Introduction
HL-60 cells, belonging to human leukemia cells, have
been widely used for differentiation investigations. Previous
studies have revealed that HL-60 cells can be induced to
differentiate into granulocytes, monocytes and macrophages by
treating the cells with various agents, including retinoic acid
(RA), dimethyl sulphoxide (DMSO), vitamin D and 12-O-tetradecanoyl
phorbol-13-acetate (TPA) (1,2).
Several previous studies have indicated that the mitogen-activated
protein kinase (MAPK) signaling pathways, c-Jun N-terminal kinase
(JNK), p38 and extracellular signal-regulated kinase (ERK), are
important for HL-60 cell differentiation (3–7). RA
and DMSO can induce HL-60 cells to differentiate into granulocytes
via the ERK phosphorylation signaling pathway (8–10),
vitamin D can induce HL-60 cells to differentiate into monocytes
via the EKR, JNK and p38 phosphorylation signaling pathways
(4,11,12)
and TPA can induce HL-60 cells to differentiate into macrophages
via the ERK phosphorylation signaling pathway (13,14).
In addition, previous studies have demonstrated that ERK5 is
associated with vitamin D-differentiated HL-60 cells, while ERK/1/2
is associated with TPA-differentiated and RA-differentiated HL-60
cells (14–17). These previous reports indicated
that ERK5 phosphorylation is required to differentiate HL-60 cells
into monocytes, while ERK1/2 is required to differentiate HL-60
cells into granulocytes and macrophages.
The MAPK signaling pathways, protein kinase C (PKC)
and oxidative stress may also be associated with HL-60
differentiation (18–21). The activation of PKC is observed in
RA-, vitamin D- and TPA-differentiated HL-60 cells (13,14,22,23).
However, oxidative stress can affect HL-60 cell differentiation. A
previous study revealed that antioxidants, catalase, superoxide
dismutase and N-acetyl cysteine increase the differentiation rate
of vitamin D-treated HL-60 cells (24). However, compared with vitamin
D-treated HL-60 cells, antioxidant inhibits cell differentiation in
TPA-treated HL-60 cells (25).
Therefore, oxidative stress exerts a dual role to promote vitamin
D-differentiated cells and to inhibit TPA-differentiated cells.
It is well known that RA and vitamin D can induce
HL-60 cells to differentiate into granulocytes and monocytes,
respectively. As with RA and vitamin D, ascorbic acid is also a
type of vitamin. Previous studies have demonstrated that ascorbic
acid can activate the ERK signaling pathway to induce progenitor
cell differentiation (26,27). Additionally, several studies have
demonstrated that high-doses of ascorbic acid (>100 μM)
can activate a caspase cascade to promote radiation-induced and
etoposide-induced apoptosis in HL-60 cells (28,29).
A previous study also demonstrated that high-doses of ascorbic can
induce HL-60 cell apoptosis and induce a fraction of HL-60 cells to
express the granulocyte marker, CD 66b (30). By contrast, low-doses of ascorbic
acid decreases levels of cellular H2O2 and
protects HL-60 cells against X ray- and
As2O3-induced apoptosis (31–34).
However, whether ascorbic acid affects TPA-differentiated HL-60
cells remains to be elucidated.
A previous study demonstrated that
H2O2 may be a secondary messenger associated
with cell differentiation (25).
Several studies have demonstrated that ascorbic acid exerts
anti-oxidative stress functions (35–38)
and a previous study demonstrated that ascorbic acid decreases
levels of cellular H2O2 (39). Previous studies have also reported
that the ERK pathway is required for TPA-differentiated HL-60 cells
(13,14).
Therefore, the aim of the present study was to
determine the cellular effects of treatment with ascorbic acid on
TPA-differentiated HL-60 cells.
Materials and methods
Chemicals
An MTT assay kit was purchased from Bio Basic Inc.
(Markham, ON, Canada). TPA, ascorbic acid and luminol were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Ac-DEVD-pNA, a
Caspase-3-like substrate, Ac-IETD-pNA, a caspase-8 substrate, and
Ac-LEHD-pNA, a caspase-9 substrate, were purchased from Anaspec
(San Jose, CA, USA). Fetal bovine serum, RPMI-1640 media,
non-essential amino acid, L-glutamine and penicillin/streptomycin
were purchased from Gibco Life Technologies (Carlsbad, CA,
USA).
Cell line and cell culture
The HL-60 cells were purchased from Bioresources
Collection and Research Center (Hsin Chu, Taiwan) and were cultured
in Dulbecco's modified Eagle's media, containing 10% fetal bovine
serum, 2 mM L-glutamine, 100 IU/ml penicillin/streptomycin and 0.1
mM non-essential amino acids. The cells were maintained in a
humidified atmosphere containing 5% CO2 at 37°C.
Cell survival rate assay
A total of 3,000 cells were cultured in each well of
a 96-well culture dish. The survival rates of the cells in the
control group (non-ascorbic acid treated-cells) and the
experimental groups (5 μM and 5 mM ascorbic acid-treated
cells) were determined for 96 h at 37°C. Every 24 h, the cells were
treated using an MTT assay kit, according to the manufacturer's
instructions. Following incubation for 3 h, the absorbance (570 nm)
was measured using a multi-well enzyme-linked immunosorbent assay
reader (SpectraMax Paradigm Multi-Mode Microplate Reader; Molecular
Devices, Sunnyvale, CA, USA). The cell survival rate was calculated
using the following formula: Absorbanceexperimental
group/Absorbancecontrol group × 100%.
Caspase activity assay
Caspase activities in the cells were determined
using a substrate cleavage assay, as previous described (40,41).
Briefly, the cells were treated with lysis buffer, containing 50 mM
Tris-HCl, 120 mM NaCl, 1 mM EDTA, 1% NP-40 (pH 7.5) and protease
inhibitors. The cell pellets were collected by centrifugation at
15,000 × g for 30 min at 4°C and the quantity of protein was
determined using a Bradford assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Subsequently, 40 μl of the cell lysates
(80 μg total protein) were prepared in 158 μl
reaction buffer, containing 20% glycerol, 0.5 mM EDTA, 5 mM
dithiothreitol, 100 mM HEPES (pH 7.5) and 2 μl fluorogenic
caspase substrate (Ac-LEHD-pNA, Ac-DEVD-pNA or Ac-IETD-pNA). The
total solution was incubated at 37°C in the dark. Following
incubation for 6 h, fluorogenic substrate cleavage was determined
at 405 nm using a FLx800™ fluorescence microplate reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA). The fold increase of caspase
activity was calculated with the following formula:
(Absorbanceexperimental group − Absorbancecontrol
group) / Absorbancecontrol group.
Measurement of
H2O2
The levels of H2O2 in the
cells was determined using a lucigenin-amplified method, as
described previously (39,42,43).
Briefly, the sample (200 μl, containing 104
cells) was added to 0.2 mmol/l luminol solution (100 ml). The
mixture was then analized using a chemiluminescence analyzing
system (CLA-FSI; Tohoku Electronic Industrial Co., Ltd., Sendal,
Japan).
