Introduction
Gastric cancer has become one of the leading causes
of cancer-related mortality in Asia (1). Therefore, an improved understanding
of its pathogenic factors and molecular mechanisms may facilitate
the identification of precise prognostic markers and effective
therapeutic targets.
MicroRNAs (miRs), a class of small non-coding RNAs
of ~22 nucleotides in the length, are critical in cell development,
proliferation, differentiation and apoptosis (2,3).
Previous studies have revealed that the dysregulation of miRs is
tightly associated with tumorigenesis, including cell cycle
progression, cell survival and angiogenesis (4,5).
Thus, the aberrant expression of miRs may provide novel strategies
for blocking the development of human cancers.
The expression and functions of miR-132 have been
clearly elucidated in various types of cancer by previous studies.
For example, miR-132 was found to be frequently downregulated in
ductal carcinoma in situ of breast and acted as a tumor
suppressor by inhibiting cell proliferation (6). Furthermore, miR-132 was shown to
inhibit colorectal cancer cell invasion and metastasis via directly
targeting zinc finger E-box binding homeobox 2 (ZEB2) (7). Notably, a recent study indicated that
miR-132 expression levels in gastric cancer tissues were
significantly higher than those in the corresponding normal tissues
(8). The increased expression
levels of miR-132 were associated with more frequent lymph node
metastasis, more lymphatic tumor emboli and a more advanced stage
(8). In addition, a functional
variant at the miR-132 binding site in the CD80 gene was observed
to alter the susceptibility to gastric cancer in a Chinese Han
population (9). However, the
precise roles and molecular mechanisms of miR-132 in the
development of gastric cancer remain undefined. The aim of the
present study was to investigate the expression and roles of
miR-132 in the regulation of gastric cancer progression.
Materials and methods
Clinical tissue specimens
A total of 35 pairs of human gastric cancer and
adjacent normal tissue specimens were obtained from routine
therapeutic surgery at our department (Department of
Gastroenterology, Medical College of Qingdao University, Qingdao,
China) between May and November 2012. Informed consent was obtained
from each patient prior to the collection of tissue samples. The
present study was approved by the Ethics Committee of the Hiser
Medical Group of Qingdao (Qingdao, China).
Cell culture and transient
transfection
The gastric cancer cell lines (NCI-N87 and MGC80-3)
were purchased from the Cell Bank of Type Culture Collection of
Chinese Academy of Sciences (Shanghai, China). Cells were
maintained at 37°C in an atmosphere of 5% CO2 in
Dulbecco's modified Eagle's medium (DMEM; Gibco Life Technologies,
Beijing, China), supplemented with 10% fetal bovine serum,
penicillin (100 IU/ml) and streptomycin (100 mg/ml; all Gibco Life
Technologies). The miR-132 mimics and antisense oligos were
purchased from Ambion Life Technologies (Carlsbad, CA, USA). All
the transient transfections were performed using Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions.
RNA isolation and quantitative polymerase
chain reaction (qPCR)
Total RNA was extracted using the mirVana™ miRNA
Isolation kit (Ambion Life Technologies, Grand Island, NY, USA)
according to the manufacturer's instructions and the expression of
mature miRs was assayed using the TaqMan MicroRNA assay (Applied
Biosystems, Shanghai, China). qPCR was performed using an Applied
Biosystems 7300 Real-Time PCR system and a TaqMan Universal PCR
Master Mix (Applied Biosystems Life Technologies, Foster City, CA,
USA). The expression of miRs was normalized to that of U6 small
nuclear RNA.
Western blot analysis
Human tissues or cells were harvested and lysed with
ice-cold lysis buffer (pH 6.8–7.0). After centrifugation at 10,000
× g for 10 min at 4°C, the proteins in the supernatant were
quantified and separated by 10% SDS-PAGE (Beyotime Institute of
Biotechnology, Nantong, China). The western blot assay was
performed using anti-ZEB2 (cat. no ab25837; 1:1,000; rabbit
anti-human polyclonal antibody; Abcam, Cambridge, MA, USA),
anti-cyclin E1 (cat. no ab7959; 1:2,000; rabbit polyclonal
antibody; Abcam), and anti-retinoblastoma 1 (RB1) antibodies (cat.
no ab24; 1:1,000; mouse anti-human monoclonal antibody; Abcam). The
protein levels were normalized to total GAPDH (cat. no. sc-365062;
1:5,000; mouse anti-human monoclonal antibody; Santa Cruz
Biotechnology, Inc., Dallas, Texas, USA).
Cell proliferation and cell cycle
analysis
The cell viability was determined by assaying the
reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) to formazan. For bromodeoxyuridine (BrdU) analysis, a
cell proliferation enzyme-linked immunosorbent assay (a BrdU kit;
Beyotime Institute of Biotechnology, Shanghai, China) was used to
analyze the incorporation of BrdU during DNA synthesis, and was
conducted according to the manufacturer's instructions. For cell
cycle analysis, the cells were labeled for 15 min with propidium
iodide and immediately analyzed by flow cytometry (BD Biosciences,
San Jose, CA, USA). The bar charts represent the percentage of
cells in each phase of the cell cycle (G0/G1,
S and G2/M).
