Introduction
Chondrocyte dysfunction results in the cervical
spondylosis associated with degeneration of the intervertebral
disc, or the joint disorder osteoarthritis (OA), which is
characterized by progressive breakdown of articular cartilage
(1–3). Chondrocytes are sensitive to
environmental and multiple cellular stresses, including
inflammation, mechanical loading, stress to the endoplasmic
reticulum and hypoxia (4–6). Reactive oxygen species (ROS), another
major determinant of stress, including hydrogen peroxide
(H2O2), hypochlorite ion, hydroxyl radical
and superoxide anion, are involved in normal intracellular
transduction and degenerative cellular processes. Increased ROS
production is considered to be an important cause of chondrocyte
dysfunction and articular cartilage degradation, which leads to the
pathogenesis of OA and the aging of cartilage (7–10).
Chondrocytes and cartilage tissues exhibit efficient protective
strategies to minimize damage induced by increased ROS production,
including H2O2 (7,11).
Previous studies have investigated various antioxidant supplements,
including resveratrol, quercetin, vitamin E, and other strategies
(12–15). However, information available on
the antioxidative status of chondrocytes is limited (16). Therefore, identifying available and
promising antioxidative strategies or antioxidant drugs to combat
oxidative stress in chondrocytes is required.
Ascorbic acid (AA) is widely used in clinical
applications and is known for its role in bone formation, wound
healing and the maintenance of healthy gums. AA is involved in
protecting the immune system and combating infection and allergic
reactions (17). AA is also
regarded as the most important antioxidant, which provides
protection against oxidative stress. It has been previously
reported that AA may be a potential drug for therapeutic
intervention and to slow the progression of age-associated
diseases, including atherogenesis and Alzheimer's disease (17,18).
Previous studies have revealed that AA attenuates oxidative stress
in diabetic aged rats (19,20).
AA also exerts a role in chondroprotection: The application of AA
and mechanical stimulation in combination was revealed to improve
the mechanical characteristics of regenerated cartilage (21). AA was revealed to markedly affect
the expression of proteins with specific functions in human
articular chondrocytes under conditions of homogentisic
acid-induced stress, which may be mediated by protein oxidation
(22). A combination of
α-tocopherol (0.1–2.5 µM), AA (10–50 µM) and selenium
(1–50 nM) provided a promising strategy to combat oxidative stress
and cytokine-induced matrix degradation in chondrocytes (23). AA supplementation of chondrocytes
following static loading was demonstrated to have the potential to
reduce the morphological and biochemical degeneration of
chondrocytes in vivo (24).
These previous findings support the hypothesis that AA may be a
promising drug or antioxidant to protect against the chondrocyte
damage induced by oxidative stress. However, no reports in this
research area currently exist.
The present study focused on the role of AA in
combating oxidative stress and the crosstalk between AA and
response transcription factors under conditions of oxidative stress
in C28/I2 human chondrocytes.
Materials and methods
Cell culture and cell treatments
The human C28/I2 chondrocyte cell line was acquired
from Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The cells were cultivated in Dulbecco's modified
Eagle's medium (DMEM), containing 10% fetal bovine serum (Gibco
Life Technologies, Carlsbad, CA, USA) and antibiotics (50 U/ml
penicillin and 50 µg/ml streptomycin; Invitrogen Life
Technologies, Carlsbad, CA, USA), in 5% CO2 at 37°C. The
cells were subcultured after reaching ~90% confluence. AA
(Sigma-Aldrich, St. Louis, MO, USA) and H2O2
(Sigma-Aldrich) were diluted in serum-free DMEM. The cells were
treated with H2O2 (100 µM) for 4 h
following incubation with AA at 100 (AA100 group) or 200 µM
(AA200 group) for 24 h after the C28/I2 cells had reached 80%
confluence. Normal C28/I2 cells without treatment and C28/I2 cells
treated with H2O2 (100 µM) were
denoted as the N group and C group, respectively.
Analysis of apoptosis
The annexin-V-fluorescein isothiocyanate (FITC)
apoptosis detection kit was used to analyze apoptosis in the cells,
according to the manufacturer's instructions (KeyGen Biotech. Co.,
Ltd., Nanjing, China). The cells were harvested following the
indicated treatments and cell suspensions were fixed overnight with
ice-cold 70% ethanol. The fixed cells were subsequently stained
with propidium iodide or annexin V-FITC following centrifugation at
70 × g for 5 min at room temperature, and resuspension. Analyses
were performed using a flow cytometer (BD FACScan; BD Biosciences,
Franklin Lakes, NJ, USA). The results were expressed as the
percentage of apoptotic cells from the total cells.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) assay
Cell viability was determined using an MTT assay
(Sigma-Aldrich), following the indicated treatments. Briefly, 50
µg/ml MTT was added to the cells at 37°C for 4 h. Following
incubation, the MTT-containing medium was discarded and dimethyl
sulfoxide was added to dissolve the formazan crystals. The optical
densities (OD) were measured at 490 nm using a Versamax microplate
reader (Molecular Devices, Sunnyvale, CA, USA). The viability of
the cells was normalized, according to the OD value/cell number,
and the quantity of normal C28/I2 cells was denoted as 100%.
