Introduction
It is well established that spinal cord injury (SCI)
produces primary damage and triggers a prolonged period of
secondary lesion. Among the factors associated with the secondary
injury, reactive oxygen species have attracted significant
attention for their role in the pathogenesis of SCI (1). Treatment with lecithinized superoxide
dismutase, an important antioxidant enzyme, was found to markedly
recover SCI-induced motor dysfunction and ameliorate neuronal
apoptosis in rats (2). In
addition, previous studies have demonstrated that certain
traditional Chinese medicinal herbs were able to attenuate neuronal
impairment via suppressing oxidative stress in experimental SCI in
rats (3,4). These findings imply that oxidative
stress may serve as a potential therapeutic target for the
amelioration of SCI.
Nitric oxide (NO) is an important
endothelium-derived relaxing factor involved in the pathophysiology
of SCI. It is well established that NO is produced from the
guanidine group of L-arginine by three types of nitric oxide
synthase (NOS) enzymes, including endothelial NOS (eNOS), neuronal
NOS and inducible NOS (5). Among
these isoforms, eNOS is the most important sub-group and increased
eNOS activity has been demonstrated to generate significant
quantities of NO, subsequently exacerbating the damage following
SCI (5).
Carvacrol (CAR) is a natural monoterpenoid phenol
compound extracted from the essential oil of the family Lamiaceae,
which includes the genera Origanum and Thymus
(6). Substantial evidence has
demonstrated that CAR possesses diverse biological activities,
including anti-oxidative (7) and
anti-apoptotic (8) properties. Two
previous studies illustrated that CAR was able to alleviate
oxidative damage in rat models of acute myocardial infarction
(8) and streptozotocin-induced
diabetes (9). However, to the best
of our knowledge, there are no studies on the effects of CAR
against SCI in rats to date. Furthermore, as oxidative stress and
the eNOS signaling pathway are important in the amelioration of
SCI, it was hypothesized that they are involved in the
neuroprotective effects of CAR. The present study aimed to assess
the neuroprotective potential of CAR in SCI-induced rats and
examine whether this neuroprotection involves oxidative stress and
eNOS pathways.
Materials and methods
Animals
Wistar rats weighing ~220–260 g were obtained from
the Animal Centre of Beijing (Beijing, China). They were kept in a
standard environment and allowed free access to water and food.
Experimental protocols were performed in accordance with the
guidelines of the Care and Use of Laboratory Animals of the
Provincial Hospital Affiliated to Shandong University (Jinan,
China). The study was approved by the ethics committee of the
Provincial Hospital Affiliated to Shandong University (Jinan,
China).
Drugs and reagents
CAR (with a purity >98%) was purchased from
Sigma-Aldrich (St. Louis, MO, USA). Commercial kits for
malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD),
glutathione peroxidase (GSH-Px) and eNOS were obtained from Nanjing
Jiancheng Biotechnology Institute (Nanjing, China). 8-Isoprostane
EIA kit (no. 516351) was obtained from Cayman Chemical (Ann Arbor,
MI, USA). Other reagents were all of analytical grade.
Establishment of an SCI rat model and
drug administration
The rat model of SCI was prepared as described
previously with minor modifications (10). Briefly, the spinal cord was injured
at the thoracic level 10 (T10) following an established spinal cord
compression model. The skin of rats above the vertebral column was
incised and a laminectomy at vertebral level T10 was performed. The
dorsal cord surface was exposed with the dura remaining intact.
Rats were assigned to five groups: i) Sham group (Sham; n=10),
which experienced sham surgery but no trauma (physiological saline
0.1 ml/100 g, i.p.); ii) SCI group (n=10), which underwent spinal
cord injuries and received saline (physiological saline 0.1 ml/100
g, i.p.); iii) CAR (25) group; iv) CAR (50) group and v) CAR (100)
group (n=10), which all had spinal cord injuries and were treated
with CAR at doses of 25, 50 and 100 mg/kg once a day for 46
consecutive days, respectively. The dosage and dosing frequency of
CAR were referred to in a previous study (9).
Evaluation of neuronal function
recovery
The motor recovery in SCI rats was evaluated by a
locomotor rating scale between 0 (complete paralysis) and 21
(normal locomotion) developed by Basso, Beattie and Bresnahan (BBB)
(11).
Assessment of water content in spinal
cord tissues
Spinal cord edema was assessed by measuring the
water content in spinal cord tissues. Following treatment with CAR
for 46 days, the impaired spinal cords were dried at 80°C for 48 h
in order to determine the dry weight. Water content of the spinal
cords was calculated using the following formula: Spinal cord water
content (%) = (wet weight − dry weight) / wet weight × 100%.
Measurement of MDA level and the activity
of CAT, SOD and GSH-Px
The oxidative markers, including MDA level and the
activities of CAT, SOD and GSH-Px in spinal cord tissues were
detected using corresponding commercial kits (Nanjing Jiancheng
Bioengineering Institute). Detection of plasma 8-isoprostane levels
was performed using the 8-Isoprostane EIA kit.