Luciferase reporter assays
The human RB1 gene 3′-untranslated region (UTR) was
amplified by reverse-transcription PCR using cDNA from NCI-N87
cells. A 3′-UTR mutant construct was also generated by replacing
the 3′-UTR with custom-made synthetic whole 3′-UTR DNAs with
mismatched seed region mutations. miR-132 mimics or negative
controls (NCs) and a pMIR-3′-UTR vector were co-transfected into
the NCI-N87 cells. The NC was a scramble sequence
(5′-TACGGATTGAACTGACTCGA-3′). Renilla and firefly luciferase
activities were measured using a Dual-Luciferase Reporter system
(Promega Corporation, Madison, WI, USA).
Statistical analysis
The data shown represent the mean ± standard error
values of at least three independent experiments. Comparisons
between groups were analyzed using the t-test and χ2
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-132 expression is upregulated in
gastric cancer tissues
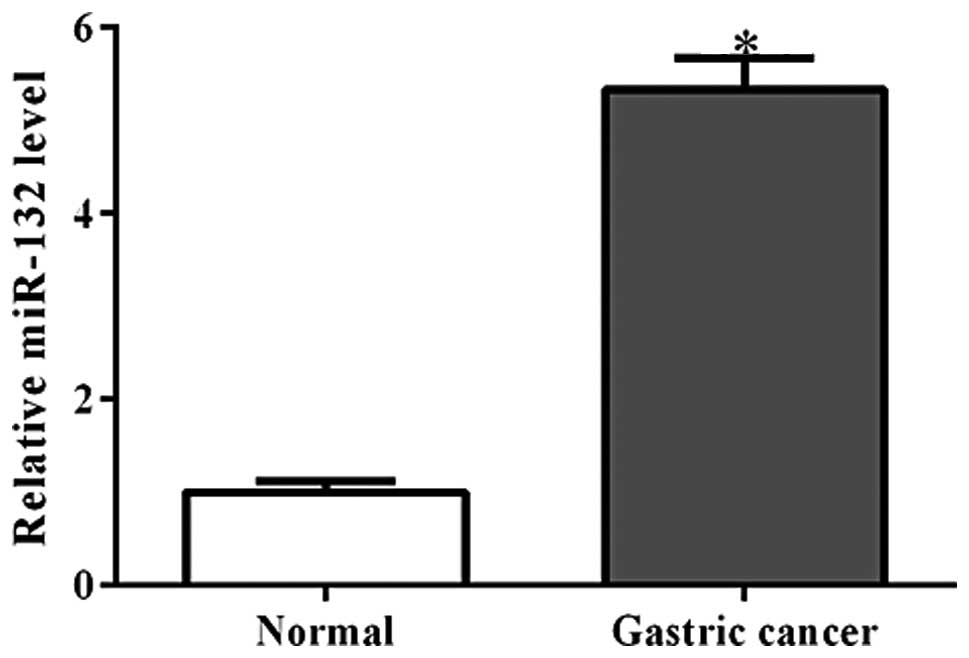
Using qPCR analysis, the miR-132 expression levels
were detected in 35 pairs of gastric cancer tissue and their
matched adjacent non-cancerous tissues. As shown in Fig. 1, miR-132 was observed to be
significantly increased in gastric cancer tissues (P<0.001).
This finding is consistent with that of a previous report (8).
