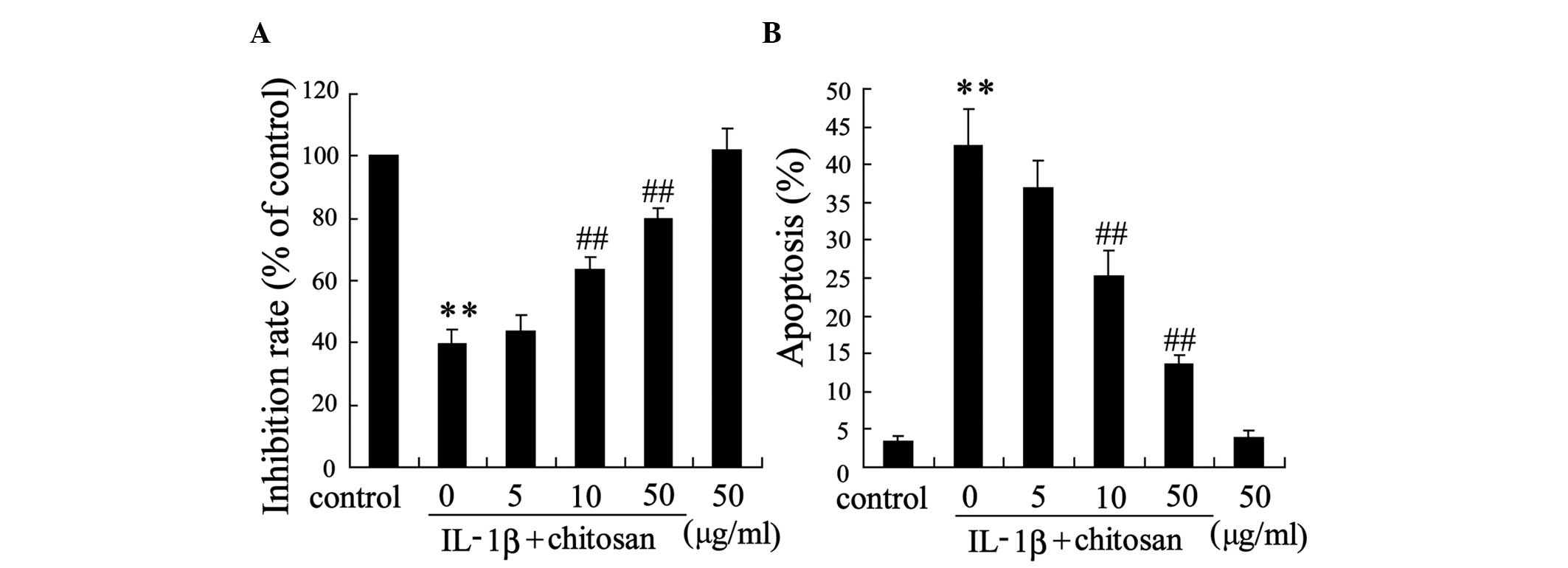
An in vitro experiment was performed in human
tenocytes to further ascertain the protective effects of chitosan
on tendon repair. Following chitosan pretreatment, the tenocytes
were incubated with IL-1β. Fig. 3
demonstrates that chitosan influenced the survival of tenocytes.
Treatment with 10 µg/ml or 50 µg/ml chitosan
attenuated IL-1β-induced cell proliferation (Fig. 3A) and apoptosis (Fig. 3B) in a dose-dependent manner,
compared with the IL-1β group.