Immunoblotting
Following treatment with CAR for 46 consecutive
days, the injured spinal cord tissues were homogenized in ice-cold
lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 10% glycerol, 1% Nonidet
P-40, 5 mM EDTA and 1 mM phenyl-methylsulfonyl fluoride).
Supernatant was collected following centrifugation at 12,000 ×g for
20 min and protein quantification was conducted using a BCA kit
(Beyotime Institute of Biotechnology, Shanghai, China). Protein (60
µg) was separated by electrophoresis on 8 or 10%
SDS-polyacrylamide gels and transferred onto nitrocellulose
membranes (Millipore, Billerica, MA, USA). The membranes were
probed with the following primary antibodies: Rabbit anti-eNOS
(SAB1305369; 1:1,000; Sigma-Aldrich), rabbit anti-Bcl-2-associated
X protein (Bax; sc-101874; 1:200; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), rabbit anti-B cell lymphoma-2 (Bcl-2; sc-492;
1:200; Santa Cruz Biotechnology, Inc.) and mouse anti-GAPDH
(KC-5G4; 1:2,000; Zhejiang Kangchen Biotech Co., Ltd., Hangzhou,
China), overnight at 4°C. Following washing with phosphate-buffered
saline, they were then incubated with horseradish
peroxidase-conjugated goat anti-rabbit antibody (sc-45101; 1:5,000;
Santa Cruz Biotechnology, Inc.) or goat anti-mouse antibody
(sc-2075; 1:5,000; Santa Cruz Biotechnology, Inc.) for 2 h.
Immunodetection was conducted with an enhanced chemiluminescence
kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Measurement of eNOS activity in spinal
cords and plasma NO production
The eNOS activity was measured according to the
manufacturer's instructions of the Nitric Oxide Synthase Assay kit
(no. S0025; Beyotime Institute of Biotechnology). Additionally, NO
production in the plasma was analyzed by measuring the supernatant
for nitrite using Griess reagent (Promega Corp., Madison, WI,
USA).
Measurement of caspase-3 activity in
spinal cord tissues
As a critical molecule in cellular apoptosis,
caspase-3 activity was measured by cleavage of chromogenic caspase
substrates, Ac-DEVD-pNA. Colorimetric analysis of the quantity of
caspase-3 was performed using a spectrophotometer (Alpha-1860S;
Shanghai Puyuan Company, Shanghai, China) at a wavelength of 405
nm.
Statistical analysis
All values are presented as the mean ± standard
deviation and were analyzed using SPSS 16.0 software (SPSS, Inc.,
Chicago, IL, USA). Statistical analysis was performed using one-way
analysis of variance (ANOVA) followed by Dunnett's test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Evaluation of neural function
The chemical structure of CAR is shown in Fig. 1. It was noted that BBB scores in
the sham group were 14.21±1.12, 18.11±1.27 and 19.88±1.44 at 24, 48
and 72 h post-surgery, respectively, as summarized in Table I. By contrast, SCI-induced rats
demonstrated severe neurological impairment with marked reductions
in BBB scores (4.98±0.87, 4.18±0.82 and 4.05±0.63; P<0.01) at
the selected time points. However, CAR at doses of 25, 50 and 100
mg/kg significantly improved neurological function (P<0.01) in
injured animals, compared with the SCI model group, particularly at
72 h post-surgery. Thus, this time point was selected for
subsequent investigations.
 | Table IEffects of CAR on the motor function
of rats 24, 48 and 72 h after SCI. |
Table I
Effects of CAR on the motor function
of rats 24, 48 and 72 h after SCI.
| Group | n | 24 h | 48 h | 72 h |
|---|
| Sham | 10 | 14.21±1.12 | 18.11±1.27 | 19.88±1.47 |
| SCI | 10 | 4.98±0.87a | 4.18±0.82a | 4.05±0.63a |
| CAR (25 mg/kg) | 10 | 9.78±0.89b | 10.12±0.97b | 11.25±0.76b |
| CAR (50 mg/kg) | 10 | 11.63±0.65b | 11.89±0.75b | 12.04±0.95b |
| CAR (100 mg/kg) | 10 | 12.85±0.96b | 14.15±0.88b | 16.21±1.06b |
Assessment of water content in spinal
cord tissues of SCI-induced rats following CAR treatment
As shown in Fig. 2,
there was a marked elevation in water content of the spinal cord
(P<0.01) in the SCI group compared with the sham group.
Following treating SCI-induced rats with CAR, the water content in
spinal cord tissues was significantly decreased in a dose-dependent
manner (P<0.01) compared with that in the control group.
