Introduction
The number of individuals with visual impairment is
estimated to be 285,000,000 worldwide, of whom 39,000,000 are blind
(1). A variety of diseases of the
eye causing blindness, including optic nerve (ON) injury caused by
trauma and glaucoma caused by optic atrophy, have no effective
treatment strategies at present. Pathological changes of the ON and
retinal ganglion cell (RGC) degeneration or damage are caused by
various factors, which lead to the complete loss of visual
function. Therefore, it is important to protect the retina and
supplement retinal nerve cells in diseases of the eye, which cause
blindness. Stem cell research and gene technology have been
developing increasingly, which may provide novel strategies for the
treatment of diseases of the eye causing blindness.
Bone marrow mesenchymal stem cells (BMSCs), a type
of multipotent stem cell from the bone marrow, are capable of
differentiating into a variety of lineages. Due to the ease of
separation and culture, avoidance of the immune rejection and
ethical issues, BMSCs have become a significant area of interest in
the investigation of adult stem cells. A previous study revealed
that, in the field of ophthalmology, BMSCs can be induced into
corneal epithelial cells in vitro (2). Wang et al (3) demonstrated that intravitreally
injected BMSCs are capable of mobilizing into cells subjected to
laser-induced retinal injury and differentiating into retinal
pigment epithelium, endothelial cells, pericytes and
photoreceptors. Investigations using rat models subjected to
ischemia/reperfusion ocular injury have demonstrated that a few
BMSCs are integrated into the ganglion cell layer, following
intravitreal injection, and express specific markers of
neuron-specific enolase (NSE), neurofilament (NF) and various
neurotrophic factors. Therefore, intravitreally injected BMSCs can
reduce damage to RGCs (4). It is
important to investigate BMSC differentiation into RG-like cells
for developments of treatment of eye disease, which cause
blindness, including retinitis pigmentosa, age-related macular
degeneration and glaucomatous optic neuropathy (5).
Growth-associated protein-43 (GAP-43), a protein
kinase C substrate, is a member of the calmodulin-binding protein
family and concentrates in the presynaptic membrane and growth
cones (6). GAP-43 is involved in
neurotransmitter release, and promotes membrane expansion by
vesicle fusion or inducing endocytosis of the growth cone and
presynaptic terminal (7). The
expression of GAP-43 is gradually increased in the process of axon
regeneration (8–10). Ivanov et al (11) revealed that GAP-43 is overexpressed
in adult RGCs, compared with other retinal cells using a DNA
microarray method. A long-term investigation of ON injury in a
zebrafish model also suggested that the phosphorylated form of
GAP-43 is important in the early and late periods of nerve
regeneration (10).
In the present study, the effects of
lentiviral-mediated overexpression and knockdown of GAP-43 on BMSC
differentiation, and the expression of phenotypic markers were
investigated in vitro. In addition, a traumatic optic
neuropathy (TON) rat model was established, and the effects of
lentiviral-mediated GAP-43 gene-modified BMSCs on the process of
nerve repair in the TON rat model were observed.
Materials and methods
Ethical statement
Approval from the Animal Ethics Committee of the
Animal Laboratory Center of the Basic Medical College of Jilin
University (Basic Medical College of Jilin University, Changchun,
China) was obtained prior to the use of the animals in the present
study.
Isolation, culture and identification of
BMSCs
Fifteen healthy newborn Wistar rats (16–18 g, male),
~10-days old, were provided by the Animal Laboratory Center of the
Basic Medical College of Jilin University. The rats were sacrificed
by cervical dislocation. BMSCs were isolated, according to
previously described procedures (12). Briefly, the bone marrow was
obtained from the bilateral femurs and tibias, flushed out with 5
ml Dulbecco's modified Eagle's Medium (DMEM)/F12 (Gibco Life
Technologies, Carlsbad, CA, USA) and mixed with equivalent percoll
separating medium (1.073 g/l; Gibco Life Technologies). Following
centrifugation at 900 xg for 20 min at room temperature, the
intermediate monolayer cells were collected and flushed twice with
phosphate-buffered saline (PBS). The collected BMSCs were
resuspended in 3 ml DMEM/F12 containing 10% fetal calf serum (Gibco
Life Technologies) and placed into 6-well plates (Corning
Incorporated, Corning, NY, USA). Following incubation for 24 h
(37°C, 5% CO2 and saturation humidity), the non-adherent
cells were removed by replacing the medium. At >90% confluence,
the cells were digested with a mixture of 0.25% Trypsin
(Sigma-Aldrich, St. Louis, MO, USA) and 0.02% EDTA (Sigma-Aldrich)
and then passaged. To identify the BMSCs, cells in the third
passage were collected for detection using flow cytometry. A total
of 5×105 cells in 50 µl PBS were stained with 10
µl mouse anti-rat CD90-fluorescein isothiocyanate (FITC),
CD44-FITC, CD11b-FITC and CD44-FITC monoclonal antibodies (1:50; BD
Biosciences, Franklin Lakes, NJ, USA) for 30 min at 4°C in the
dark, respectively. Subsequently, the cells were washed with PBS
three times and detected using a FACSCalibur II flow cytometer (BD
Biosciences). The data were analyzed using CellQuest software (BD
Biosciences).
Preparation of lentiviral vectors
The GAP-43 gene overexpression lentivirus was
constructed and termed LV5-GAP-43. The GAP-43 gene was amplified
from rat cDNA. The polymerase chain reaction (PCR) program was as
follows: 95°C for 3 min, 30 cycles of 95°C for 30 sec, 60°C for 30
sec and 72°C for 90 sec, with a final elongation at 72°C for 10min
using the Geneamp PCR (Applied Biosystems, Foster City, CA, USA).
Then GAP-43 gene was cloned into the pGLV5 lentiviral vector. The
GAP-43 gene silencing lentiviral vector was also constructed and
termed LV3-short hairpin (sh)RNA-GAP-43. A total of four target
shRNAs (Gap43-rat-619, Gap43-rat-844, Gap43-rat-1215 and
Gap43-rat-678) and one negative scrambled shRNA (Gap43-rat-NC), as
listed in Table I, were designed
using siRNA Target Finder and Design Tools software (Ambion Life
Technologies, Carlsbad, CA, USA). The cloned DNA segment was
inserted into the pGLV3 lentiviral vector. Sequence analysis
confirmed that the inserted GAP-43 and shRNA-GAP-43 sequences were
correct (Shanghai Sangon Biotech Co. Ltd., Shanghai, China). To
screen for specific small interfering (si)RNAs against GAP-43, 293T
cells (Sangon Biotech, Shanghai, China) were co-transfected with
LV5-GAP-43 and LV3-shRNA-GAP-43-619/844/1215/678, respectively. The
expression of GAP-43 was detected using western blotting and the
most effective siRNAs were used for further investigation. The
successfully constructed lentiviral vector and packaging plasmid
(mix) were co-transfected into the 293T cells using Lipofectamine
2000 (Invitrogen Life Technologies, Carlsbad, CA, USA). Packaging
and titering of the lentiviral vectors were performed, according to
previously described procedures (13). After transfection for 8 h, the
culture medium was changed to complete medium. The supernatant was
harvested after culturing for 48 h and concentrated by
ultrafiltration. The virus titer was measured using the dilution
gradient method and calculated as follows: Virus titer (TU/ml) =
counted fluorescent cells/corresponding volume of virus stock
solution. Ultimately, the titer of the lentivirus was
2×108 TU/ml and the lentivirus was stored at −80°C for
later use.