Cell senescence assay
For the senescence-associated β-galactosidase
(SA-β-gal) assay, cell senescence was measured using a cellular
senescence assay kit (Cell Biolabs, San Diego, CA, USA), according
to the manufacturer's instructions. Briefly, the cells
(5×104) were treated as indicated and following
treatment, the cells were washed with phosphate-buffered saline
(PBS), harvested and collected by centrifugation at 70 × g for 5
min at room temperature. The cells were treated with freshly
prepared fluorimetric substrate for 2 h at 37°C in the dark. The
fluorescence intensity of each reaction mixture was determined and
quantified using Image J software (NIH, Bethesda, MD, USA). The
average fluorescence intensity was analyzed from five fields for
each treatment using ImageJ 1.38.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was isolated from the cells using an
RNeasy kit (Qiagen, Valencia, CA, USA). RT-qPCR was performed on an
Applied Biosystems 7500 instrument with SYBR Green PCR kits
(Applied Biosystems, Foster City, CA, USA). First-strand cDNA was
synthesized using reverse transcription reagents (PrimeScript™ RT
reagent kit; Takara Biotechnology Co., Ltd., Dalian, China). The
reactions were conducted with the following thermocycling
conditions: at 95°C for 10 min, followed by 45 cycles of 95°C for
10 s, 56°C for 30 s and extension for 10 s at 72°C. The data were
assessed using the 2−ΔΔCt method for relative
quantification (21,22). The GAPDH gene was used as the
endogenous control for normalization. The primer sequences
(synthesized by Sangon Biotech Co., Ltd, Beijing, China) were as
follows: Col1a1, forward: 5′-CAAGATGGTGGCCGTTACTAC-3′ and reverse:
5′-TTAGTCCTTACCGCTCTTCCAG-3′; Col2a1, forward:
5′-GACTTTCCTCCGTCTACTGTCC-3′ and reverse:
5′-GTGTACGTGAACCTGCTGTTG-3′; Agc1, forward:
5′-ACTGAAGGACAGGTTCGAGTG-3′ and reverse:
5′-CACACCGATAGATCCCAGAGT-3′; Nrf2, forward:
5′-TTCAAAGCGTCCGAACTCCA-3′ and reverse: 5′-AATGTCTGCGCCAAAAGCTG-3′;
matrix metallopro-teinase-3 (MMP-3), forward: 5′-CTGGACTCCGACACTCTG
GA-3′ and reverse: 5′-CAGGAAAGGTTCTGAAGTGACC-3′; AP1, forward:
5′-TGTCTGTGGCTTCCCTTGATCTGA-3′ and reverse:
5′-TGGATGATGCTGGGAACAGGAAGT-3′; GAPDH, forward:
5′-GCACCGTCAAGGCTGAGAAC-3′; reverse:
5′-ATGGTGGTGAAGACGCCAGT-3′.
Nuclear fraction preparation
The nuclear and cytosolic fractions were separated
using a Nuclear and Cytoplasmic Protein Extraction kit obtained
from the Beyotime Institute of Biotechnology (Jiangsu, China). The
cell pellets were resuspended in 100 µl cytosolic extract A
reagent, containing 1 mM PMSF and vortexed for 5 sec. Subsequently,
5 µl cytosolic extract B reagent was added to the lysates
and the samples were vortexed for 5 sec. The supernatant (cytosolic
fractions) was acquired following centrifugation at 13,000 × g at
4°C for 5 min, after which the pellets were resuspended in 30
µl nuclear extract reagent, containing 1 mM PMSF, and
centrifuged again at 13,000 × g at 4°C for 10 min. The resulting
supernatants (nuclear fraction) were extracted and subsequently
analyzed to assess the protein expression of nrf2 in the nuclear
fraction.
Immunoblotting
Western blotting was performed using a standard
procedure, as described previously (6). Briefly, the C28/I2 chondrocytes were
lysed using a lysis reagent (Promega Corporation, Madison, WI, USA)
and the proteins were fractionated on 10% SDS-PAGE gels
(Sigma-Aldrich) and electrotransferred onto nitrocellulose
membranes (Millipore, Billerica, MA, USA). The membranes were
incubated with primary antibodies for 2 h at 37°C and then blocked
with PBS containing 1% bovine serum albumin and 0.02% Tween 20
(Sigma-Aldrich). The signals were acquired using an enhanced
chemiluminescence detection system (SuperSignal West Femto; Pierce
Biotechnology, Inc., Rockford, IL, USA) following incubation with
horseradish-peroxidase-conjugated goat anti-rabbit immunoglobulin G
secondary antibody (1:500; cat. no. G-21079; Pierce Biotechnology,
Inc.). The primary antibodies used in the present study were as
follows: Rabbit monoclonal anti-NF-κB (1:500; cat. no. 8242; Cell
Signaling Technology, Inc., Beverly, MA, USA), rabbit
phosphorylated (p)-STAT3 (Tyr705; 1:500; p-NF-κB; cat. no. 3033;
Cell Signaling Technology, Inc.), rabbit monoclonal anti-nrf2
(1:500; cat. no. SAB4501984; Sigma-Aldrich) and rabbit anti-β-actin
antibody (1:1,000; cat. no. A2066; Sigma-Aldrich).