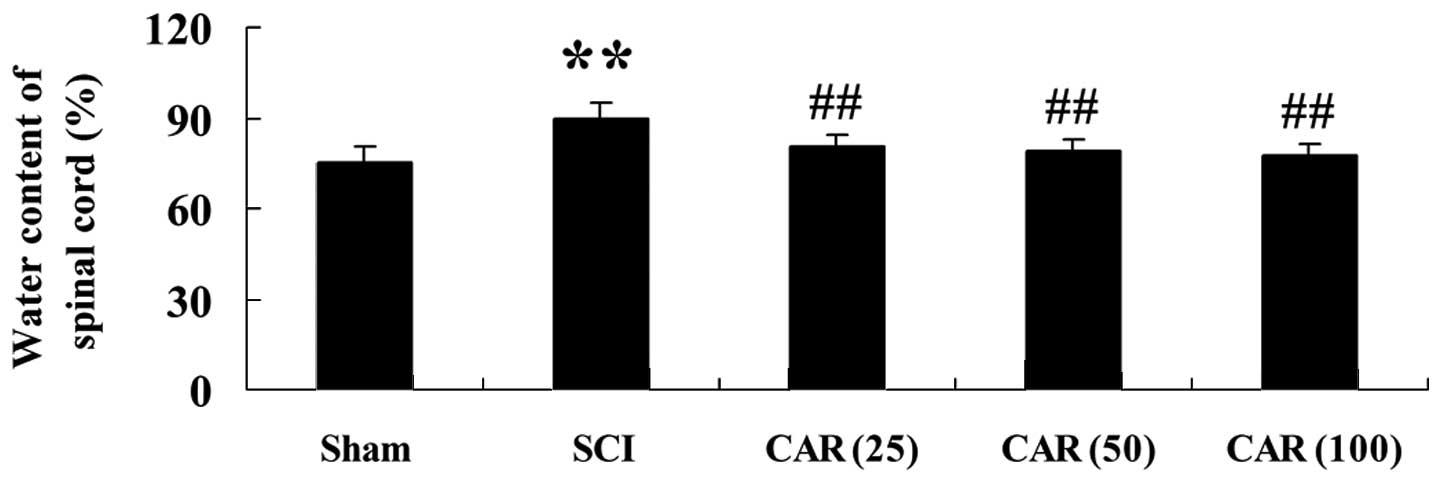 | Figure 2Effects of CAR on the water content of
the spinal cord following SCI (n=10, mean ± standard deviation).
**P<0.01, compared with the sham group;
##P<0.01, compared with the SCI group. Sham, sham
group; SCI, spinal cord injury group; CAR (25), carvacrol (25
mg/kg)-treated group; CAR (50), carvacrol (50 mg/kg)-treated group
and CAR (100), carvacrol (100 mg/kg)-treated group. CAR, carvacrol;
SCI, spinal cord injury. |
Effects of CAR on oxidative stress in
SCI-induced rats
At 72 h post-surgery, it was observed that MDA
levels were markedly increased and the antioxidant enzymes,
including CAT, SOD and GSH-Px were all decreased in spinal cord
tissues (P<0.01), compared with the sham control (Fig. 3A–D). The alterations in MDA
content, CAT, SOD and GSH-Px activities were all significantly
reversed following administration of CAR (25, 50 and 100 mg/kg). In
order to confirm the inhibitory effect of CAR on oxidative damage
in SCI-subjected rats, another marker reflecting the oxidative
stress, namely, the plasma 8-isoprotane level, was measured in the
present study. Fig. 4 shows an
evident increase in 8-isoprotane content in the SCI group and
administration of CAR dose dependently reversed this
phenomenon.
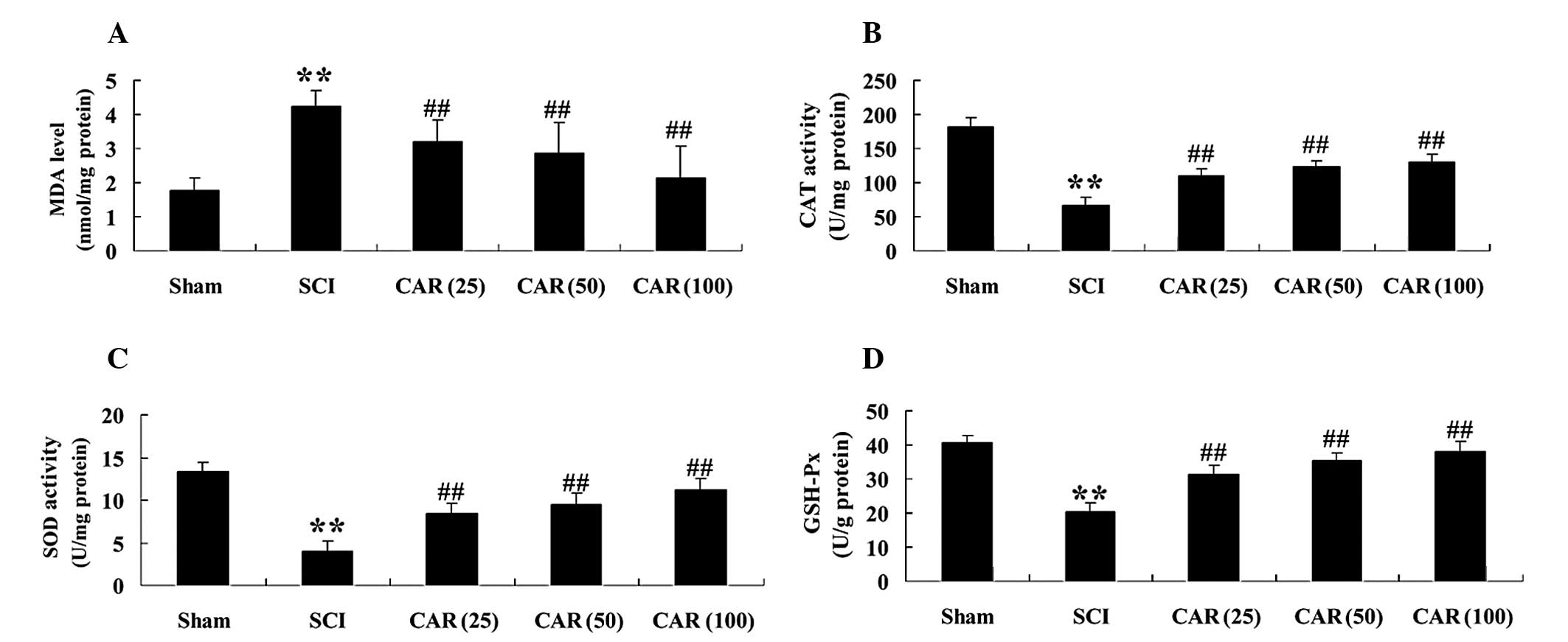 | Figure 3Effects of CAR on the concentration of
(A) MDA and on the activities of antioxidant enzymes (B) CAT, (C)
SOD and (D) GSH-Px in spinal cord tissues of rats from different
groups (n=10, mean ± standard deviation). **P<0.01,
