Introduction
Pancreatic cancer is one of the most deadly forms of
carcinoma, estimated to be the seventh leading cause of
cancer-related mortality in China and the fourth leading cause of
cancer-related mortality in the United States (1,2). In
the United States alone, >250,000 patients succumb to pancreatic
cancer annually (3). Although
progress has been made in understanding the molecular mechanisms
and developing suitable treatment strategies, the overall prognosis
of patients with pancreatic cancer remains poor (4,5).
Therefore, it is of great interest to further understand the
development of pancreatic cancer and to seek novel methods for
early detection and effective therapeutic strategies to treat
patients with pancreatic cancer.
MicroRNAs (miRNAs) are a group of non-coding,
small-size RNA nucleotides involved in regulating gene expression
through translational cleavage or repression (6). In previous decades, it has been
demonstrated that miRNAs are widely involved in various
developmental stages, including embryogenesis, organ maturation,
cell differentiation and carcinoma, in human and animal tissue
(7–9). In cancer, mounting evidence has
revealed that miRNAs are directly involved in several aspects of
cancer development, including carcinogenesis, tumor growth, tumor
differentiation, carcinoma metastasis and cancer apoptosis
(7,10–15).
Among several of the cancer-associated miRNAs (miR),
miR-138 is a family of microRNA precursors. The overexpression or
underexpression of miR-138 have been demonstrated to be important
in regulating tumor development or cancer apoptosis in various
forms of carcinoma, including lung cancer (16), hepatocellular carcinoma (17) and leukemia (18). However, little is known regarding
the expression pattern or functional role of miR-138 in modulating
pancreatic cancer development.
In the present study, the expression levels of the
human isoform of miR-138, miR-138-5p, were examined in human
pancreatic cancer cell lines and primary pancreatic carcinoma,
obtained from patient samples. The possible biological functions of
miR-138-5p in regulating cell invasion or sensitivity to the
chemotherapeutic agent, 5-fluorouracil (5-FU), were investigated
through lentivirus-mediated miR-138-5p over- or downregulation in
the PANC-1 pancreatic cancer cell line. The possible molecular
target, vimentin (VIM), was examined to determine whether it was
directly regulated by miR-138-5p in pancreatic cancer. Small
interfering (si) RNA-mediated genetic downregulation of VIM was
also performed to observe whether VIM exerted any functional role
in regulating pancreatic cancer development.
Materials and methods
Cell lines and culture
A total of eight pancreatic cancer cell lines were
used: AsPC-1, BxPc-3, Capan-1, Capan-2, CFPAC-1, PANC-1, MIA PaCa-2
and SW1990 (American Type Culture Collection, Shanghai, China).
Human pancreatic ductal epithelial (HPDE) cells were obtained from
Dr M.S. Tsao at the Ontario Cancer Institute (Ontario, Canada) and
maintained as described previously (19). Primary human normal pancreatic
epithelial cells were obtained from Shanghai Genomics (Shanghai,
China) and cultured in CS-C medium (Cell Systems Corporation,
Kirkland, WA, USA), containing 10% fetal bovine serum (FBS;
Sigma-Aldrich, St. Louis, MO, USA).
Tissue samples
The human pancreatic tissue samples were obtained
from patients following a surgical procedure to resect a portion of
the pancreas, performed at the Department of Hepatobiliary Surgery,
Affiliated Hospital of Guiyang Medical College (Guiyang, China)
between January 2013 and March 2014. The normal pancreatic tissue
samples were obtained from areas of peripheral tissue adjacent to
tumors. The tissues were rapidly removed and a section of each
sample was embedded in Optimal Cutting Temperature compound (Miles
Laboratories, Elkhart, IN, USA). Histologic examination was
subsequently performed on all tissue samples adjacent to the
specimens (BX51WI; Olympus Corporation, Tokyo, Japan). Consent
forms were obtained from all patients and all procedures in the
present study were reviewed and approved by the Ethics Committees
of the Affiliated Hospital of Guiyang Medical College and
Affiliated Tongji Hospital.
Lentivirus construction and
transfection
The oligonucleotides of the hsa-miR-138-5p mimics,
hsa-miR-138-5p inhibitor, and their non-specific control were
synthesized by RiboBio (RiboBio, Shanghai, China). The coding
sequences were subsequently amplified and cloned into
pCDH-CMV-MCS-EF1-coGFP constructs (System Biosciences, Mountain
View, CA, USA). The lentiviral expression constructs and pPACK
packaging plasmid mix (System Biosciences) were co-transfected into
293 T cells (American Type Culture Collection), according to the
manufacturer's instructions, and viral particles of the miR-138-5p
mimics (lv-miR-138-m), miR-138-5p inhibitor (lv-miR-138-i) and
non-specific control (lv-control) were collected and the titers
were determined by fluorescence-activated cell sorting (FACSCanto
II flow cytometer; BD Biosciences, San Jose, CA, USA). The
pancreatic cells were subsequently transfected with lentiviruses
using Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad,
CA, USA), according to manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA and miRNA fractions were isolated from
tissues and cell lines using TRIzol reagent, according to the
manufacturer's instructions (Invitrogen Life Technologies). The
total RNA concentrations were measured using a NanoDrop ND-1000
spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) at
260 and 280 nm (A260/280) and examined with an Agilent 2100
Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The
expression of miRNA was quantified using SYBR Green. The primers
for amplifying miR-138-5p were a commercial product from RiboBio.