Observation of cell morphology and
suspension cell counts
Undifferentiated HL-60 cells grow as suspension
cells and TPA-differentiated HL-60 cells (macrophages) grow as
attached cells (14,44). The morphologies of the suspension
cells and attached cells were observed under a phase-contrast
microscope (Olympus CK40, Olypmus Corporation, Tokyo, Japan;
magnification, ×200). The suspended cells located in the media were
collected using a pipette, whereas the attached cells remained in
the bottom of the culture dish. The cells in suspension were
counted using a trypan blue exclusion assay (0.4% in PBS), as
described previously (45).
Briefly, the media containing the suspended cells were mixed with
trypan blue and placed in a CBC Customized Logo Hemocytometer Blood
Counting Chamber (VIC Science, Xixiang City, China). The number of
cells were then counted under a light microscope (Olympus, CK40;
Olympus Corporation).
Western blotting
The cells were treated with lysis buffer and
centrifuged at 16,000 × g for 10 min at 4°C. The proteins were
located in the supernatant layer and were collected, concentrated
and determined using a Bradford assay (Bio-Rad Laboratories, Inc.).
Equal quantities of protein were separated on a 13.3%
SDS-polyacrylamide gel (GHE320 Mini-STD Vertical Gel
Electrophoresis Tank; Genepure Technology, Co., Ltd, Taichung,
Taiwan). Following separation, the proteins were transferred onto a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). The membranes were placed in phosphate-buffered saline (PBS)
containing 5% non-fat milk. Following incubation for 2 h at 25°C,
the membranes were washed with PBS. The membranes were further
incubated in PBS buffer, containing 5% non-fat milk with primary
anti-human monoclonal antibodies to ERK (cat. no. 4965) and p-ERK
(cat. no. 4370) (1:400; Cell Signaling Technology, Inc., Danvers,
MA, USA) for 2 h at 25°C. Following incubation, the membranes were
washed with PBS and incubated with secondary mouse anti-human
antibodies (1:2,000; cat. no. 10702-MM01E-50; Sino Biological Inc.
Beijing, China) for 1 h at 25°C. The proteins were detected using
Western Lightning Chemiluminescence Reagent Plus (PerkinElmer,
Waltham, MA, USA)
Statistical analysis
The data were calculated from four independent
experiments and are presented as the mean ± standard deviation.
Student's t-test was used to analyze the statistical differences.
P<0.05 was considered to indicate a statistically significant
difference.
Results
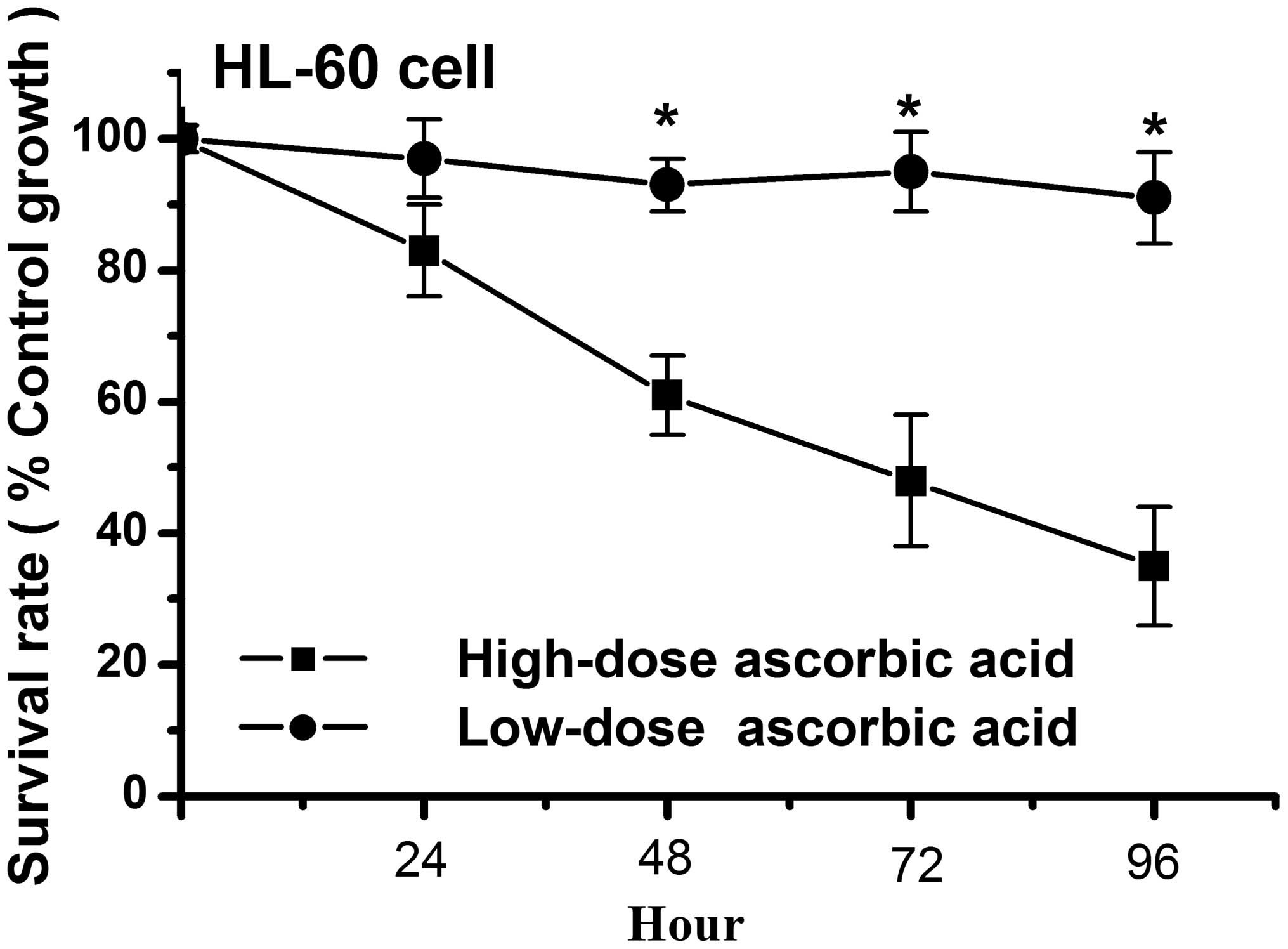
High-dose ascorbic acid inhibits HL-60
cell growth, whereas a low-dose does not
Previous studies have demonstrated that a high-dose
of ascorbic acid can increase radiation-induced and
etoposide-induced apoptosis in HL-60 cells (28,29).
Similar to these studies, the present study demonstrated that a
high-dose (5 μM) of ascorbic acid inhibited cell growth in
the HL-60 cells, whereas a low-dose (5 μM) had no affect on
the growth of the HL-60 cells (Fig.
1). As shown in Fig. 1, the
cell survival rate was <50% in the high-dose ascorbic
acid-treated HL-60 cells at 72 h, however, the survival rate was
>90% in the low-dose ascorbic acid-treated HL-60 cells at 96 h.
