Introduction
Reactive oxygen species (ROS) are oxygen-derived
radicals and include members such as the highly reactive superoxide
(O2−), hydroxyl (OH−) and peroxyl
(RO2−), as well as non-radicals, including hydrogen
peroxide (H2O2) and peroxynitrite
(ONOO−). In healthy individuals, ROS and antioxidants
remain in balance; however, an ROS overabundance results in
oxidative stress. Previous studies have demonstrated that oxidative
stress can alter microRNA (miRNA) expression. miRNAs are non-coding
RNAs, ~22 nucleotide (nt) in length, that are evolutionarily
conserved and function as sequence-specific regulators of gene
expression through translational repression and/or transcriptional
cleavage (1–6). In addition, miRNAs have a role in
cellular oxidative damage caused by ROS (7,8).
The association between miRNAs and ROS has been
investigated in various diseases, including cancer, vascular
diseases and cardiometabolic diseases (9–11).
UV, H2O2, ionizing radiation and anticancer
drugs that produce ROS are known to modulate miRNA expression
(12–14). Numerous studies have focused on
miRNA profiling following oxidative stress exposure in various
tissues and have demonstrated the importance of miRNA modulation in
the cellular response to a redox imbalance (10). The interaction between ROS and
miRNAs remains to be elucidated, with certain studies suggesting
that miRNA expression levels could be regulated by ROS, including
miR-17-92 (15), while others
suggest that miRNAs, including miR-34a and miR-23b, are able to
modulate ROS production (8,16).
Additionally, miR-23b can either inhibit or promote ROS during
transcriptional regulatory processes, thus causing an anti- or
pro-oxidant effect (16).
Spinal cord injuries (SCI) are one of the most
debilitating pathologies and lead to huge rehabilitation challenges
(17,18). SCI is a comprehensive consequence
of a primary mechanical insult followed by a sequence of
progressive secondary pathophysiological events, with experimental
evidence indicating that ROS are important mediators of secondary
damage (19–22). Furthermore, increased ROS levels
can cause oxidative damage leading to neuronal death and
neurological dysfunction (23–25)
in uninjured rat spinal cords. Therefore, finding a regulator to
reduce ROS damage of the central nervous system may reduce
secondary SCI.
The present study aimed to investigate the
expression of miR-146a, miR-21 and miR-150 in
H2O2-stimulated rat spinal cord neurons
(RN-sc). In addition, the present study assessed whether inhibition
of miR-21 and miR-150 affects cell proliferation and apoptosis.
Materials and methods
Cell culture
RN-sc cells (ScienCell Research Laboratories, San
Diego, CA, USA) isolated from the E14 rat spinal cord were and
cultured in neuronal medium (ScienCell Research Laboratories; cat.
no. 1521).
H2O2 treatment,
miRNA mimics synthesis and transfection
RN-scs were seeded in 6-well plates, treated 24 h
post-seeding with 100 mM H2O2 for 6 h and
harvested for quantitative polymerase chain reaction (qPCR). Cells
were plated to 50% confluency and transfected with 200 nM miR-150,
miR-21 mimic or negative control (NC; Guangzhou RiboBio Co., Ltd.,
Guangzhou, China) using HiPerFect HTS Reagent (Qiagen, Valencia,
CA, USA) according to the manufacturer's instructions. Cells were
exposed to 50 mM H2O2 24 h post-transfection
for varying lengths of time and harvested for further
experimentation. For the MTT assay, RN-sc cells were pre-treated
with anti-sense-miR-21 or anti-sense-miR-150 mimics for 24 h prior
to exposure to 50 µM H2O2 for 0, 2, 4
or 6 days. For flow cytometric analysis, RN-sc cells were
pre-treated with anti-sense-miR-21 or anti-sense-miR-150 mimics for
24 h, stimulated with a high concentration of
H2O2 (200 µM) for 12 h and harvested
for flow cytometric analysis
Reverse transcription (RT)-qPCR
Total RNA was extracted using TRIzol reagent and
reverse transcribed to cDNA using stem loop RT primers specific to
miR-146a, miR-150 or miR-21 (Guangzhou RiboBio Co., Ltd.,
Guangzhou, China). The following PCR primer sequences were used:
Smad, forward 5′-TTTTGAGGTGTGGTGGGT-3′ and reverse
5′-GAGGCAGTAAGACAGGGATGA-3′. All reactions were performed using
SYBR Green mix (Takara Bio Inc., Shiga, Japan) under the following
PCR conditions: 94°C for 5 min, 40 cycles of 94°C for 30 sec, 55°C
for 30 sec and a final incubation at 72°C for 20 sec, with
fluorescence detected following each cycle and continuously traced
using an Applied Biosystems 7500 system (Applied Biosystems; Thermo
Fisher Scientific, Waltham, MA, USA). Relative expression levels
were calculated as ratios normalized to β-actin. All
experimentation was performed in triplicate with the results
expressed as the mean ± standard deviation.