Statistical analysis
All experiments were performed, at least, in
triplicate. All the data were expressed as the mean ± standard
deviation or as the mean ± standard error. The PASW Statistics 18
software package (formerly SPSS Statistics) was used for
statistical analysis (SPSS, Inc., Chicago, IL, USA). Comparison
among multiple samples was performed by one-way analysis of
variance and Student's t-test was used to compare two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
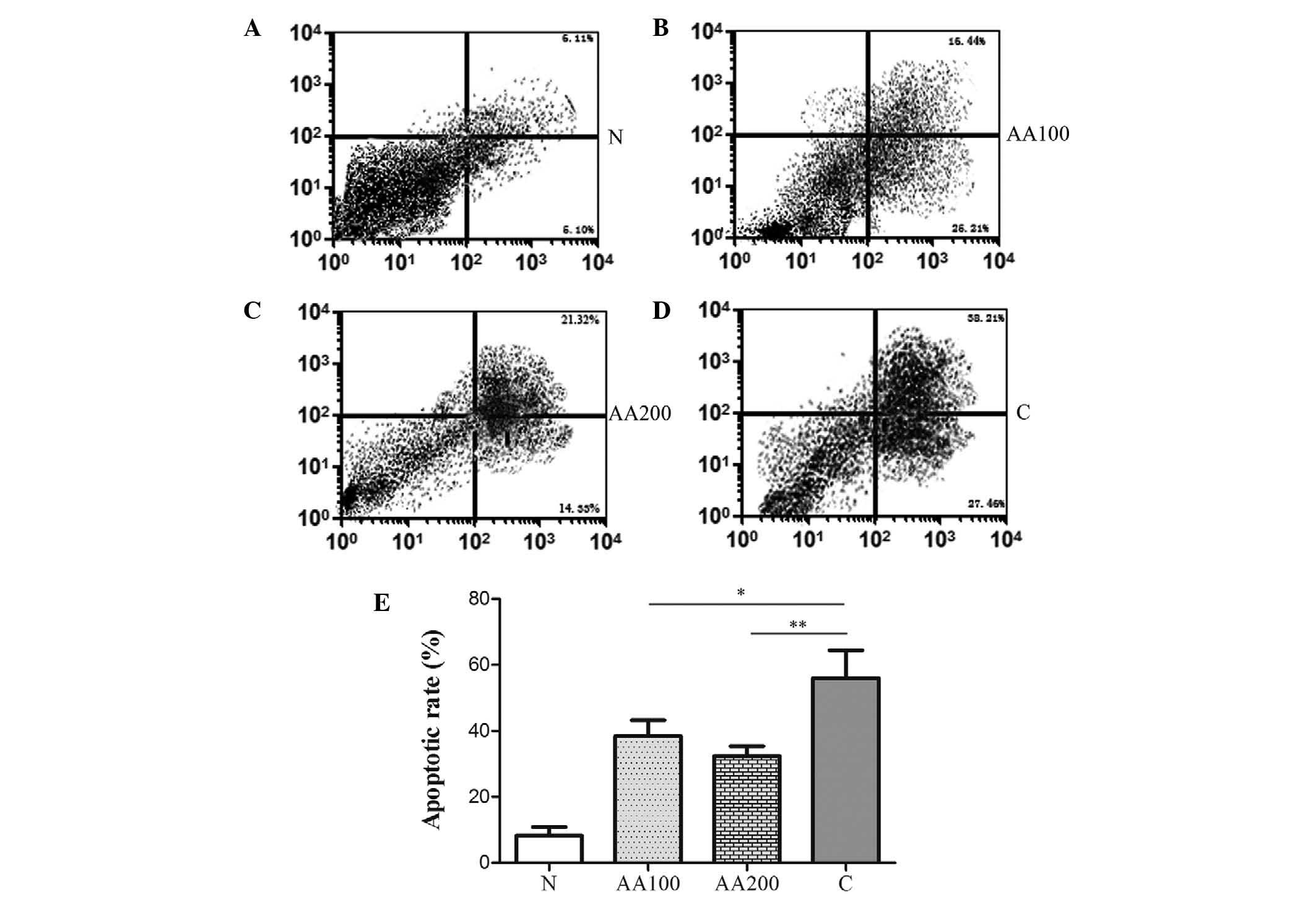
AA reduces
H2O2-mediated apoptosis
The C28/I2 human chon-drocytes were treated with
H2O2 to simulate pathophysiological oxidative
stress as it occurs in vivo. The treatment groups were as
follows: N group, normal C28/I2 cells; C group, cells treated with
100 µM H2O2 for 4 h; AA100 group,
cells preincubated with 100 µM AA for 24 h and treated with
100 µM H2O2 for 4 h; AA200 group,
cells preincubated with 200 µM AA for 24 h and treated with
100 µM H2O2 for 4 h. It was revealed
that H2O2 significantly increased the levels
of apoptosis when compared with the normal cells (N group,
P<0.001), as shown in Fig. 1.
notably, preincubation with 100 µM AA resulted in a
significant decrease in the levels of apoptosis compared with the C
group (P=0.034). An increased concentration of AA (200 µM)
led to a modest decrease in the levels of apoptosis compared with
the AA100 group. Significant differences in the levels of apoptosis
between the AA groups and the N group were observed (P<0.001).
These data suggested that AA markedly reduced
H2O2-mediated apoptosis, however,
preincubation with AA (100 and 200 µM) failed to completely
abolish the effects of H2O2 on cellular
apoptosis.
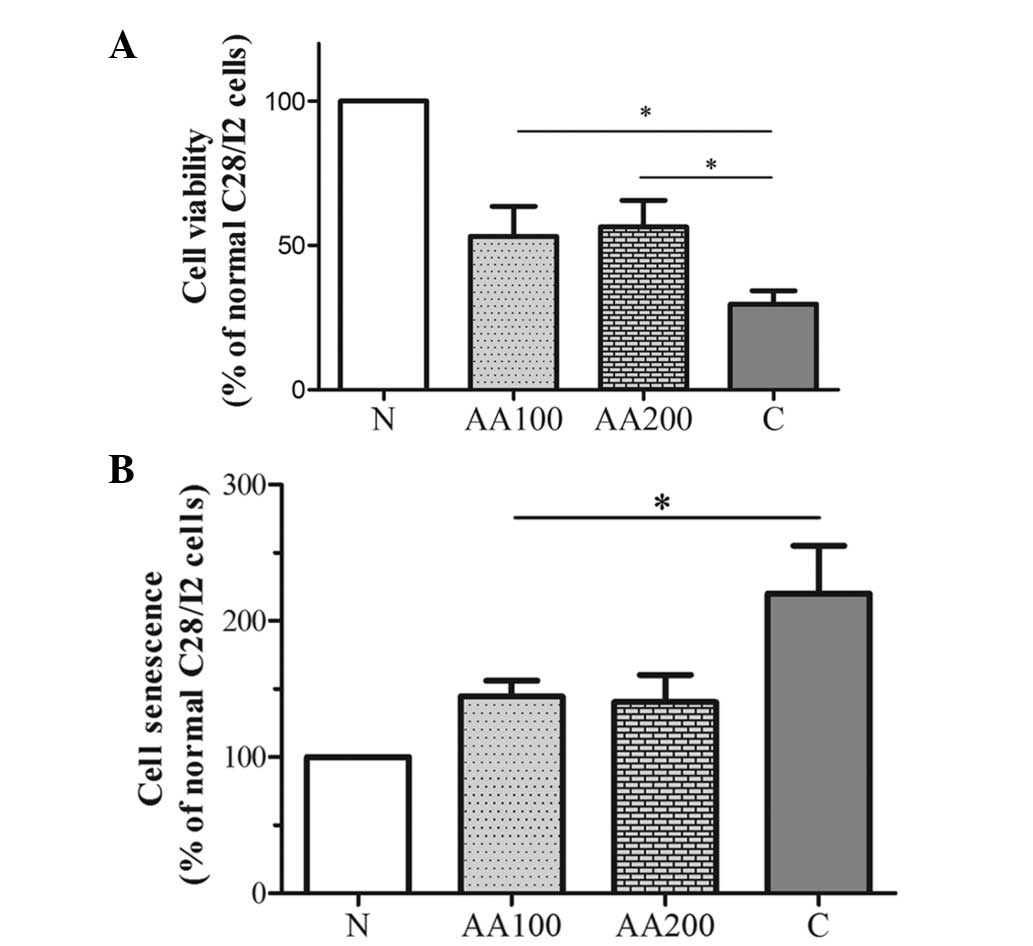
AA protects chondrocytes from
H2O2-induced loss of viability and
senescence
Cell viability and senescence are characterized by a
loss of function and integrity, which contribute to the progressive
degeneration of tissues (25,26).
