Introduction
Alzheimer's disease (AD), which is the most common
age-associated neurodegenerative disorder, causes progressive
dementia, and microglia-mediated neuroinflammation is key in the
pathogenesis of AD (1). Microglia
are the resident immune cells of the brain, which are important in
host defense and tissue repair in the central nervous system
(2). In response to brain injury
or immunological stimuli, including amyloid β (Aβ) or
lipopolysaccharide (LPS), microglia are activated, producing
various proinflammatory mediators, including tumor necrosis
factor-α (TNF-α), interleukin-1β (IL-1β), nitric oxide (NO), and
reactive oxygen species (ROS) (3).
Accumulation of these mediators contributes to neuronal damage and
aggravates AD progression. Therefore, the production of
inflammatory factors by microglia requires inhibition to prevent
neuroinflammation and neurodegeneration in AD.
Resveratrol
(3,4′,5-trihydroxy-trans-stilbene), which is a natural,
nonflavonoid, polyphenolic compound, is present in several plant
species, including giant knotweeds, peanuts, mulberries and grapes,
and is found in red wine (4).
Evidence suggests that resveratrol exerts neuroprotective effects
against neurodegenerative diseases, due to its anti-inflammatory
properties (5,6). In vitro studies have
demonstrated that resveratrol inhibits the production of
LPS-induced NO and TNF-α in murine microglial cells (7,8), as
well as the production of prostaglandin E2 and free radicals in rat
primary microglia (9). In
addition, previous studies have demonstrated that resveratrol
prevents microglial activation and subsequent inflammatory-mediator
release by inhibiting transcriptional factors, including nuclear
factor-κB (10,11). The neuroprotective effects of
resveratrol have been detected in numerous studies; however, the
mechanisms underlying its beneficial effects remain poorly
understood.
NADPH oxidase has been confirmed as an important
contributor to microglia-mediated neuroinflammation and
neurodegeneration (12,13). Previous studies have demonstrated
that soluble oligomeric (o)Aβ, which is present in the cortex of
patients with AD, exhibits more marked correlation with AD
symptoms, compared with fibrillar Aβ in amyloid plaques (14,15).
Using the BV-2 murine microglial cell line, our previous study
demonstrated that oAβ induced the activated properties of
microglia, and activation was inhibited by the NADPH oxidase
inhibitors, diphenyleneiodonium (DPI) and apocynin. These results
suggested that NADPH oxidase may be involved in the activation of
oAβ-induced microglia (16). The
present study aimed to use BV-2 microglia cultures to investigate
the inhibitory effects of resveratrol on the activation of
oAβ-induced microglia and further investigate the role of NADPH
oxidase.
Materials and methods
Regents
Dulbecco's modified Eagle's medium and fetal bovine
serum were purchased from Gibco Life Technologies (Grand Island,
NY, USA). Resveratrol, 2′, 7′-dichlorodihydrofluorescein diacetate
(DCFH-DA), DPI, Hoechst 33258 and Hoechst 33342 were purchased from
Sigma–Aldrich (St. Louis, MO, USA). Mouse monoclonal
anti-bromodeoxyuridine (BrdU) antibody (1:200; cat. no. MS-1508;
Lab Vision, Fremont, CA, USA), polyclonal fluorescein isothioyanate
(FITC)-conjugated goat anti-mouse immunoglobulin G (1:200; cat. no.
F9006; Sigma–Aldrich) were used, and
1,1,1,3,3,3-hexafluoro-2-propanol (used in oAβ preparation) was
obtained from J&K Scientific Ltd. (Beijing, China). Mouse
monoclonal anti-gp91phox and rabbit anti-p47phox (1:200, cat. no.
sc-74514 Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). TNF-α
and IL-1β ELISA kits were purchased from Diaclone (Besançon,
France), and the BrdU cell proliferation ELISA kit was purchased
from Roche Diagnostics (Mannheim, Germany).
Preparation of peptides
The Aβ1-42 peptide was purchased from AnaSpec
(Fremont, CA, USA). OAβ was prepared as follows. Briefly, Aβ1-42
peptide was dissolved in 1 mM 1,1,1,3,3,3 hexafluoro-2-propanol,
immediately aliquoted, and dried under a vacuum. The residual
peptide was stored at −20°C and subsequently dissolved in DMSO to 5
mM, which was then diluted with Millipore water (EMD Millipore,
Billerica, MA, USA) to a final concentration of 50 μM prior
to use. The oAβ fraction was obtained following 24 h of gentle
agitation at 4°C and the conformation was confirmed using atomic
force microscopy (Dimension 3100; Veeco, Plainview, NY, USA), as
described in our previous study (16).
Cell culture
BV-2 murine microglial cells were provided by
Professor Y. C. Kim (Seoul National University, Seoul, South
Korea). The cells (5×104 cells/ml) were cultured, as
described in our previous study (16). The BV-2 murine microglial cells
were obtained by immortalization of primary murine microglial
cultures with a v-raf/v-myconcogene-containing retrovirus (J2),
which retained the majority of the morphological, phenotypic and
functional properties of freshly isolated microglia. The BV-2 cells
were cultured in Dulbecco's modified Eagle's medium supplemented
with 10% heat-inactivated fetal bovine serum at 37°C in a
humidified 5% CO2 atmosphere. Stock cells were passaged
two to three times per week with a 1:5 split ratio and used within
six passages.
Measurement of BrdU incorporation using
ELISA
The BV-2 cells (5×104 cells/ml) were
plated into 96 -well microtiter plates. The microglial cells were
then treated with 20 μg/ml oAβ, either alone or in
combination with resveratrol (0.3, 1, 3, 10 or 30 μM).
Following 24 h incubation, the cells were assessed, as described
previously (16). Briefly,
following 24 h incubation, the cells were assessed for novel DNA
synthesis using a BrdU cell proliferation ELISA kit (Roche
Diagnostics, Mannheim, Germany). BrdU (10 μM) was added to
the plate for 2 h, following which the cells were fixed, according
to the manufacturer's instructions. BrdU incorporation was detected
by the addition of anti-BrdU antibody with peroxidase activity.