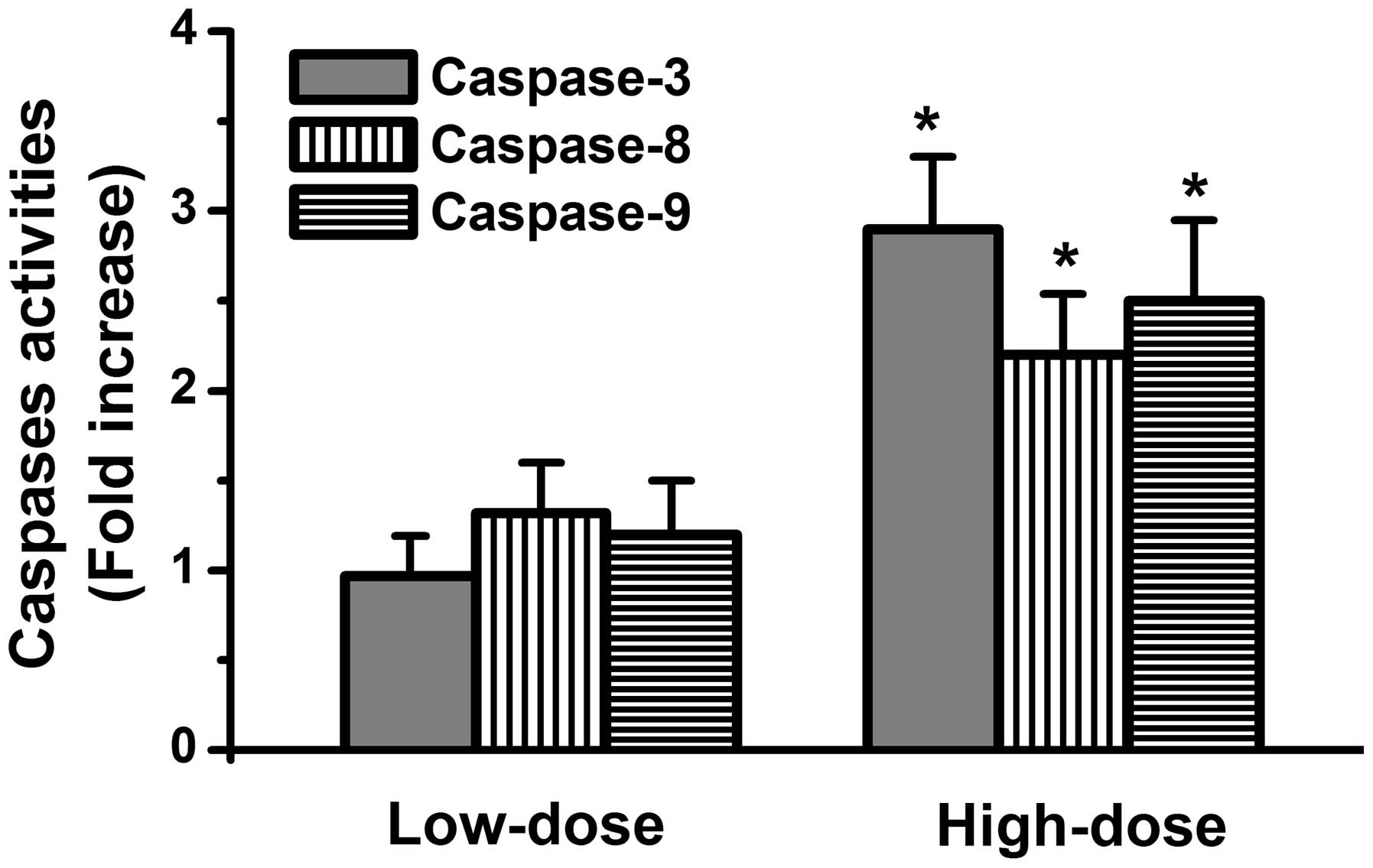
Whether ascorbic acid activates caspase death signals in the HL-60
cells was subsequently investigated. The results demonstrated that
activation of caspase-8, caspase-9 and caspase-3 occurred in the
high-dose ascorbic acid-treated HL-60 cells, but not in low-dose
ascorbic acid-treated HL-60 cells (Fig. 2). These findings indicated that a
high-dose of ascorbic acid exerted antitumor activities in the
HL-60 cells.
Low-dose ascorbic acid reduces cellular
levels of H2O2 in TPA-treated HL-60
cells
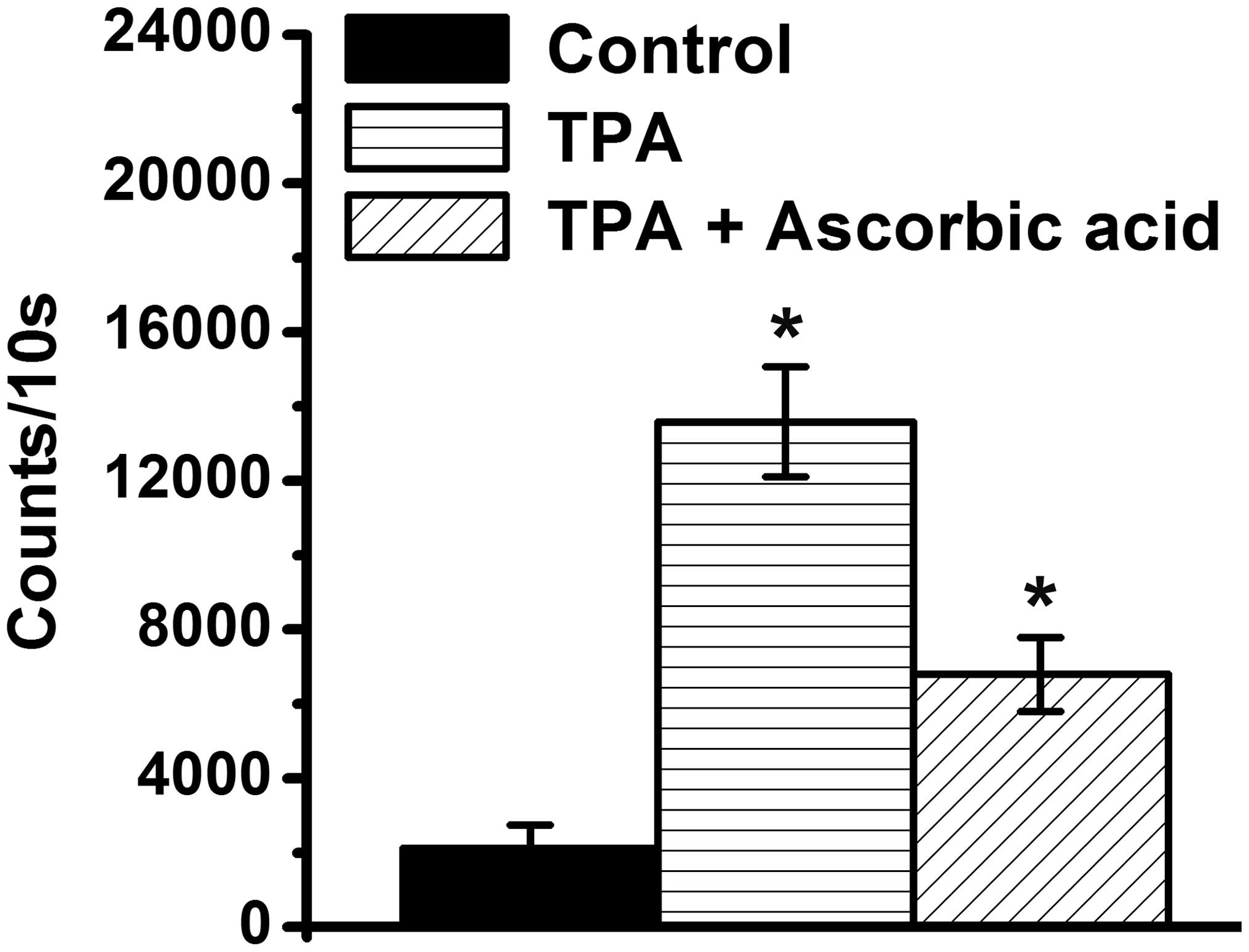
H2O2 is important in cell
differentiation (25). The present
study demonstrated that the levels of H2O2
increased in the TPA-differentiated HL-60 cells, compared with the
TPA-treated HL-60 cells (P<0.05; Fig. 3), which suggested that
H2O2 may be associated with HL-60 cells
differentiation by TPA. Anti-oxidative functions of ascorbic acid
have been demonstrated (35–38),
therefore, the present study further determined whether ascorbic
acid reduces the levels of H2O2 in
TPA-treated HL-60 cells. The results revealed that a low-dose of
ascorbic acid inhibited the increased levels of
H2O2 levels the TPA-treated HL-60 cells
(Fig. 3).