RT-qPCR was performed using the TaqMan miRNA assay (Applied
Biosystems), according to the manufacturer's instructions. The
amplification conditions were as follows: 35 cycles of denaturing
(at 94°C for 60 sec), annealing (at 60°C for 60 sec), and chain
extension (at 72°C for 1 min), followed by a final extension step
at 72°C for 10 min. The expression levels of miR-138-5p and
vimentin (VIM) were normalized against the expression levels of the
house keeping genes, U6 and GAPDH, respectively.
MTT migration assay
For the MTT assays, the PANC-1 cells were initially
plated (10,000 cells/well) into 96-well plates for 24 h at 37°C.
The lentiviruses of lv-miR-138-m, lv-miR-138-I and lv-Control were
subsequently added into the culture for 3 days, followed by
replacing the culture medium with 100 µl Dulbecco's modified
Eagle's medium (Invitrogen Life Technologies) + 10% FBS. MTT
solution (20 µl; Sigma-Aldrich) was added for 2 h, according
to the manufacturer's instructions. The absorbance at 490 nm was
measured using a multifunctional plate reader (Victor 3,
PerkinElmer, Turku, Finland), in order to determine the total
number of viable cells.
Cell proliferation assay and 5-FU
treatment
The pancreatic cancer cells were transfected with
either lv-miR-138-m, lv-miR-138-i or lv-control lentivirus, and
were seeded into 6-well plates for 24 h (1×106/well).
The viable cells were subsequently transferred at 2×104
cells/well into 24-well plates and cultured for a further 48 h.
Cell proliferation was determined by measuring the fluorescence
intensity of propidium iodide (PI), as described previously
(20). At 24 and 48 h, the
fluorescence intensities were measured using a CytoFluor II
multiwell plate reader (PerSeptive Biosystems, Inc., Framingham,
MA, USA) and normalized against the intensity at 0 h. All
experiments were performed in triplicate. For chemo-treatment,
between 1 and 100 µg/ml 5-FU was added to the culture.
Luciferase reporter assays
Bioinformatic methods, including TargetScan
(www.targetscan.org), Pictar
(pictar.mdc-berlin.de) and miRANDA (www.microrna.org), were used to identify the
downstream target of miR-138-5p. Regular PCR was performed on the
cDNA (5 ng) of PANC-1 cells to amplify the wild-type
3′-untranslated region (UTR) and mutant 3′-UTR (modified
hsa-miR-138-5p binding site) of VIM. The wild-type 3′-UTR and the
mutant (mu) 3′-UTR of VIM were subsequently inserted into a
luciferase reporter vector (pmiR-REPORT; Ambion Life Technologies,
Carlsbad, CA, USA) to produce constructs of Luc-VIM and Luc-VIM-mu,
and were verified by DNA sequencing. The pmiR-REPORT control
vector, Luc-VIM and Luc-VIM-mu were co-transfected with
β-galactosidase and Lv-miR-138-M into HEK293 cells (American Type
Culture Collection) in 12-well plates using Lipofectamine 2000
(Invitrogen Life Technologies), according to the manufacturer's
instructions. The luciferase activity was examined following
culturing for 24 h, using a luciferase reporter assay system
(Promega Corporation, Madison, WI, USA), according to the
manufacturer's instructions. The signals were normalized against
the β-galactosidase activity of the control vector. The experiment
was performed at least three times.
Western blotting
For western blotting, lysates of PANC-1 cells were
extracted using lysis buffer (Sigma-Aldrich), containing 50 mM Tris
(pH 7.6), 150 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% NP-40 and
protease inhibitor cocktail (Sigma-Aldrich). The protein products
were subsequently resolved on 10% SDS-PAGE gels and transferred
onto nitrocellulose membranes (Invitrogen Life Technologies). The
membranes were incubated with primary antibody against VIM (1:250;
SC-5565; Santa Cruz Biotechnology, Santa Cruz, CA, USA), according
to the manufacturer's instructions. Following incubation, the
membranes were washed with phosphate-buffered saline containing
Tween and incubated with horseradish peroxidase-conjugated
secondary antibodies (166-2408EDU; Bio-Rad Laboratories, Hercules,
CA, USA). The western blots were visualized using an enhanced
chemiluminesence system (Amersham Biosciences, Piscataway, NJ,
USA), according to the manufacturer's instructions.
Transfection of siRNA
The specific VIM siRNA (VIM_siRNA) and its
non-specific scramble siRNA (NC_siRNA) were purchased from Santa
Cruz Biotechnology Inc., and the transfection of siRNAs was
performed using Lipofectamine 2000 (Invitrogen Life Technologies),
according to the manufacturer's instructions. Briefly, PANC-1 cells
were transfected with either VIM_siRNA (100 nM) or NC_siRNA (100
nM) for 48 h, followed by examination of cell proliferation. The
efficiency of siRNA on the knock down of VIM was confirmed by
RT-qPCR 48 h following transfection.
Statistical analysis
In vivo tumor volumes were analyzed using a
one-way analysis of variance. The data are expressed as the mean ±
standard error of the mean and evaluated using Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference. All experiments were repeated at least three times.