 | Table IShort hairpin RNA sequences of
GAP-43. |
Table I
Short hairpin RNA sequences of
GAP-43.
| Name | Sequence |
|---|
| Gap43-rat-619 Top
strand |
5′-GATCCGCCCGACAGGATGAGG GTAAATTCA |
|
AGAGATTTACCCTCATCCTGTCGGGCTTTTTTG-3′ |
| Gap43-rat-619
Bottom strand |
5′-AATTCAAAAAAGCTAAAGCTA CCA CTG
ATAACT |
|
CTCTTGAAGTTATCAGTGGTAGCTTTAGCG-3′ |
| Gap43-rat-844 Top
strand | 5′-GATCCGCCC
GACAGGATGAGG GTA AAT TCAAGA |
|
GATTTACCCTCATCCTGTCGGGCTTTTTTG-3′ |
| Gap43-rat-844
Bottom strand | 5′-AAT
TCAAAAAAGCCCGACAGGATG AGGGTAAAT |
|
CTCTTGAATTTACCCTCATCCTGTCGGGCG-3′ |
| Gap43-rat-1215 Top
strand |
5′-GATCCGAGTCCACTTTCCTCTCTATTTCAAGAG |
|
AATAGAGAGGAAAGTGGACTCTTTTTTG-3′ |
| Gap43-rat-1215
Bottom strand | 5′-AAT TCA AAA AAG
AGT CCA CTT TCC TCTCTA |
| TTC
TCTTGAAAGGAAAGTGGACTCG-3′ |
| Gap43-rat-678 Top
strand | 5′-GAT CCG GAG CCT
AAA CAA GCC GATGTTTCA |
|
AGAGAACATCGGCTTGTTTAGGCTCCTTTTTTG-3′ |
| Gap43-rat-678
Bottom strand |
5′-AATTCAAAAAAGGAGCCTAAACAAGCCGATGTT |
|
CTCTTGAAACATCGGCTTGTTTAGGCTCCG-3′ |
| Gap43-rat-NC Top
strand |
5′-GATCCGTTCTCCGAACGTGTCACGTTTCAAGAG |
|
AACGTGACACGTTCGGAGAACTTTTTTG-3′ |
| Gap43-rat-NC Bottom
strand |
5′-AATTCAAAAAAGTTCTCCGAACGTGTCACG |
|
TTCTCTTGAAACGCACGTTCGGAGAACG-3′ |
Lentivirus transduction
The third passage BMSCs were transduced with the
negative control lentiviral vector, LV5-GAP-43 and
LV3-shRNA-GAP-43s, with a multiplicity of infection (MOI) of 20,
and 5 µl polybrene (10 mg/ml) was added, respectively. The
stable control, GAP-43-overexpression and GAP-43-knockdown cell
lines, termed GFP/BMSCs, GAP-43/BMSCs and shGAP-43/BMSCs, were
established in culture medium containing puromycin (10
µl/ml).
Effects of Gap-43 on BMSC
differentiation
To analyze the effects of the overexpression of
GAP-43 on BMSC differentiation, the third passage BMSCs were
divided into three groups: Blank, NC (transduced with negative
control lentiviral vector) and GAP-43 (transduced with LV5-GAP-43).
The expression levels of GAP-43, NSE, nestin, NF and βIII-tubulin
were detected using semi-quantitative PCR and western blotting. The
morphology of the BMSCs in each group was observed under a
fluorescence microscope (Olympus Corp., Tokyo, Japan).
To analyze the effects of the silencing of GAP-43 on
BMSC differentiation, the third passage BMSCs were divided into
three groups: Blank, NC (transduced with negative control
lentiviral vector) and shGAP-43 (transduced with LV3-shRNA-GAP-43),
which were all induced by retinal cell-conditioned differentiation
medium containing 10 ng/ml brain-derived neurotrophic factor (BDNF;
Sigma-Aldrich) and 5 ng/ml basic fibroblast growth factor
(Sigma-Aldrich). The expression levels of GAP-43, NSE, NF,
neuron-specific nuclear-binding protein (NeuN) and βIII-tubulin
were detected using western blotting and cell immunofluorescence.
The morphology of the BMSCs was observed under a fluorescence
microscope.
Animal models of TON and BMSC
transplantation
A total of 120 healthy male Sprague Dawley rats
(180–200 g) were provided by the Animal Laboratory Center of the
Basic Medical College of Jilin University, and acclimated to a
natural day-night cycle and given free access to food and water at
21°C together for 1 week prior to the trial. The rats were randomly
divided into five groups with 24 rats in each group. The groups
were as follows: Sham group; PBS group; GFP/BMSCgroup; GAP-43/BMSC
group and shGAP-43/BMSC group. The rats in each group were randomly
divided into four subgroups (six rats in each subgroup): 3, 7, 14
and 28 days after surgery, respectively. The TON rat model was
established using the method described by Jiang et al
(14). In brief, the rats were
anesthetized with 10% chloral hydrate (3 ml/kg; GE Healthcare Life
Sciences, Uppsala, Sweden). An incision was made on the temporal
eyelid, and the ON was exposed and isolated. The ON 2 mm from the
eyeball was crushed using cross action forceps with a 40 g holding
force for 30 sec. Sham surgery was performed using the same
procedures but without crushing of the ON. In the GFP/BMSC,
GAP-43/BMSC and shGAP-43/BMSC groups, 105 GFP/BMSCs,
GAP-43/BMSCs and shGAP-43/BMSCs were injected into the rat vitreous
cavity on day 3, 7, 14 and 28 post-surgery, respectively, and an
equal volume of PBS was administered to the sham and PBS groups. At
5 weeks post-BMSC injection, the retinas were post-fixed in 4%
paraformaldehyde (Dingguo Changsheng Biotech, Beijing, China) for
24 h.
Retrograde labeling of RGCs with
fluorogold (FG) and RGC counts
The numbers of RGCs were counted using retrograde
labeled with FG. Briefly, the rats were anesthetized and their
heads were immobilized. The skin overlying the skull was incised.
The lambda and bregma sutures served as landmarks for drilling two
holes, following which 5 µl 5% FG (Sigma-Aldrich) in saline
was injected into the superior colliculus once. The rats were
sacrificed by cervical dislocation following injections for 7 days
and the retinal tissues were harvested. The numbers of labeled RGCs
were determined using an inverted fluorescence microscope (IX70;
Olympus Corp.). The rate of RGC labeling was calculated as follows:
RGCs from injured eye/RGCs from uninjured eye × 100%.
Reverse
transcription-quantitative/semi-quantitative PCR
Cells and tissues were homogenized on ice with
TRIzol reagent (Invitrogen Life Technologies) and total RNA was
extracted, according to the manufacturer's instructions.
High-quality RNA was reverse transcribed into complementary DNA
(cDNA) with a Reverse Transcription kit (cat no. DRR036A; Takara
Biotechnology Co., Ltd., Dalian, China). GAP-43, NSE, nestin, NF,
βIII-tubulin and glyceral-dehyde-3-phosphate dehydrogenase (GAPDH)
primers were used (Table II). The
PCR reaction system comprised cDNA (10 ng), primers (0.1
µM), deoxy-ribonucleoside triphosphate (1 mM), Taq DNA
polymerase (5 U), 10X buffer (2.5 µl), and double distilled
water to achieve a final volume of 25 µl. PCR amplification
was performed using SYBR® Premix Ex Taq™ (cat. no.