compared with the sham group; ##P<0.01, compared with
the SCI group. Sham, sham group; SCI, spinal cord injury group; CAR
(25), carvacrol (25 mg/kg)-treated group; CAR (50), carvacrol (50
mg/kg)-treated group and CAR (100), carvacrol (100 mg/kg)-treated
group. CAR, carvacrol; SCI, spinal cord injury; SOD, superoxide
dismutase; GSH-Px, glutathione peroxidase; CAT, catalase; MDA,
malondialdehyde. |
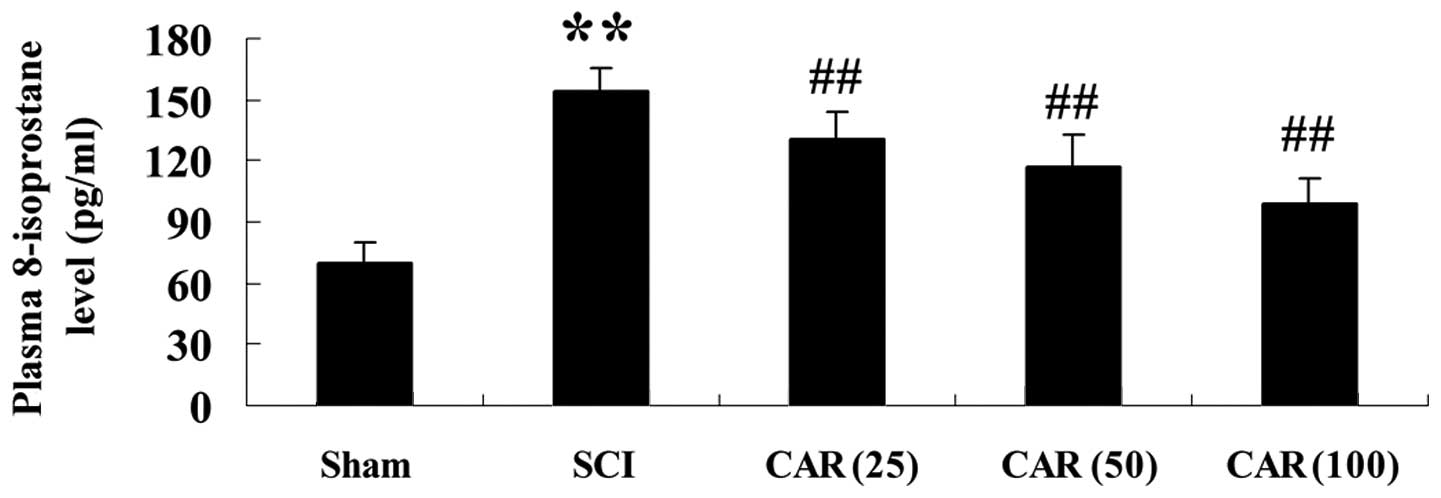 | Figure 4Effects of CAR on the plasma
8-isoprostane level following SCI (n=10, mean ± standard
deviation). **P<0.01, compared with the sham group;
##P<0.01, compared with the SCI group. Sham, sham
group; SCI, spinal cord injury group; CAR (25), carvacrol (25
mg/kg)-treated group; CAR (50), carvacrol (50 mg/kg)-treated group
and CAR (100), carvacrol (100 mg/kg)-treated group. CAR, carvacrol;
SCI, spinal cord injury. |
Effects of CAR on the protein expression
and activity of eNOS as well as plasma NO concentration following
SCI
The present study further examined whether CAR
exerted a protective effect through mediating the eNOS pathway.
Fig. 5A revealed that western blot
analysis with eNOS antibody exhibited an anticipated band of 133
kDa. Quantitative analysis demonstrated that there was a
significant elevation in the protein level of eNOS (P<0.01) in
injured rats, versus the control group. However, markedly reduced
eNOS protein expression was observed following CAR treatment in
SCI-induced animals in a dose-dependent manner (P<0.01; Fig. 5B). Similar results were observed in
eNOS activity (Fig. 5C).