Chitosan attenuates IL-1β-induced
expression of signaling proteins in human tenocytes
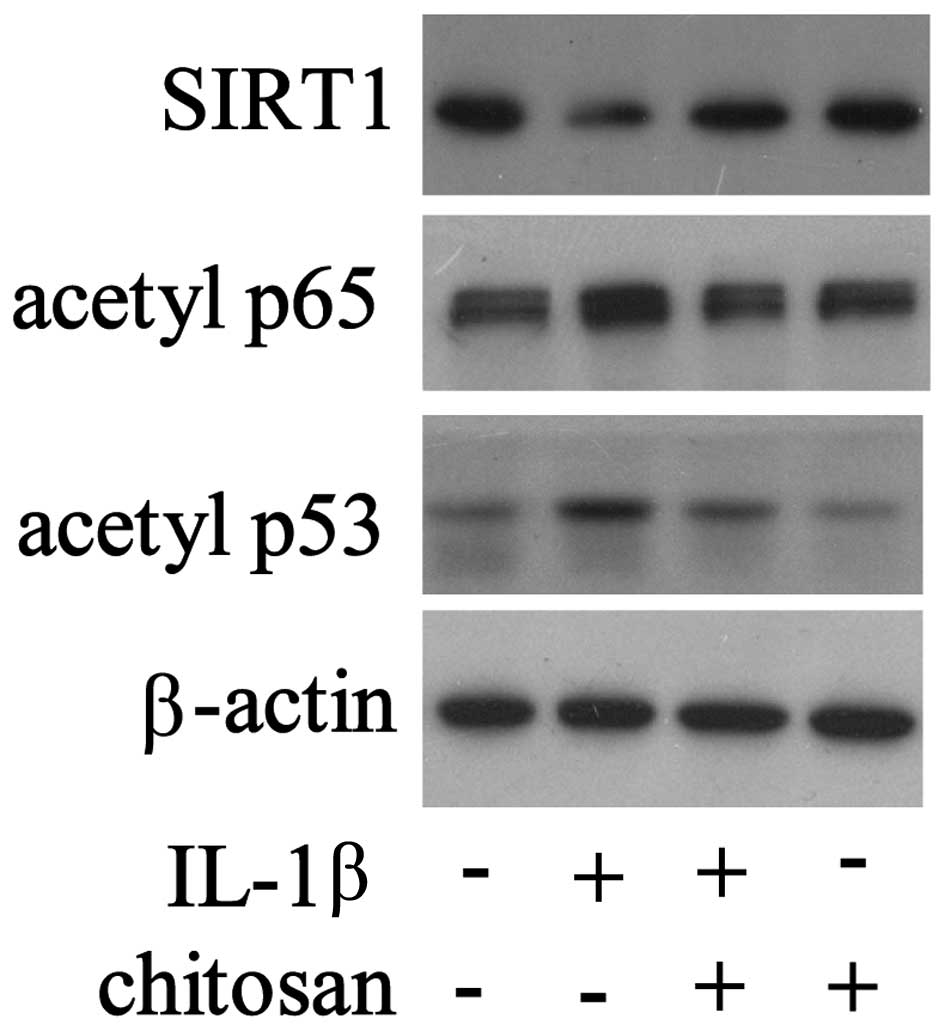
Following chitosan pretreatment, the cells were
exposed to IL-1β, and the expression levels of various signaling
molecules were detected. As shown in Fig. 4, the expression levels of SIRT1
were downregulated and the expression levels of acetylated p65 and
p53 were upregulated in the tenocytes stimulated with IL-1β.
However, chitosan reversed the changes in protein expression that
had been induced by IL-1β. These results demonstrate the
fundamental regulatory role of SIRT1 in tendon repair by
chitosan.
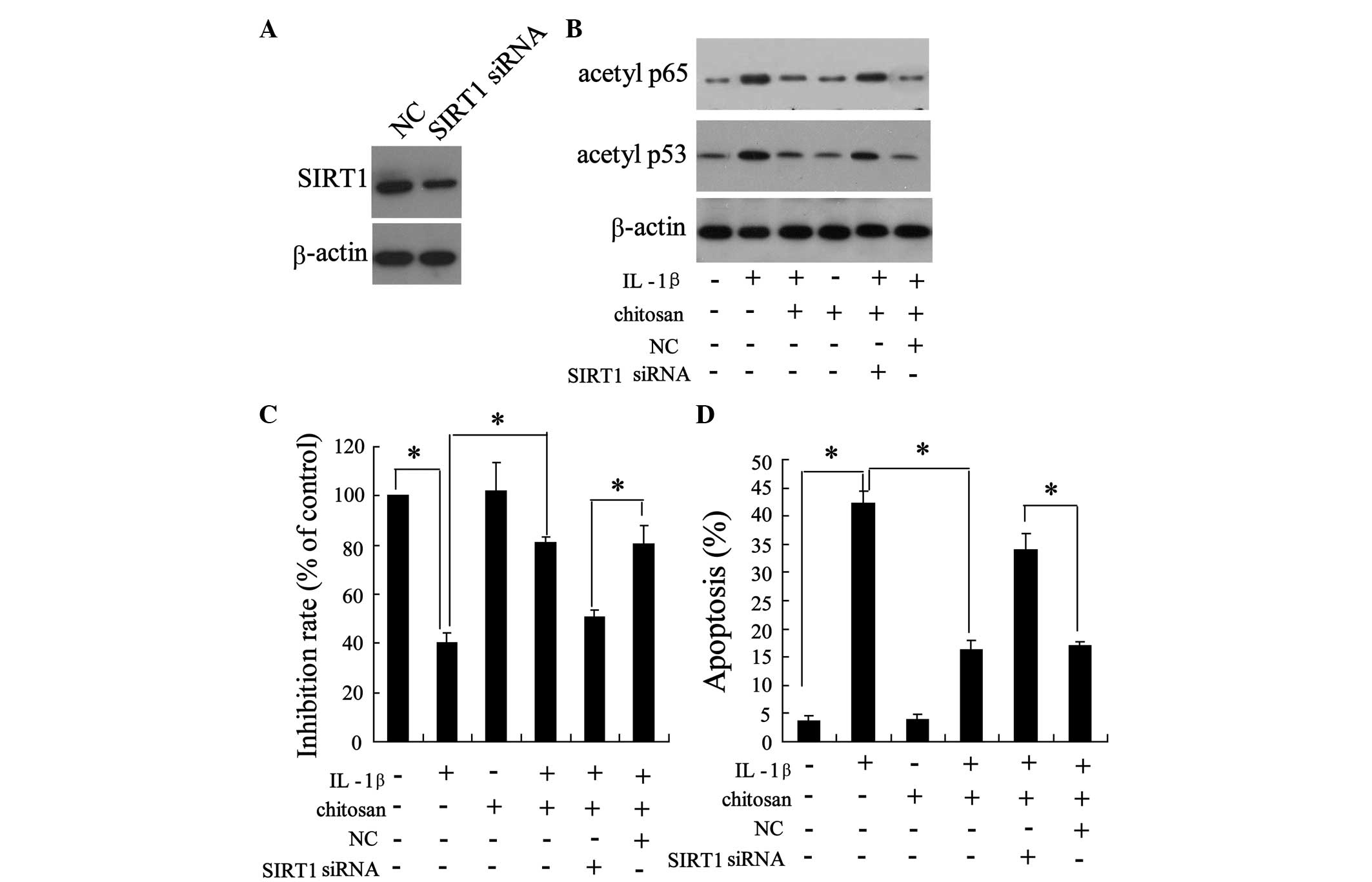
Knockdown of SIRT1 reduces the
bioactivity of chitosan on human tenocytes
To determine the role of SIRT1 in the protection of
tendons from adhesion, the tenocytes were transfected with small
interfering (si)-SIRT1. As shown in Fig. 5A, transfection with si-SIRT1
resulted in a down-regulation of SIRT1 expression, indicating the
successful knockdown of SIRT1. As shown in Fig. 4, chitosan reversed the
IL-1β-induced downregulation of acetylated p65 and p53. Notably,
the action of chitosan on the IL-1β-induced expression of
acetylated p65 and p53 was abrogated following SIRT1 knockdown
(Fig. 5B). Furthermore, silencing
of SIRT1 expression abolished the effects of chitosan on
IL-1β-induced cell proliferation (Fig.
5C) and apoptosis (Fig. 5D),
when compared with the IL-1β + chitosan negative control group.
Discussion
Adhesion formation is a common clinical problem,
which is characteristic of alignment and maturation of tenoblasts
and collagen fibers, and inadequate attachment of the tendon to its
location (20). It has previously