DRR420A; Takara Biotechnology) on an ABI StepOnePlus Real-time PCR
system (Applied Biosystems). The PCR protocol was as follows: 95°C
for 2 min, 40 cycles of 95°C for 10 sec and 60°C for 40 sec.
Relative quantification and calculations were performed using the
comparative threshold (Ct) cycle method (2−ΔΔCt). For
semi-quantitative PCR, products were analyzed using agarose gel
electrophoresis and the corresponding optical density ratio was
measured (optical density value of the specific gene/optical
density value of GAPDH).
 | Table IIPrimer sequences of GAP-43, NSE,
nestin, NF, βIII-tubulin and GAPDH primers. |
Table II
Primer sequences of GAP-43, NSE,
nestin, NF, βIII-tubulin and GAPDH primers.
| Gene | Primer
sequence | Product size
(bp) |
|---|
| GAP-43 sense |
5′-CATGAGAAGTATGACAACAGCCT-3′ | 113 |
| GAP-43
antisense |
5′-AGTCCTTCCACGATACCAAAGT-3′ | |
| NSE sense |
5′-CAACAGCACCATCGCACCG-3′ | 436 |
| NSE antisense |
5′-GGCAAAGCCGCCTTCATC-3′ | |
| nestin sense |
5′-CAGGCTTCTCTTGGCTTTCTGG-3′ | 431 |
| nestin
antisense |
5′-TGGTGAGGGTTGAGGTTTGT-3′ | |
| NF sense |
5′-TGGAGAATGAGCTGCGAAGC-3′ | 325 |
| NF antisense |
5′-TTCGTAGCCTCAATGGTCTC-3′ | |
| βIII-tubulin
sense |
5′-TGAGACCTACTGCATCGACA-3′ | 447 |
| βIII-tubulin
antisense |
5′-GGGATCCACTCCACGAAGTA-3′ | |
| GAPDH sense |
5′-CTGCAACCAAAATTCAGGCTA-3′ | 129 |
| GAPDH
antisense |
5′-CATCAGCAACGGGAGCAT-3′ | |
Western blotting
The cells were collected and lysed in
radio-immunoprecipitation assay lysis buffer (Dingguo Changsheng
Biotech). Supernatants were acquired by centrifugation at 12,000×g
for 15 min at 4°C. Subsequently, the protein concentration was
detected using the Bicinchoninic Protein Quantitative Assay
(Shanghai Sangon Biotech). A total of 40 µg protein was
separated by 10% SDS-PAGE and then transferred onto polyvinylidene
difluoride membranes (Amersham Pharmacia, Piscataway, NJ, USA),
which were blocked in 5% defatted milk for 1 h at room temperature.
The membranes were incubated with rabbit anti-rat polyclonal
antibodies against GAP-43, NSE, nestin, NF, βIII-tubulin and GAPDH
(1:500; Wuhan Boster Biological Technology, Ltd., Wuhan, China)
overnight at 4°C, washed three times with PBS and then incubated
with goat anti-mouse immunoglobulin G (H+L)-horseradish peroxidase
(1:5,000; Wuhan Boster Biological Technology, Ltd.) for 2 h at room
temperature. Proteins were detected by enhanced chemiluminescence
(Amersham Pharmacia) and the corresponding gray value ratios
(specific genes/GAPDH) were measured.
Hematoxylin and eosin (H&E)
staining
The retinal tissues were harvested, fixed in 4%
paraformaldehyde for 24 h, processed and embedded into paraffin
blocks. The sections of retinal tissues were dipped into gradient
ethanol, stained with hematoxylin for 5 min, differentiated in 1%
hydrochloric acid alcohol for 2 sec, incubated in ammonia water for
2 min and then stained with eosin for 1 min. The sections were
rinsed with distilled water at each interval step. The sections
were then dehydrated with gradient ethanol, cleared with xylene
(Sinopharm Chemical Reagent, Beijing, China), mounted with neutral
resin (Beijing Dingguo Changsheng), and observed using light
microscopy (BX41; Olympus Corp.).
Cell immunofluorescence
The cells were washed twice with Hanks' balanced
salt solution at 37°C and then fixed with 4% paraformaldehyde
(precooling) for 15 min at 37°C, followed by blocking in PBS
containing 1% bovine serum albumin and 1% goat serum for 1 h at
room temperature. The samples were then incubated with rabbit
anti-rat nestin, NeuN and GAP-43 polyclonal antibodies (Wuhan
Boster Biological Technology, Ltd.) at a dilution of 1:100 in PBS
at 4°C overnight, washed twice with PBS and incubated with FITC- or
Cy3-labeled rabbit anti-goat IgG (H+L; Wuhan Boster Biological
Technology, Ltd.) at a dilution of 1:200 in PBS at room temperature
for 1 h. Finally, images were captured using an inverted
fluorescence microscope (Olympus Corp.).
Statistical analysis
Statistical analysis was performed using SPSS 12.0
statistical analysis software (SPSS, Inc., Chicago, IL, USA). The
data are expressed as the mean ± standard deviation and were
analyzed using one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphology and identification of
BMSCs
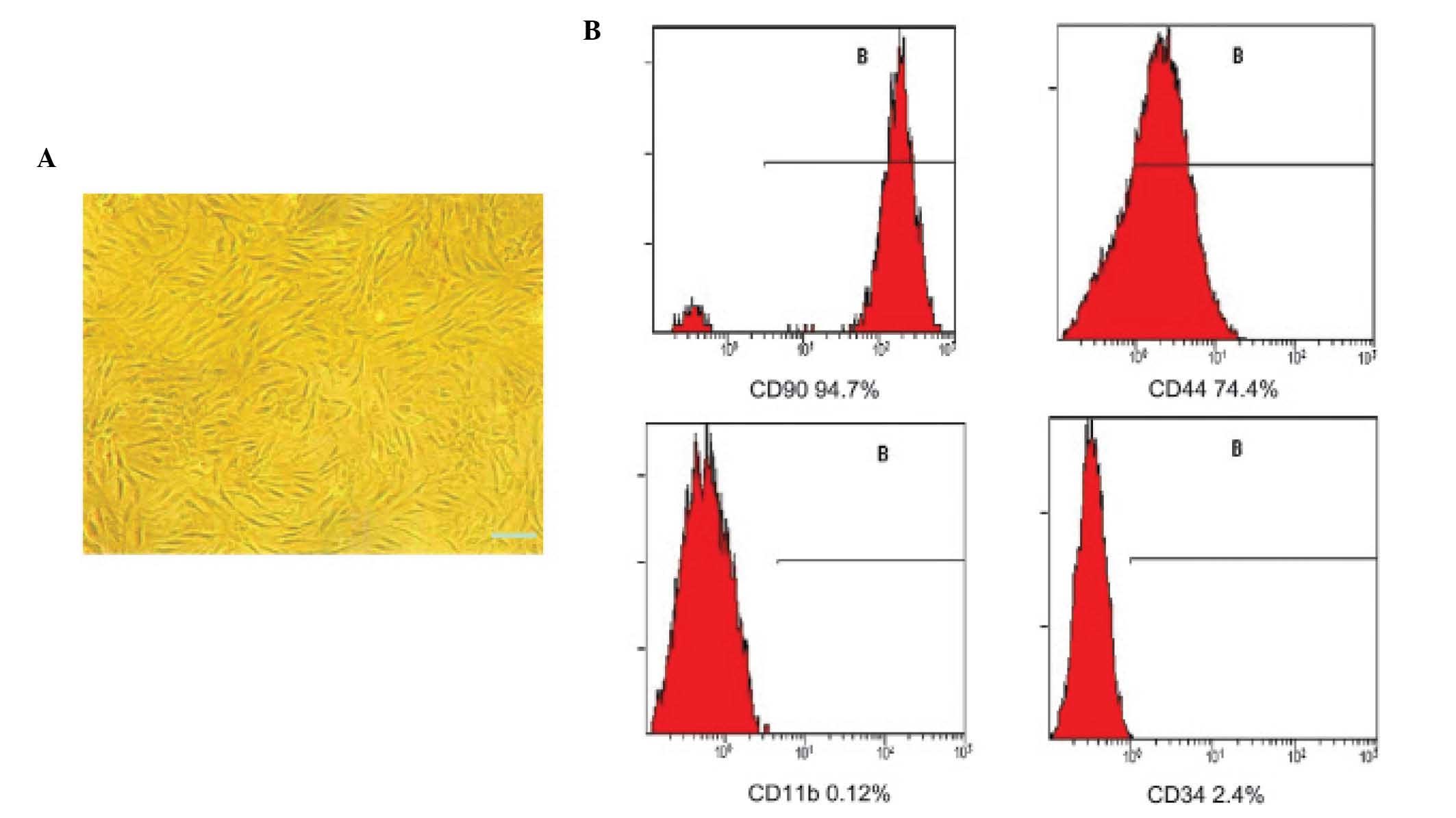
The third passage BMSCs uniformly assumed a
vortex-like or radiating appearance (Fig. 1A). The cells were positive for CD90
(94.7%) and CD44 (74.4%), and negative for CD11b (0.12%) and CD34
(2.4%; Fig. 1B). Therefore, the
third passage BMSCs were used in the subsequent experiments.