The cell viability and senescence of chondrocytes were determined
under oxidative stress conditions mediated by
H2O2 or AA pretreatment using an MTT assay or
the SA-β-gal assay, as shown in Fig.
2. Incubation with H2O2 stimulated a loss
in cell viability and promoted the senescence-like phenotype of
chondrocytes when compared with the normal C28/I2 cells
(P<0.001). Addition of 100 µM AA significantly reduced
the loss of viability (P=0.023) and good agreement was obtained
with the SA-β-gal activity assay used to measure senescence induced
by H2O2 (P=0.026) compared with the C group.
Following treatment with a higher concentration of AA (200
µM), the effects of AA were further enhanced (AA200 group
vs. AA100 group, 56.48±9.17 vs. 53.17±10.40% with the MTT assay and
130.33±10.69 vs. 144.71±11.39% with the SA-β-gal assay; Fig. 2). These results clearly revealed
that AA significantly protected chondrocytes from a loss of
viability and from senescence induced by
H2O2.
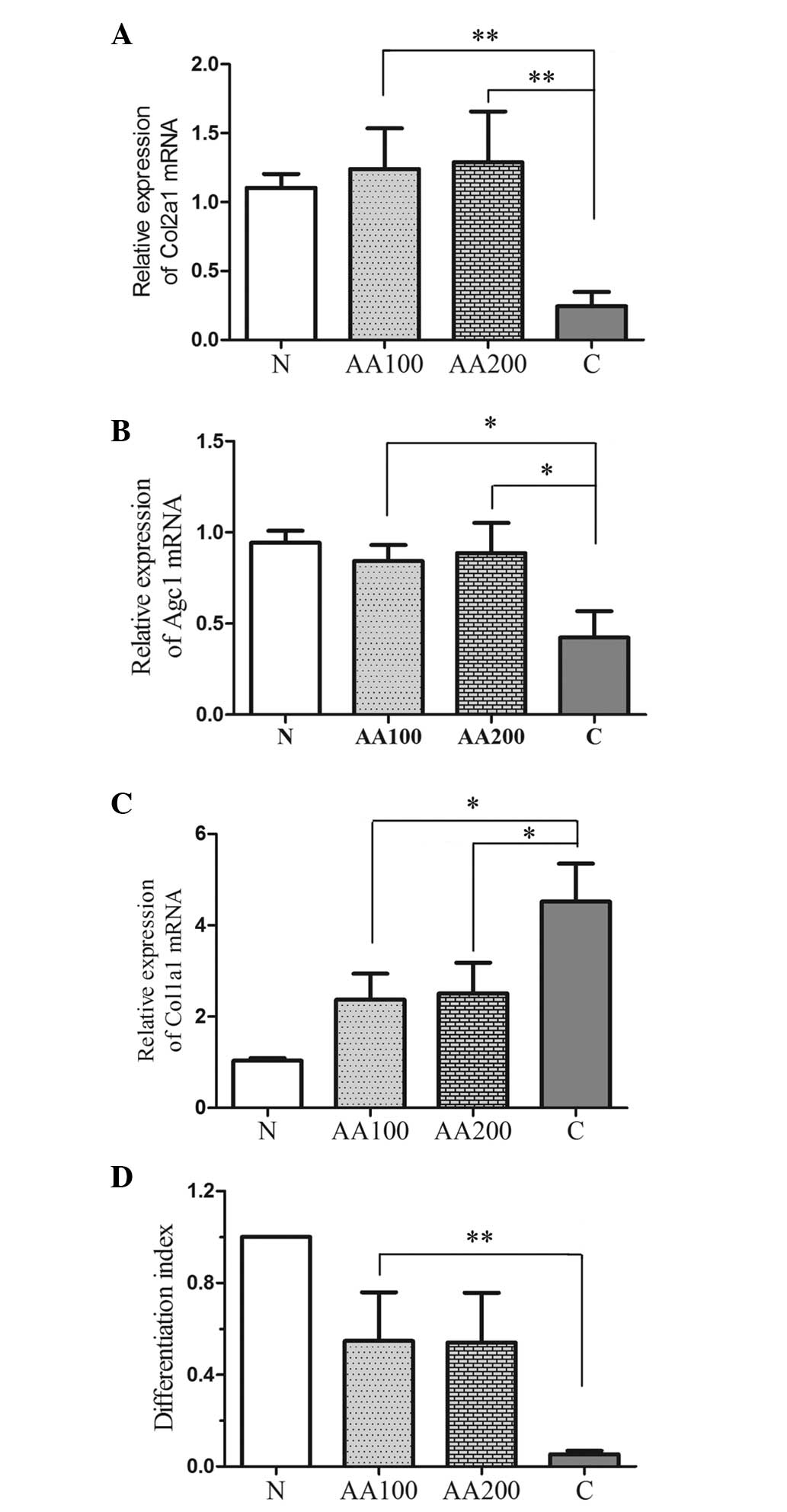
AA affects the differentiation and
expression of proteoglycans and collagens in human chondrocytes
exposed to H2O2
To investigate the role of AA in the expression and
differentiation of collagens and proteoglycans in chondrocytes when
exposed to oxidative stress, the expression levels of the major
factors, Col1a1, Col2a1 and Agc1, were detected by RT-qPCR, as
shown in Fig. 3A–C. Oxidative
stress not only suppressed the expression levels of Col2a1 and Agc1
(P<0.001), but also increased the expression of Col1a1
(P<0.001) compared with the normal cells (N group).