miR-132 promotes cell proliferation and
invasion in vitro
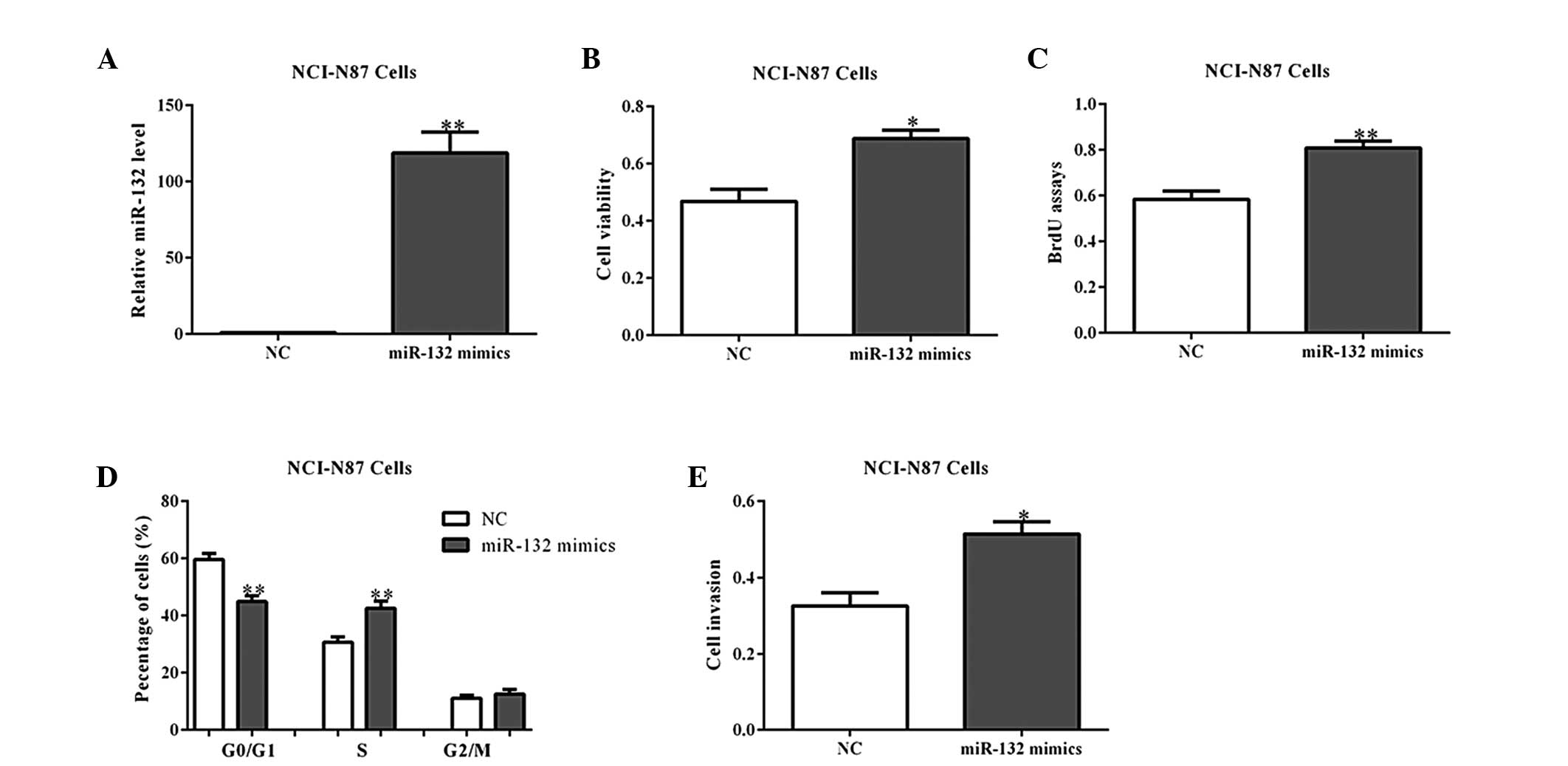
To further determine the roles of miR-132 in
tumorigenesis, its mimics or a NC were transfected into NCI-N87
cells (Fig. 2A). As expected,
overexpression of miR-132 mimics promoted cell viability and
proliferation, as shown by the MTT and BrdU incorporation assays
(Fig. 2B and C). Cell cycle
analysis also indicated that miR-132 overexpression led to a
reduced percentage of cells in the G1/G0
phase and increased percentage of cells in the S phase, when
compared with the NC-transfected cells (Fig. 2D; P<0.01). Additionally, the
cell invasion abilities were observed to be increased by miR-132
mimics in the NCI-N87 cells (Fig.