Additionally, NO production was also assessed in the present study.
NO production, which was indicated as nitrite formation, was found
to be significantly increased following SCI (P<0.01). CAR
treatment markedly reduced the nitrite level (P<0.01) in a
dose-dependent manner, as shown in Fig. 5D.
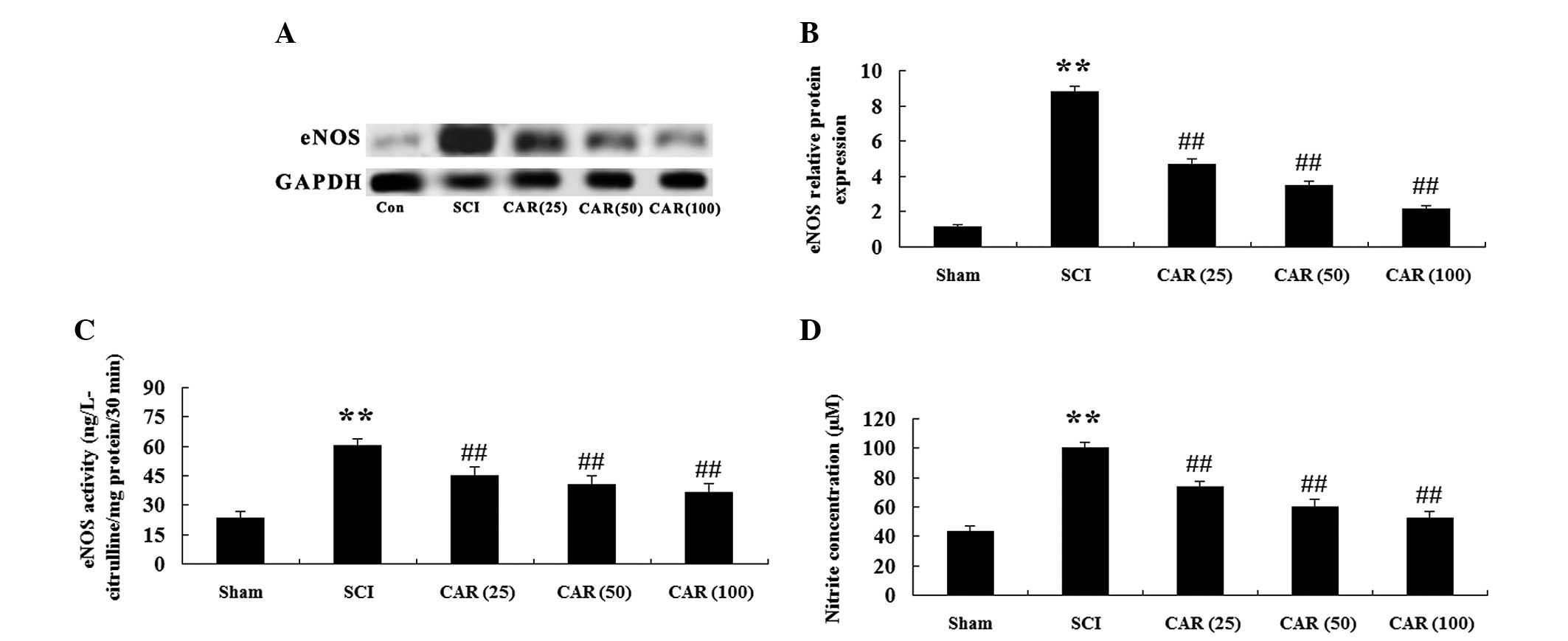 | Figure 5Effects of CAR on the protein level
and activity of eNOS as well as plasma NO concentration following
SCI (n=10, mean ± standard deviation). (A) Representative images of
immunoblots with antibodies against eNOS in injured spinal cords
from different groups. eNOS: 133 kDa; GAPDH: 36 kDa. (B)
Quantitative analysis of the protein level of eNOS in spinal cords
from different groups. The data were normalized to the loading
control GAPDH. (C) Measurement of eNOS activity. (D) NO production
was detected spectrophotometrically by measuring its metabolite,
nitrite. **P<0.01, compared with the sham group;
##P<0.01, compared with the SCI group. Sham, sham
group; SCI, spinal cord injury group; CAR (25), carvacrol (25
mg/kg)-treated group; CAR (50), carvacrol (50 mg/kg)-treated group
and CAR (100), carvacrol (100 mg/kg)-treated group. CAR, carvacrol;
SCI, spinal cord injury; eNOS, endothelial nitric oxide synthase;
NO, nitric oxide. |
Effects of CAR on cellular apoptosis
following SCI
In order to determine the effect of CAR on cellular
apoptosis following SCI, the protein expression of
apoptosis-regulated proteins, including Bcl-2 and Bax was detected
by western blot analysis. As shown in Fig. 6A, Bcl-2 and Bax exhibited specific
bands of 26 and 23 kDa, respectively. Following one-way ANOVA
analysis, there was evident decreases in Bcl-2 expression and
increases in Bax expression following SCI (P<0.01), versus the
control. CAR treatment to the SCI-induced rats increased the
expression of Bcl-2 and decreased Bax at the protein level in a
dose-dependent manner (Fig. 6B and
C). The activity of caspase-3, an executive molecule in the
apoptotic cascade, was found to be increased in the SCI group
(P<0.01) and CAR significantly suppressed this increase, as
shown in Fig. 7.