Results
miR-138-5p is downregulated in pancreatic
cancer cell lines and tissues
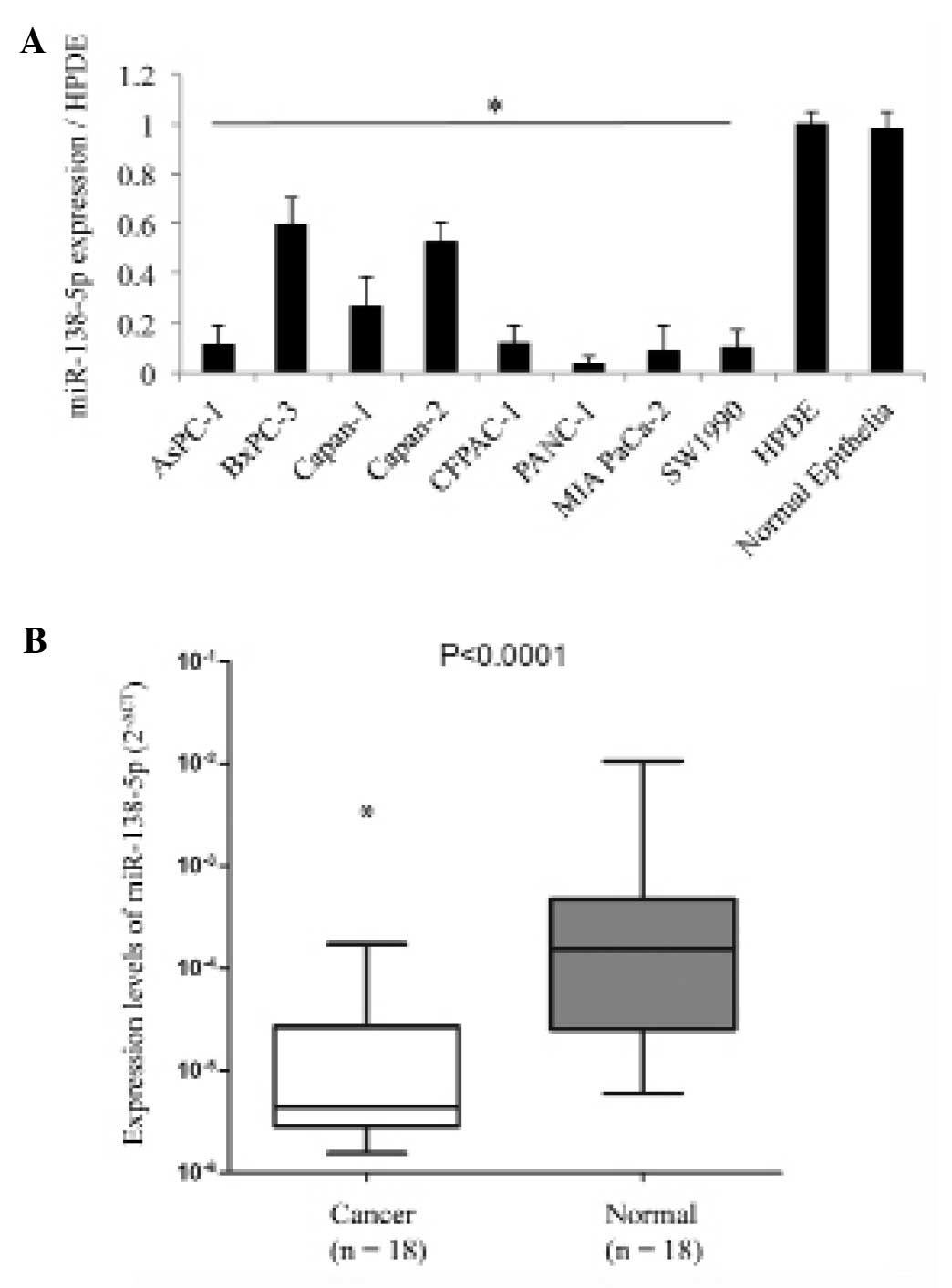
The present study used RT-qPCR to examine the
expression levels of miR-138-5p in the eight pancreatic cancer cell
lines, non-carcinoma epithelia and HPDE cells. It was demonstrated
that the expression levels of miR-138-5p were significantly lower
in all the pancreatic cancer cell lines compared with the
non-carcinoma cells (Fig. 1A;
P<0.05).
The expression levels of miR-138-5p in primary
pancreatic carcinoma tissues and adjacent normal pancreatic
epithelial tissues from 18 patients was subsequently assessed. It
was demonstrated that the expression levels of miR-138-5p in cancer
tissue samples was notably lower compared with the normal tissue
samples (Fig. 1B; P<0.05).