been demonstrated that the prevention of tendon adhesion is an
important objective of hand surgery (21). The results of the present study
demonstrate that administration of chitosan relieves the structural
and biomechanical properties during an experimental model of flexor
tendon wound repair. In addition, SIRT1 was observed to be a key
regulator affected by chitosan treatment in the prevention of
tendon adhesion.
Tendon repair may occur intrinsically, via
proliferation of epitenon and endotenon tenocytes, which results in
improved biomechanics and fewer complications; or extrinsically,
via invasion of cells from the surrounding sheath and synovium,
which causes adhesion formation (22). It has previously been reported that
inflammation is a common initial event of tendon repair. During
inflammation of the tendons, vasoactive and chemotactic factors are
released resulting in increased vascular permeability, initiation
of angiogenesis, stimulation of tenocyte proliferation, and
recruitment of more inflammatory cells (23). It has been suggested that
protective tenocytes, released during inflammation in intrinsic
repair, may be effective in the prevention of tendon adhesion.
Accordingly, the present study demonstrated that chitosan exerted
an anti-adhesive effect on flexor tendons, and abolished tenocyte
apoptosis, which had been induced by IL-1β (an inflammatory
factor). Chitosan is a type of biomate-rial that possesses
hemostatic and anti-inflammatory properties, which has been shown
(experimentally and clinically) to prevent adhesion (24). However, the underlying mechanism of
the anti-adhesive effect of chitosan and the associated
intracellular signaling pathway has yet to be fully elucidated.