Substrate solution was added, and the resultant color was measured
using a Biotek Synergy HT plate reader (Biotek Instruments,
Winooski, VT, USA) at absorbance wavelengths of 370 and 492 nm.
Cell count determination using a
high-content screening (HCS) system
The BV-2 cells were incubated under the same
conditions and with the same treatments as were used for the
measurement of BrdU incorporation. The number of microglial cells
was measured using an IN Cell Analyzer 2000 HCS system (GE
Healthcare Life Sciences, Little Chalfont, UK), as described
previously (16).
Fluorescence imaging of the
double-labeled microglial cells, acquired using the HCS system
The BV-2 cells (5×104 cells/ml) were
plated into 96-well microtiter plates. The microglial cells were
then treated with oAβ (20 μg/ml), either alone or with
resveratrol (3, 10 or 30 μM). Following 24 h incubation, new
DNA synthesis of the microglia was examined, as described
previously (16). Briefly,
following 24 h incubation, novel DNA synthesis of microglia was
examined by adding BrdU (10 μM) to the culture medium.
Following another 24 h culture, the cells were fixed in 4%
paraformaldehyde at 4°C. After 30 min, the paraformaldehyde was
removed, followed by three washes with PBS. The preparations were
treated with HCl (2 M) for 20 min, sodium borate (0.1 M) for 15
min, and 0.2% Triton X-100 for 10 min at room temperature (HCl,
sodium borate and Triton X-100 were all from Sigma–Aldrich).
Following each step, the plates were washed three times with PBS.
Subsequently, nonspecific binding sites were blocked with 5% bovine
serum albumin in PBS. Microglial cells were then successively
incubated in mouse monoclonal anti-BrdU antibody overnight at 4°C
and FITC-conjugated goat anti-mouse IgG for 1 h. Cultures processed
without the primary antibody or without BrdU were devoid of
labeling, which indicated the absence of nonspecific labeling. Cell
nuclei were stained with Hoechst 33342. Fluorescence images were
acquired using the IN Cell Analyzer 2000 system with the following
filter sets: Excitation, 360 and 480 nm; emission, 460 and 535 nm.
Fluorescence images were captured using the IN Cell Analyzer 2000
system with the following filter sets: Excitation, 360 and 480 nm;
emission, 460 and 535 nm.
Measurement of intracellular ROS
The levels of intracellular ROS were measured using
a DCFH-DA oxidation assay. The BV-2 cells were plated into 96-well
microtiter plates at a density of 3×105 cells/ml and
treated with oAβ (20 μg/ml) for 2 h, either alone or in
combination with resveratrol (1, 3, 10 or 30 μM) or DPI (5
μM). Following treatment, the cells were washed with
phosphate-buffered saline. DCFH-DA (20 μM) was then added,
and the cells were incubated for 40 min at 37°C. Fluorescence was
measured using a Biotek Synergy 2 plate reader (Biotek Instruments)
at an excitation wavelength of 485 nm and an emission wavelength of
528 nm.
Determination of nitric oxide (NO)
release
The concentration of nitrite (NO2), which
accumulated in the culture supernatant fraction was measured,
according to the Griess reaction (17). The BV-2 microglia were plated into
96-well microtiter plates at a density of 3×105 cells/ml
and treated with oAβ (20 μg/ml) for 24 h, either alone or in
combination with resveratrol (1, 3, 10 or 30 μM μM)
or DPI (5 μM). Minocycline (30 μM; Sigma–Aldrich) was
used as a positive control. The culture supernatant fluid (50
μl) was then collected from the cells and mixed with 50
μl Griess reagent (part I, 1% sulfanilamide; part II, 0.1%
naphthylethylene diamide dihydrochloride and 2% phosphoric acid;
Sigma–Aldrich) at room temperature. Following 15 min incubation,
the absorbance was measured at 540 nm using a Biotek Synergy HT
plate reader (Biotek Instruments).
Determination of TNF-a and IL-1β
The BV-2 cells (3×104 cells/ml) were
incubated under the same conditions and with the same treatments as
for the measurement of NO release. The concentrations of TNF-α and
IL-1β in the culture medium were measured using ELISA kits,
according to the manufacturer's instructions. Briefy, 100 μl
of each standard, sample and zero were added in duplicate to the
appropriate number of wells. Subsequently, 50 μl diluted
biotinylated anti-mTNF-α or biotinylated anti-mIL-1β was added to
all wells, covered with a plastic plate cover and incubated at room
temperature for 3 h. The cover was then removed and the plate
washed, as follows: Liquid was aspirated from each well; 0.3 ml 1X
washing solution was dispensed into each well; contents of each
well were aspirated; and the first two steps were repeated another
two times. Subsequently, 100 μl streptavidin-horseadish
peroxidase solution was added to each well, covered with a plastic
plate cover and incubated at room temperature for 30 min. Following
incubation, the washing step was repeated and 100 μl of
ready-to-use 3,3′,5,5′-tetramethylbenzidine substrate solution was
added into all wells. Following incubation at room temperature in
the dark for 10–20 min, 100 μl Stop reagent was added to
each well, and the absorbance at 450 nm was measured using a Biotek
Synergy HT plate reader.