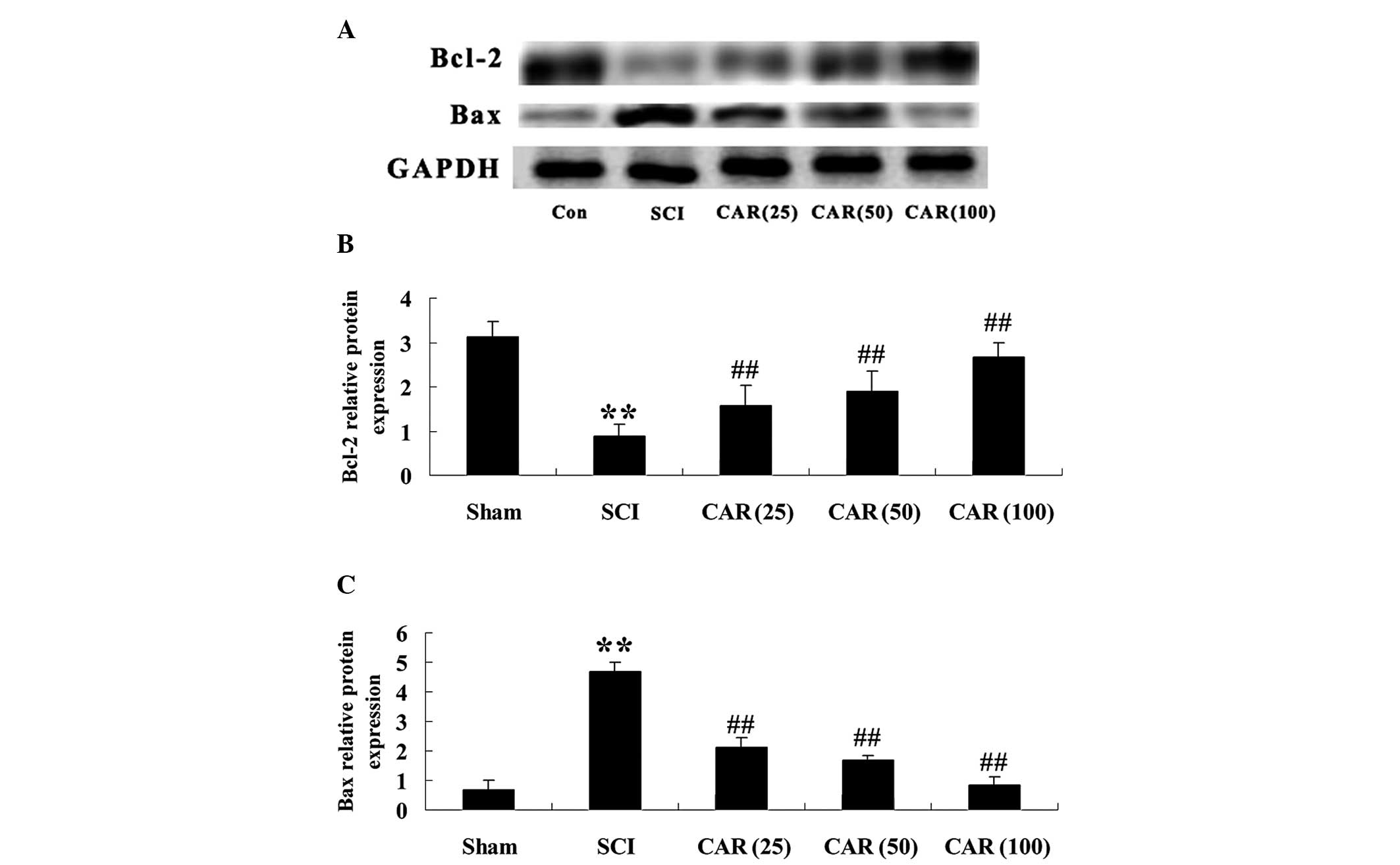 | Figure 6Effects of CAR on the protein
expression of Bcl-2 and Bax following SCI (n=10, mean ± standard
deviation). (A) Representative images of immunoblots with
antibodies against Bcl-2 and Bax in injured spinal cords from
different groups. Bcl-2: 26 kDa; Bax: 23 kDa; GAPDH: 36 kDa. (B and
C) Quantitative analysis of the protein levels of Bcl-2 and Bax,
respectively in spinal cords from different groups. The data were
normalized to the loading control GAPDH. **P<0.01,
compared with the sham group; ##P<0.01 compared with
the SCI group. Sham, sham group; SCI, spinal cord injury group; CAR
(25), carvacrol (25 mg/kg)-treated group; CAR (50), carvacrol (50
mg/kg)-treated group and CAR (100), carvacrol (100 mg/kg)-treated
group. SCI, spinal cord injury; CAR, carvacrol; Bcl-2, B cell
lymphoma-2; Bax, Bcl-2-associated X protein. |
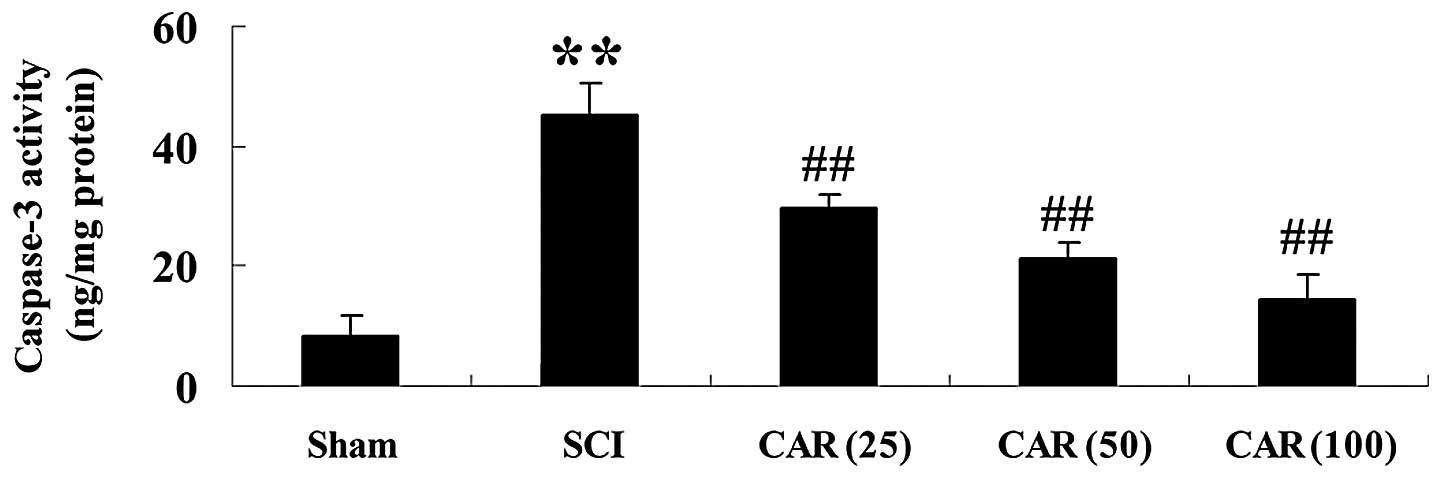 | Figure 7Effects of CAR on caspase-3 activity
following SCI (n=10, mean ± standard deviation).
**P<0.01, compared with the sham group;
##P<0.01, compared with the SCI group. Sham, sham
group; SCI, spinal cord injury group; CAR (25), carvacrol (25
mg/kg)-treated group; CAR (50), carvacrol (50 mg/kg)-treated group
and CAR (100), carvacrol (100 mg/kg)-treated group. SCI, spinal
cord injury; CAR, carvacrol. |
Discussion
The major findings of the present study illustrated
that CAR facilitated the recovery of motor function in SCI-induced
rats and that its neuroprotection may be associated with
suppressing oxidative stress and the eNOS signaling pathway.
It has been demonstrated that SCI-induced primary
damage is irreversible and appears to not be amenable to
neuroprotective therapy. However, the secondary impairment occurs
in response to the deleterious substances produced following
primary trauma (12). Among these