Effects of lentiviral-mediated
overexpression of GAP-43 on BMSC differentiation
Western blot analysis revealed that the protein
expression of GAP-43 was significantly increased in the LV5-GAP-43
group, compared with in the NC and blank groups (P<0.05;
Fig. 2A), suggesting that the
GAP-43/BMSC line was successfully established. BMSCs were observed
under fluorescence microscopy 3, 5 and 7 days after transduction
with LV5-GAP-43. The bodies of the BMSCs were contracted with
protruding processes at day 3, and the typical neuron-like cells
appeared at day 5. On day 7, the majority of the BMSCs exhibited a
neuronal phenotype and the prominences formed a network structure
(Fig. 2B). According to
semi-quantitative PCR, the BMSCs exhibited positive expression of
NSE, NF, nestin and βIII-tubulin in the LV5-GAP-43 group (Fig. 2C).
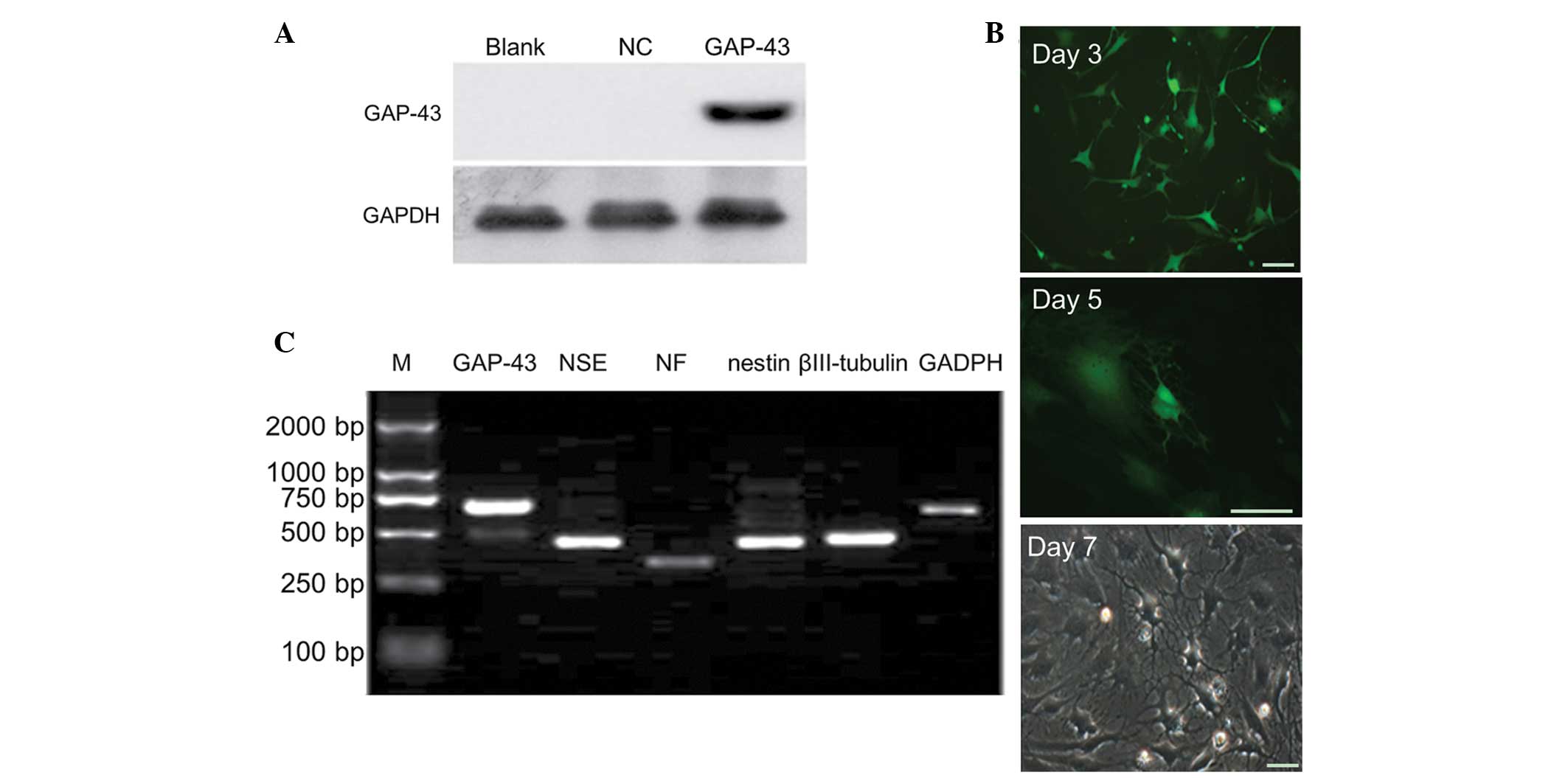 | Figure 2Overexpression of GAP-43 promotes the
differentiation of BMSCs into neuron-like cells. (A) Western blot
analysis demonstrated that the protein expression of the GAP-43 was
significantly increased in the LV5-GAP-43 group, compared with the
NC and Blank groups. (B) BMSCs were observed using microscopy and
it was revealed that BMSCs exhibited a neuronal phenotype following
transduction with LV5-GAP-43. (C) BMSCs expressed positive specific
neural markers for NSE, NF, nestin and βIII-tubulin in the
LV5-GAP-43 group, determined using semi-quantitative polymerase
chain reaction detection. Scale bar=50 µm. GAP-43,
growth-associated protein-43; BMSCs, bone marrow mesenchymal stem
cells; NSE, neuron-specific enolase; NF, neurofilament; NC,
transduced with negative control scramble lentiviral vector;
LV5-GAP-43, transduced with LV5-GAP-43; M, marker. |
Effects of lentiviral-mediated knockdown
of GAP-43 on BMSC differentiation
Western blot analysis revealed that
LV3-shRNA-GAP-43-619 was the most effective siRNA against GAP-43
(Fig. 3A) and was used for the
subsequent investigations. Following induction by retinal
cell-conditioned differentiation medium, western blot analysis
demonstrated that GAP-43 was expressed in the NC and Blank groups,
but not in the LV3-shRNA-GAP-43-619 group (Fig. 3B), suggesting that the
shGAP-43/BMSC line was successfully established. The BMSCs
exhibited a neuronal phenotype in the NC and Blank group; however,
the majority of BMSCs exhibited no marked morphological changes
(Fig. 3C) in the
LV3-shRNA-GAP-43-619 group, suggesting that neuron-like cells were
successfully induced by retinal cell-conditioned differentiation
medium. The expression levels of NSE, NF, nestin and βIII-tubulin
were significantly reduced in the LV3-shRNA-GAP-43-619 group,
compared with the NC and Blank groups (P<0.05; Fig. 3D). In addition, cell
immunofluorescence analysis revealed that ~80% of BMSCs expressed
GAP-43, nestin and NeuN in the NC group under induction conditions,
whereas this value was <30% in the LV3-shRNA-GAP-43-619 group
(Fig. 3E).