Pretreatment with ascorbic acid inhibits
the differentiation of HL-60 cells into macrophages following TPA
treatment
As shown in Fig. 3,
a low-dose of ascorbic acid reduced the levels of
H2O2 in the TPA-treated HL-60 cells. In
addition, a previous study demonstrated that
H2O2 may be an important messenger for cell
differentiation (25). Therefore,
whether low-dose ascorbic acid inhibits the differentiation of
HL-60 cells into macrophages treated with TPA was determined.
Previous studies have revealed that TPA-differentiated HL-60 cells
(macrophages) are attached cells, whereas the undifferentiated
HL-60 cells are suspensed (14,44).
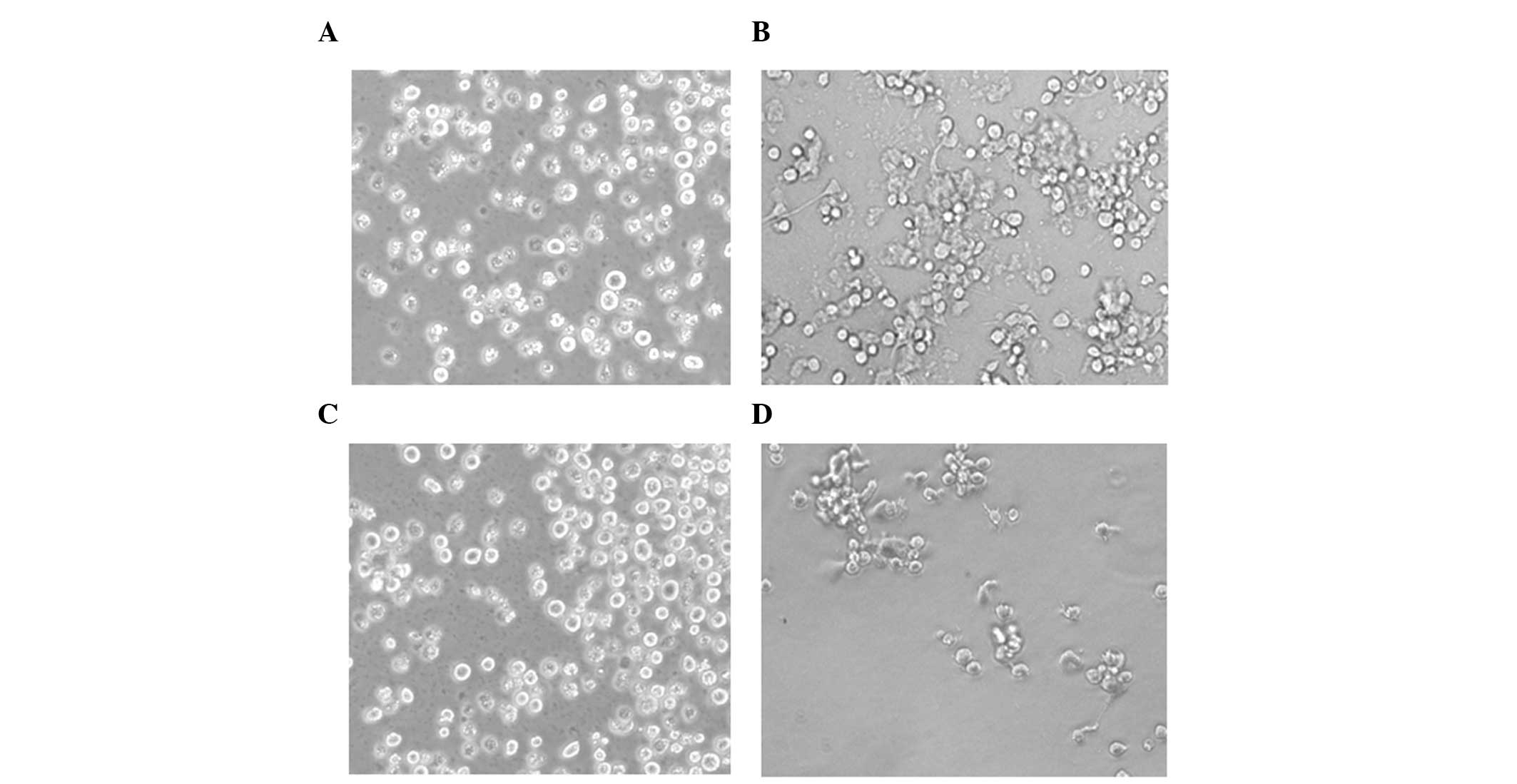
The present study assessed the morphology of the cells using a
phase contrast microscope, and observed that the control HL-60
cells were in suspension (Fig. 4A)
and the TPA-treated HL-60 cells were attached (Fig. 4B). These data suggested that TPA
induced the HL-60 cells to differentiate into macrophages. In
addition, suspended cells were observed in the TPA-treated HL-60
cells pretreated with ascorbic acid (Fig. 4C), whereas attached cells were
observed in the TPA-treated HL-60 cells post-treated with ascorbic
acid (Fig. 4D). These data
indicated that ascorbic acid pretreatment inhibited the TPA-induced
differentiation of HL-60 cells into macrophages, however,
post-treatment did not inhibit the ability of TPA to induce HL-60
cell differentiation into macrophages. The number of cells in