miR-138-5p regulates pancreatic cancer
cell invasion
In order to investigate the molecular function of
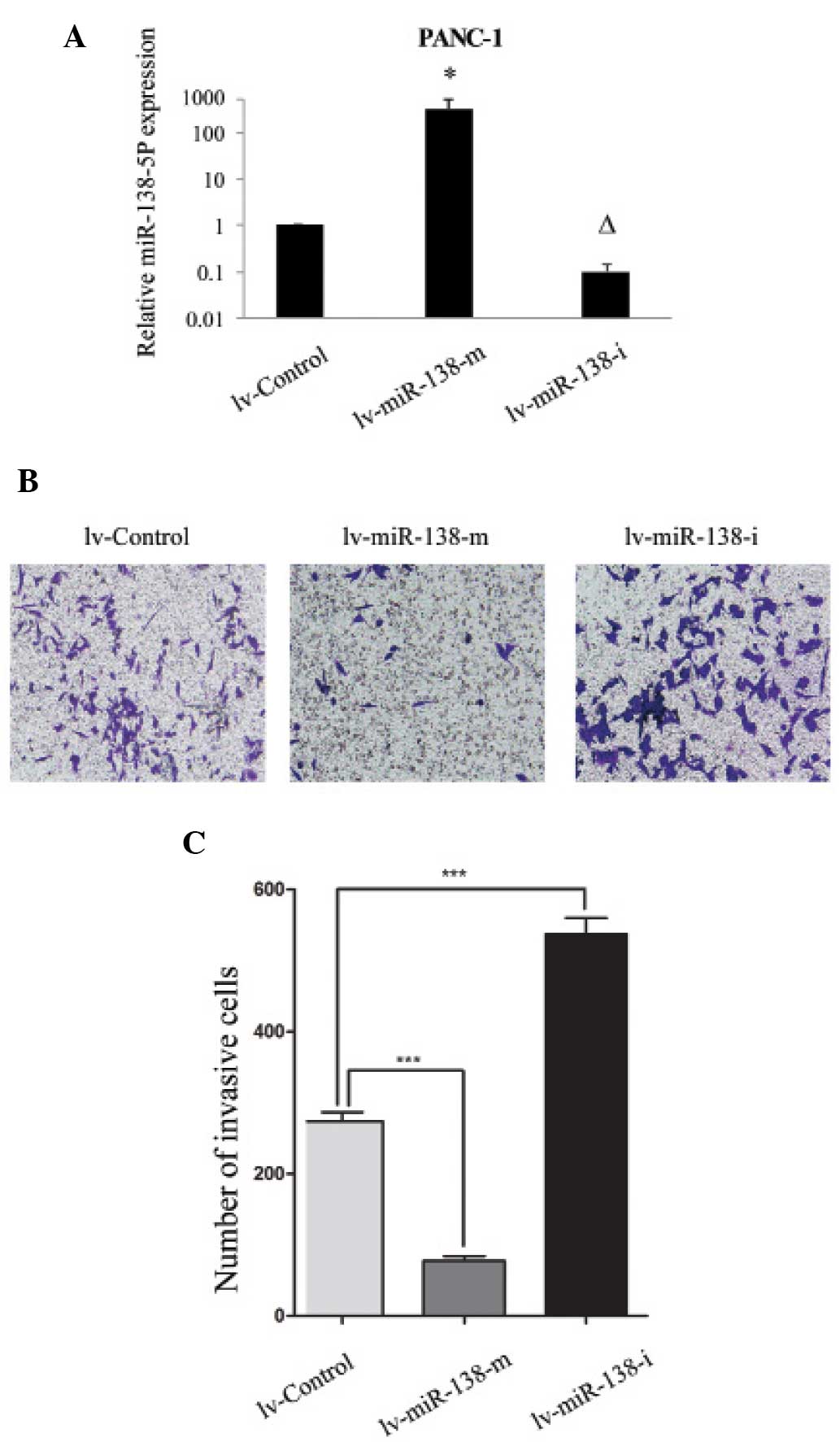
miR-138-5p in pancreatic cancer, PANC-1 cells were transfected with
lentiviral vectors of miR-138-5p mimics (lv-miR-138-m) or
miR-138-5p inhibitor (lv-miR-138-i) to genetically upregulate or
down-regulate the expression levels of miR-138-5p, respectively.
The transfection efficiency was confirmed using RT-qPCR and
demonstrated that the expression levels of miR-138-5p were
significantly upregulated by lv-miR-138-m and significantly
downregulated by lv-miR-138-i, compared with the expression levels
of miR-138-5p in PANC-1 cells transfected with non-specific control
lentivirus (lv-Control) (Fig. 2A;
P<0.05).
The effect of miR-138-5p regulation on the invasive
capability of PANC-1 cells was investigated. The PANC-1 cells were
transfected with either lv-miR-138-m or lv-miR-138-i, to upregulate
or downregulate, the endogenous expression levels of miR-138-5p,
respectively. The control cells were transfected with non-specific
lv-Control. Following lentiviral transfection for 3 days, an MTT
Transwell assay revealed that the upregulation of miR-138-5p
significantly reduced pancreatic cancer cell invasion and
downregulation significantly increased pancreatic cancer cell
invasion, in the PANC-1 cells (Fig. 2B
and C; P<0.05).