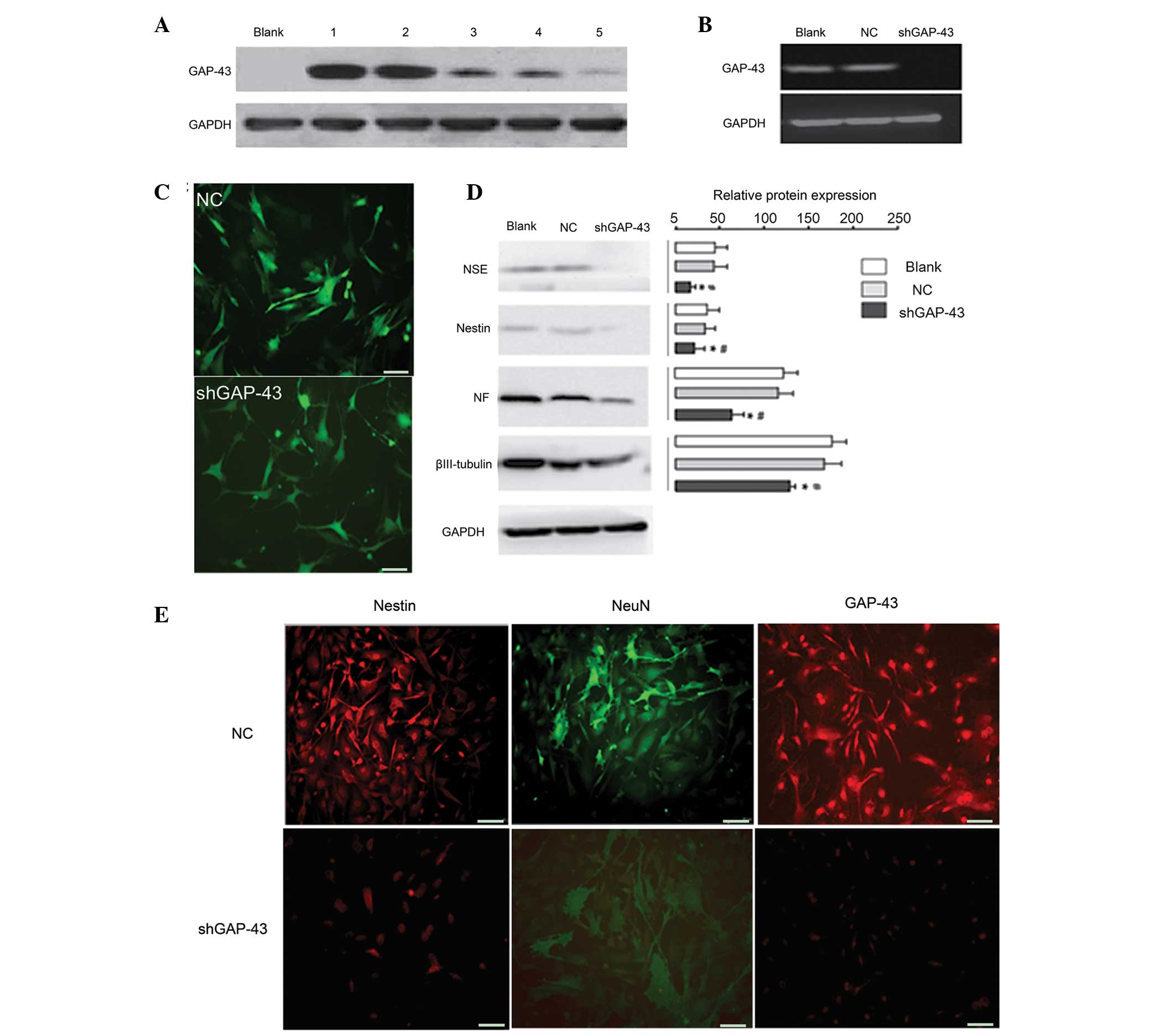 | Figure 3Ability of BMSCs to differentiate into
neuron-like cells is significantly weakened when the expression of
GAP-43 in BMSCs is inhibited. (A) Western blot analysis
demonstrated that LV3-shRNA-GAP-43-619 was the most effective siRNA
against GAP-43. 1, transduced with LV5-GAP-43; 2, transduced with
LV5-GAP-43 and LV3-shRNA-GAP-43-844; 3, transduced with LV5-GAP-43
and LV3-shRNA-GAP-43-1215; 4, transduced with LV5-GAP-43 and
LV3-shRNA-GAP-43-678; 5, transduced with LV5-GAP-43 and
LV3-shRNA-GAP-43-619. (B) Western blot analysis revealed that the
protein expression of the GAP-43 was significantly decreased in the
LV3-shRNA-GAP-43-619 group, compared with the NC and Blank groups.
(C) BMSCs exhibited a neuronal phenotype in the NC and Blank
groups; however, the majority of BMSCs exhibited no marked
morphological changes. (D) Western blot analysis demonstrated that
the expression levels of NSE, NF, nestin and βIII-tubulin were
decreased in the LV3-shRNA-GAP-43-619 group compared with NC and
Blank groups. (E) Cell immunofluorescence analysis revealed that
the expression of GAP-43, nestin and NeuN were decreased in the
LV3-shRNA-GAP-43-619 group, compared with the NC group under
induction conditions. Scale bar=50 µm.
*P<0.05, vs. Blank group; #P<0.05, vs.