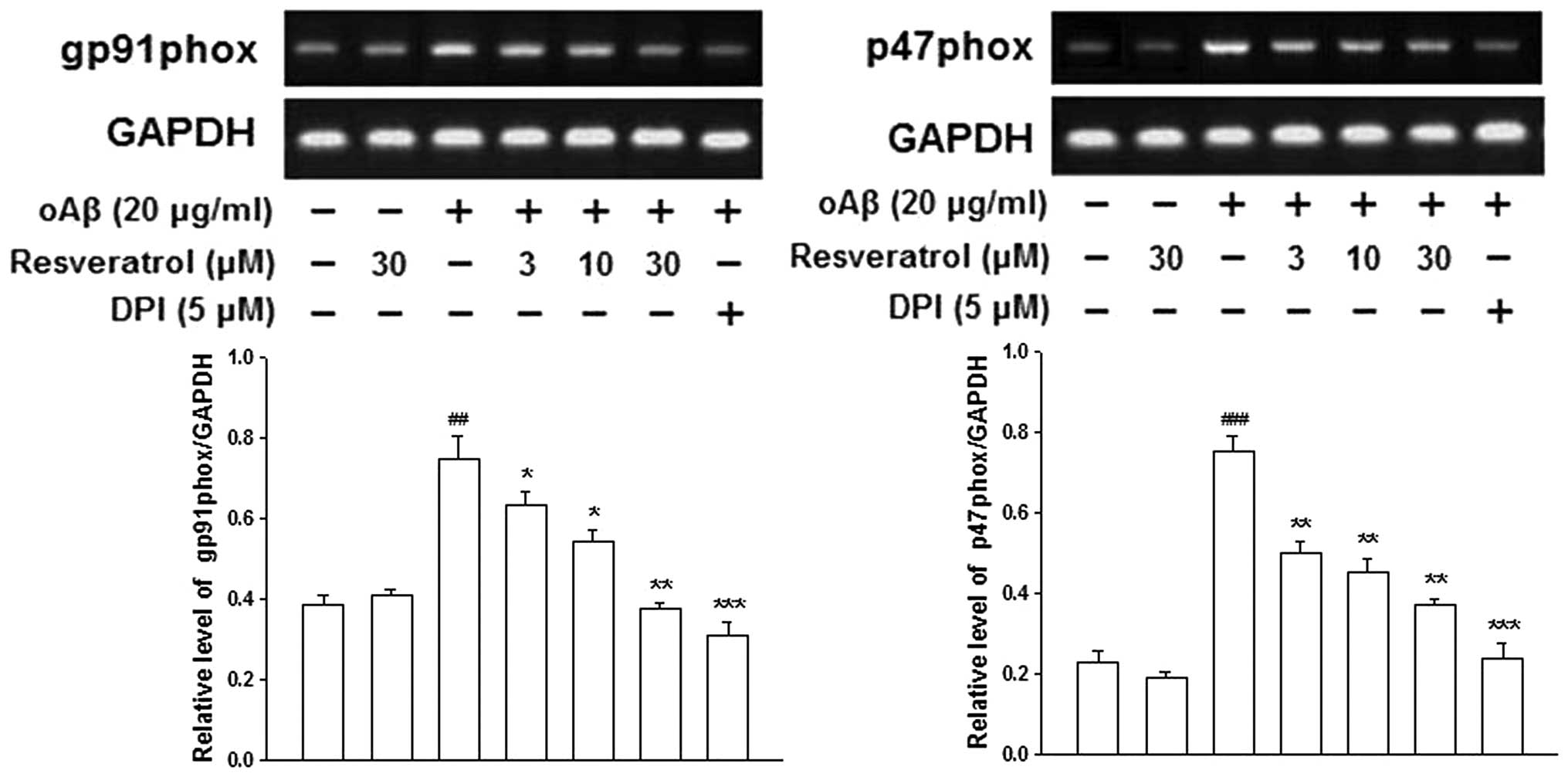
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR) analysis
The BV-2 microglial cells were treated with oAβ (20
μg/ml), either alone or together with resveratrol (3, 10 or
30 μM) or DPI (5 μM) for 12 h. Total RNA was
extracted from the cells using TRIzol® (Invitrogen Life
Technologies, Carlsbad, CA, USA). Total RNA was reverse transcribed
using a cDNA First-Strand Synthesis system (Fermentas, Thermo
Fisher Scientific, Inc., Pittsburgh, PA, USA). The PTC-200 PCR
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for
the PCR analysis. The quantity of sample analyzed was as follows:
cDNA (1 μl), 1X PCR buffer (2.5 μl), dNTPs (2.5 mM; 2
μl); primers (10 pmol; 1 μl). The cDNA was amplified
by PCR using specific primers for gp91phox, p47phox and GAPDH. The
primer sequences were as follows: gp91phox, sense
5′-GCACTGGAACCCCTGAGAAA-3′ and antisense
5′-GGTTTATGATGATGGGCCTAA-3′; p47phox, sense
5′-ACATCACAGGCCCCATCATCCTTC-3′ and anti-sense
5′-ATGGATTGTCCTTTGTGCC-3′; and GAPDH, sense
5′-GGTGCTGAGTATGTCATGGA-3′ and antisense
5′-TTCAGCTCTGGGATGACCTT-3′. Primers were from Huamei Biotechnology,
Ltd. (Wuhan China). The following PCR cycling conditions were
applied: gp91phox, 34 cycles of denaturation at 94°C for 30 sec,
annealing at 56°C for 30 sec and extension at 72°C for 45 sec;
p47phox, 28 cycles of denaturation at 94°C for 30 sec, annealing at
58°C for 30 sec and extension at 72°C for 45 sec; GAPDH, 32 cycles
of denaturation at 94°C for 30 sec, annealing at 55°C for 45 sec
and extension at 72°C for 45 sec. The PCR products were then
separated on 1.2% agarose gels, and visualized with ethidium
bromide (0.5 μg/mL; Sigma–Aldrich). The mRNA expression
levels of GAPDH were used for standardization.