Cell proliferation assay
Cell proliferation was monitored using the MTT assay
kit (Promega Corporation, Madison, WI, USA) according to the
manufacturer's instructions. Rat spinal neurons cells were seeded
at 1×103 per well in 96-well plates 24 h
post-transfection, with the cellular proliferation assay performed
on days 0, 2, 4 and 6. To perform the assay, 10 µl MTT
reagent was added to each well and the plate was incubated for 4 h
at 37°C. Prior to the end of the incubation period, the absorbance
was measured at 570 nm using a Vmax microplate spectrophotometer
(Molecular Devices, Sunnyvale, CA, USA), with each sample assayed
in triplicate.
Cell apoptosis assay
Rat spinal neuron cells were harvested 48 h
post-transfection, with 1×106 cells (500 µl)
added into FACS tubes, mixed with 25 ng/ml Annexin V-fluorescein
isothiocyanate and 10 mg/ml propidium iodide and incubated for 15
min at room temperature in the dark. The cells were then
immediately analyzed by flow cytometry on a BD Accuri™ C6 flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
RN-sc cells (2×106) were collected and
washed twice with ice-cold phosphate-buffered saline. The cell
pellets were suspended in RIPA lysis buffer, incubated on ice for
40 min and the lysates were centrifuged at 12,000 × g for 15 min at
4°C. Proteins were isolated by 10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis and transferred onto a
polyvinylidene fluoride membrane. Membranes were blocked for 1 h at
37°C with 5% non-fat milk and incubated with rabbit anti-human
Smad7 monoclonal antibody (1:1,000; cat no. ab124890; Abcam,
Cambridge, MA, USA) or rabbit anti-human β-actin monoclonal
antibody (1:2,000; cat no. ab119716; Abcam). Following washing with