Preincubation with AA resulted in a significant increase in the
relative expression levels of Col2a1 (AA100, P=0.005; AA200,
P<0.001) and Agc1 (AA100, P=0.013; AA200, P=0.022), and
significantly decreased the expression of Col1a1 (AA100, P=0.015;
AA200, P=0.031) compared with the C group. The differentiation
index (Fig. 3D) was calculated as
the ratio of the expression levels between Col2a1 and Col1a1
(27). Addition of
H2O2 decreased the differentiation index,
whereas preincubation with AA increased the differentiation index
(Fig. 3D). These findings revealed
that AA not only stimulated the expression of collagens and
proteoglycans, but also inhibited the differentiation of
chondrocytes in the presence of oxidative stress.
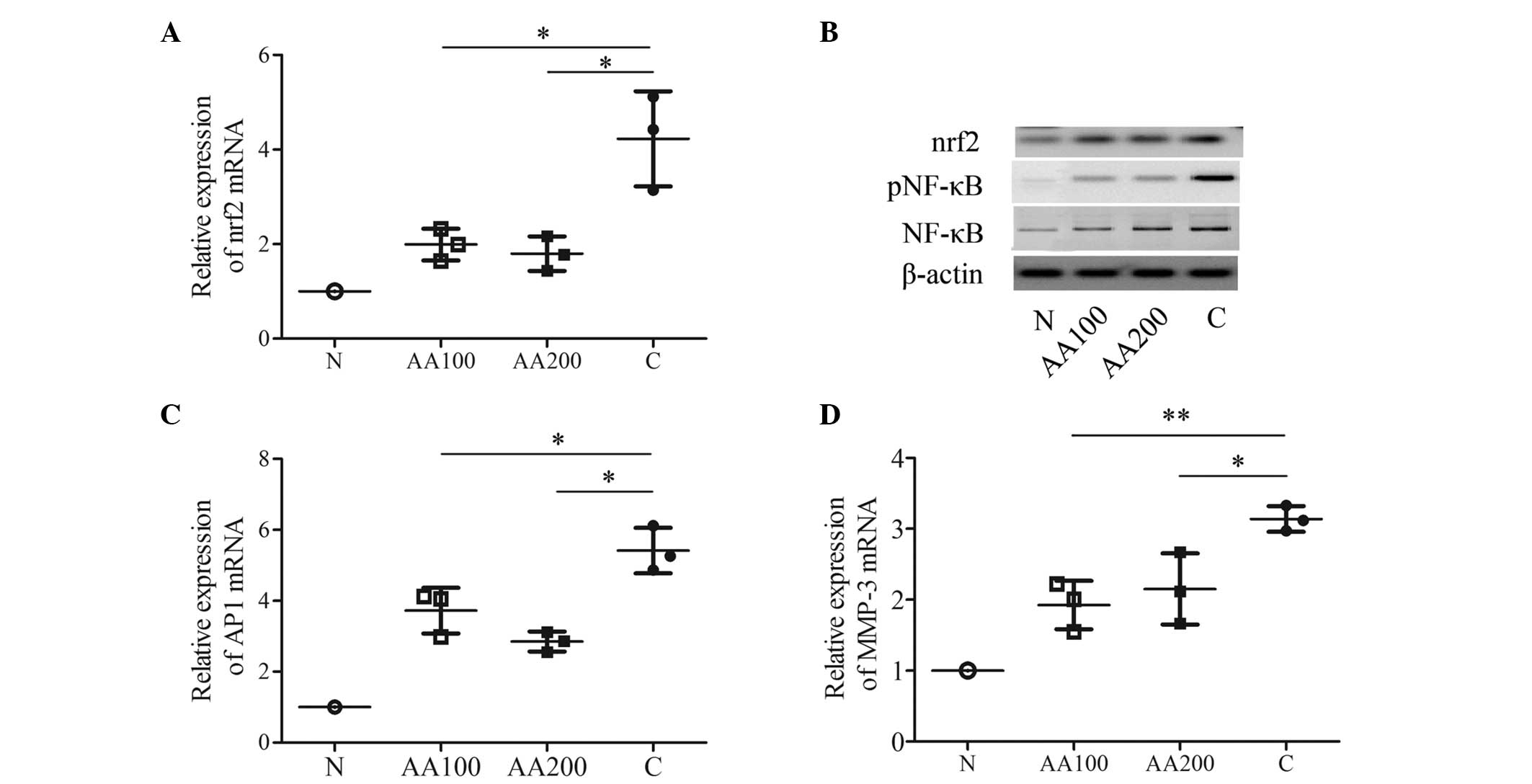
AA contributes to the inhibition of
multiple transcription factors in C28/I2 cells exposed to
H2O2
In order to assess transcription factor(s), which
are modulated by AA, certain known stress and toxicity responding
factors (nrf2, NF-κB, AP1 and MMP-3) found in four different
signaling pathways were investigated. H2O2
markedly upregulated the mRNA expression levels of nrf2, AP1 and
MMP-3 (P<0.001), in addition to stimulating the activation of
NF-κB when compared with the N group (Fig. 4). Preincubation with 100 µM
AA attenuated the increase in the expression of nrf2 (P=0.022) and
the protein expression of nrf2 induced by
H2O2 (Figs. 4A
and B). Furthermore, 100 µM AA decreased the activation
of NF-κB compared with the C group (Fig. 4B). In addition, 100 µM AA
decreased the mRNA expression levels of AP1 (P=0.032) and MMP-3
(P=0.006) compared with the C group (Figs. 4C and D). A higher concentration of
AA (200 µM) elicited similar effects on the mRNA expression
levels of the three transcription factors (nrf2, P=0.017; AP1,
P=0.045; MMP-3, P=0.033) and on the activation of NF-κB (Fig. 4). These data suggested that AA may
protect human chondrocytes from injuries induced by
H2O2 by exerting regulatory roles in the
antioxidant response via the protein kinase C (PKC)/nrf2, NF-κB and
c-Jun N-terminal kinase (JNK)/AP1 signaling pathways, and in the
inflammatory process. In other words, the effects of AA may be
mediated via multiple regulatory pathways in C28/I2 cells when
exposed to oxidative stress.