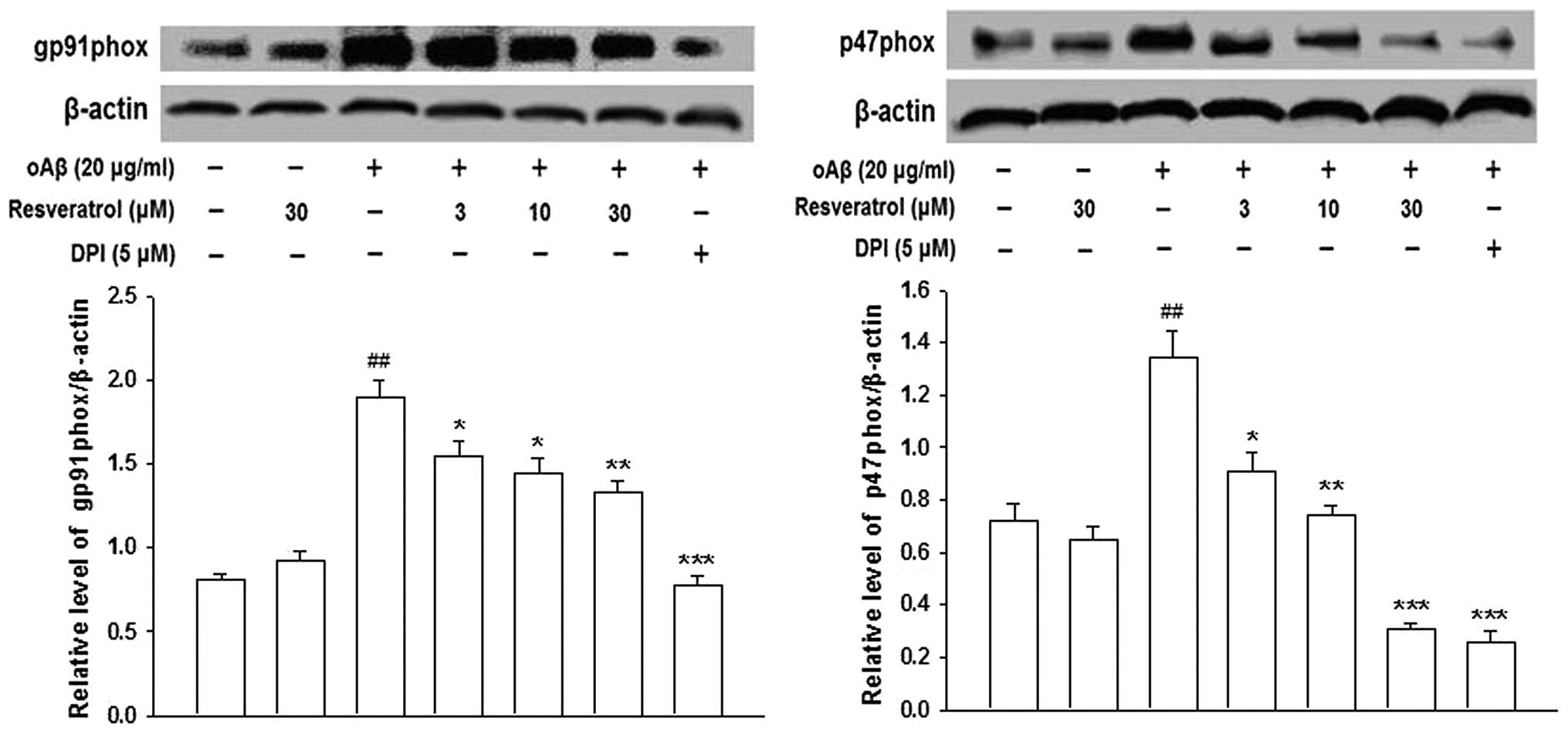
Western blot analysis
In order to determine the protein expression levels
of gp91phox and p47phox, the BV-2 microglial cells were treated
with oAβ (20 μg/ml), either alone or together with
resveratrol (3–30 μM) or DPI (5 μM) for 24 h. The
cells were then washed with ice-cold PBS and lysed for 10 min using
radioimmunoprecipitation lysis buffer (Santa Cruz Biotechnology,
Inc.), containing 50 mM Tris-Cl (pH 7.4), 150 mM NaCl, 0.1% SDS, 1%
NP-40, 1% sodiumdeoxycholate, 1 mM phenylmethyl-sulfonylfluoride, 1
mM EDTA, 1 μg/ml pepstatin, 1 μg/ml leupeptin and 1
μg/ml aprotinin. The protein concentration in the
supernatant fluid of the lysate was measured using a bicinchoninic
acid protein assay (Pierce Biotechnology, Inc., Rockford, IL, USA).
Equal quantities (60 μg) of protein were separated by 12%
SDS-PAGE (Ameresco, Solon, OH, USA) and were then transferred onto
0.45 μm polyvinylidene fuoride membranes (EMD Millipore,
Bedford, MA, USA). The membranes were then blocked in blocking
buffer (5% skimmed milk) and incubated overnight with primary
antibodies at 4°C, followed by incubation with horseradish
peroxidase-conjugated goat anti-mouse (1:2,000; cat. no. A4416) and
horseradish peroxidase-conjugated goat anti-rabbit (1:2,000; cat.
no. A6154) secondary antibodies for 1 h at room temperature,
obtained from Sigma Aldrich. Following three washes in
tris-buffered saline containing 0.1% Tween 20 (Amaresco),
immunoreactive bands were visualized using enhanced
chemiluminescence reagent (Beyotime Institute of Biotechnology,
Shanghai, China). The protein expression levels of β-actin were
used for standardization.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean of three experiments performed in triplicate. One-way
analysis of variance and Student's t-test were used for statistical
analysis (SPSS 16.0; SPSS, Inc., Chicago, IL, USA). ImageJ software
(version 1.44; National Institutes of Health, Bethesda, MA, USA)
was used to quantify mRNA and protein levels in the RT-PCR and
western blot assays, respectively. P<0.05 was considered to
indicate a statistically significant difference.