NC group. GAP-43, growth-associated protein-43; shRNA, short
hairpin RNA; NC, transduced with negative control scramble
lentiviral vector; LV3-shGAP-43, transduced with
LV3-shRNA-GAP-43-619; BMSCs, bone marrow mesenchymal stem cells;
siRNA, small interfering RNA; NSE, neuron-specific enolase; NF,
neurofilament; NeuN, neuron-specific nuclear-binding protein. |
Effects of lentiviral-mediated GAP-43
gene-modified BMSCs on a rat model of TON
FG staining revealed that the labeled RGCs were
significantly decreased in the TON model rats, compared with the
normal rats (P<0.05; Fig. 4A).
This suggested that the TON model had been successfully
established. The labeled RGCs began to reduce at day 3 and reached
the lowest point at day 7, with no further reduction with longer
periods of time (Fig. 4B),
suggesting that the optimum period of nerve repair was limited to 3
days. At each time-point, the mRNA level of GAP-43 in the retinal
tissues was significantly increased in the GAP-43/BMSC group
(P<0.01), compared with the GFP/BMSC group, and decreased in the
shGAP-43/BMSC group, compared with the GFP/BMSC group (P<0.05;
Fig. 4C). Similar results were
observed for GAP-43 protein (P<0.05; Fig. 4D). The retinal tissue was harvested
at day 3 post-surgery (early injection) and subjected to H&E
staining. As observed in the sections from the sham group (Fig. 4E), all layers were normally
organized, the thickness of the retina was normal and no cell
apoptosis was evident. In the PBS group, the outer nuclear layer of
the retina became thinner, the inner nuclear layer of retina did
not change and the ganglion cell layer became gradually thinner. In
the GAP-43/BMSC group, all layers exhibited normal thicknesses and
no significant decrease in ganglion cells was observed. The
pathological changes in the GFP/BMSC group were similar to those in
the GAP-43/BMSC group, with the exception that ganglion cells
decreased, compared with the GAP-43/BMSC group. In the
shGAP-43/BMSC group, all layers became thinner and ganglion cells
exhibited marginal nuclear pyknosis, loss of nucleoli and
cytoplasmic vacuolation. The degree of pathological changes was
markedly improved in the GAP-43/BMSC group, compared with the
GFP/BMSC and shGAP-43/BMSC groups (Fig. 4E).
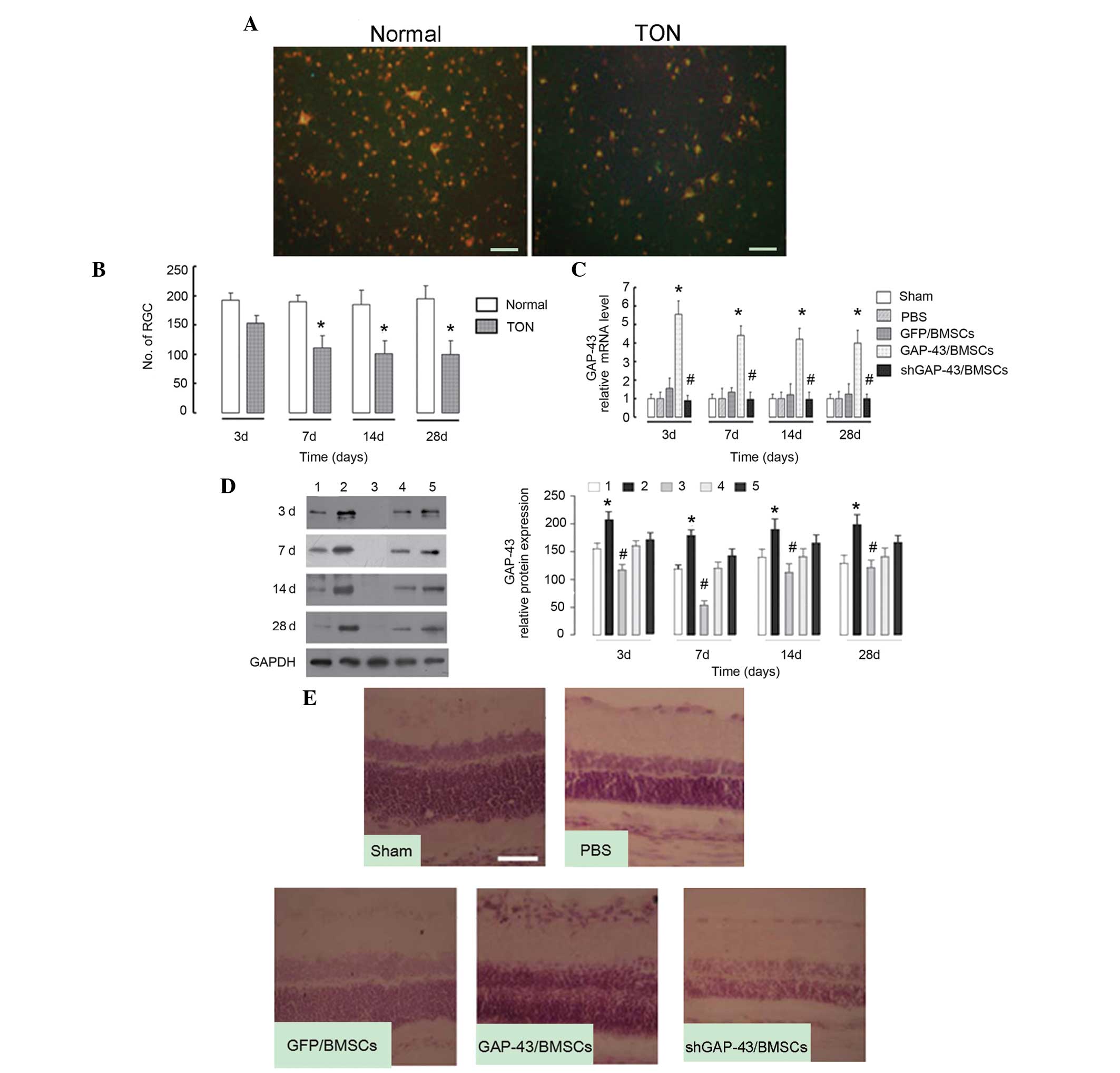 | Figure 4Intravitreally injected BMSCs
overexpressing GAP-43 promote the nerve repair process in a rat
model of TON. (A) Fluorogold staining revealed that the number of
labeled RGCs was decreased in the TON model rats, compared with
normal rats at day 7. (B) Number of labeled RGCs began to reduce at
day 3 and reached the lowest point at day 7, with no further
reduction as the duration extended in TON model rats, compared with
the normal rats. *P<0.05, vs. normal group. (C) mRNA
levels of GAP-43 in the retinal tissues was significantly increased
in the GAP-43/BMSC group, compared with the GFP/BMSC control group,
and decreased in the shGAP-43/BMSC group, compared with the
GFP/BMSCs group at days 3, 7, 14 and 28, determined using
quantitative polymerase chain reaction. (D) Protein expression of
GAP-43 was significantly increased in the GAP-43/BMSC group,
compared with the GFP/BMSCs group, and decreased in the
shGAP-43/BMSC group, compared with the GFP/BMSCs group at days 3,
7, 14 and 28, determined using western blotting. The graph depicts
the results of the densitometric quantification of the expression
of GAP-43. 1, sham group; 2, GAP-43/BMSC group; 3, shGAP-43/BMSCE
group; 4, PBS group; 5, GFP/BMSC group. (E) Hematoxylin and eosin
staining revealed that pathological changes in the retina were
markedly improved in the GAP-43/BMSC group, compared with the
GFP/BMSC and shGAP-43/BMSC groups at day 3. Scale bar=50 µm.