miR-138-5p regulates the chemosensitivity
of pancreatic cancer cells to 5-FU
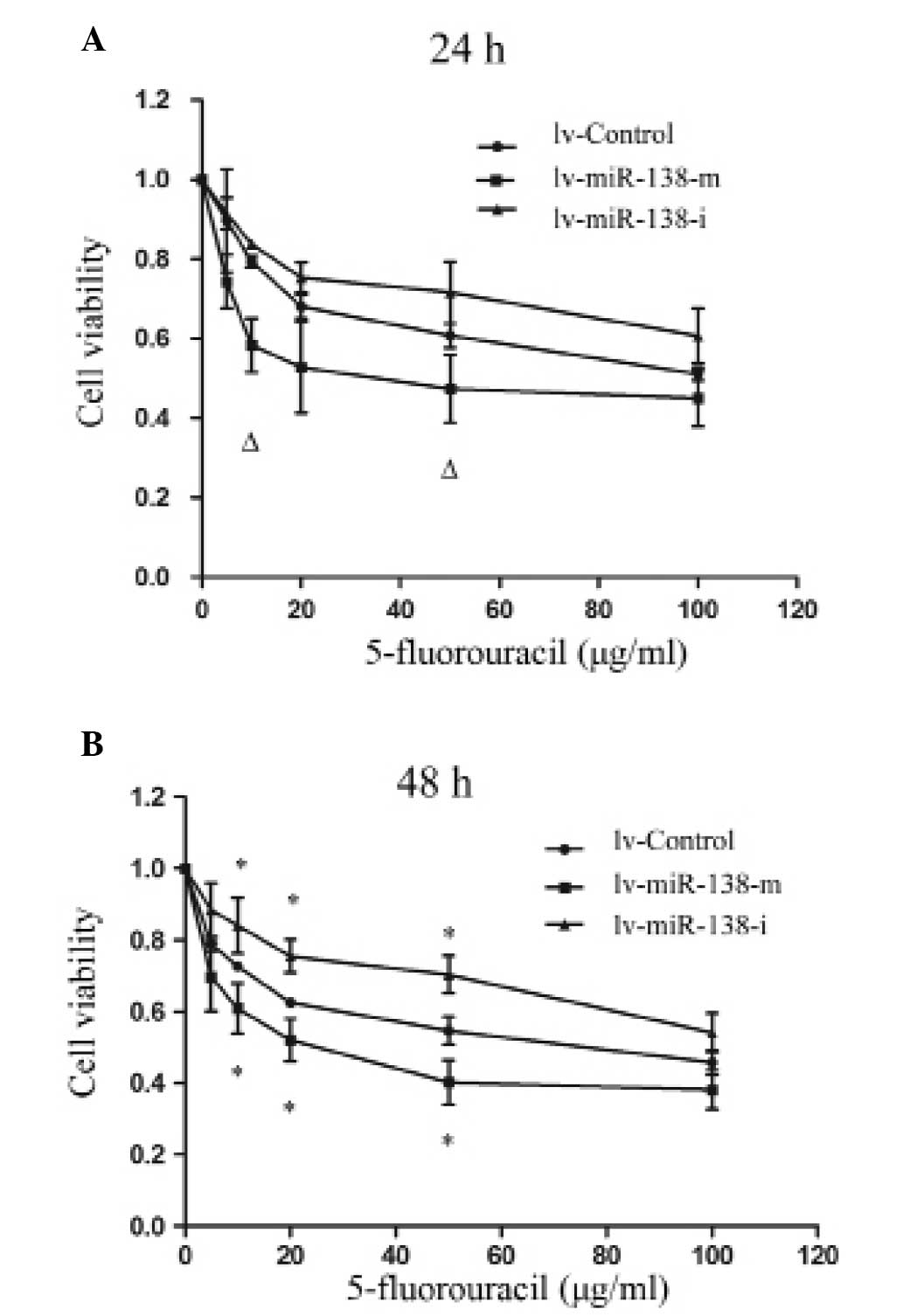
The present study next determined whether miR-138-5p
affected the chemotherapy sensitivity exhibited in pancreatic
cancer. PANC-1 cells were treated with various concentrations of
the chemotherapeutic agent, 5-FU (between 5 and 100 µg/ml),
and either upregulated or downregulated expression levels of
miR-138-5p. Following transfection for 24 h, it was revealed that
the upregulation of miR-138-5p significantly increased cancer cell
chemosensitivity to 5-FU at concentrations of 10 and 50
µg/ml (Fig. 3A; P<0.05).
After 48 h, the effect of miR-138-5p regulation was more clear. The
upregulation of miR-138-5p significantly increased the
chemosensitivity, whereas downregulation of miR-138-5p
significantly decreased the chemosensitivity to 5-FU at
concentrations of 10, 20 and 50 µg/ml (Fig. 3B; P<0.05).