Results
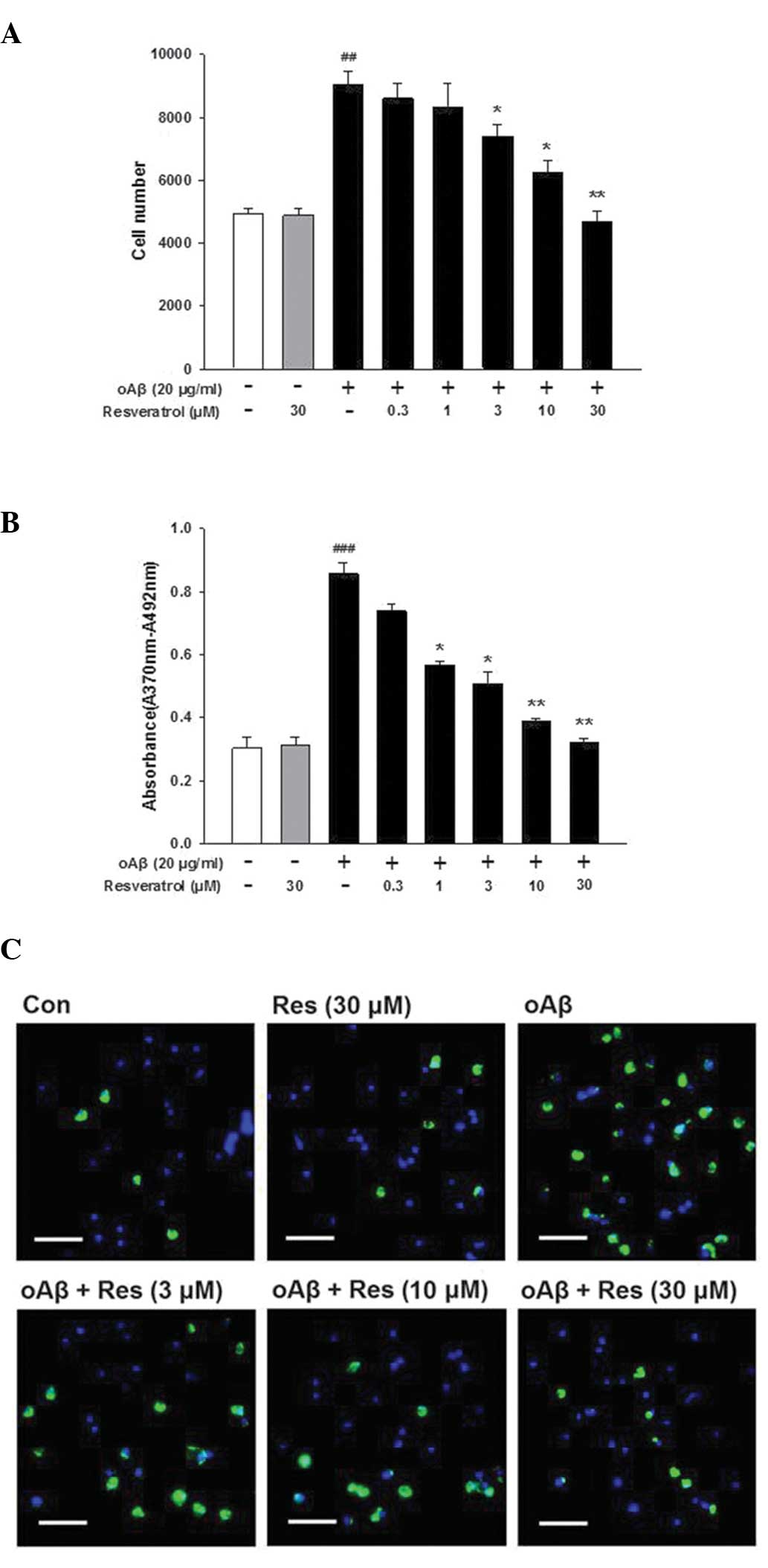
Inhibitory effects of resveratrol on
oAβ-induced BV-2 microglial cell proliferation
Treatment of the cells with oAβ (20 μg/ml)
for 24 h induced the proliferation of the cultured microglia;
however, this effect was inhibited by various concentrations of
resveratrol (Fig. 1A).
Furthermore, higher levels of BrdU incorporation were observed when
the microglial cells were treated with oAβ (20 μg/ml) for 24
h, and this effect was also inhibited by resveratrol (Fig. 1B). In controlled trials,
resveratrol alone (30 μM) did not affect microglial
proliferation (Fig. 1A and B); and
did not decrease microglial viability, as assessed using an MTT
reduction assay (data not shown). The fluorescence images of the
microglial cells double-labeled with Hoechst 33342 and BrdU were
concordant with the results obtained from the HCS and BrdU assays
(Fig. 1C). These results indicated
that resveratrol inhibited the proliferation of oAβ-induced
microglia.
Inhibitory effects of resveratrol on
oAβ-induced BV-2 microglial proinflammatory mediator release
In the present study, resveratrol decreased the
levels of oAβ-induced ROS in a dose-dependent manner (Fig. 2A). The NADPH-oxidase inhibitor,
DPI, was used as a positive control. Neither resveratrol nor DPI
had an effect on the production of microglial ROS in the absence of
oAβ. In addition, NO secretion increased in response to oAβ
treatment, but was inhibited by resveratrol in a dose-dependent
manner (Fig. 2B). MINO was used as
a positive control. These data indicated that oAβ-induced NO
secretion was inhibited by resveratrol. Furthermore, when the cells
were treated with resveratrol in combination with oAβ, a
significant inhibition in the production of oAβ-induced TNF-α and
IL-1β was observed. By contrast, resveratrol exerted no effect on
microglial TNF-α and IL-1β production in the absence of oAβ
(Fig. 2C and D). These results
suggested that oAβ peptide activated the microglia to produce and
release ROS, NO, TNF-α and IL-1β, and these effects were inhibited
by treatment with resveratrol.