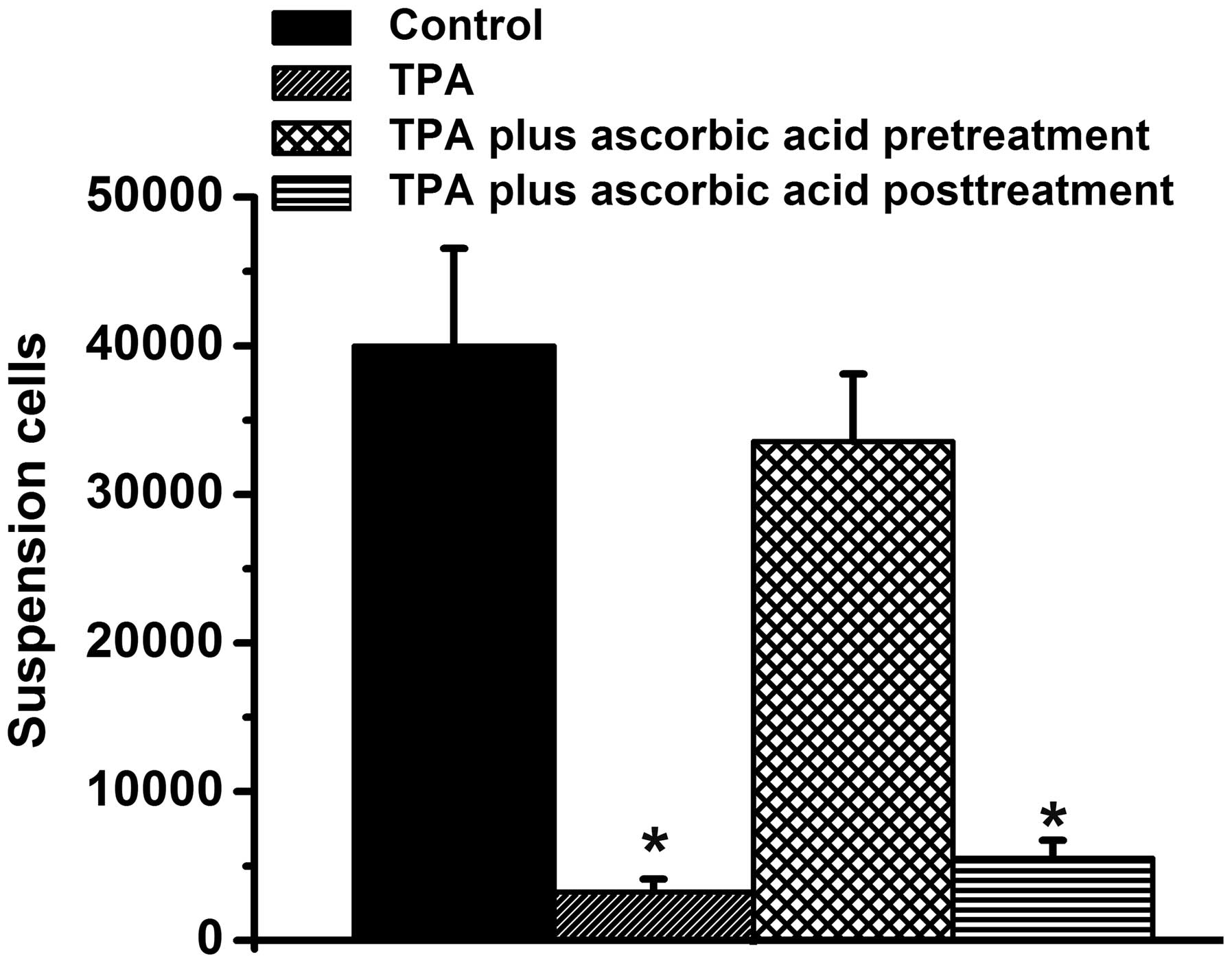
suspension were also quantified in the present study (Fig. 5). There were ~35,000 suspended
cells in the control group and the TPA + ascorbic acid pretreatment
group, however, very few suspended cells were observed in the
TPA-treated group and the TPA + ascorbic acid post-treatment group,
compared with the control group (P<0.05; Fig. 5) Taken together, these results
suggested that pretreatment with ascorbic acid inhibited the
ability of TPA to induce the differentiation of HL-60 cells into
macrophages.
Ascorbic acid inhibits TPA-induced HL-60
cell differentiation via ERK phosphorylation
Previous studies have revealed that the induction of
HL-60 cells to differentiate into macrophages by TPA requires ERK
phosphorylation (13,14). These studies demonstrated that the
inhibition of p-ERK inhibits TPA-induced HL-60 cell
differentiation. In the present study, as shown in Figs. 4 and 5, pretreatment with ascorbic acid
inhibited the differentiation of the HL-60 cells into macrophages
following TPA treatment. Whether pretreatment with ascorbic acid
inhibited TPA-differentiated HL-60 cells via ERK phosphorylation
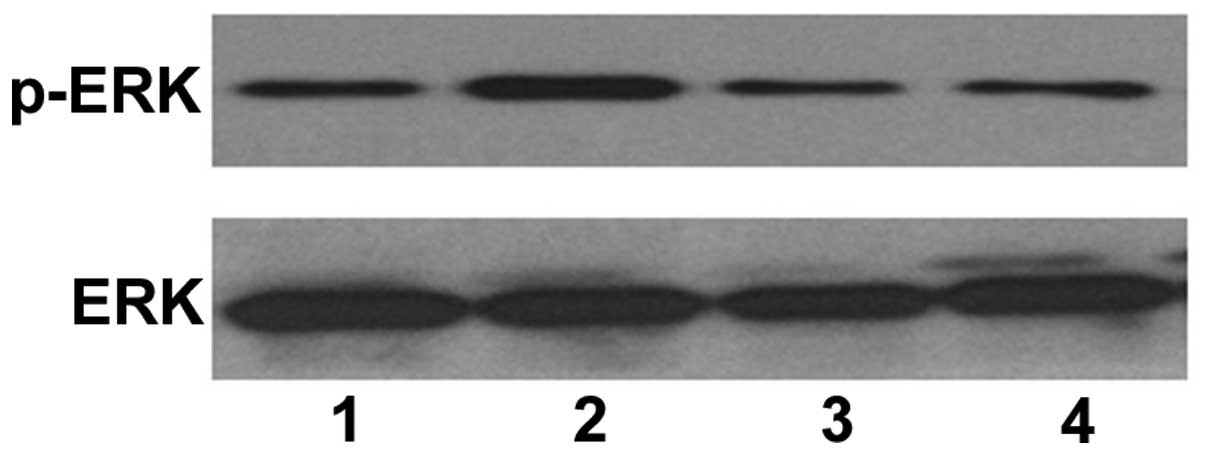
was subsequently investigated, and western blotting revealed that
TPA induced an increase in the protein expression of p-ERK
(Fig. 6; lane 2). In addition,
pretreatment with ascorbic acid reduced the expression of p-ERK in
the TPA-treated HL-60 cells (Fig.