Tris-buffered saline with 0.5% Tween 20 (TBST), the membrane was
incubated with horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G (H+L) secondary antibody (1:10,000, cat no.
ab97080; Abcam) at room temperature for 40 min, washed again with
TBST and imaged with enhanced chemiluminescence captured on X-ray
films.
Plasmid construction and luciferase
reporter assay
To construct a luciferase reporter vector, the
wild-type 3′ untranslated region (UTR) and mutant 3′UTR of Smad7
containing putative binding sites for miR-21 were subcloned into
the psi-CHECK-2 vector. For the luciferase reporter assay, 293T
cells (Cell Bank of the Chinese Academy of Sciences, Shanghai,
China) were plated at 5×104 cells per well in 24 well
plates. On the following day, psiCHECK-2 luciferase vectors
containing the 3′UTR of Smad7 and the miR-21 mimic or the negative
control oligonucleotides were transfected into cells using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA). The luciferase assay was performed 48 h post-transfection
using the dual luciferase reporter assay system (Promega
Corporation) according to the manufacturer's instructions.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). All numerical data were
analyzed using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of H2O2 on
miR-146a, miR-21 and miR-150 expression in RN-sc cells
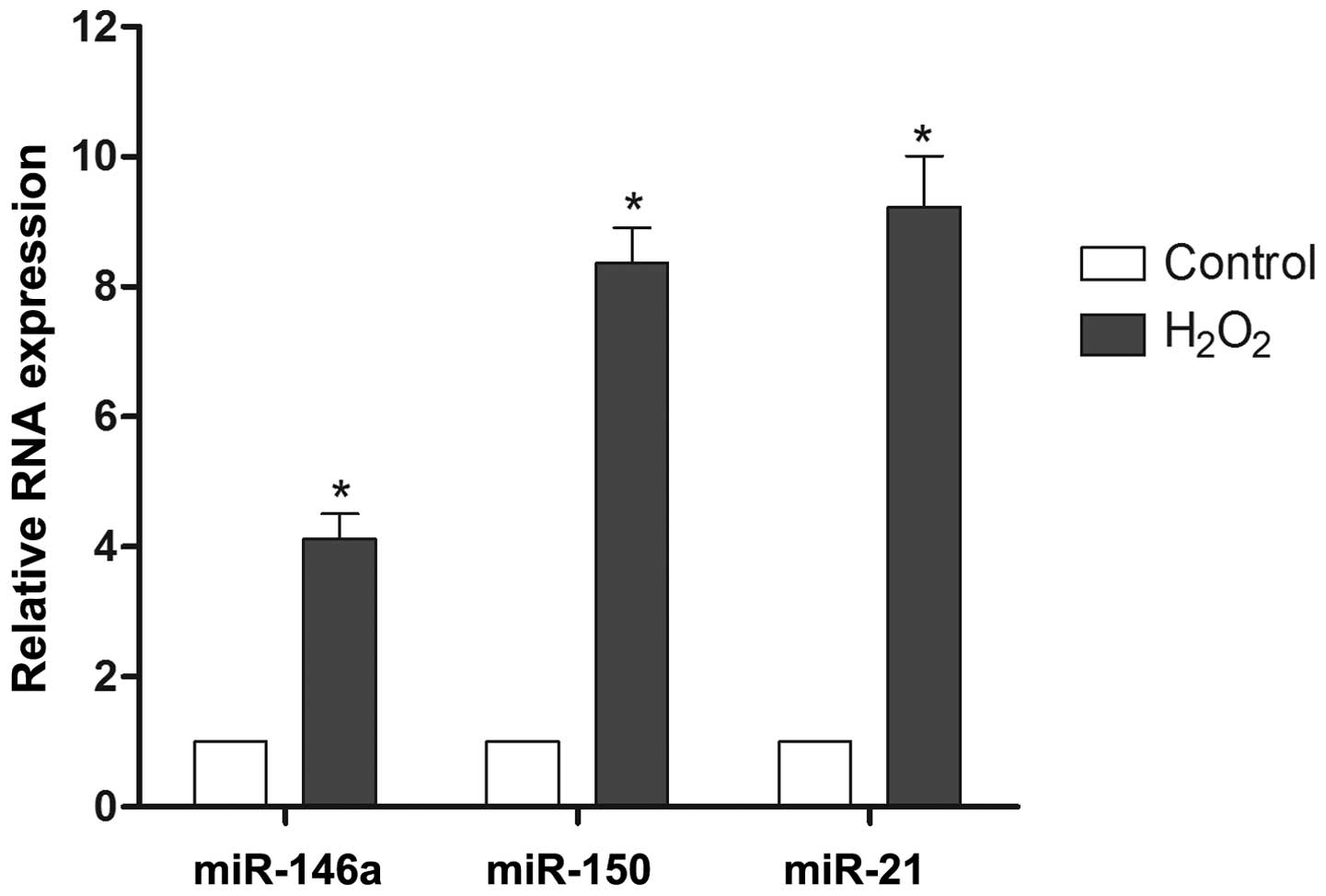
To investigate the possibility of miRNAs modulating
reactive oxygen injury, the expression levels of miR-146a, miR-21
and miR-150 were monitored during H2O2
exposure. The results demonstrated that the expression of miR-146a,
miR-150 and miR-21 was upregulated 4.1-fold, 8.4-fold and 9.2-fold,
respectively, during H2O2 treatment relative