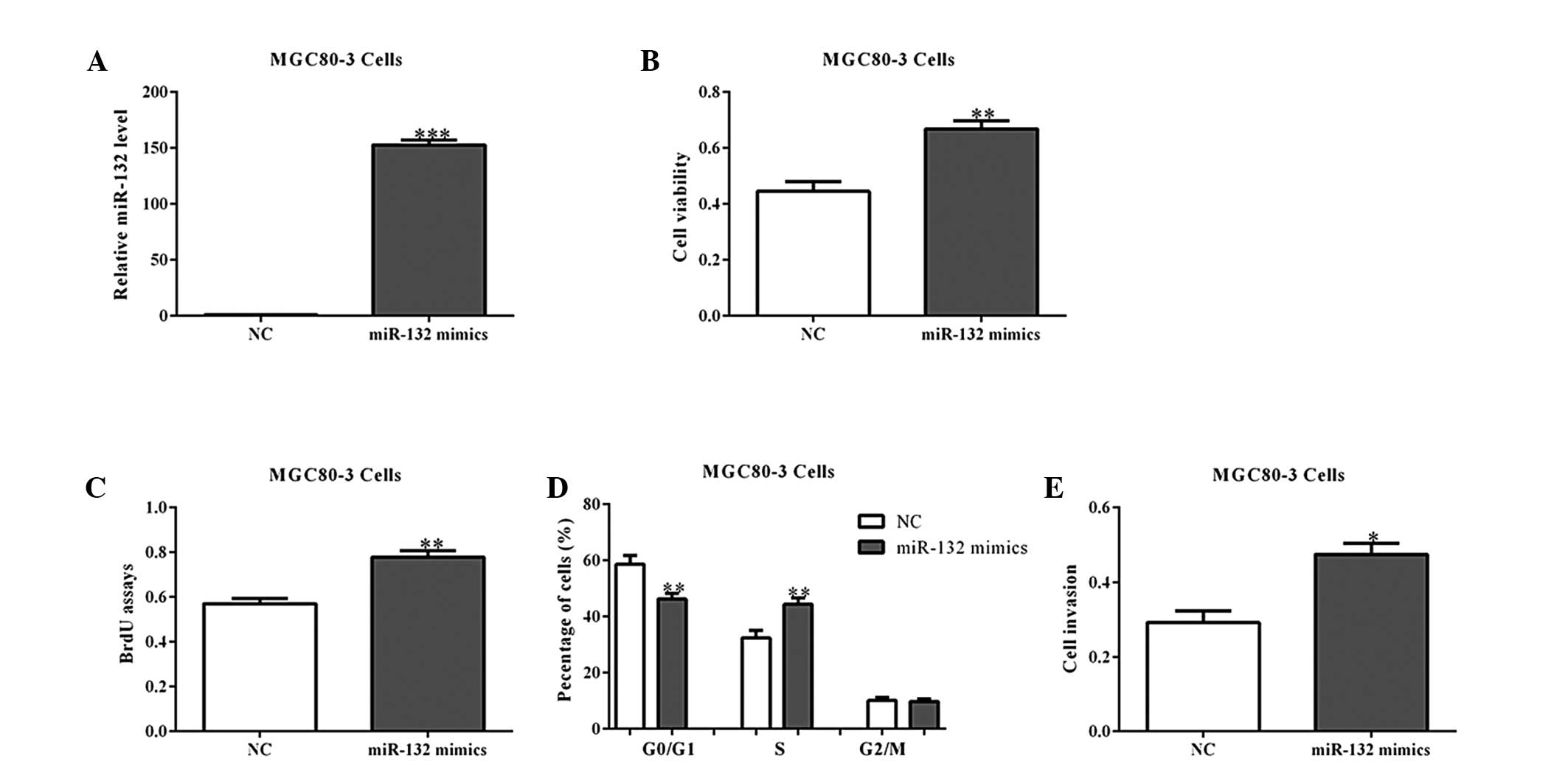
2E). Similar results were observed in the MGC80-3 cells with
miR-132 overexpression (Fig.
3A–E).
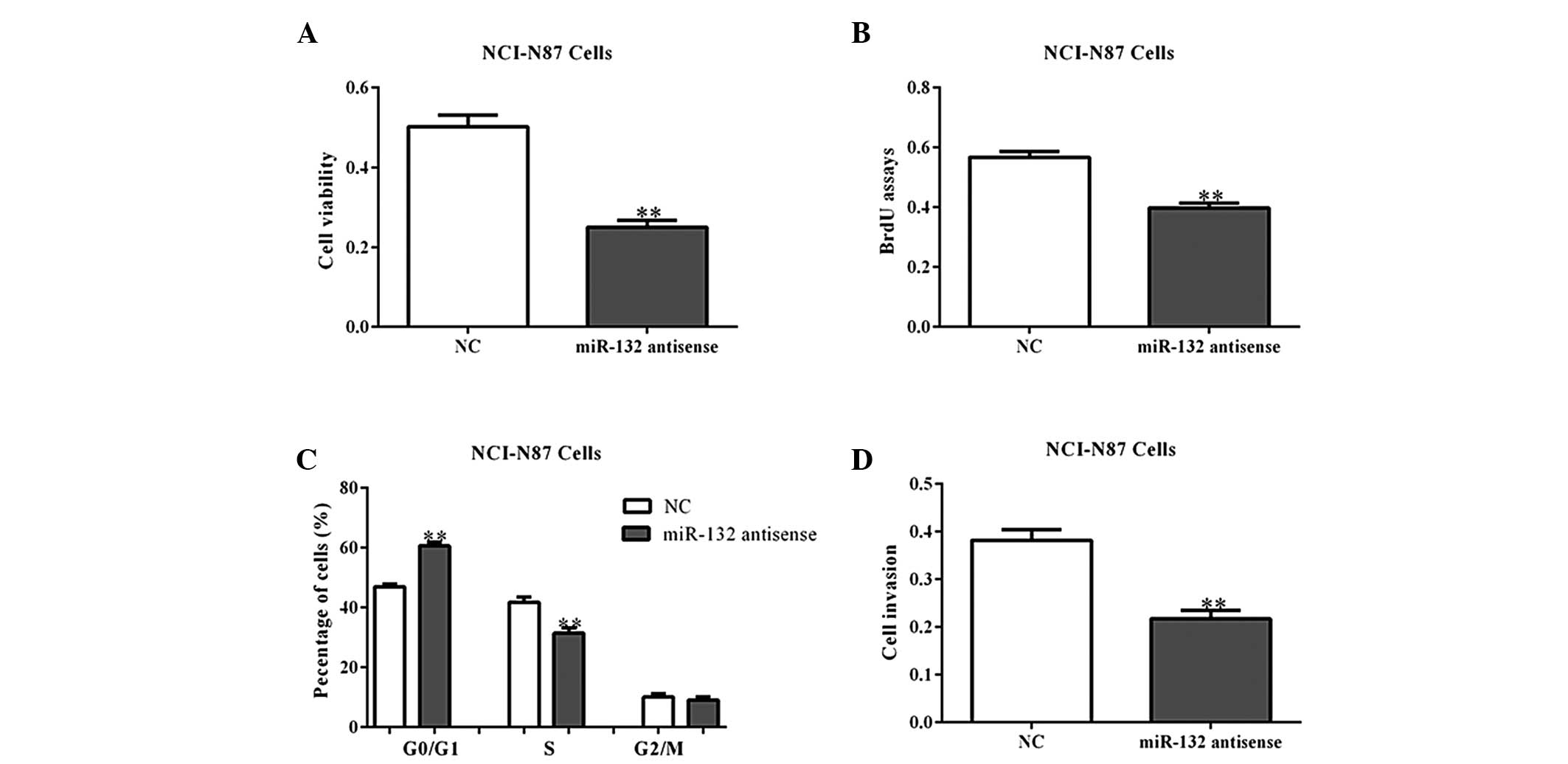
Next, the NCI-N87 and MGC80-3 cells were transfected
with miR-132 antisense oligos, which block the functions of
endogenous miR-203, to assess whether the inhibition of miR-132
inhibits cell growth. As expected, miR-132 antisense oligos
inhibited the growth and invasion of gastric cancer cells, when
compared with the NC-transfected cells (Fig. 4A–D; P<0.05). These data indicate
that miR-132 may promote cell proliferation and invasion.
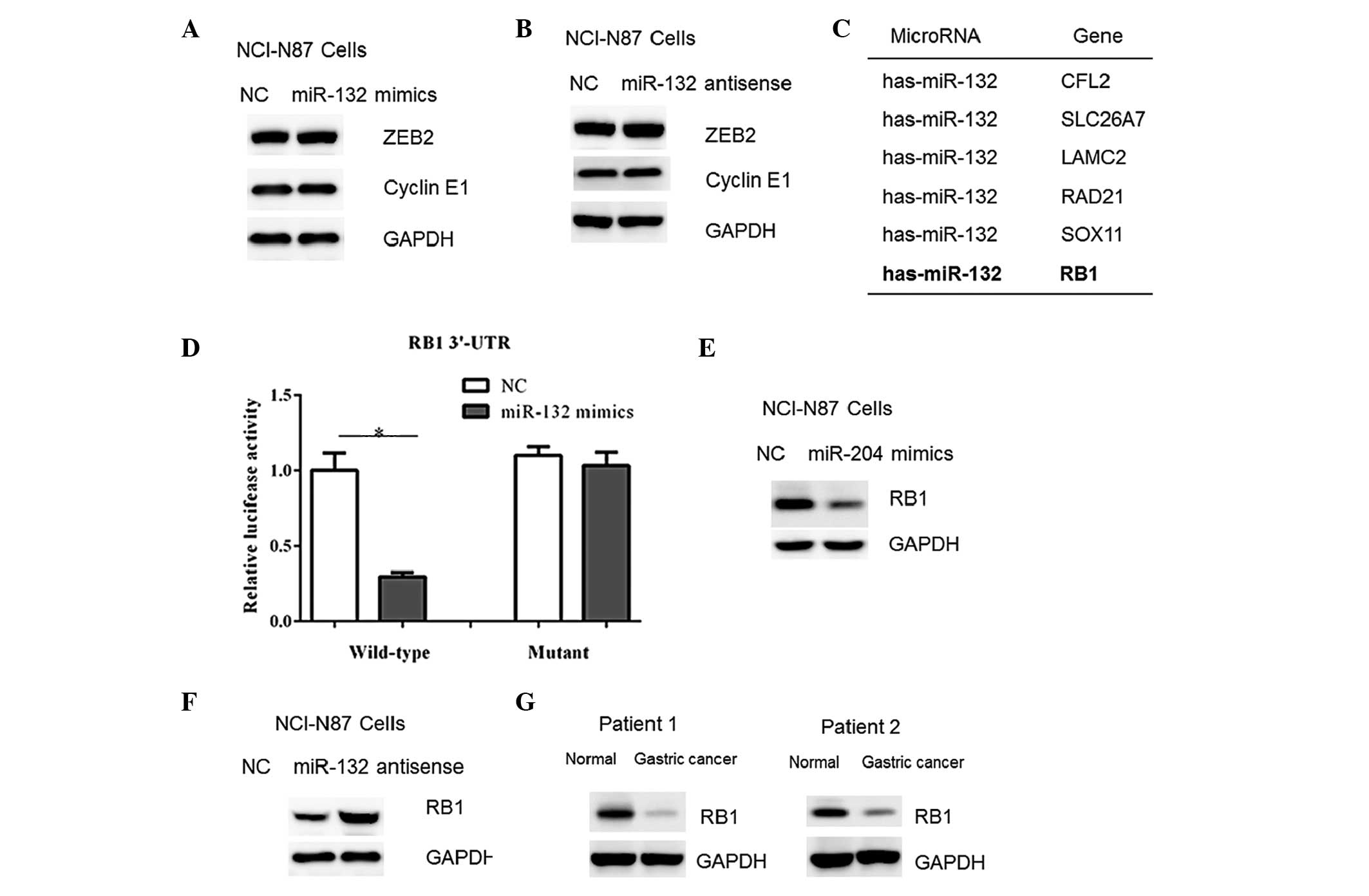
miR-132 directly targets RB1 in gastric
cancer cells
Previous studies have shown that various genes, such
as ZEB2 and cyclin E1 are negatively regulated by miR-132 in human
cancer cells (7,10). However, in the present study, no
changes were detected in the protein contents of ZEB2 and cyclin E1
in NCI-N87 cells transfected with miR-132 mimics or antisense
oligos (Fig. 5A–B). Therefore, a
TargetScan algorithm (http://www.targetscan.org) was used to facilitate the
identification of miR-132 targets in human gastric cancer cells.