6; lane 3). This data suggested that ascorbic acid inhibited
the ability of TPA to induce HL-60 cell differentiation via ERK
phosphorylation.
Discussion
Previous studies have demonstrated that TPA induces
ERK phosphorylation, which in turn causes HL-60 cells to
differentiate into macrophages (13,14).
In addition, a previous study indicated that
H2O2 accumulation is important for macrophage
differentiation following TPA treatment (25). Similar to previous findings, the
present study demonstrated that TPA induced an increase in the
levels of H2O2 and induced ERK
phos-phorylation (Figs. 3 and
6). These results suggested that
TPA induced HL-60 cells to differentiate into macrophages via the
accumulation of H2O2 and phosphorylation of
ERK. However, the association between H2O2
and the phosphorylation of ERK remains to be elucidated. The
present study demonstrated that pretreatment with ascorbic acid
reduced TPA-induced H2O2 accumulation
(Fig. 3) and inhibited TPA-induced
HL-60 cell differentiation into macrophages (Figs. 4 and 5). However, the data also revealed that
post-treatment with ascorbic acid did not have an inhibitory effect
of TPA (Figs. 4 and 5). The results of the present study
indicated H2O2 accumulation as an upstream
signal, affecting HL-60 cell differentiation by TPA at an early
stage. In addition, several previous studies have demonstrated that
H2O2 induces the phosphorylation of EKR in
various types of cell (46–48).
Therefore, the present study indicated that TPA induced an increase
in the levels of H2O2 initially, and
subsequently induced the phosphorylation of ERK, leading to HL-60
cell differentiation. However, pretreatment with ascorbic acid
inhibited TPA-induced H2O2 accumulation at an
early stage, preventing HL-60 cell differentiation.
The dual role of ascorbic acid in promoting cell
death and preventing cell damage have been previously reported.
Generally, a high-dose of ascorbic acid induces cell cytotoxicity
(28,29), whereas a low-dose of ascorbic acid
protects cells against oxidative stress-induced damage (32–34).
Similar to these studies, the present study demonstrated that a
high-dose of ascorbic acid inhibited cell growth and activated the
caspase-death pathway in the HL-60 cells (Figs. 1 and 2). However, a low-dose of ascorbic acid
reduced TPA-induced increases in H2O2 levels
(Fig. 3). Therefore, high-dose and
low-dose ascorbic acids exerted different mechanisms to affect cell
growth. Previous studies have also reported that ascorbic acid
induces ERK phosphorylation in various types of cell, including
acute myeloid leukemia cells and human endothelial cells (49,50).
By contrast, ascorbic acid inhibits ERK phosphorylation in human
dermal fibroblasts (51). The
present study demonstrated that a low-dose of ascorbic acid
inhibited the TPA-induced phosphorylation of ERK (Fig. 6; lane 3). Therefore, it was
hypothesized that ascorbic acid induces different signaling
pathways to affect cell growth in a dose-dependent and
cell-dependent manner.
Regarding the association between ascorbic acid and
cell differentiation, several studies have demonstrated that
ascorbic acid can promote cell differentiation in various types of
cell, including periodontal ligament progenitor cells, osteoblastic
cells and embryonic stem cells (26,27,52–56).
However, the present study demonstrated that low-doses of ascorbic
acid inhibited the HL-60 cells from differentiating into
macrophages following TPA treatment. The possible reason may be
that TPA-induced cell differentiation requires increases in
cellular oxidative stress (25),
while ascorbic acid can reduce cellular H2O2
levels to inhibit TPA-treated cells. Another possible reason is
that ascorbic acid induces a small fraction of HL-60 cells to
express the granulocyte marker, CD66b (30) and induces a small fraction of HL-60
cells to differentiate into granulocytes, therefore, inhibiting the
differentiation of HL-60 cells into macrophages, induced by
TPA.
In conclusion, the present study demonstrated for
the first time, to the best of our knowledge, that low-doses of
ascorbic acid inhibited TPA-treated HL-60 cells from
differentiating into macrophages by decreasing TPA-induced levels
of H2O2 and ERK phosphorylation.
Acknowledgments
This study was supported by the Ministry of Science
and Technology (grant. no. MOST103 2320-B-039–052-MY3), the
National Health Research Institute (grant. no. N HRI-EX102–10245BI)
and Taipei Tzu Chi Hospital (grant. nos. TCRD-TPE-102–26 and
TCRD-TPE-103–48).
References
|
1
|
Trayner ID, Bustorff T, Etches AE, Mufti
GJ, Foss Y and Farzaneh F: Changes in antigen expression on
differentiating HL60 cells treated with dimethylsulphoxide,
all-trans retinoic acid, alpha1,25-dihydroxyvitamin D3 or
12-O-tetradecanoyl phorbol-13-acetate. Leuk Res. 22:537–547. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zylber-Katz E and Glazer RI: Phospholipid-
and Ca2+-dependent protein kinase activity and protein
phosphorylation patterns in the differentiation of human
promyelocytic leukemia cell line HL-60. Cancer Res. 45:5159–5164.
1985.PubMed/NCBI
|
|
3
|
Congleton J, MacDonald R and Yen A: Src
inhibitors, PP2 and dasatinib, increase retinoic acid-induced
association of Lyn and c-Raf (S259) and enhance MAPK-dependent
differentiation of myeloid leukemia cells. Leukemia. 26:1180–1188.
2012. View Article : Google Scholar :
|
|
4
|
Ji Y, Kutner A, Verstuyf A, Verlinden L
and Studzinski GP: Derivatives of vitamins D2 and D3 activate three
MAPK pathways and upregulate pRb expression in differentiating HL60
cells. Cell Cycle. 1:410–415. 2002. View Article : Google Scholar
|
|
5
|
Wang N, Wang LW, Gou BD, Zhang TL and Wang
K: Realgar-induced differentiation is associated with MAPK pathways
in HL-60 cells. Cell Biol Int. 32:1497–1505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SH, Yoo JC and Kim TS: Nargenicin
enhances 1,25-dihy-droxyvitamin D (3)- and all-trans retinoic
acid-induced leukemia cell differentiation via PKCbetaI/MAPK
pathways. Biochem Pharmacol. 77:1694–1701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stixová L, Procházková J, Soucek K,
Hofmanová J and Kozubík A: 5-Lipoxygenase inhibitors potentiate
1alpha,25-dihy-droxyvitamin D3-induced monocytic differentiation by
activating p38 MAPK pathway. Mol Cell Biochem. 330:229–238. 2009.