substances, oxidative stress is regarded as an important factor
during traumatic SCI. Evidence supported that marked generation of
reactive oxygen species was observed in rats following SCI and
treatment with antioxidant compounds attenuated edema formation and
cellular apoptosis (13). It was
previously reported that CAR protected against acute myocardial
infarction and diabetes-associated cognitive deficits in rats via
antioxidative mechanisms (8,9). In
addition, results from the current study demonstrated that CAR
markedly decreased the concentration of MDA, an important and
reliable index for determining the extent of the peroxidation
reaction (14), and increased the
activities of antioxidant enzymes, including CAT, SOD and GSH-Px as
well as reduced the levels of the oxidative marker, 8-isoprotane,
following SCI in rats. This implies that the protective role of CAR
against SCI may be associated with the inhibition of free radical
and oxidant formation.
NO is considered to be one of the major regulators
of spinal damage. A previous study revealed that eNOS-derived NO
production aggravated the impairment caused by SCI in rats
(5). Additionally, a previous
study reported the attenuation of injury in rats exposed to
traumatic SCI following treatment with rosuvastatin via reducing NO
production (15). The present
study revealed that the protein expression level and the activity
of eNOS together with NO concentration were all elevated in the SCI
group. In addition, CAR treatment significantly decreased eNOS
levels and correspondingly suppressed the elevated quantity of NO
in rats with SCI. CAR was previously found to have NO-scavenging
activity (7). Taken together,
these findings supported that CAR protects the spinal cord from
injury via suppressing eNOS and concomitantly decreasing NO
bioavailability.