SIRT1 has been identified as a modulator in the
development and progression of inflammation through the
deacetylation of histones and critical transcription factors, thus
leading to transcriptional repression of various
inflammation-associated genes (17). In addition, SIRT1 has been shown to
inhibit apoptosis and the inflammatory response of tenocytes
(18). Therefore, the present
study examined whether SIRT1 signaling may be involved in the
prevention of adhesion by chitosan treatment in rabbit flexor
tendons and human tenocytes. The results indicated that chitosan
increased the expression of SIRT1 in adhesive tendons in
vivo and reversed the IL-1β-mediated downregulation of SIRT1 in
tenocytes. In addition, it was demonstrated that inhibition of
SIRT1 by nicotinamide partly suppressed the ability of chitosan to
prevent adhesion in rabbits. These results indicated a regulatory
role of SIRT1 signaling in the anti-adhesive properties of
chitosan.
Nuclear factor (NF)-κB is a nuclear transcription
factor, which has been reported to regulate the gene expression of
numerous proinflammatory proteins (17). The present study demonstrated that
chitosan reversed proinflammatory IL-1β-induced upregulation of
acetylated p65 (a subunit of NF-κB) and upregulated SIRT1. It has
previously been reported that deacetylation of NF-κB subunit p65
may lead to a decrease in NF-κB transcriptional activity, thereby
resulting in cell apoptosis (17,25).
In this context the results of the present study indicated that
knockdown of SIRT1 abrogated the inhibitory effects of chitosan on
IL-1β-induced p65 acetylation and apoptosis. These results indicate
that chitosan may inhibit inflammation-associated NF-κB activation
via SIRT1 signaling during adhesion repair.
The present study also demonstrated that the
expression of p53 was upregulated in adhesive tendons and tenocytes
that had been exposed to IL-1β. As a tumor suppressor gene, p53 is
stimulated by various stress signals and is significant in
modulating the cell cycle and apoptosis (26,27).
p53 has been characterized as one of the numerous substrates of
SIRT1, and deacetylation at the lysine residue of p53 reduces its
DNA binding activity, thus affecting co-activator recruitment,
which is required for cell survival (28). The present study also demonstrated
that chitosan inhibited p53 activation in rabbit adhesive tendons
and IL-1β-induced tenocytes. In addition, knockdown of SIRT1
resulted in increased acetylation of p53, as well as increased cell
viability and reduced apoptosis in human tenocytes. To the best of
our knowledge, the present study is the first to demonstrate a
regulatory role of SIRT1 signaling (via NF-κB, a subunit of p53,
and p53) in the prevention of adhesion using chitosan, in
vivo.
In conclusion, the present study provided important
insights regarding the underlying mechanisms of adhesion prevention
via the administration of chitosan. Prevention of adhesion was
shown to be associated with SIRT1 signaling in a flexor tendon
repair model. Furthermore, the results indicated that chitosan may
inhibit inflammation and protect tenocytes from apoptosis, via
suppression of NF-κB and activation of p53 by SIRT1. The
upregulation of SIRT1 as a result of chitosan treatment in injured
tendons may be useful in the development of future therapeutic
strategies for the treatment of tendon injury.
References
|
1
|
Moshiri A and Oryan A: Structural and
functional modulation of early healing of full-thickness
superficial digital flexor tendon rupture in rabbits by repeated
subcutaneous administration of exogenous human recombinant basic
fibroblast growth factor. J Foot Ankle Surg. 50:654–662. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chalmers J: Review article: Treatment of
Achilles tendon ruptures. J Orthop Surg (Hong Kong). 8:97–99.
2000.
|
|
3
|
Peterson RK, Shelton WR and Bomboy AL:
Allograft versus autograft patellar tendon anterior cruciate
ligament reconstruction: A 5-year follow-up. Arthroscopy. 17:9–13.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Woo SL, Lee TQ and Abramowitch SD:
Structure and function of ligaments and tendons. Basic Orthopaedic
Biomechanics and Mechanobiology. Mow VC and Huiskes R: 3rd.
Lippincott Williams & Wilkins; Philadelphia: pp. 301–342.
2005
|
|
5
|
Kulick MI, Smith S and Hadler K: Oral
ibuprofen: Evaluation of its effect on peritendinous adhesions and
the breaking strength of a tenorrhaphy. J Hand Surg Am. 11:110–120.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amiel D, Ishizue K, Billings E Jr, Wiig M,
Vande Berg J, Akeson WH and Gelberman R: Hyaluronan in flexor
tendon repair. J Hand Surg Am. 14:837–843. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujita M, Hukuda S and Dodia Y: The effect
of constant direct electrical current on intrinsic healing in the
flexor tendon in vitro. An ultrastructural study of differing
attitudes in epitenon cells and tenocytes. J Hand Surg Br.