View Article : Google Scholar
|
|
8
|
Battle TE, Levine RA and Yen A: Retinoic
acid-induced blr1 expression promotes ERK2 activation and cell
differentiation in HL-60 cells. Exp Cell Res. 254:287–298. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yen A, Roberson MS and Varvayanis S:
Retinoic acid selectively activates the ERK2 but not JNK/SAPK or
p38 MAP kinases when inducing myeloid differentiation. In Vitro
Cell Dev Biol Anim. 35:527–532. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu HN, Lee YR, Noh EM, Lee KS, Song EK,
Han MK, Lee YC, Yim CY, Park J, Kim BS, et al: Tumor necrosis
factor-alpha enhances DMSO-induced differentiation of HL-60 cells
through the activation of ERK/MAPK pathway. Int J Hematol.
87:189–194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li C, Yu Y, Wang Y, Liu L, Zhang M, Sugano
S, Wang Z and Chang Z: Both ERK and JNK are required for
enhancement of MD-2 gene expression during differentiation of HL-60
cells. Biol Cell. 100:365–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Harrison JS and Studzinski GP:
Isoforms of p38MAPK gamma and delta contribute to differentiation
of human AML cells induced by 1,25-dihydroxyvitamin D (3). Exp Cell
Res. 317:117–130. 2011. View Article : Google Scholar
|
|
13
|
Matsumoto E, Hatanaka M, Bohgaki M and
Maeda S: PKC pathway and ERK/MAPK pathway are required for
induction of cyclin D1 and p21Waf1 during 12-o-tetradecanoylphorbol
13-acetate-induced differentiation of myeloleukemia cells. Kobe J
Med Sci. 52:181–194. 2006.
|
|
14
|
Yiang GT, Yu YL, Hu SC, Chen MH, Wang JJ
and Wei CW: PKC and MEK pathways inhibit caspase-9/-3-mediated
cytotoxicity in differentiated cells. FEBS Lett. 582:881–885. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Pesakhov S, Harrison JS, Danilenko
M and Studzinski GP: ERK5 pathway regulates transcription factors
important for monocytic differentiation of human myeloid leukemia
cells. J Cell Physiol. 229:856–867. 2014. View Article : Google Scholar
|
|
16
|
Jamshidi F, Zhang J, Harrison JS, Wang X
and Studzinski GP: Induction of differentiation of human leukemia
cells by combinations of COX inhibitors and 1,25-dihydroxyvitamin
D3 involves Raf1 but not Erk 1/2 signaling. Cell Cycle. 7:917–924.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Pesakhov S, Weng A, Kafka M, Gocek
E, Nguyen M, Harrison JS, Danilenko M and Studzinski GP: ERK 5/MAPK
pathway has a major role in 1α,25-(oh) vitamin D3-induced terminal
differentiation of myeloid leukemia cells. J Steroid Biochem Mol
Biol. 144:223–227. 2013. View Article : Google Scholar
|
|
18
|
Uruno A, Noguchi N, Matsuda K, Nata K,
Yoshikawa T, Chikamatsu Y, Kagechika H, Harigae H, Ito S, Okamoto
H, et al: All-trans retinoic acid and a novel synthetic retinoid
tami-barotene (Am80) differentially regulate CD38 expression in
human leukemia HL-60 cells: possible involvement of protein kinase
C-delta. J Leukoc Biol. 90:235–247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ju SM, Kang JG, Pae HO, Lee GS, Kim WS,
Lyu YS and Jeon BH: Nardostachys chinensis induces the
differentiation of human promyelocytic leukemic cells through the
activation of the protein kinase C-dependent extracellular
signal-regulated kinase signaling pathway. Int J Mol Med.
33:573–580. 2014.
|
|
20
|
Ogino T, Ozaki M and Matsukawa A:
Oxidative stress enhances granulocytic differentiation in HL 60
cells, an acute promy-elocytic leukemia cell line. Free Radic Res.
44:1328–1337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu XM, Yuan B, Tanaka S, Zhou Q, Onda K,
Toyoda H and Hirano T: Involvement of oxidative stress associated
with glutathione depletion and p38 mitogen-activated protein kinase
activation in arsenic disulfide-induced differentiation in HL-60
cells. Leuk Lymphoma. 55:392–404. 2014. View Article : Google Scholar
|
|
22
|
Kambhampati S, Li Y, Verma A, Sassano A,
Majchrzak B, Deb DK, Parmar S, Giafis N, Kalvakolanu DV, Rahman A,
et al: Activation of protein kinase C delta by all-trans-retinoic
acid. J Biol Chem. 278:32544–32551. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SH, Kang SN, Kim HJ and Kim TS:
Potentiation of 1,25-dihydroxyvitamin D (3)-induced differentiation
of human promyelocytic leukemia cells into monocytes by
costunolide, a germacranolide sesquiterpene lactone. Biochem
Pharmacol. 64:1233–1242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bondza-Kibangou P, Millot C, El Khoury V
and Millot JM: Antioxidants and doxorubicin supplementation to
modulate CD14 expression and oxidative stress induced by vitamin D3
and seocalcitol in HL60 cells. Oncol Rep. 18:1513–1519.
2007.PubMed/NCBI
|
|
25
|
Yamamoto T, Sakaguchi N, Hachiya M,
Nakayama F, Yamakawa M and Akashi M: Role of catalase in monocytic
differentiation of U937 cells by TPA: hydrogen peroxide as a second
messenger. Leukemia. 23:761–769. 2009. View Article : Google Scholar
|
|
26
|
Yan Y, Zeng W, Song S, Zhang F, He W,
Liang W and Niu Z: Vitamin C induces periodontal ligament
progenitor cell differentiation via activation of ERK pathway
mediated by PELP1. Protein Cell. 4:620–627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mimori K, Komaki M, Iwasaki K and Ishikawa
I: Extracellular signal-regulated kinase 1/2 is involved in
ascorbic acid-induced osteoblastic differentiation in periodontal
ligament cells. J Periodontol. 78:328–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shinozaki K, Hosokawa Y, Hazawa M,
Kashiwakura I, Okumura K, Kaku T and Nakayama E: Ascorbic acid
enhances radiation-induced apoptosis in an HL60 human leukemia cell
line. J Radiat Res. 52:229–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gokhalé P, Patel T, Morrison MJ and
Vissers MC: The effect of intracellular ascorbate on the
susceptibility of HL60 and Jurkat cells to chemotherapy agents.
Apoptosis. 11:1737–1746. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang HK, Suh JH, Lee JJ, Yoon SH, Hyun JW,
Choi SW, Choi JY, Ryu KH and Chung MH: Induction of the
differentiation of HL-60 promyelocytic leukemia cells by L-ascorbic
acid. Free Radic Res. 37:773–779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Witenberg B, Kletter Y, Kalir HH, Raviv Z,
Fenig E, Nagler A, Halperin D and Fabian I: Ascorbic acid inhibits
apoptosis induced by X irradiation in HL60 myeloid leukemia cells.
Radiat Res. 152:468–478. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karasavvas N, Carcamo JM, Stratis G and
Golde DW: Vitamin C protects HL60 and U266 cells from arsenic
toxicity. Blood. 105:4004–4012. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cuddihy SL, Parker A, Harwood DT, Vissers
MC and Winterbourn CC: Ascorbate interacts with reduced glutathione
to scavenge phenoxyl radicals in HL60 cells. Free Radic Biol Med.