The secondary lesion caused by SCI can damage spinal
neurons and trigger apoptotic cascades. The pharmacological
inhibition of apoptosis may function as a potential therapeutic
strategy. Targeted retrograde gene delivery of brain-derived
neurotrophic factor was reported to inhibit cellular apoptosis and
restore neurological function following SCI (16). Bcl-2 family proteins have been
demonstrated to be important in the modulation of cellular
apoptosis. Under normal circumstances, Bcl-2 itself serves as an
anti-apoptotic protein, whereas another member of the family, Bax,
acts as a pro-apoptotic molecule (17). The results of the present study
demonstrated that the evident reduction of Bcl-2 and increased Bax
protein levels were observed in the spinal cord tissues of SCI.
However, treatment with CAR dose dependently caused elevated levels
of Bcl-2 and reduced Bax protein in SCI-induced rats. In addition,
the activity of caspase-3, an executioner molecule in the apoptotic
signaling pathway, was found to be markedly elevated in rats
following SCI and CAR significantly inhibited this index.
Collectively, these findings indicated that the neuroprotective
action of CAR may involve the regulation of Bcl-2/Bax and caspase-3
pathways in the spinal cord following SCI.
Taking these results into account, it was concluded
that CAR protected the rat spinal cord from injury. The
neuroprotective effect of CAR may be associated with suppressing
oxidative stress and inhibiting the eNOS signaling pathway
following SCI in rats.
References
|
1
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: the in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takenaga M, Ohta Y, Tokura Y, et al:
Lecithinized superoxide dismutase (PC-SOD) improved spinal cord
injury-induced motor dysfunction through suppression of oxidative
stress and enhancement of neurotrophic factor production. J Control
Release. 110:283–289. 2006. View Article : Google Scholar
|
|
3
|
Fu J, Fan HB, Guo Z, et al: Salvianolic
acid B attenuates spinal cord ischemia-reperfusion-induced neuronal
injury and oxidative stress by activating the extracellular
signal-regulated kinase pathway in rats. J Surg Res. 188:222–230.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou YF, Li L, Feng F, et al: Osthole
attenuates spinal cord ischemia-reperfusion injury through
mitochondrial biogenesis-independent inhibition of mitochondrial
dysfunction in rats. J Surg Res. 185:805–814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Diaz-Ruiz A, Vergara P, Perez-Severiano F,
et al: Cyclosporin-A inhibits constitutive nitric oxide synthase
activity and neuronal and endothelial nitric oxide synthase
expressions after spinal cord injury in rats. Neurochem Res.
30:245–251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guimarães AG, Xavier MA, de Santana MT, et
al: Carvacrol attenuates mechanical hypernociception and
inflammatory response. Naunyn Schmiedebergs Arch Pharmacol.
385:253–263. 2012. View Article : Google Scholar
|
|
7
|
Guimarães AG, Oliveira GF, Melo MS, et al:
Bioassay-guided evaluation of antioxidant and antinociceptive
activities of carvacrol. Basic Clin Pharmacol Toxicol. 107:949–957.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu W, Liu Q and Zhu S: Carvacrol protects
against acute myocardial infarction of rats via anti-oxidative and
anti-apoptotic pathways. Biol Pharm Bull. 36:579–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng W, Lu H and Teng J: Carvacrol
attenuates diabetes-associated cognitive deficits in rats. J Mol
Neurosci. 51:813–819. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ravikumar R, Fugaccia I, Scheff SW, Geddes
JW, Srinivasan C and Toborek M: Nicotine attenuates morphological
deficits in a contusion model of spinal cord injury. J Neurotrauma.
22:240–251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Basso DM, Beattie MS, Bresnahan JC, et al:
MASCIS evaluation of open field locomotor scores: effects of
experience and teamwork on reliability. Multicenter Animal Spinal
Cord Injury Study. J Neurotrauma. 13:343–359. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Springer JE, Azbill RD and Knapp PE:
Activation of the caspase-3 apoptotic cascade in traumatic spinal
cord injury. Nat Med. 5:943–946. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharma HS, Gordh T, Wiklund L, Mohanty S
and Sjöquist PO: Spinal cord injury induced heat shock protein
expression is reduced by an antioxidant compound H-290/51. An
experimental study using light and electron microscopy in the rat.
J Neural Transm. 113:521–536. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harman D: Free radical theory of aging: an
update: increasing the functional life span. Ann NY Acad Sci.
1067:10–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Die J, Wang K, Fan L, Jiang Y and Shi Z:
Rosuvastatin preconditioning provides neuroprotection against
spinal cord ischemia in rats through modulating nitric oxide
synthase expressions. Brain Res. 1346:251–261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakajima H, Uchida K, Yayama T, et al:
Targeted retrograde gene delivery of brain-derived neurotrophic
factor suppresses apoptosis of neurons and oligodendroglia after
spinal cord injury in rats. Spine (Phila Pa 1976). 35:497–504.
2010. View Article : Google Scholar
|
|
17
|
Shacka JJ and Roth KA: Regulation of
neuronal cell death and neurodegeneration by members of the Bcl-2
family: therapeutic implications. Curr Drug Targets CNS Neurol
Disord. 4:25–39. 2005. View Article : Google Scholar : PubMed/NCBI
|