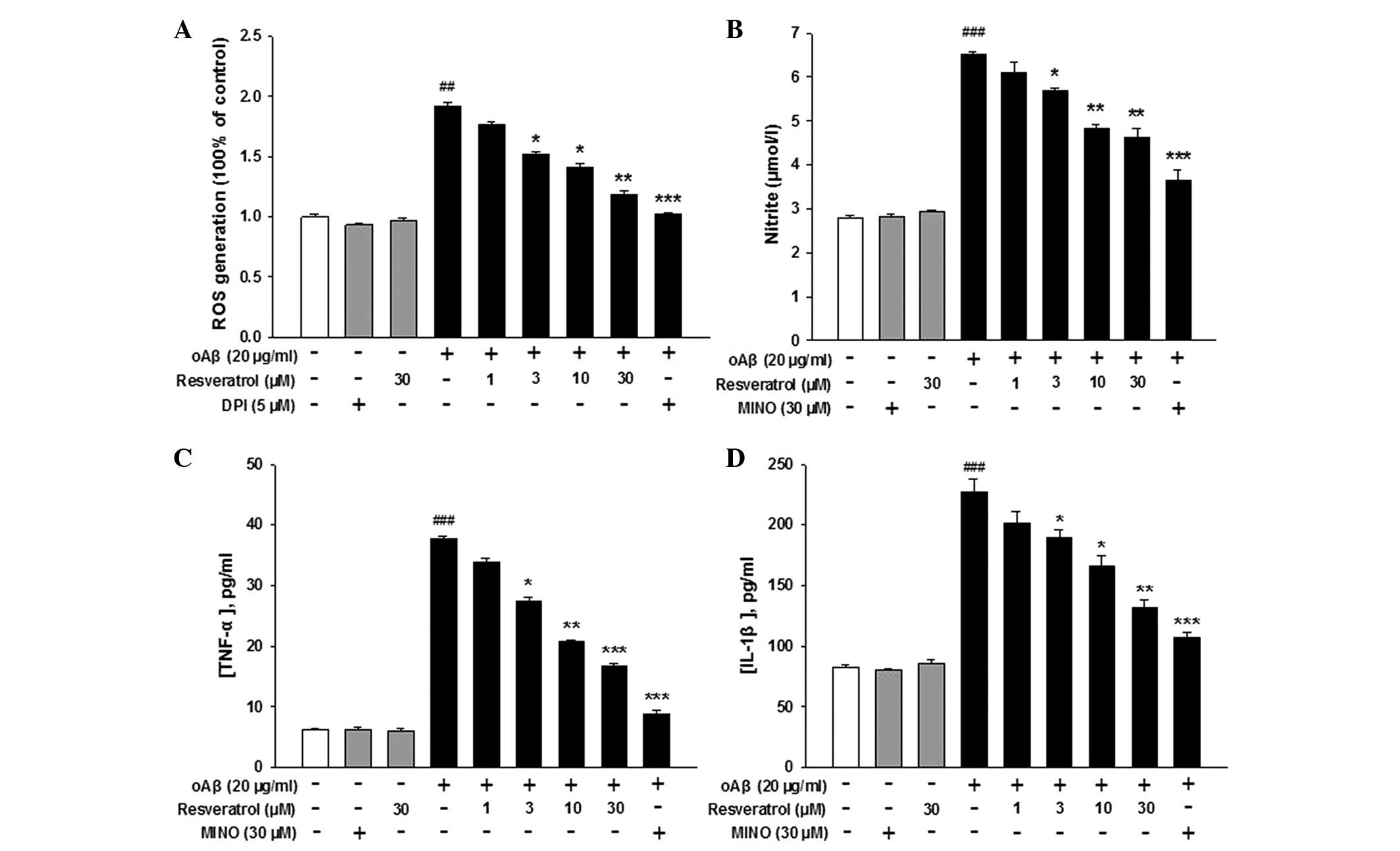 | Figure 2Effects of resveratol on oAβ-induced
pro-inflammatory mediator release in BV-2 microglia. Microglial
cultures were incubated with 20 μg/ml oAβ, with or without
various concentrations of resveratrol for 2 h to measure ROS, and
for 24 h to measure the levels of NO, TNF-α and IL-1β. DPI (5
μM) or MINO (30 μM) were used as positive controls.
Following incubation, the cell media were collected for measurement
of the levels of (A) ROS, (B) NO, (C) TNF-α and (D) IL-1β. Data are
presented as the mean ± standard error of the mean from three
independent experiments. ##P<0.01 and
###P<0.001, compared with the control group;
*P<0.05 **P<0.01 and
***P<0.001, compared with the oAβ group. oAβ,
oligomeric amyloid β; ROS, reactive oxygen species; NO, nitric
oxide; TNF, tumor necrosis factor; IL, interleukin; DPI,
diphenyleneiodonium; MINO, minocycline. |
Inhibitory effects of resveratrol on
oAβ-induced microglial activation may be mediated by NADPH
oxidase
NADPH oxidase is the key enzyme, which is required
for the production of ROS in activated microglia. The activation of
NADPH oxidase requires the p47phox, p67phox and p40phox cytosolic
subunits and the p22phox and gp91phox catalytic subunits (18). RT-PCR and western blotting
demonstrated that the presence of oAβ increased the mRNA and
protein expression levels of gp91phox and p47phox in the microglia;
however, this increase was prevented by pre-treatment with
resveratrol (Figs. 3 and 4). These results suggested that
resveratrol inhibited oAβ-induced microglial activation by
inhibiting the expression of NADPH oxidase, and that the gp91phox
and p47phox NADPH oxidase subunits were involved in this
reaction.
Discussion
Cell proliferation is a key aspect in microglial
activation in response to brain damage or injury; proliferation can
be quantified by cell counting or incorporation experiments,
including BrdU or 3H-TdR assays (19). In addition, it has been reported
that oAβ induces the proliferation of microglia (20). A previous study demonstrated that
the pro-proliferative activity of oAβ is regulated by NADPH oxidase
(21). The present study
investigated the effects of resveratrol on oAβ-stimulated
microglial proliferation, using an HCS system and BrdU assay. The
results demonstrated that oAβ induced the proliferation of
microglia, and this effect was markedly inhibited by resveratrol,
suggesting that the anti-inflammatory effect of resveratrol may
contribute to the inhibition of microglial proliferation. To the
best of our knowledge, this is the first study to report these
findings.