to the control samples (Fig. 1).
Based on these findings, miR-21 and miR-150 were selected for
further evaluation.
Effect of miR-21 and miR-150 on cell
proliferation during H2O2 treatment
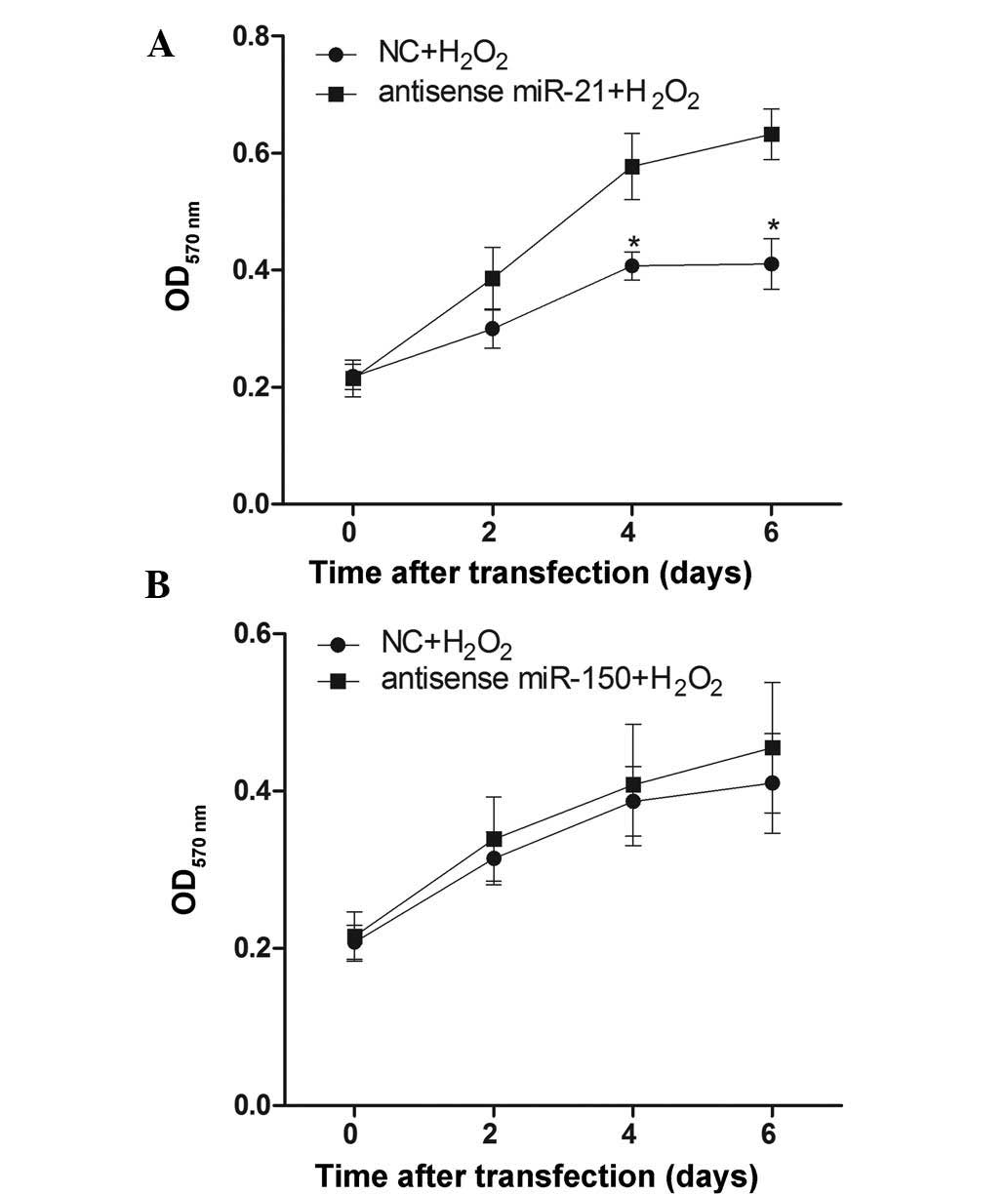
To verify whether decreasing miR-21 and miR-150 has
an effect on cellular proliferation following
H2O2 treatment, RN-sc cells were pre-treated
with anti-sense-miR-21 or anti-sense-miR-150 mimics for 24 h,
stimulated with 50 µM H2O2 for 6 days
and harvested for MTT assays at different time points. The results
demonstrated that pre-treatment with the anti-sense-miR-21 mimic
could reduce H2O2-induced inhibition of
cellular proliferation (Fig. 2A),
while the anti-sense-miR-150 mimic pre-treatment had no pronounced
effect (Fig. 2B).
Effect of miR-21 and miR-150 on cell
apoptosis during H2O2 treatment
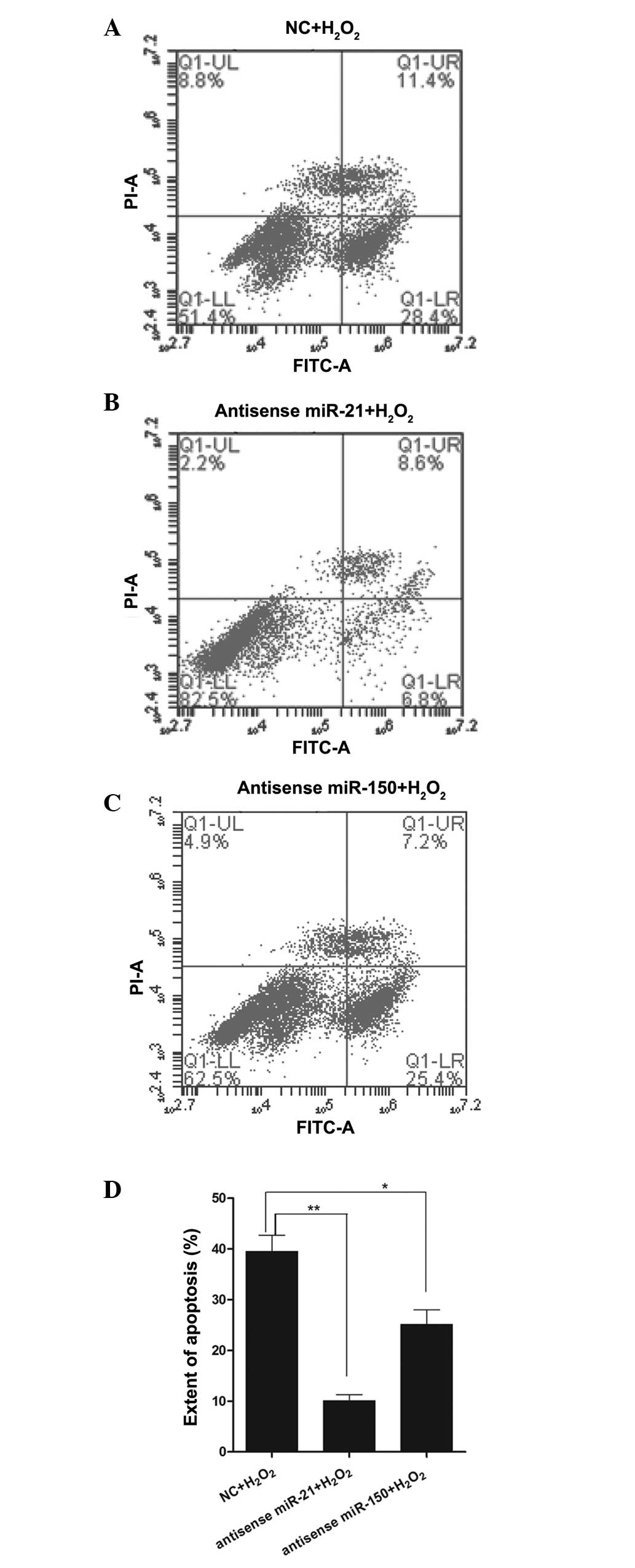
To verify whether a decrease in miR-21 and miR-150
affects apoptosis following H2O2 treatment,
RN-sc cells were pre-treated with anti-sense-miR-21 or
anti-sense-miR-150 mimics for 24 h and stimulated with a high
concentration of H2O2 (200 µM) for 12
h. Subsequently, the cells were harvested for flow cytometric
analysis. The results demonstrated that anti-sense-miR-21 mimic
pre-treatment markedly reduced H2O2-induced
cellular apoptosis relative to the control samples (Fig. 3A, B and D), while
anti-sense-miR-150 mimic pre-treatment reduced apoptosis to a
lesser extent than anti-sense-miR-21 pre-treatment (Fig. 3A, C and D).
Smad7 is a direct target of miR-21 under
H2O2 treatment
The above results demonstrated that miR-21, but not
miR-150, could regulate proliferation and apoptosis during
H2O2 treatment in RN-sc cells. To understand