Discussion
Oxidative stress is regarded as a major cause of the
degradation of chondrocytes and articular cartilage, which results
in the pathogenesis of OA and the aging of cartilage (9,10,16).
Several previous reports have focussed on the various strategies
used to resist oxidative stress (14,28).
Exploiting antioxidant treatment has been demonstrated as a
promising strategy for the protection of chondrocytes against
oxidative stress. Antioxidants were identified to exert
cytoprotective effects in vitro and in vivo (29,30).
AA, a widely used antioxidant, was revealed to delay the
progression of age-associated diseases, which are highly
susceptible to oxidative stress (19,21).
However, it is unknown whether AA can provide any resistance to
oxidative stress for the protection of chondrocytes. The present
study hypothesized that AA may confer protective effects against
chondrocyte damage, which is induced by H2O2.
By examining the apoptotic rate, viability and senescence of
chondrocytes, AA was revealed to effectively reduce
H2O2-induced damage by attenuating the
increase of apoptosis, loss of viability and increase of senescence
(Fig. 1 and 2). Graeser et al (23) reported that administering a single
dose of AA (10–50 µM) failed to protect chondrocytes from
the damage induced by t-Butyl hydroperoxide. By contrast, Sharma
et al (24) found that AA
supplementation reduced the morphological and biochemical
degeneration of chondrocytes in vivo. Notably, another
previous report revealed that lower concentrations of AA did not
protect against oxidative stress in retinal pigment epithelium
cells until the concentration of AA reached 100 µM (31). The present study surmised that any
resistance to oxidative stress exerted by AA was likely to be
associated with the concentration of AA.
Collagens and proteoglycans (in chondrocytes, Col2a1
and Agc1 are expressed) are the predominant constituents of the
extracellular matrix, which are important in chondrocytes competing
against various stress factors (32,33).
Previous studies confirmed that H2O2 reduces
the level of proteoglycans in chondrocytes (15,34,35).
In the present study, the data supported that of previous studies
by identifying that Col2a1 and Agc1 are, respectively, the major
collagen and proteoglycan expressed by chondrocytes (Fig. 3A–B). In addition, the present
results have also demonstrated that the administration of AA
efficiently counteracts the inhibition of Col2a1 and Agc1 by
H2O2 in C28/I2 cells. In normal cartilage,
the differentiation index is high as a result of the large quantity
of Col2a1 and low quantity of Col1a1 that are present. An augmented
differentiation index is indicative of the active maintenance of
the hyaline phenotype of chondrocytes (36,37).
By contrast, an upregulated expression of Col1a1 or a decline in
the differentiation index represented the formation of
fibrocartilage rather than hyaline cartilage (37,38).
The present study revealed that H2O2 not only
upregulated the expression of the Col1a1 gene, but also inhibited
the expression of Col2a1 (Figs. 3A, C
and D). Furthermore, AA pretreatment inhibited the upregulation
of the expression of the Col1a1 gene and downregulation of Col2a1
induced by H2O2 (Figs. 3A, C and D). Consequently, these
findings demonstrated that AA not only stimulates the expression of
collagens and proteoglycans, but also inhibits the differentiation
of chondrocytes into fibrocartilage.
Several response transcription factors and signaling
pathways, including MMP-3 (39),
NF-κB (13), the PKC/Nrf2 pathway
(40) and the JNK/AP1 signaling
pathway (41,42), have been demonstrated to exert key
effects on the protection against environmental and multiple
cellular stresses in chondrocytes (13,39–42).
Theoretically, the specific regulation of these response
transcription factors can cause various changes in pathologies and
physiologies. Previous studies have identified the crosstalk
between AA and MMP-3 in non-Hodgkin's lymphoma (43), the AA-mediated inhibition of NF-κB
leading to the suppression of liver fibrosis (44), the regulatory role of AA in the
PKC/Nrf2 pathway (45) and a
moderate AA-mediated attenuation of the elevated levels of the AP1
gene induced by H2O2 in the retinal pigment
epithelium (31). These findings
demonstrate that AA may affect multiple signaling pathways and that
the regulatory mechanism in which AA is involved is highly
dependent on the micro-environment. In order to identify the
transcription factor(s), which AA modulates, four response
transcription factors were selected, including MMP-3, NF-κB, the
PKC/Nrf2 pathway and the JNK/AP1 signaling pathway. AA was revealed
to repress the elevated transcriptional activities of the response
transcription factors induced by H2O2,
lowering them to basal activities (Fig. 4). This demonstrated that AA can
regulate multiple factors and signaling pathways. These findings
also revealed how promising novel therapeutic methods, which lack
any substantial untoward effects due to the inhibition of any given
transcription factors may be acquired.
Overall, the present study demonstrated that AA
protected cultured chondrocytes from apoptosis, loss of viability
and an increase in senescence under conditions of
H2O2-induced oxidative stress in
vitro. In addition, AA not only stimulated the expression of
collagens and proteoglycans, but also inhibited the differentiation
of chondrocytes in the presence of oxidative stress. The effects of
AA were mediated by multiple regulatory pathways (or factors).
Future studies will focus on improving the efficacy of AA for
alleviating damage to chondrocytes induced by oxidative stress, and
an investigation of AA pretreatment in vivo.
Acknowledgments
This study was funded by The Second Affiliated
Hospital of Inner Mongolia Medical University.