Among which, it was found that the RB1 gene, a tumor suppressor,
harbored a potential miR-132 binding site in its 3′-UTR (Fig. 5C). Therefore, the full-length RB1
3′-UTR was cloned into a luciferase reporter vector. Overexpression
of the miR-132 mimics reduced the luciferase activity using the
wild-type RB1 3′-UTR (Fig. 5D).
However, mutation of the putative miR-132 binding site abrogated
the luciferase responsiveness to miR-132 (Fig. 5D). Furthermore, transfection of
miR-132 mimics in NCI-N87 cells resulted in a reduced RB1 protein
abundance (Fig. 5E). In accordance
with this, a marked increase in RB1 content was observed in the
cells with miR-132 inhibition (Fig.
5F). Furthermore, the RB1 protein content was observed to be
decreased in the gastric cancer tissue samples (Fig. 5G), which correlated with the
upregulation of miR-132 in the tumor tissue samples (Fig. 1). Taken together, these results
indicate that RB1 may be a downstream target of miR-132 in gastric
cancer cells.
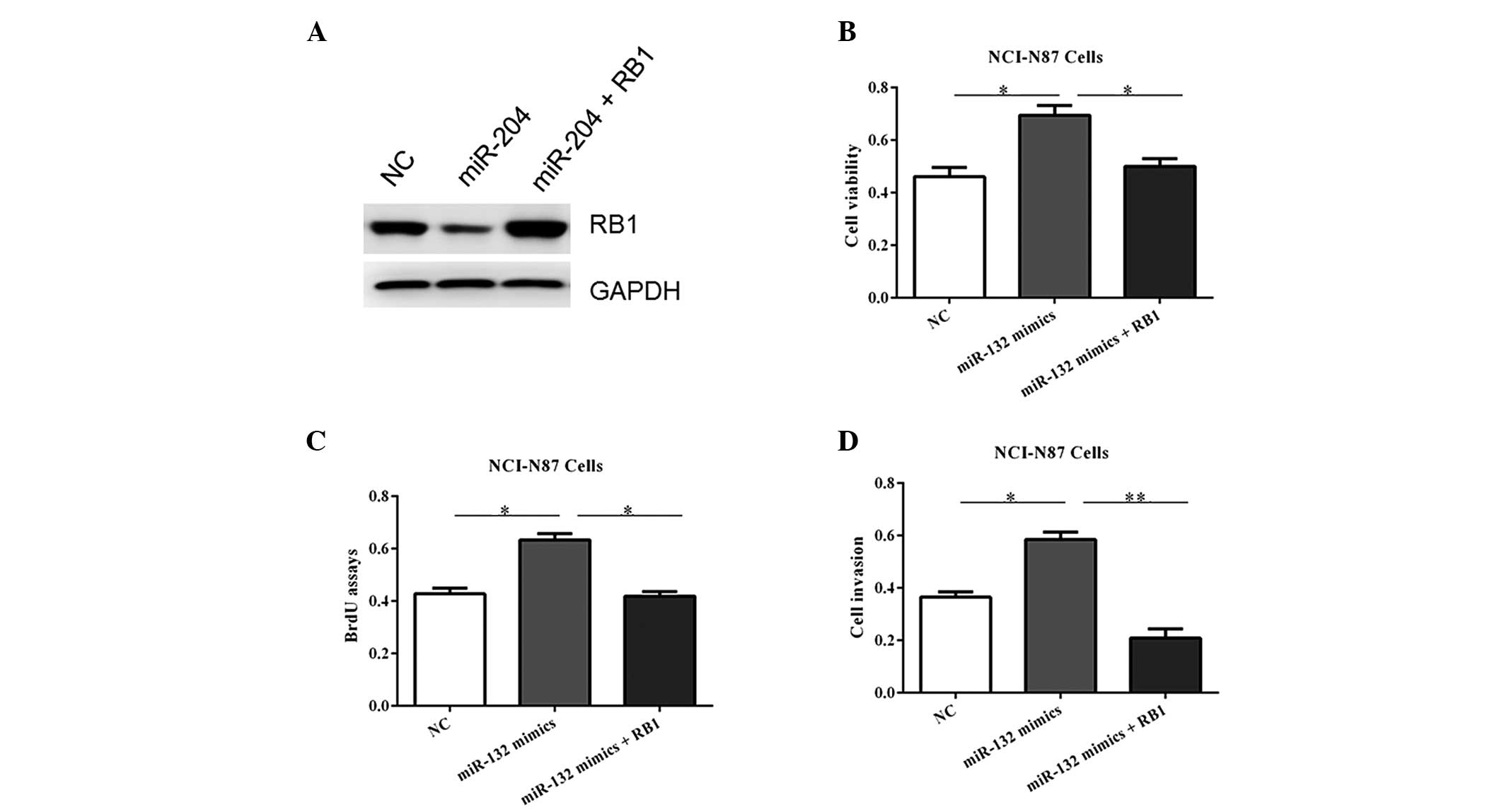
RB1 overexpression blocks the
proliferative role of miR-132
To verify the functional connection between miR-132