Previous studies have demonstrated that microglia
are the predominant source of NADPH oxidase in the brain (22,23).
Among various neurotoxic factors produced by activated microglia,
NADPH oxidase-derived ROS are important in microglia-mediated
neuroinflammation. ROS are involved in host defense systems by
destroying invading pathogens and inducing the production of
various antioxidant enzymes in host cells (24). Previous studies have revealed that
ROS also act as secondary messengers to enhance gene expression by
encoding a variety of pro-inflammatory factors (25). The present study demonstrated that
resveratrol reduced ROS production in oAβ-activated BV-2 microglial
cells. Although resveratrol has previously been reported to reduce
oxidative effects by functioning as a ROS scavenger (26), a novel finding in the present study
was that resveratrol inhibited oAβ-induced activation of microglial
NADPH oxidase and the consequent production of ROS.
Previous studies have reported that resveratrol
downregulates the mRNA expression of NADPH oxidase 4, which is a
homolog of gp91phox and is the most abundant NADPH
oxidase-catalytic subunit in human umbilical vein endothelial cells
(27). However, the role of
resveratrol in the oAβ-induced microglial expression of NADPH
oxidase subunits remains to be elucidated. The molecular
mechanistic experiments performed in the present study demonstrated
that resveratrol inhibited the oAβ-induced mRNA and protein
expression of the gp91phox and p47phox NADPH oxidase subunits,
leading to decreased ROS production. Although resveratrol affects
the expression of gp91phox, which is the dominant NADPH oxidase and
the major superoxide-generating enzyme in inflamed microglia
(28), the effects of resveratrol
on the phosphorylation and translocation of NADPH oxidase subunits
and on NADPH oxidase activity require further investigation.
The results of the present study demonstrated that
resveratrol exerted potent inhibitory effects on the oAβ-induced
production of NO, TNF-α and IL-1β in the BV-2 microglia cultures.
These findings were concordant with the results of previous
studies, in which resveratrol was found to inhibit the LPS-induced
production of pro-inflammatory factors in primary microglia or
microglial cell lines (8,9). NO, TNF-α and IL-1β are regarded as
important substances in microglial activation (29,30).
Our previous study demonstrated that oAβ alone induces the
production of these pro-inflammatory factors in microglia, and
NADPH oxidase is important in these effects (16). Accordingly, in the present study,
inhibition of NADPH oxidase by resveratrol decreased the levels of
pro-inflammatory factors released by the oAβ-activated microglial
cells. It has been widely accepted that increased levels of
cytokines and chemokines are released by activated microglia, which
result in chronic neuroinflammation and are partially responsible
for neuronal damage and neurodegeneration in the brains of patients
with AD (31). This suggests that
the inhibitory effects of resveratrol on oAβ-induced microglial
pro-inflammatory factor release partly contribute to its
neuroprotective and cognitive improvement effects in AD.
In conclusion, the present study demonstrated that
resveratrol inhibited oAβ-induced BV-2 microglial activation,
resulting in the inhibition of cell proliferation and reductions in
the secretion of various pro-inflammatory factors. Subsequent
mechanistic investigation demonstrated that the inhibitory effects
of resveratrol on microglial activation were mediated by NADPH
oxidase. Furthermore, the gp91phox and p47phox NADPH oxidase
subunits were important in these effects. These results suggest
that resveratrol is a valuable natural product, possessing
therapeutic potential against AD.
Acknowledgments
The present study was supported by the China
Postdoctoral Science Foundation (grant no. 2014T70204), the
National Natural Science Foundation of China (grant no. 81460665),
the Natural Science Foundation of Ningxia (grant no. NZ14059) and
the Special Talent Research Project of Ningxia Medical University
(grant no. XT201316).
References
|
1
|
Glass CK, Saijo K, Winner B, Marchetto MC
and Gage FH: Mechanisms underlying inflammation in
neurodegeneration. Cell. 140:918–934. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kawabori M and Yenari MA: The role of the
microglia in acute CNS injury. Metab Brain Dis. 30:381–392. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neurotoxicity: Uncovering the molecular
mechanisms. Nat Rev Neurosci. 8:57–69. 2007. View Article : Google Scholar
|
|
4
|
Markus MA and Morris BJ: Resveratrol in
prevention and treatment of common clinical conditions of aging.
Clin Interv Aging. 3:331–339. 2008.PubMed/NCBI
|
|
5
|
Ponzo V, Soldati L and Bo S: Resveratrol:
a supplementation for men or for mice? J Transl Med. 12:1582014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de la Lastra CA and Villegas I:
Resveratrol as an anti-inflammatory and anti-aging agent:
Mechanisms and clinical implications. Mol Nutr Food Res.
49:405–430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lorenz P, Roychowdhurys, Engelmann M, Wolf
G and Horn TF: Oxyresveratrol and resveratrol are potent
antioxidants and free radical scavengers: Effect on nitrosative and
oxidative stress derived from microglial cells. Nitric Oxide.
9:64–76. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bi XL, Yang JY, Dong YX, Wang JM, Cui YH,
Ikeshima T, Zhao YQ and Wu CF: Resveratrol inhibits nitric oxide
and TNF-alpha production by lipopolysaccharide-activated microglia.