the mechanisms by which miR-21 affects cellular apoptosis and
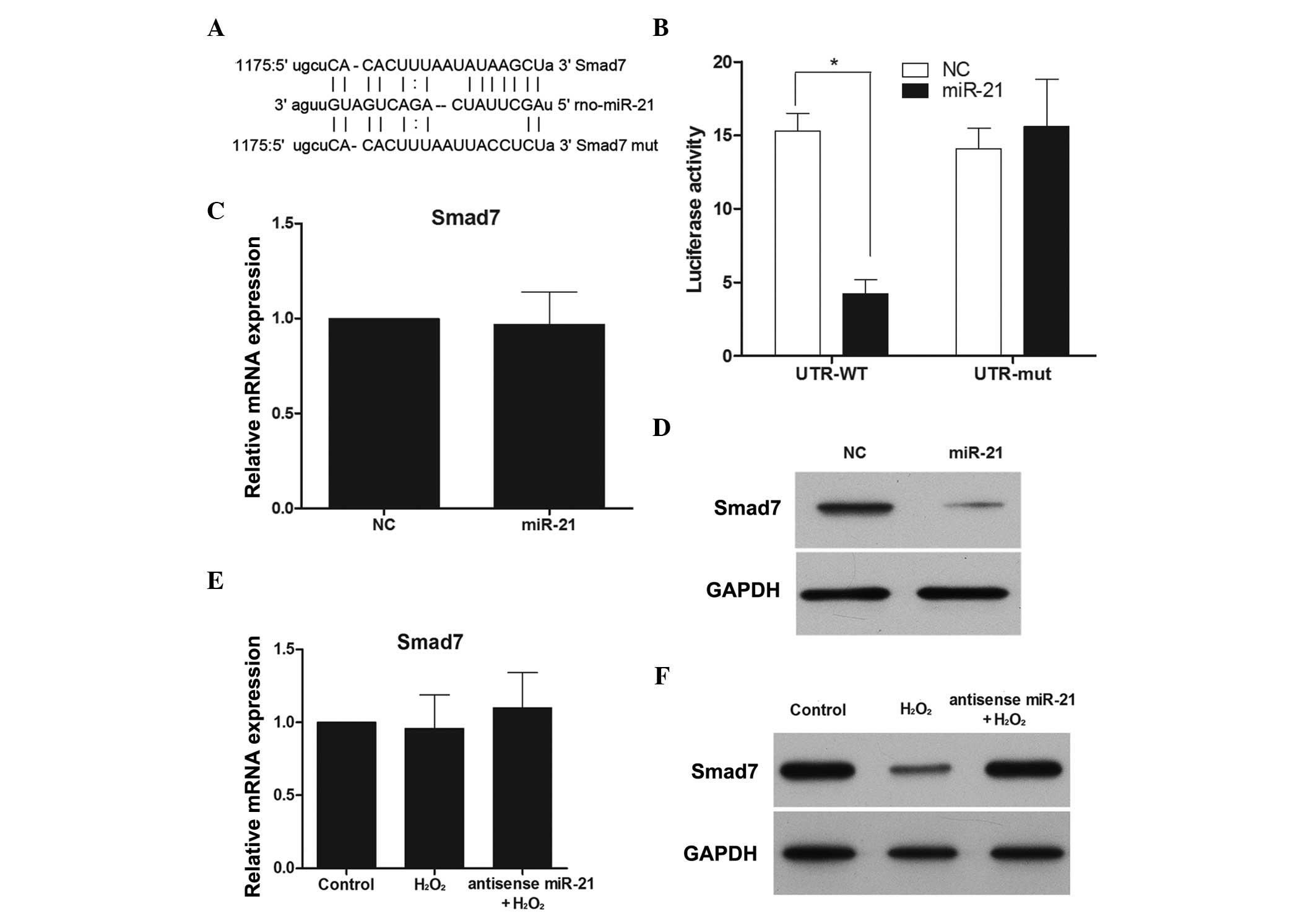
proliferation, several computational methods on MIRANDA (http://www.microrna.org) were employed to identify
miR-21 targets in rats. Among the predicted targets, Smad7 was of
particular interest (Fig. 4A). To
assess whether Smad7 was a direct target of miR-21, a luciferase
reporter assay was performed. Luciferase inhibition was observed
following transfection with the wild-type Smad7 3′-UTR, while no
inhibition was noted in the presence of the mut-type Smad7 3′UTR
(Fig. 4B). Subsequently, the
effect of miR-21 over-expression on Smad7 mRNA and protein
expression was examined. Although miR-21 overexpression did not
cause degradation of Smad7 mRNA (Fig.
4C), it did reduce the activity of the luciferase reporter gene
fused to the wild-type Smad7 3′-UTR, thus indicating that miR-21
targets Smad7 through translational inhibition. In support of these
results, a clear reduction in the levels of endogenous Smad7
protein in miR-21-overexpressing RN-sc cells was observed (Fig. 4D).
To further establish interactions between miR-21 and
Smad7 during H2O2 treatment, Smad7 mRNA and
protein expression was monitored in the presence and absence of an
anti-sense miR-21 mimic and H2O2. While
H2O2 had no effect on Smad7 mRNA expression,
Smad7 protein expression was downregulated (Fig. 4E and F), and this effect was
reversed when miR-21 expression was inhibited (Fig. 4F).
Discussion
In the present study, it was found that
H2O2 could clearly alter the expression level
of miR-21 and miR-150 in RN-sc cells. Previous studies examining
cardiac myocytes found that miR-150 and miR-21 expression was