and RB1, the NCI-N87 cells were transfected with RB1 expression
plasmids or an empty vector following transfection of miR-132
mimics (Fig. 6A). As shown in
Fig. 6B–D, RB1 reintroduction
reversed the oncogenic roles of miR-132, underlining the specific
importance of RB1 for miR-132 action in tumorigenesis.
Discussion
Numerous studies have demonstrated that various
miRs, such as miR-375, -200 and -516a-3p are dysregulated in
gastric cancer tissues or cell lines (11–13).
In addition, abnormal expression of certain miRs in gastric cancer
is associated with progression, poor prognosis and sensitivity to
chemotherapy (14,15). Therefore, identification and
understanding of the roles of miRs will expand their application in
clinical practice, to be used in the detection, diagnosis,
prognosis and treatment of gastric cancer.
Previous studies have shown that miR-132 is
downregulated and may serve as a tumor suppressor in prostate, lung
and breast cancer (16–18). In the present study, evidence
demonstrating that miR-132 is upregulated in gastric cancer tissues
is presented. In addition, in vitro studies further
indicated that the overexpression of miR-132 mimics promoted (while
its antisense oligos suppressed) cell proliferation and invasion.
Although the reason why miR-132 could either inhibit or promote
cell proliferation remains unknown, the role of miR-132 in
tumorigenesis may be cell or tissue-specific. However, the
functions of miR-132 in gastric cancer development require further
investigation in vivo; thus, analysis in miR-132 knockout
mice or overexpression of miR-132 in nude mice may be
facilitative.
At the molecular level, computer-assisted analysis
with TargetScan revealed that RB1 was a direct target of miR-132 in
gastric cells. RB1 is the first tumor suppressor gene that was
identified and is a negative regulator of the cell cycle (19). It has been shown that RB1
expression is inhibited by various miRs in human cancer cells
(20–22), indicating that downregulation of
RB1 may be a key feature of tumor initiation or progression.
In conclusion, miR-132 was identified as a novel
positive regulator of gastric cancer development via suppression of
RB1 protein expression. These data may be beneficial for
determining novel therapeutic strategies for future treatment of
gastric cancer.