Int Immunopharmacol. 5:185–193. 2005. View Article : Google Scholar
|
|
9
|
Candelario-Jalil E, de Oliveira AC, Gräfs,
Bhatia HS, Hüll M, Muñoz E and Fiebich BL: Resveratrol potently
reduces prostaglandin E2 production and free radical formation in
lipopolysaccharide-activated primary rat microglia. J
Neuroinfammation. 4:252007. View Article : Google Scholar
|
|
10
|
Lu X, Ma L, Ruan L, Kong Y, Mou H, Zhang
Z, Wang Z, Wang JM and Le Y: Resveratrol differentially modulates
inflammatory responses of microglia and astrocytes. J
Neuroinfammation. 7:462010. View Article : Google Scholar
|
|
11
|
Zekry D, Epperson TK and Krause KH: A role
for NOX NADPH oxidases in Alzheimer's disease and other types of
dementia? IUBMB Life. 55:307–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Capiralla H, Vingtdeux V, Zhao H,
Sankowski R, Al-Abed Y, Davies P and Marambaud P: Resveratrol
mitigates lipopolysaccharide- and Aβ-mediated microglial
infammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J
Neurochem. 120:461–472. 2012. View Article : Google Scholar :
|
|
13
|
Choi SH, Aid S, Kim HW, Jackson SH and
Bosetti F: Inhibition of NADPH oxidase promotes alternative and
anti-inflammatory microglial activation during neuroinflammation. J
Neurochem. 120:292–301. 2012. View Article : Google Scholar :
|
|
14
|
McLean CA, Cherny RA, Fraser FW, Fullers
J, Smith MJ, Beyreuther K, Bush AI and Masters CL: Soluble pool of
Abeta amyloid as a determinant of severity of neurodegeneration in
Alzheimer's disease. Ann Neurol. 46:860–866. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mc Donald JM, Savva GM, Brayne C, Welzel
AT, Forster G, Shankar GM, Selkoe DJ, Ince PG and Walsh DM: Medical
Research Council Cognitive Function and Ageing Study: The presence
of sodium dodecyl sulphate-stable Abeta dimers is strongly
associated with Alzheimer-type dementia. Brain. 133:1328–1341.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Yang JY, Yao XC, Xue X, Zhang QC,
Wang XX, Ding LL and Wu CF: Oligomeric Aβ-induced microglial
activation is possibly mediated by NADPH oxidase. Neurochem Res.
38:443–452. 2013. View Article : Google Scholar
|
|
17
|
Bargers W and Harmon AD: Microglial
activation by Alzheimer amyloid precursor protein and modulation by
apolipoprotein E. Nature. 388:878–881. 1997. View Article : Google Scholar
|
|
18
|
Groemping Y and Rittinger K: Activation
and assembly of the NADPH oxidase: A structural perspective.
Biochem J. 386:401–416. 2005. View Article : Google Scholar :
|
|
19
|
Giuliani F, Hader W and Yong VW:
Minocycline attenuates T cell and microglia activity to impair
cytokine production in T cell-microglia interaction. J Leukoc Biol.
78:135–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maezawa I, Zimin PI, Wulff H and Jin LW:
Amyloid-beta protein oligomer at low nanomolar concentrations
activates microglia and induces microglial neurotoxicity. J Biol
Chem. 286:3693–3706. 2011. View Article : Google Scholar :
|
|
21
|
Jekabsone A, Mander PK, Tickler A, Sharpe
M and Brown GC: Fibrillar beta-amyloid peptide Abeta1–40 activates
microglial proliferation via stimulating TNF-alpha release and
H2O2 derived from NADPH oxidase: A cell
culture study. J Neuroinflamm. 3:242006. View Article : Google Scholar
|
|
22
|
Green SP, Cairns B, Rae J,
Errett-Baroncini C, Hongo JA, Erickson RW and Curnutte JT:
Induction of gp91-phox, a component of the phagocyte NADPH oxidase,
in microglial cells during central nervous system inflammation. J
Cereb Blood Flow Metab. 21:374–384. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klegeris A and McGeer PL: Rat brain
microglia and peritoneal macrophages show similar responses to
respiratory burst stimulants. J Neuroimmunol. 53:83–90. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Babior BM: Oxidants from phagocytes:
Agents of defense and destruction. Blood. 64:959–966.
1984.PubMed/NCBI
|
|
25
|
Qin L, Liu Y, Wang T, Wei SJ, Block ML,
Wilson B, Liu B and Hong JS: NADPH oxidase mediates
lipopolysaccharide-induced neurotoxicity and proinflammatory gene
expression in activated microglia. J Biol Chem. 279:1415–1421.
2004. View Article : Google Scholar
|
|
26
|
Leonard SS, Xia C, Jiang BH, Stinefelt B,
Klandorf H, Harris GK and Shi X: Resveratrol scavenges reactive
oxygen species and effects radical-induced cellular responses.
Biochem Biophys Res Commun. 309:1017–1026. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spanier G, Xu H, Xia N, Tobias S, Deng S,
Wojnowski L, Forstermann U and Li H: Resveratrol reduces
endothelial oxidative stress by modulating the gene expression of
superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and
NADPH oxidase subunit (Nox4). J Physiol Pharmacol. 60(Suppl 4):
111–116. 2009.
|
|
28
|
Banati RB, Gehrmann J, Schubert P and
Kreutzberg GW: Cytotoxicity of microglia. Glia. 7:111–118. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hur J, Lee P, Kim MJ, Kim Y and Cho YW:
Ischemia-activated microglia induces neuronal injury via activation
of gp91phox NADPH oxidase. Biochem Biophys Res Commun.
391:1526–1530. 2010. View Article : Google Scholar
|
|
30
|
Wilms H, Sievers J, Rickert U,
Rostami-Yazdi M, Mrowietz U and Lucius R: Dimethylflumarate
inhibits microglial and astrocytic inflammation by suppressing the
synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an
in-vitro model of brain inflammation. J Neuroinfammation. 7:302010.
View Article : Google Scholar
|
|
31
|
Eikelenboom P, Veerhuis R, Scheper W,
Rozemuller AJ, van Gool WA and Hoozemans JJ: The significance of
neuroinflammation in understanding Alzheimer's disease. J Neural
Transm. 113:1685–1695. 2006. View Article : Google Scholar : PubMed/NCBI
|