*P<0.01 GAP-43/BMSC, vs. GFP/BMSC group,
#P<0.05 shGAP-43/BMSC, vs. GFP/BMSC group. GAP-43,
growth-associated protein-43; shRNA, short hairpin RNA; NC,
transduced with negative control scramble lentiviral vector;
shGAP-43, transduced with shRNA-GAP-43-619; BMSCs, bone marrow
mesenchymal stem cells; RGCs, retinal ganglion cells; TON,
traumatic optic neuropathy; PBS, phosphate-buffered saline. |
Discussion
Diseases of the eye causing blindness can result in
irreversible damage to retinal nerve cells (15). The present study aimed to
transplant lentiviral-mediated GAP-43 gene-modified BMSCs into the
rat eye of a TON model in order to compensate for the loss of
retinal nerve cells. The results demonstrated that overexpression
of GAP-43 promoted the differentiation of BMSCs into neuron-like
cells; however, the ability of BMSCs to differentiate into
neuron-like cells was significantly weakened when the expression of
GAP-43 in BMSCs was inhibited. Intravitreally injected GAP-43/BMSCs
promoted the nerve repair process in the rat TON model.
In the present study, lentiviral-mediated
GAP-43/shGAP-43 transfection into BMSCs was performed and the
effect of GAP-43 on the differentiation of BMSCs was investigated.
In previous years, investigation into the differentiation of BMSCs
into nerve cells has received great attention. Experiments have
demonstrated that BMSCs can be differentiated into neural cells
under the action of appropriate inducers and cytokines in
vitro (16). The present study
demonstrated that the expression levels of specific neural markers,
including NSE, nestin, NF and βIII-tubulin were increased in the
GAP-43/BMSCs, and the morphology of the GAP-43/BMSCs was similar to
that of neuronal cells, which indicated that GAP-43 induced the
differentiation of BMSCs into neuron-like cells. GAP-43 is
important in the developmental process of the visual nervous system
(17–19) and is involved in retinal light
damage repair and regeneration. Meyer et al (20) observed that GAP-43 is continuously
expressed for an extended duration in mature RGCs in vitro.
These results offer an explanation for the directional
differentiation of GAP-43/BMSCs into neuron-like cells.
To further investigate the promoting effect of
GAP-43 on ON repair, lentiviral-mediated GAP-43 gene-modified BMSCs
were injected into the eye of a rat TON model at different
time-points. The TON model was successfully established and defined
using FG staining. It was observed that the number of RGCs
decreased with the extension of survival rate. The number of RGCs
began to reduce at day 3 and reached the lowest point at day 7,
with no further reduction with extended duration. Thus, it was
hypothesized that the loss of RGCs, caused by ON injury, might be
inhibited when rats were treated the injection of neurotrophic
factors or hormonal drugs within the first 3 days. However, beyond
a certain range (>7 or 14 days in the present study), nerve
damage is irreversible. It has been reported that transplanted
BMSCs survive, migrate and integrate in injured retinal tissue
(21–24). A previous study has indicated that
the proliferative ability of BMSCs transfected with exogenous genes
remains high (25). The present
study observed that lentiviral-mediated GAP-43 was successfully
expressed in the rats. Kurozumi et al (26) found that, following BDNF-modified
BMSC transplantation into the brain of a rat middle cerebral artery
occlusion model, the infarction area was markedly reduced and
neural function exhibited a degree of recovery. In addition, Haider
et al (27) also suggested
that adenovirus-mediated insulin-like growth factor 1 (IGF-1)
gene-modified BMSCs secrete IGF-1, and the area of myocardial
infarction was significantly reduced following transplantation of
gene-modified BMSCs into the rat model of myocardial infarction.
These data suggest that it is feasible to transplant gene-modified
BMSCs into rats.
Notably, in the early treatment group (day 3), the
degree of pathological changes was markedly improved in the
GAP-43/BMSC group, compared with the GFP/BMSC and shGAP-43/BMSC
groups, which indicated that intravitreally injected BMSCs
overexpressing GAP-43 promoted the process of ON repair in the rat
model of TON. GAP-43 is considered to be a molecular marker of
neural plasticity, including nerve growth and damage repair
(28–30). Ju et al (31) found that GAP-43 immunoreactivity is
present in the inner plexiform layer in the normal retina; however,
it appears in ganglion cells following ischemia and reperfusion,
and certain ganglion cells can regenerate through the upregulation
of GAP-43 in the ischemic rat retina. Vanselow et al
(32) observed RGCs of embryonic
and adult chickens in vitro and found that axonal forms
exhibited marked differences, but expressed GAP-43. Following
transplant of a segment of peripheral nerve to the vitreous body
and proximal stump of the ON, regenerative RGCs expressing GAP-43
are significantly increased, which confirms there is a correlation
between GAP-43 and RGC axonal regeneration (33,34).
Thus, the present study hypothesized that the transplantation of
lentiviral-mediated GAP-43 gene-modified BMSCs into the rat eye of
the TON model may compensate for the loss of RGCs and repair ON
injury to a certain extent. In addition, the pathological changes
in the GFP/BMSC group were similar to those in the GAP-43/BMSC
group, with the exception of the decrease in ganglion cells,
compared with the GAP-43/BMSCs group. Inoue et al (35) demonstrated that BMSCs injected into
an area of retinal degeneration in a Royal College of Surgeons rat,
successfully delayed the degeneration of the retina and preserved
some function of the retina. Arnhold et al (36) suggested the introduction of
adenovirally-transduced BMSCs into Wistar rats, which were able
differentiate into pigment epithelial cells. These data indicated
that BMSCs are involved in promoting ON repair without gene
modification.
In conclusion, the present study demonstrated that
GAP-43 promoted the differentiation of BMSCs into neuron-like
cells, and intravitreally injected BMSCs overexpressing GAP-43
promoted the process of nerve repair in a rat model of TON. These
findings suggested that lentiviral-mediated GAP-43 gene-modified
BMSCs may be a valuable method for protection of the retina and
from ON disease.
Acknowledgments
This study was supported by a grant from the General
Programs of National Natural Science Foundation (grant no.
30973265).
References
|
1
|
Pascolini D and Mariotti SP: Global
estimates of visual impairment: 2010. Br J Ophthalmol. 96:614–618.
2012. View Article : Google Scholar
|
|
2
|
Gu S, Xing C, Han J, Tso MO and Hong J:
Differentiation of rabbit bone marrow mesenchymal stem cells into
corneal epithelial cells in vivo and ex vivo. Mol Vis. 15:99–107.
2009.PubMed/NCBI
|
|
3
|
Wang HC, Brown J, Alayon H and Stuck BE:
Transplantation of quantum dot-labelled bone marrow-derived stem
cells into the vitreous of mice with laser-induced retinal injury:
survival, integration and differentiation. Vision Res. 50:665–673.