VIM is directly regulated by miR-138-5p
in pancreatic cancer
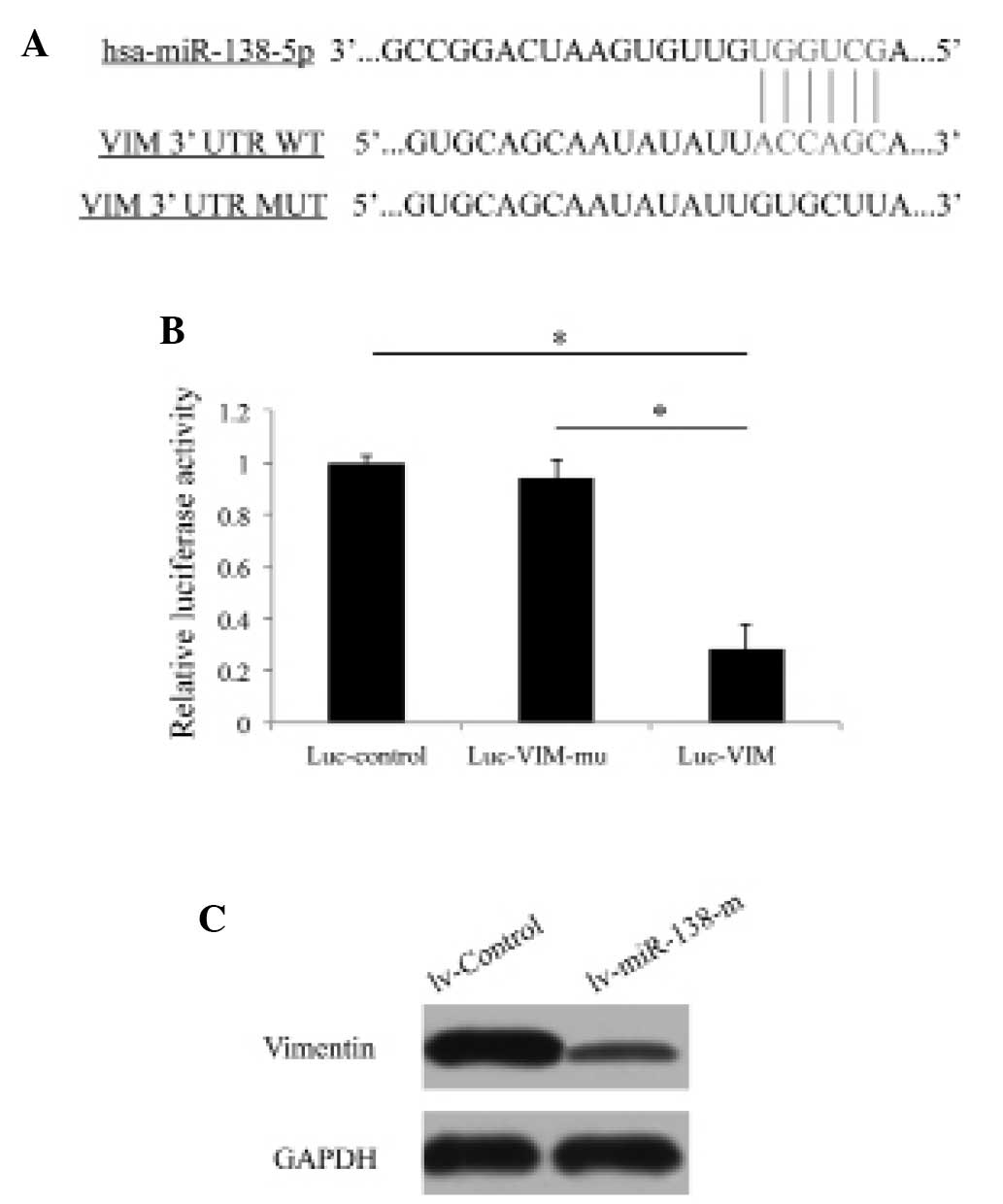
The present study next investigated the possible
molecular targets associated with the regulation of miR-138-5p in
pancreatic cancer. Using bioinformatic methods, including
TargetScan, Pictar and miRANDA, it was demonstrated that VIM was
highly likely to be the direct target of miR-138-5p (Fig. 4A). A luciferase assay was performed
to confirm that miR-138-5p was directly targeting VIM in pancreatic
cancer cells (Fig. 4B; P<0.05).
Furthermore, western blotting revealed that lentivirus-mediated
upregulation of miR-138-5p decreased the protein expression levels
of VIM in PANC-1 cells, confirming that VIM is directly regulated
by miR-138-5p in pancreatic cancer (Fig. 4C).
miR-138-5p-mediated pancreatic cancer
invasion is also regulated by VIM
Finally, it was investigated whether the molecular
target of miR-138-5p, VIM, exhibited a functional role in
regulating pancreatic cancer development. siRNA was used to
genetically knock down the expression levels of VIM in PANC-1 cells
and cancer cell invasion was subsequently examined using an MTT
migration assay. The results demonstrated that downregulation of
VIM significantly reduced the invasive capability (Fig. 5A and B; NC_siRNA, vs. VIM_siRNA).
These results also demonstrated that, following the downregulation
of miR-138-5p in pancreatic cancer cells, knock down of VIM
remained effective in reducing cancer cell invasion (Fig. 5A and B; lv-miR-138-i/NC_siRNA, vs.
lv-miR-138-i/VIM_siRNA). These results suggested that VIM may act
either independently, or coordinately through miR-138-5p mediation,
to regulate the development of pancreatic cancer.