44:1637–1644. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Parker A, Cuddihy SL, Son TG, Vissers MC
and Winterbourn CC: Roles of superoxide and myeloperoxidase in
ascorbate oxidation in stimulated neutrophils and H2O2-treated HL60
cells. Free Radic Biol Med. 51:1399–1405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Taniguchi M, A rai N, Kohno K, Ushio S and
Fukuda S: Anti-oxidative and anti-aging activities of
2-O-alpha-glucopyranosyl-L-ascorbic acid on human dermal
fibroblasts. Eur J Pharmacol. 674:126–131. 2012. View Article : Google Scholar
|
|
36
|
Naziroglu M, Akkus S, Soyupek F, Yalman K,
Çelik Ö, Eriş S and Uslusoy GA: Vitamins C and E treatment combined
with exercise modulates oxidative stress markers in blood of
patients with fibromyalgia: A controlled clinical pilot study.
Stress. 13:498–505. 2010.PubMed/NCBI
|
|
37
|
Yokoo S, Furumoto K, Hiyama E and Miwa N:
Slow-down of age-dependent telomere shortening is executed in human
skin keratinocytes by hormesis-like-effects of trace hydrogen
peroxide or by anti-oxidative effects of pro-vitamin C in common
concurrently with reduction of intracellular oxidative stress. J
Cell Biochem. 93:588–597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim YH, Kim CH, Cho MK, Kim KM, Lee SY,
Ahn BW, Yang SY, Kim SM and Song TB: Total peroxyl radical-trapping
ability and anti-oxidant vitamins of the umbilical venous plasma
and the placenta in pre-eclampsia. J Obstet Gynaecol Res. 32:32–41.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yiang GT, Chou PL, Hung YT, Chen JN, Chang
WJ, Yu YL and Wei CW: Vitamin C enhances anticancer activity in
methotrexa-tetreated Hep3B hepatocellular carcinoma cells. Oncol
Rep. 32:1057–1063. 2014.PubMed/NCBI
|
|
40
|
Yu YL, Yiang GT, Chou PL, Tseng HH, Wu TK,
Hung YT, Lin PS, Lin SY, Liu HC, Chang WJ, et al: Dual role of
acetaminophen in promoting hepatoma cell apoptosis and kidney
fibroblast proliferation. Mol Med Rep. 9:2077–2084. 2014.PubMed/NCBI
|
|
41
|
Yiang GT, Chen YH, Chou PL, Chang WJ, Wei
CW and Yu YL: The NS3 protease and helicase domains of Japanese
encephalitis virus trigger cell death via caspasedependent and
independent pathways. Mol Med Rep. 7:826–830. 2013.PubMed/NCBI
|
|
42
|
Chen KH, Li PC, Lin WH, Chien CT and Low
BH: Depression by a green tea extract of alcohol-induced oxidative
stress and lipogenesis in rat liver. Biosci Biotechnol Biochem.
75:1668–1676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lin BR, Yu CJ, Chen WC, Lee HS, Chang HM,
Lee YC, Chien CT and Chen CF: Green tea extract supplement reduces
D-galactosamine-induced acute liver injury by inhibition of
apoptotic and proinflammatory signaling. J Biomed Sci. 16:352009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Das D, Pintucci G and Stern A:
MAPK-dependent expression of p21 (WAF) and p27 (kip1) in
PMA-induced differentiation of HL60 cells. FEBS Lett. 472:50–52.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wei CW, Hu CC, Tang CH, Lee MC and Wang
JJ: Induction of differentiation rescues HL-60 cells from Rana
catesbeiana ribonuclease-induced cell death. FEBS Lett.
531:421–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Siebel A, Cubillos-Rojas M, Santos RC,
Schneider T, Bonan CD, Bartrons R, Ventura F, Rodrigues de Oliveira
J and Rosa JL: Contribution of S6K1/MAPK signaling pathways in the
response to oxidative stress: Activation of RSK and MSK by hydrogen
peroxide. PLoS One. 8:e755232013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moslehi M, Meshkini A and Yazdanparast R:
Flavonoid baicalein modulates H2O2-induced mitogen-activated
protein kinases activation and cell death in SK-N-MC cells. Cell
Mol Neurobiol. 32:549–560. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kang KA, Lee KH, Zhang R, Piao MJ, Kang
MY, Kwak YS, Yoo BS, You HJ and Hyun JW: Protective effects of
Castanopsis cuspidate through activation of ERK and NF-kappaB on
oxidative cell death induced by hydrogen peroxide. J Toxicol
Environ Health A. 70:1319–1328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ulrich-Merzenich G, Zeitler H, Panek D,
Bokemeyer D and Vetter H: Vitamin C promotes human endothelial cell
growth via the ERK-signaling pathway. Eur J Nutr. 46:87–94. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Park S, Park CH, Hahm ER, Kim K, Kimler
BF, Lee SJ, Park HK, Lee SH, Kim WS, Jung CW, et al: Activation of
Raf1 and the ERK pathway in response to l-ascorbic acid in acute
myeloid leukemia cells. Cell Signal. 17:111–119. 2005. View Article : Google Scholar
|
|
51
|
Park HJ, Ock SM, Kim HJ, Park HJ, Lee YB,
Choi JM, Cho CS, Lee JY, Cho BK and Cho DH: Vitamin C attenuates
ERK signalling to inhibit the regulation of collagen production by
LL-37 in human dermal fibroblasts. Exp Dermatol. 19:e258–e264.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cuaranta-Monroy I, Simandi Z, Kolostyak Z,
Doan-Xuan QM, Poliska S, Horvath A, Nagy G, Bacso Z and Nagy L:
Highly efficient differentiation of embryonic stem cells into
adipocytes by ascorbic acid. Stem Cell Res. 13:88–97. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yu J, Tu YK, Tang YB and Cheng NC:
Stemness and transdif-ferentiation of adipose-derived stem cells
using L-ascorbic acid 2-phosphate-induced cell sheet formation.
Biomaterials. 35:3516–3526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Valenti MT, Zanatta M, Donatelli L,
Viviano G, Cavallini C, Scupoli MT and Dalle Carbonare L: Ascorbic
acid induces either differentiation or apoptosis in MG-63
osteosarcoma lineage. Anticancer Res. 34:1617–1627. 2014.PubMed/NCBI
|
|
55
|
Langenbach F and Handschel J: Effects of
dexamethasone, ascorbic acid and beta-glycerophosphate on the
osteogenic differentiation of stem cells in vitro. Stem Cell Res
Ther. 4:1172013. View Article : Google Scholar
|
|
56
|
Hadzir SN, Ibrahim SN, Abdul Wahab RM,
Zaino Abidin IZ, Senafi S, Ariffin ZZ, Abdul Razak M and Zainal
Ariffin SH: Ascorbic acid induces osteoblast differentiation of
human suspension mononuclear cells. Cytotherapy. 16:674–682. 2014.
View Article : Google Scholar
|