2010. View Article : Google Scholar
|
|
4
|
Li N, Li XR and Yuan JQ: Effects of
bone-marrow mesenchymal stem cells transplanted into vitreous
cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin
Exp Ophthalmol. 247:503–514. 2009. View Article : Google Scholar
|
|
5
|
Vijaya L, Asokan R, Panday M, Choudhari
NS, Ramesh SV, Velumuri L, Boddupalli SD, Sunil GT and George R:
Baseline risk factors for incidence of blindness in a South Indian
population: The chennai eye disease incidence study. Invest
Ophthalmol Vis Sci. 55:5545–5550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Basi GS, Jacobson RD, Virág I, Schilling J
and Skene JP: Primary structure and transcriptional regulation of
GAP-43, a protein associated with nerve growth. Cell. 49:785–791.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caprini M, Gomis A, Cabedo H,
Planells-Cases R, Belmonte C, Viana F and Ferrer-Montiel A: GAP43
stimulates inositol trisphosphate-mediated calcium release in
response to hypotonicity. EMBO J. 22:3004–3014. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Donovan SL, Mamounas LA, Andrews AM, Blue
ME and McCasland JS: GAP-43 is critical for normal development of
the serotonergic innervation in forebrain. J Neurosci.
22:3543–3552. 2002.PubMed/NCBI
|
|
9
|
Koutcherov Y, Mai JK and Paxinos G:
Hypothalamus of the human fetus. J Chem Neuroanat. 26:253–270.
2003. View Article : Google Scholar
|
|
10
|
Kaneda M, Nagashima M, Nunome T, Muramatsu
T, Yamada Y, Kubo M, Muramoto K, Matsukawa T, Koriyama Y, Sugitani
K, et al: Changes of phospho-growth-associated protein 43
(phospho-GAP43) in the zebrafish retina after optic nerve injury: a
long-term observation. Neurosci Res. 61:281–288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ivanov D, Dvoriantchikova G, Nathanson L,
Mckinnon SJ and Shestopalov VI: Microarray analysis of gene
expression in adult retinal ganglion cells. FEBS Lett. 580:331–335.
2006. View Article : Google Scholar
|
|
12
|
Hiyama A, Mochida J, Iwashina T, Omi H,
Watanabe T, Serigano K, Tamura F and Sakai D: Transplantation of
mesenchymal stem cells in a canine disc degeneration model. J
Orthop Res. 26:589–600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng Z, Zhang C and Chen J:
Lentivirus-mediated RNA interference of DC-STAMP expression
inhibits the fusion and resorptive activity of human osteoclasts. J
Bone Miner Metab. 31:409–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang B, Zhang P, Zhou D, Zhang J, Xu X
and Tang L: Intravitreal transplantation of human umbilical cord
blood stem cells protects rats from traumatic optic neuropathy.
PLoS One. 8:e699382013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zrenner E: Will retinal implants restore
vision? Science. 295:1022–1025. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Woodbury D, Schwarz EJ, Prockop DJ and
Black IB: Adult rat and human bone marrow stromal cells
differentiate into neurons. J Neurosci Res. 61:364–370. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moya KL, Jhaveri S, Schneider GE and
Benowitz LI: Immunohistochemical localization of GAP-43 in the
developing hamster retinofugal pathway. J Comp Neurol. 288:51–58.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reh TA, Tetzlaff W, Ertlmaier A and Zwiers
H: Developmental study of the expression of B50/GAP-43 in rat
retina. J Neurobiol. 24:949–958. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moya KL, Benowitz LI, Jhaveri S and
Schneider GE: Changes in rapidly transported proteins in developing
hamster reti-nofugal axons. J Neurosci. 8:4445–4454.
1988.PubMed/NCBI
|
|
20
|
Meyer RL, Miotke JA and Benowitz LI:
Injury induced expression of growth-associated protein-43 in adult
mouse retinal ganglion cells in vitro. Neuroscience. 63:591–602.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tomita M, Adachi Y, Yamada H, Takahashi K,
Kiuchi K, Oyaizu H, Ikebukuro K, Kaneda H, Matsumura M and Ikehara
S: Bone marrow-derived stem cells can differentiate into retinal
cells in injured rat retina. Stem Cells. 20:279–283. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kicic A, Shen WY, Wilson AS, Constable IJ,
Robertson T and Rakoczy PE: Differentiation of marrow stromal cells
into photoreceptors in the rat eye. J Neurosci. 23:7742–7749.
2003.PubMed/NCBI
|
|
23
|
Yu S, Tanabe T, Dezawa M, Ishikawa H and
Yoshimura N: Effects of bone marrow stromal cell injection in an
experimental glaucoma model. Biochem Biophys Res Commun.
344:1071–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sengupta N, Caballero S, Mames RN, Butler
JM, Scott EW and Grant MB: The role of adult bone marrow-derived
stem cells in choroidal neovascularization. Invest Ophthalmol Vis
Sci. 44:4908–4913. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee K, Majumdar MK, Buyaner D, Hendricks
JK, Pittenger MF and Mosca JD: Human mesenchymal stem cells
maintain transgene expression during expansion and differentiation.
Mol Ther. 3:857–866. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kurozumi K, Nakamura K, Tamiya T, Kawano
Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, et al:
BDNF gene-modified mesenchymal stem cells promote functional
recovery and reduce infarct size in the rat middle cerebral artery
occlusion model. Mol Ther. 9:189–197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haider HK, Jiang S, Idris NM and Ashraf M:
IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow
stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4
signaling to promote myocardial repair. Circ Res. 103:1300–1308.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Benowitz LI and Routtenberg A: GAP-43: An
intrinsic determinant of neuronal development and plasticity.
Trends Neurosci. 20:84–91. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Strittmatter SM, Vartanian T and Fishman
MC: GAP-43 as a plasticity protein in neuronal form and repair. J
Neurobiol. 23:507–520. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dinocourt C, Gallagher SE and Thompson SM:
Injury-induced axonal sprouting in the hippocampus is initiated by
activation of trkB receptors. Eur J Neurosci. 24:1857–1866. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ju WK, Gwon JS, Park SJ, Kim KY, Moon JI,
Lee MY, Oh SJ and Chun MH: Growth-associated protein 43 is
up-regulated in the ganglion cells of the ischemic rat retina.
Neuroreport. 13:861–865. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vanselow J, Müller B and Thanos S:
Regenerating axons from adult chick retinal ganglion cells
recognize topographic cues from embryonic central targets. Vis
Neurosci. 6:569–576. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schaden H, Stuermer CA and Bähr M: GAP-43
immunoreac-tivity and axon regeneration in retinal ganglion cells
of the rat. J Neurobiol. 25:1570–1578. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ng TF, So KF and Chung SK: Influence of
peripheral nerve grafts on the expression of GAP-43 in regenerating
retinal ganglion cells in adult hamsters. J Neurocytol. 24:487–496.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Inoue Y, Iriyama A, Ueno S, Takahashi H,
Kondo M, Tamaki Y, Araie M and Yanagi Y: Subretinal transplantation
of bone marrow mesenchymal stem cells delays retinal degeneration
in the RCS rat model of retinal degeneration. Exp Eye Res.
85:234–241. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Arnhold S, Heiduschka P, Klein H, Absenger
Y, Basnaoglu S, Kreppel F, Henke-Fahle S, Kochanek S, Bartz-Schmidt
KU, Addicks K and Schraermeyer U: Adenovirally transduced bone
marrow stromal cells differentiate into pigment epithelial cells
and induce rescue effects in RCS rats. Invest Ophthalmol Vis Sci.
47:4121–4129. 2006. View Article : Google Scholar : PubMed/NCBI
|