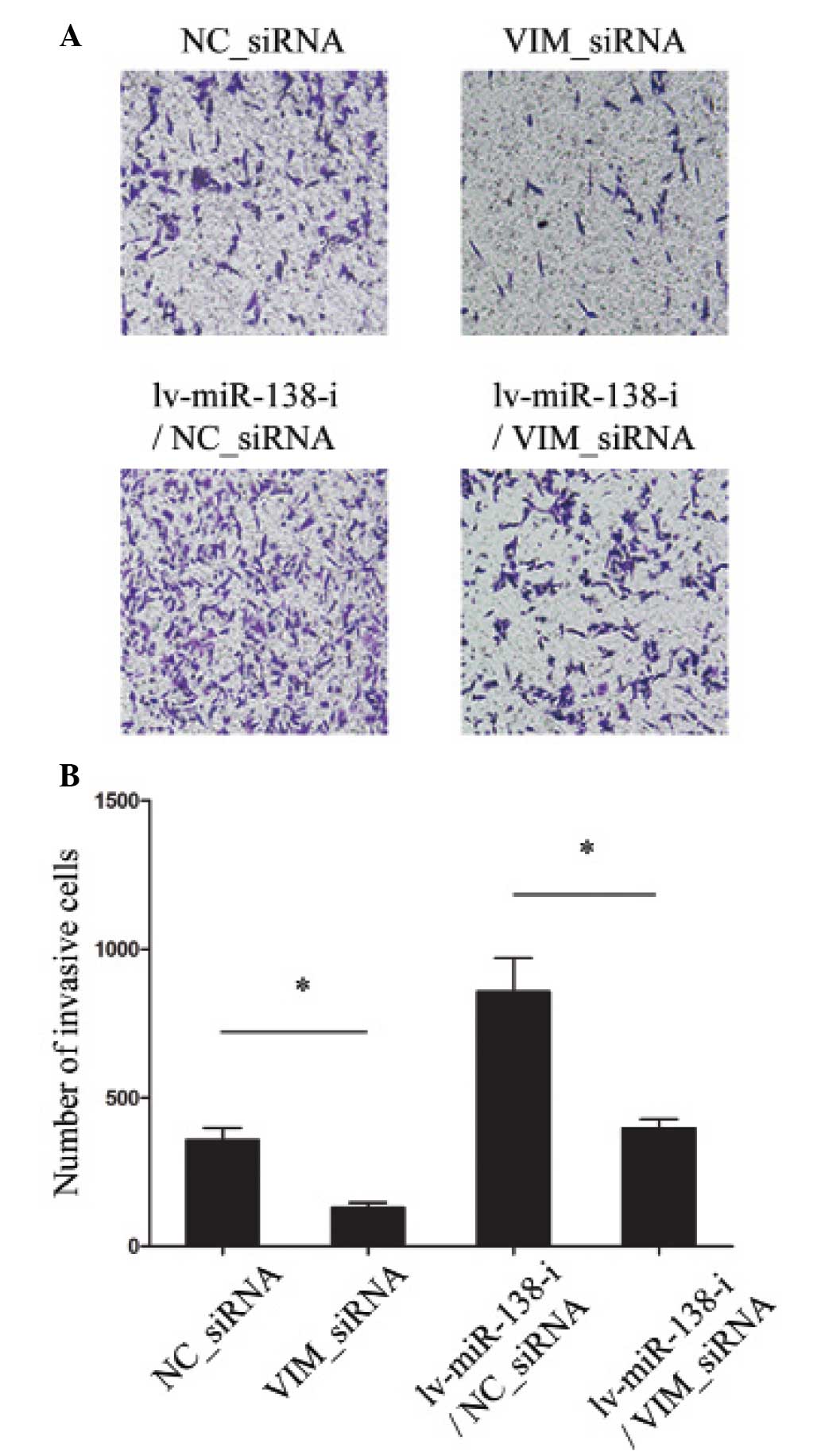 | Figure 5Knock down of VIM reduced pancreatic
cancer cell invasion. (A) PANC-1 cells were transfected with either
VIM siRNA (VIM_siRNA, 100 nM) or non-specific control siRNA
(NC_siRNA, 100 nM), and PANC-1 cells were initially transfected
with lv-miR-138-i to downregulated the expression of miR-138-3p for
24 h, followed by either VIM siRNA (VIM_siRNA, 100 nM) or
non-specific control siRNA (NC_siRNA, 100 nM) treatment. The
invasion of PANC-1 cells was examined by an MTT assay
(magnification, ×100). (B) The quantification of the numbers of
invasive cells under the four experimental conditions were also
quantified (*P<0.05). VIM, vimentin; siRNA, small
interfering RNA; miR, microRNA. |
Discussion
In the present study, it was demonstrated that
miR-138-5p was functionally involved in the development of
pancreatic cancer cells. The results demonstrated that the
expression of miR-138-5p was generally downregulated in pancreatic
cancer cell lines and primary pancreatic carcinoma tissue samples.
The subsequent functional assay revealed that overexpression of
miR-138-5p significantly inhibited cancer cell invasion, whereas
the downregulation of miR-138-5p significantly promoted invasion.
These results are consistent with previous studies demonstrating
that overexpressing miR-138-5p decreased cancer cell growth and
increased chemosensitivity in other types of cancer (16–18).
Therefore, it is likely that in pancreatic cancer, miR-138-5p
exerts a similar functional role, acting as a tumor suppressor, as
in other types of cancer, with its upregulation inhibiting and its
downregulation facilitating cancer development.
The present study also demonstrated that VIM is the
molecular target of miR-138-5p, by revealing that the expression
level of VIM was significantly downregulated upon the
overexpression of miR-138-5p in pancreatic cancer cells. It was
revealed that VIM was directly involved in the regulation of
pancreatic cancer development, by demonstrating that genetic knock
down of VIM was also able to decrease pancreatic cancer invasion.