upregulated following H2O2 treatment and that
miR-150 and miR-21 silencing could decrease
H2O2-induced cardiac cell death and apoptosis
(26,27). Another study found that ROS promote
gastric carcinogenesis via upregulating miR-21 expression, which in
turn downregulates the expression of programmed cell death protein
4 (PDCD4) (28). In a previous
study examining cardiac myocytes,
H2O2-mediated upregulation of miR-21 was
confirmed by qPCR, with H2O2-induced cardiac
cell death and apoptosis increased by a miR-21 inhibitor and
decreased by pre-miR-21 (27).
Based on these results, it was proposed that miR-21 and miR-150 may
have a role in oxidative stress damage in rat spinal cord
neurons.
To assess the potential role of miR-21 and miR-150
in H2O2-mediated SCI in rat neurons, miR-21
and miR-150 expression was modulated via targeted inhibition.
Notably, downregulation of miR-21 expression inhibited
H2O2-mediated apoptosis and proliferative
inhibition in RN-sc cells, while downregulation of miR-150 had no
effect. These results suggest that miR-21 downregulation has a
protective effect in H2O2-mediated apoptosis
and proliferative inhibition.
It is clear that miRNAs downregulate the expression
of target genes by either inducing mRNA degradation or inhibiting
mRNA translation. In lung squamous carcinomas, miR-21
simultaneously regulates multiple pathways that enhance cell
proliferation, apoptosis and tumor invasiveness by targeting
phosphatase and tensin homolog, reversion-inducing cysteine-rich
protein with Kazal motifs and B-cell lymphoma-2 (29). In addition, PDCD4 is an important
target gene of miR-21 and miR-21-PDCD4 signaling has been
demonstrated to be involved in the regulation of ROS-induced
physiological processes (30).