17:94–98. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Turner SM, Powell ES and Ng CS: The effect
of ultrasound on the healing of repaired cockerel tendon: Is
collagen cross-linkage a factor? J Hand Surg Br. 14:428–433. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishiyama N, Moro T, Ohe T, Miura T,
Ishihara K, Konno T, Ohyama T, Kimura M, Kyomoto M, Saito T,
Nakamura K and Kawaguchi H: Reduction of Peritendinous adhesions by
hydrogel containing biocompatible phospholipid polymer MPC for
tendon repair. J Bone Joint Surg Am. 93:142–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Skardal A, Shu XZ and Prestwich GD:
Prevention of peritendinous adhesions using a hyaluronan-derived
hydrogel film following partial-thickness flexor tendon injury. J
Orthop Res. 26:562–569. 2008. View Article : Google Scholar
|
|
11
|
Illum L: Chitosan and its use as a
pharmaceutical excipient. Pharm Res. 15:1326–1331. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raafat D and Sahl HG: Chitosan and its
antimicrobial potential – a critical literature survey. Microb
Biotechnol. 2:186–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaats GR, Michalek JE and Preuss HG:
Evaluating efficacy of a chitosan product using a double-blinded,
placebo-controlled protocol. J Am Coll Nutr. 25:389–394. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Sheng ZJ and Hou CL: Effect of
chitosan membrane on tendon adhesion and healing. Zhongguo Xiu Fu
Chong Jian Wai Ke Za Zhi. 13:382–385. 1999.In Chinese.
|
|
15
|
Majima T, Funakosi T, Iwasaki N, Yamane
ST, Harada K, Nonaka S, Minami A and Nishimura S: Alginate and
chitosan polyion complex hybrid fibers for scaffolds in ligament
and tendon tissue engineering. J Orthop Sci. 10:302–307. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia CS, Hong GX, Dou RR and Yang XY:
Effects of chitosan on cell proliferation and collagen production
of tendon sheath fibroblasts, epitenon tenocytes, and endotenon
tenocytes. Chin J Traumatol. 8:369–374. 2005.PubMed/NCBI
|
|
17
|
Yeung F, Hoberg JE, Ramsey CS, Keller MD,
Jones DR, Frye RA and Mayo MW: Modulation of NF-kappaB-dependent
transcription and cell survival by the SIRT1 deacetylase. EMBO J.
23:2369–2380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Busch F, Mobasheri A, Shayan P, Stahlmann
R and Shakibaei M: Sirt-1 is required for the inhibition of
apoptosis and inflammatory responses in human tenocytes. J Biol
Chem. 287:25770–25781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang JB, Shi D and Zhang QG: Biomechanical
and histologic evaluation of tendon sheath management. J Hand Surg
Am. 21:900–908. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharma P and Maffulli N: Tendon injury and
tendinopathy: Healing and repair. J Bone Joint Surg Am. 87:187–202.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khanna A, Gougoulias N and Maffulli N:
Modalities in prevention of flexor tendon adhesion in the hand:
what have we achieved so far? Acta Orthop Belg. 75:433–444.
2009.PubMed/NCBI
|
|
22
|
Gelberman RH, Manske PR, Vande Berg JS,
Lesker PA and Akeson WH: Flexor tendon repair in vitro: A
comparative histologic study of the rabbit, chicken, dog, and
monkey. J Orthop Res. 2:39–48. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma P and Maffulli N: Biology of tendon
injury: Healing, modeling and remodeling. J Musculoskelet Neuronal
Interact. 6:181–190. 2006.PubMed/NCBI
|
|
24
|
Moutzouri AG and Athanassiou GM: Insights
into the alteration of osteoblast mechanical properties upon
adhesion on chitosan. Biomed Res Int. 2014:7407262014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tak PP, Gerlag DM, Aupperle KR, van de
Geest DA, Overbeek M, Bennett BL, Boyle DL, Manning AM and
Firestein GS: Inhibitor of nuclear factor kappaB kinase beta is a
key regulator of synovial inflammation. Arthritis Rheum.
44:1897–1907. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ryan KM, Phillips AC and Vousden KH:
Regulation and function of the p53 tumor suppressor protein. Curr
Opin Cell Biol. 13:332–337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakaguchi K, Herrera JE, Saito S, Miki T,
Bustin M, Vassilev A, Anderson CW and Appella E: DNA damage
activates p53 through a phosphorylation-acetylation cascade. Genes
Dev. 12:2831–2841. 1998. View Article : Google Scholar : PubMed/NCBI
|