It has been demonstrated in a previous study that VIM is commonly
overexpressed in cancer tissues and is associated with cancer
proliferation, migration or invasion (21). In pancreatic cancer, it was
demonstrated that a high expression level of FOXC1 was notably
associated with poor clinical outcomes in patients with ductal
adenocarcinoma (22–25). Therefore, the results of the
present study demonstrating that VIM was normally highly expressed
in pancreatic cancer cell lines and cancer tissues from patients,
and that downregulating VIM had an inhibitory (or
anti-proliferative) effect on pancreatic cancer cell invasion,
supported the hypothesis that VIM is likely a proliferative factor
involved in pancreatic cancer.
In conclusion, the present study identified a novel
miRNA regulator, miR-138-5p, in pancreatic cancer and demonstrated
a novel mechanism of how miR-138-5p may act as a tumor suppressor
to regulate pancreatic cancer cell development. The method of
upregulating miR-138-5p may provide a novel therapeutic approach
for patients with pancreatic cancer.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81160311) to JJX and the
Science and Technology Foundation of Guizhou Province [grant no.
Qian He J Zi (2015) 2013].
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zhang S, et al: Report of
incidence and mortality in China cancer registries, 2009. Chin J
Cancer Res. 25:10–21. 2013.PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirata K, Egawa S, Kimura Y, et al:
Current status of surgery for pancreatic cancer. Dig Surg.
24:137–147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gillen S, Schuster T, Meyer Zum
Büschenfelde C, Friess H and Kleeff J: Preoperative/neoadjuvant
therapy in pancreatic cancer: a systematic review and meta-analysis
of response and resection percentages. PLoS Med. 7:e10002672010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pillai RS: MicroRNA function: Multiple
mechanisms for a tiny RNA? RNA. 11:1753–1761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng W and Feng Y: MicroRNAs in neural
cell development and brain diseases. Sci China Life Sci.
54:1103–1112. 2011. View Article : Google Scholar
|
|
9
|
Bian S and Sun T: Functions of noncoding
RNAs in neural development and neurological diseases. Mol
Neurobiol. 44:359–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Papagiannakopoulos T and Kosik KS:
MicroRNAs: Regulators of oncogenesis and stemness. BMC Med.
6:152008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blandino G, Fazi F, Donzelli S, et al:
Tumor suppressor microRNAs: A novel non-coding alliance against
cancer. FEBS Lett. 588:2639–2652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palanichamy JK and Rao DS: miRNA
dysregulation in cancer: Towards a mechanistic understanding. Front
Genet. 5:542014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Kim S and Kim IM: Regulation of
metastasis by microRNAs in ovarian cancer. Front Oncol. 4:1432014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Othman N and Nagoor NH: The role of
microRNAs in the regulation of apoptosis in lung cancer and its
application in cancer treatment. Biomed Res Int. 2014:3180302014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han C, Yu Z, Duan Z and Kan Q: Role of
microRNA-1 in human cancer and its therapeutic potentials. Biomed
Res Int. 2014:4283712014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao Y, Fan X, Li W, Ping W, Deng Y and Fu
X: miR-138-5p reverses gefitinib resistance in non-small cell lung
cancer cells via negatively regulating G protein-coupled receptor
124. Biochem Biophys Res Commun. 446:179–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Zhao LJ, Tan YX, Ren H and Qi ZT:
MiR-138 induces cell cycle arrest by targeting cyclin D3 in
hepatocellular carcinoma. Carcinogenesis. 33:1113–1120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao X, Yang L, Hu J and Ruan J: miR-138
might reverse multidrug resistance of leukemia cells. Leuk Res.
34:1078–1082. 2010. View Article : Google Scholar
|
|
19
|
Radulovich N, Qian JY and Tsao MS: Human
pancreatic duct epithelial cell model for KRAS transformation.
Methods Enzymol. 439:1–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Mizumoto K, Sato N, et al:
Quantitative determination of apoptotic death in cultured human
pancreatic cancer cells by propidium iodide and digitonin. Cancer
Lett. 142:129–137. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Singh S, Sadacharan S, Su S, Belldegrun A,
Persad S and Singh G: Overexpression of vimentin: Role in the
invasive phenotype in an androgen-independent model of prostate
cancer. Cancer Res. 63:2306–2311. 2003.PubMed/NCBI
|
|
22
|
Wang L, Gu F, Liu CY, Wang RJ, Li J and Xu
JY: High level of FOXC1 expression is associated with poor
prognosis in pancreatic ductal adenocarcinoma. Tumour Biol.
34:853–858. 2013. View Article : Google Scholar
|
|
23
|
Li ZM, Wen YJ, Yang HB, et al: Enhanced
expression of human vimentin intermediate filaments in
hepatocellular carcinoma cells decreases their proliferative and
invasive abilities in vitro. Zhonghua Zhong Liu Za Zhi. 30:408–412.
2008.In Chinese. PubMed/NCBI
|
|
24
|
Gilles C, Polette M, Mestdagt M, et al:
Transactivation of vimentin by beta-catenin in human breast cancer
cells. Cancer Res. 63:2658–2664. 2003.PubMed/NCBI
|
|
25
|
Korsching E, Packeisen J, Liedtke C, et
al: The origin of vimentin expression in invasive breast cancer:
Epithelial-mesenchymal transition, myoepithelial histogenesis or
histogenesis from progenitor cells with bilinear differentiation
potential? J Oathol. 206:451–457. 2005. View Article : Google Scholar
|