References
|
1
|
Shen L, Shan YS, Hu HM, Price TJ, Sirohi
B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, et al: Management of
gastric cancer in Asia: Resource-stratified guidelines. Lancet
Oncol. 14:e535–e547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun K and Lai EC: Adult-specific functions
of animal microRNAs. Nat Rev Genet. 14:535–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ling H, Fabbri M and Calin GA: MicroRNAs
and other non-coding RNAs as targets for anticancer drug
development. Nat Rev Drug Discov. 12:847–865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song S and Ajani JA: The role of microRNAs
in cancers of the upper gastrointestinal tract. Nat Rev
Gastroenterol Hepatol. 10:109–118. 2013. View Article : Google Scholar
|
|
6
|
Li S, Meng H, Zhou F, Zhai L, Zhang L, Gu
F, Fan Y, Lang R, Fu L, Gu L, et al: MicroRNA-132 is frequently
downregulated in ductal carcinoma in situ (DCIS) of breast and acts
as a tumor suppressor by inhibiting cell proliferation. Pathol Res
Pract. 209:179–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng YB, Luo HP, Shi Q, Hao ZN, Ding Y,
Wang QS, Li SB, Xiao GC and Tong SL: MiR-132 inhibits colorectal
cancer invasion and metastasis via directly targeting ZEB2. World J
Gastroenterol. 20:6515–6522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, Yu H, Cai H and Wang Y: The
expression and clinical significance of miR-132 in gastric cancer
patients. Diagn Pathol. 9:572014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu R, Li F, Zhu J, Tang R, Qi Q, Zhou X,
Li R, Wang W, Hua D and Chen W: A functional variant at miR-132-3p,
miR-212-3p, and miR-361-5p binding site in CD80 gene alters
susceptibility to gastric cancer in a Chinese Han population. Med
Oncol. 31:602014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Xu G, Shen F and Kang Y: MiR-132
targeting cyclin E1 suppresses cell proliferation in osteosarcoma
cells. Tumour Biol. 35:4859–4865. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsukamoto Y, Nakada C, Noguchi T, Tanigawa
M, Nguyen LT, Uchida T, Hijiya N, Matsuura K, Fujioka T, Seto M, et
al: MicroRNA-375 is downregulated in gastric carcinomas and
regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer
Res. 70:2339–2349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shinozaki A, Sakatani T, Ushiku T, Hino R,
Isogai M, Ishikawa S, Uozaki H, Takada K and Fukayama M:
Downregulation of microRNA-200 in EBV-associated gastric carcinoma.
Cancer Res. 70:4719–4727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takei Y, Takigahira M, Mihara K, Tarumi Y
and Yanagihara K: The metastasis-associated microRNA miR-516a-3p is
a novel therapeutic target for inhibiting peritoneal dissemination
of human scirrhous gastric cancer. Cancer Res. 71:1442–1453. 2011.
View Article : Google Scholar
|
|
14
|
Shin VY and Chu KM: MiRNA as potential
biomarkers and therapeutic targets for gastric cancer. World J
Gastroenterol. 20:10432–10439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma J, Hong L, Chen Z, Nie Y and Fan D:
Epigenetic regulation of microRNAs in gastric cancer. Dig Dis Sci.
59:716–723. 2014. View Article : Google Scholar
|
|
16
|
Formosa A, Lena AM, Markert EK, Cortelli
S, Miano R, Mauriello A, Croce N, Vandesompele J, Mestdagh P,
Finazzi-Agrò E, et al: DNA methylation silences miR-132 in prostate
cancer. Oncogene. 32:127–134. 2013. View Article : Google Scholar
|
|
17
|
You J, Li Y, Fang N, Liu B, Zu L, Chang R,
Li X and Zhou Q: MiR-132 suppresses the migration and invasion of
lung cancer cells via targeting the EMT regulator ZEB2. PLoS One.
9:e918272014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tahiri A, Leivonen SK, Luders T, Steinfeld
I, Ragle Aure M, Geisler J, Mäkelä R, Nord S, Riis ML, Yakhini Z,
et al: Deregulation of cancer-related miRNAs is a common event in
both benign and malignant human breast tumors. Carcinogenesis.
35:76–85. 2014. View Article : Google Scholar
|
|
19
|
Chen HZ, Tsai SY and Leone G: Emerging
roles of E2Fs in cancer: An exit from cell cycle control. Nat Rev
Cancer. 9:785–797. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deng Y, Huang Z, Xu Y, Jin J, Zhuo W,
Zhang C, Zhang X, Shen M, Yan X, Wang L, et al: MiR-215 modulates
gastric cancer cell proliferation by targeting RB1. Cancer Lett.
342:27–35. 2014. View Article : Google Scholar
|
|
21
|
Zhang YF, Zhang AR, Zhang BC, Rao ZG, Gao
JF, Lv MH, Wu YY, Wang SM, Wang RQ and Fang DC: MiR-26a regulates
cell cycle and anoikis of human esophageal adenocarcinoma cells
through Rb1-E2F1 signaling pathway. Mol Biol Rep. 40:1711–1720.
2013. View Article : Google Scholar
|
|
22
|
Feng S, Cong S, Zhang X, Bao X, Wang W, Li
H, Wang Z, Wang G, Xu J, Du B, et al: MicroRNA-192 targeting
retinoblastoma 1 inhibits cell proliferation and induces cell
apoptosis in lung cancer cells. Nucleic Acids Res. 39:6669–6678.
2011. View Article : Google Scholar : PubMed/NCBI
|