However, the exact mechanisms of a given miRNA can remain elusive
due to their numerous target genes and potential differences in the
role of each target gene. In the present study, Smad7 was
identified as a new target of miR-21 in rat spinal cord neurons.
Furthermore, Smad7 protein level could be inhibited by
H2O2, with this effect reversed following the
suppression of miR-21 expression. Another study also noted
inhibition of Smad7 expression following H2O2
exposure and demonstrated that Smad7 inhibited ROS production in
angiotensin II-induced cardiac fibroblasts (31). Therefore, it was predicted that
miR-21 may be important in ROS production through the regulation of
Smad7 in rat spinal cord neurons.
In conclusion, the present study demonstrated that
H2O2 could upregulate the expression of
miR-21 and downregulate the expression of Smad7. Additionally,
Smad7 is a target gene of miR-21 and it was predicted that miR-21
may be important in ROS production through Smad7 regulation in rat
spinal cord neurons. Future studies are required to further examine
the association between SCI, ROS, miR-21 and Smad7 in
vivo.
Acknowledgments
The present study was supported by the Scientific
Research Cultivation and Innovation Fund (Jinan University,
Guangzhou, China, 2015; grant no. 21615468), and the Administration
of Traditional Chinese Medicine of Guangdong Province, China (no.
20141067). The authors would like to thank LetPub (www.letpub.com) for its linguistic assistance during
the preparation of this manuscript.
References
|
1
|
Cha HJ, Kim OY, Lee GT, Lee KS, Lee JH,
Park IC, Lee SJ, Kim YR, Ahn KJ, An IS, et al: Identification of
ultraviolet B radiation-induced microRNAs in normal human dermal
papilla cells. Mol Med Rep. 10:1663–1670. 2014.PubMed/NCBI
|
|
2
|
Fatemi N, Sanati MH, Shamsara M, Moayer F,
Zavarehei MJ, Pouya A, Sayyahpour F, Ayat H and Gourabi H:
TBHP-induced oxidative stress alters microRNAs expression in mouse
testis. J Assist Reprod Genet. 31:1287–1293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Du YY, Lin YF, Chen YT, Yang L,
Wang HJ and Ma D: The cell growth suppressor, mir-126, targets
IRS-1. Biochem Biophys Res Commun. 377:136–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luzi E, Marini F, Sala SC, Tognarini I,
Galli G and Brandi ML: Osteogenic differentiation of human adipose
tissue-derived stem cells is modulated by the miR-26a targeting of
the SMAD1 transcription factor. J Bone Miner Res. 23:287–295. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu B, Chapman EJ, Yang Z, Carrington JC
and Chen X: Transgenically expressed viral RNA silencing
suppressors interfere with microRNA methylation in Arabidopsis.
FEBS Lett. 580:3117–3120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Q, Chen W, Bai L, Chen W, Padilla MT,
Lin AS, Shi S, Wang X and Lin Y: Receptor-interacting protein 1
increases chemoresistance by maintaining inhibitor of apoptosis
protein levels and reducing reactive oxygen species through a
microRNA-146a-mediated catalase pathway. J Biol Chem.
289:5654–5663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li SZ, Hu YY, Zhao J, Zhao YB, Sun JD,
Yang YF, Ji CC, Liu ZB, Cao WD, Qu Y, et al: MicroRNA-34a induces
apoptosis in the human glioma cell line, A172, through enhanced ROS
production and NOX2 expression. Biochem Biophys Res Commun.
444:6–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bao B, Azmi AS, Li Y, Ahmad A, Ali S,
Banerjee S, Kong D and Sarkar FH: Targeting CSCs in tumor
microenvironment: The potential role of ROS-associated miRNAs in
tumor aggressiveness. Curr Stem Cell Res Ther. 9:22–35. 2014.
View Article : Google Scholar
|
|
10
|
Magenta A, Greco S, Gaetano C and Martelli
F: Oxidative stress and microRNAs in vascular diseases. Int J Mol
Sci. 14:17319–17346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aranda JF, Madrigal-Matute J, Rotllan N
and Fernández-Hernando C: MicroRNA modulation of lipid metabolism
and oxidative stress in cardiometabolic diseases. Free Radic Biol
Med. 64:31–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simone NL, Soule BP, Ly D, Saleh AD,
Savage JE, Degraff W, Cook J, Harris CC, Gius D and Mitchell JB:
Ionizing radiation-induced oxidative stress alters miRNA
expression. PLoS One. 4:e63772009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Magenta A, Cencioni C, Fasanaro P,
Zaccagnini G, Greco S, Sarra-Ferraris G, Antonini A, Martelli F and
Capogrossi MC: miR-200c is upregulated by oxidative stress and
induces endothelial cell apoptosis and senescence via ZEB1
inhibition. Cell Death Differ. 18:1628–1639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan G, Shi Y and Wu ZH: MicroRNA-22
promotes cell survival upon UV radiation by repressing PTEN.