References
|
1
|
Miyaki S and Asahara H: Macro view of
microRNA function in osteoarthritis. Nat Rev Rheumatol. 8:543–552.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Triantafillou KM, Lauerman W and Kalantar
SB: Degenerative disease of the cervical spine and its relationship
to athletes. Clin Sports Med. 31:509–520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun YQ, Zheng S, Yu J, Yan K and Tian W:
Effect of total disc replacement on atypical symptoms associated
with cervical spondylosis. Eur Spine J. 22:1553–1557. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leong DJ, Li YH, Gu XI, et al:
Physiological loading of joints prevents cartilage degradation
through CITED2. FASEB J. 25:182–191. 2011. View Article : Google Scholar :
|
|
5
|
Horton WE Jr, Bennion P and Yang L:
Cellular, molecular, and matrix changes in cartilage during aging
and osteoarthritis. J Musculoskelet Neuronal Interact. 6:379–381.
2006.PubMed/NCBI
|
|
6
|
Chang Z, Huo L, Wu Y and Zhang P: HIF-1 α
had pivotal effects on downregulation of miR-210 decreasing
viability and inducing apoptosis in hypoxic chondrocytes.
Scientific World Journal. 2014:8763632014. View Article : Google Scholar
|
|
7
|
Henrotin Y, Kurz B and Aigner T: Oxygen
and reactive oxygen species in cartilage degradation: Friends or
foes? Osteoarthritis Cartilage. 13:643–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Henrotin YE, Bruckner P and Pujol JP: The
role of reactive oxygen species in homeostasis and degradation of
cartilage. Osteoarthritis Cartilage. 11:747–755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruiz-Romero C, Calamia V, Mateos J,
Carreira V, Martínez-Gomariz M, Fernández M and Blanco FJ:
Mitochondrial dysregulation of osteoarthritic human articular
chondrocytes analyzed by proteomics: a decrease in mitochondrial
superoxide dismutase points to a redox imbalance. Mol Cell
Proteomics. 8:172–189. 2009. View Article : Google Scholar :
|
|
10
|
Yu CJ, Ko CJ, Hsieh CH, Chien CT, Huang
LH, Lee CW and Jiang CC: Proteomic analysis of osteoarthritic
chondrocyte reveals the hyaluronic acid-regulated proteins involved
in chondroprotective effect under oxidative stress. J Proteomics.
99:40–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asada S, Fukuda K, Nishisaka F, Matsukawa
M and Hamanisi C: Hydrogen peroxide induces apoptosis of
chondrocytes; involvement of calcium ion and extracellular
signal-regulated protein kinase. Inflamm Res. 50:19–23. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji JJ, Lin Y, Huang SS, Zhang HL, Diao YP
and Li K: Quercetin: A potential natural drug for adjuvant
treatment of rheumatoid arthritis. Afr J Tradit Complement Altern
Med. 10:418–421. 2013.PubMed/NCBI
|
|
13
|
Eo SH, Cho H and Kim SJ: Resveratrol
Inhibits Nitric Oxide-Induced Apoptosis via the NF-Kappa B Pathway
in Rabbit Articular Chondrocytes. Biomol Ther (Seoul). 21:364–370.
2013. View Article : Google Scholar
|
|
14
|
Yoda M, Sakai T, Mitsuyama H, Hiraiwa H
and Ishiguro N: Geranylgeranylacetone suppresses hydrogen
peroxide-induced apoptosis of osteoarthritic chondrocytes. J Orthop
Sci. 16:791–798. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhatti FU, Mehmood A, Wajid N, Rauf M,
Khan SN and Riazuddin S: Vitamin E protects chondrocytes against
hydrogen peroxide-induced oxidative stress in vitro. Inflamm Res.
62:781–789. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Henrotin Y and Kurz B: Antioxidant to
treat osteoarthritis: Dream or reality? Curr Drug Targets.
8:347–357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chambial S, Dwivedi S, Shukla KK, John PJ
and Sharma P: Vitamin C in disease prevention and cure: An
overview. Indian J Clin Biochem. 28:314–328. 2013. View Article : Google Scholar :
|
|
18
|
Heo JH, Hyon L and Lee KM: The possible
role of antioxidant vitamin C in Alzheimer's disease treatment and
prevention. Am J Alzheimers Dis Other Demen. 28:120–125. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Özkaya D, Naziroğlu M, Armağan A, Demirel
A, Köroglu BK, Çolakoğlu N, Kükner A and Sönmez TT: Dietary vitamin
C and E modulates oxidative stress induced-kidney and lens injury
in diabetic aged male rats through modulating glucose homeostasis
and antioxidant systems. Cell Biochem Funct. 29:287–293. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naziroğlu M, Butterworth PJ and Sonmez TT:
Dietary vitamin C and E modulates antioxidant levels in blood,
brain, liver, muscle, and testes in diabetic aged rats. Int J Vitam
Nutr Res. 81:347–357. 2011. View Article : Google Scholar
|
|
21
|
Omata S, Sonokawa S, Sawae Y and Murakami
T: Effects of both vitamin C and mechanical stimulation on
improving the mechanical characteristics of regenerated cartilage.
Biochem Biophys Res Commun. 424:724–729. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Braconi D, Laschi M, Taylor AM, Bernardini
G, Spreafico A, Tinti L, Gallagher JA and Santucci A: Proteomic and
redox-proteomic evaluation of homogentisic acid and ascorbic acid
effects on human articular chondrocytes. J Cell Biochem.
111:922–932. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Graeser AC, Giller K, Wiegand H, Barella
L, Boesch Saadatmandi C and Rimbach G: Synergistic
chondroprotective effect of alpha-tocopherol, PCR, and selenium as
well as glucosamine and chondroitin on oxidant induced cell death
and inhibition of matrix metalloproteinase-3–studies in cultured
chondrocytes. Molecules. 15:27–39. 2010. View Article : Google Scholar
|
|
24
|
Sharma G, Saxena RK and Mishra P:
Regeneration of static-load-degenerated articular cartilage
extracellular matrix by vitamin C supplementation. Cell Tissue Res.