Biochem Biophys Res Commun. 417:546–551. 2012. View Article : Google Scholar :
|
|
15
|
Strickertsson JA, Rasmussen LJ and
Friis-Hansen L: Enterococcus faecalis infection and reactive oxygen
species downregulates the miR-17-92 cluster in gastric
adenocarcinoma cell culture. Genes (Basel). 5:726–738. 2014.
|
|
16
|
Donadelli M, Dando I, Fiorini C and
Palmieri M: Regulation of miR-23b expression and its dual role on
ROS production and tumour development. Cancer Lett. 349:107–113.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Campagnolo DI, Bartlett JA and Keller SE:
Influence of neurological level on immune function following spinal
cord injury: A review. J Spinal Cord Med. 23:121–128.
2000.PubMed/NCBI
|
|
18
|
Wu B and Ren X: Promoting axonal myelation
for improving neurological recovery in spinal cord injury. J
Neurotrauma. 26:1847–1856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Young W: Secondary injury mechanisms in
acute spinal cord injury. J Emerg Med. 11(Suppl 1): 13–22.
1993.PubMed/NCBI
|
|
20
|
Lin Y, Vreman HJ, Wong RJ, Tjoa T,
Yamauchi T and Noble-Haeusslein LJ: Heme oxygenase-1 stabilizes the
blood-spinal cord barrier and limits oxidative stress and white
matter damage in the acutely injured murine spinal cord. J Cereb
Blood Flow Metab. 27:1010–1021. 2007.
|
|
21
|
Genovese T, Mazzon E, Esposito E, Muià C,
Di Paola R, Bramanti P and Cuzzocrea S: Beneficial effects of
FeTSPP, a peroxynitrite decomposition catalyst, in a mouse model of
spinal cord injury. Free Radic Biol Med. 43:763–780. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Genovese T, Mazzon E, Esposito E, Di Paola
R, Murthy K, Neville L, Bramanti P and Cuzzocrea S: Effects of a
metalloporphyrinic peroxynitrite decomposition catalyst, ww-85, in
a mouse model of spinal cord injury. Free Radic Res. 43:631–645.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bao F and Liu D: Peroxynitrite generated
in the rat spinal cord induces neuron death and neurological
deficits. Neuroscience. 115:839–849. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bao F and Liu D: Peroxynitrite generated
in the rat spinal cord induces apoptotic cell death and activates
caspase-3. Neuroscience. 116:59–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bao F and Liu D: Hydroxyl radicals
generated in the rat spinal cord at the level produced by impact
injury induce cell death by necrosis and apoptosis: Protection by a
metalloporphyrin. Neuroscience. 126:285–295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Kong M, Jiang D, Qian J, Duan Q and
Dong A: MicroRNA-150 aggravates H2O2-induced cardiac myocyte injury
by downregulating c-myb gene. Acta Biochim Biophys Sin (Shanghai).
45:734–741. 2013. View Article : Google Scholar
|
|
27
|
Cheng Y, Liu X, Zhang S, Lin Y, Yang J and
Zhang C: MicroRNA-21 protects against the H(2)O(2)-induced injury
on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol.
47:5–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tu H, Sun H, Lin Y, Ding J, Nan K, Li Z,
Shen Q and Wei Y: Oxidative stress upregulates PDCD4 expression in
patients with gastric cancer via miR-21. Curr Pharm Des.
20:1917–1923. 2014. View Article : Google Scholar
|
|
29
|
Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S and
Navab R: MicroRNA-21 (miR-21) regulates cellular proliferation,
invasion, migration and apoptosis by targeting PTEN, RECK and Bcl-2
in lung squamous carcinoma, Gejiu city, China. PLoS One.
9:e1036982014. View Article : Google Scholar
|
|
30
|
Wei C, Li L, Kim IK, Sun P and Gupta S:
NF-κB mediated miR-21 regulation in cardiomyocytes apoptosis under
oxidative stress. Free Radic Res. 48:282–291. 2014. View Article : Google Scholar
|
|
31
|
Yu H, Huang J, Wang S, Zhao G, Jiao X and
Zhu L: Overexpression of Smad7 suppressed ROS/MMP9-dependent
collagen synthesis through regulation of heme oxygenase-1. Mol Biol
Rep. 40:5307–5314. 2013. View Article : Google Scholar : PubMed/NCBI
|