334:111–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coppé JP, Desprez PY, Krtolica A and
Campisi J: The senescence-associated secretory phenotype: The dark
side of tumor suppression. Annu Rev Pathol. 5:99–118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Philipot D, Guérit D, Platano D, Chuchana
P, Olivotto E, Espinoza F, Dorandeu A, Pers YM, Piette J, Borzi RM,
et al: p16INK4a and its regulator miR-24 link senescence and
chondrocyte terminal differentiation-associated matrix remodeling
in osteoarthritis. Arthritis Res Ther. 16:R582014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Desando G, Cavallo C, Tschon M, Giavaresi
G, Martini L, Fini M, Giardino R, Facchini A and Grigolo B:
Early-term effect of adult chondrocyte transplantation in an
osteoarthritis animal model. Tissue Eng Part A. 18:1617–1627. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mirza R, Qiao S, Tateyama K, Miyamoto T,
Xiuli L and Seo H: 3β-Hydroxysterol-Delta24 reductase plays an
important role in long bone growth by protecting chondrocytes from
reactive oxygen species. J Bone Miner Metab. 30:144–153. 2012.
View Article : Google Scholar
|
|
29
|
Henrotin Y, Clutterbuck AL, Allaway D,
Lodwig EM, Harris P, Mathy-Hartert M, Shakibaei M and Mobasheri A:
Biological actions of curcumin on articular chondroc§ytes.
Osteoarthritis Cartilage. 18:141–149. 2010. View Article : Google Scholar
|
|
30
|
Lim HD, Kim YS, Ko SH, Yoon IJ, Cho SG,
Chun YH, Choi BJ and Kim EC: Cytoprotective and anti-inflammatory
effects of melatonin in hydrogen peroxide-stimulated CHON-001 human
chondrocyte cell line and rabbit model of osteoarthritis via the
SIRT1 pathway. J Pineal Res. 53:225–237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin J, Thomas F, Lang JC and Chaum E:
Modulation of oxidative stress responses in the human retinal
pigment epithelium following treatment with vitamin C. J Cell
Physiol. 226:2025–2032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhosale AM and Richardson JB: Articular
cartilage: Structure, injuries and review of management. Br Med
Bull. 87:77–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nugent AE, McBurney DL and Horton WE Jr:
The presence of extracellular matrix alters the chondrocyte
response to endoplasmic reticulum stress. J Cell Biochem.
112:1118–1129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jallali N, Ridha H, Thrasivoulou C,
Underwood C, Butler PE and Cowen T: Vulnerability to ROS-induced
cell death in ageing articular cartilage: The role of antioxidant
enzyme activity. Osteoarthritis Cartilage. 13:614–622. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tiku ML, Gupta S and Deshmukh DR: Aggrecan
degradation in chondrocytes is mediated by reactive oxygen species
and protected by antioxidants. Free Radic Res. 30:395–405. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marlovits S, Hombauer M, Truppe M, Vècsei
V and Schlegel W: Changes in the ratio of type-I and type-II
collagen expression during monolayer culture of human chondrocytes.
J Bone Joint Surg Br. 86:286–295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Diaz-Romero J, Nesic D, Grogan SP, Heini P
and Mainil-Varlet P: Immunophenotypic changes of human articular
chondrocytes during monolayer culture reflect bona fide
dedifferentiation rather than amplification of progenitor cells. J
Cell Physiol. 214:75–83. 2008. View Article : Google Scholar
|
|
38
|
Aini H, Ochi H, Iwata M, Okawa A, Koga D,
Okazaki M, Sano A and Asou Y: Procyanidin B3 prevents articular
cartilage degeneration and heterotopic cartilage formation in a
mouse surgical osteoarthritis model. PLoS One. 7:e377282012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
de Seny D, Cobraiville G, Charlier E,
Neuville S, Esser N, Malaise D, Malaise O, Calvo FQ, Relic B and
Malaise MG: Acute-phase serum amyloid a in osteoarthritis:
Regulatory mechanism and proinflammatory properties. PLoS One.
8:e667692013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wruck CJ, Fragoulis A, Gurzynski A,
Brandenburg LO, Kan YW, Chan K, Hassenpflug J, Freitag-Wolf S,
Varoga D, Lippross S, et al: Role of oxidative stress in rheumatoid
arthritis: Insights from the Nrf2-knockout mice. Ann Rheum Dis.
70:844–850. 2011. View Article : Google Scholar
|
|
41
|
Karreth F, Hoebertz A, Scheuch H, Eferl R
and Wagner EF: The AP1 transcription factor Fra2 is required for
efficient cartilage development. Development. 131:5717–5725. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hiyama A, Gogate SS, Gajghate S, Mochida
J, Shapiro IM and Risbud MV: BMP-2 and TGF-beta stimulate
expression of beta1,3-glucuronosyl transferase 1 (GlcAT-1) in
nucleus pulposus cells through AP1, TonEBP, and Sp1: Role of MAPKs.
J Bone Miner Res. 25:1179–1190. 2010.
|
|
43
|
Skibola CF, Bracci PM, Halperin E, Nieters
A, Hubbard A, Paynter RA, Skibola DR, Agana L, Becker N, Tressler
P, et al: Polymorphisms in the estrogen receptor 1 and vitamin C
and matrix metalloproteinase gene families are associated with
susceptibility to lymphoma. PLoS One. 3:e28162008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abhilash PA, Harikrishnan R and Indira M:
Ascorbic acid suppresses endotoxemia and NF-κB signaling cascade in
alcoholic liver fibrosis in guinea pigs: A mechanistic approach.
Toxicol Appl Pharmacol. 274:215–224. 2014. View Article : Google Scholar
|
|
45
|
Jin M, Kumar A and Kumar S:
Ethanol-mediated regulation of cytochrome P450 2A6 expression in
monocytes: Role of oxidative stress-mediated PKC/MEK/Nrf2 pathway.
PLoS One. 7:e355052012. View Article : Google Scholar : PubMed/NCBI
|