Introduction
Total joint replacement (TJR), by the implantation
of permanent prosthetic components, is currently considered to be a
particularly successful clinical procedure in orthopedic surgery
(1). However, wear
particle-induced periprosthetic osteolysis and subsequent aseptic
loosening continue to be major causes of arthroplasty failure
(2). Implant-derived wear
particles activate and recruit macrophages and osteoclasts around
and at the implant-host interface (3). These cells secrete high levels of
inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and
tumor necrosis factor (TNF)-α that mediate and accelerate the
inflammatory and osteolytic responses, which result in the
loosening and subsequent failure of bone implants (4). Thus, the application of
pharmacological agents for the prevention of this osteolytic
response to wear debris has attracted significant interest, with
particular emphasis on anti-inflammatory agents.
Pitavastatin, a 3-hydroxy-3-methylglutaryl coenzyme
A (HMG CoA) reductase inhibitor, effectively modifies atherogenic
lipid profiles and thereby reduces cardiovascular risk in
individuals presenting with dyslipidemia and cardiometabolic
diseases (5). Unlike other
statins, the characteristic structure of pitavastatin provides
improved pharmacokinetics and significant low-density lipoprotein
cholesterol-lowering efficacy at low doses (6). In addition, increasing evidence
indicates that pitavastatin exhibits numerous pleiotropic effects
beyond its lipid-lowering potencies. In a previous study,
pitavastatin at a low dose (1 µM) inhibited nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB) activation and
decreased IL-6 production, which was induced by TNF-α in human
breast cancer cells (7). Takano
et al (8) demonstrated that
pitavastatin prevented acute lung injury development in septic mice
by increasing glucocorticoid receptor expression and downregulating
NF-κB activation in alveolar macrophages. Katsuki et al
(9) showed that pitavastatin
treatment inhibited atherosclerotic plaque destabilization and
rupture in a mouse model of diet-induced lipodystrophy by
regulating monocyte chemoattractant protein-1/C-C chemokine
receptor type 2-dependent monocyte recruitment. Furthermore,
various clinical studies identified that pitavastatin therapy
significantly decreased in-hospital or 28-day mortality in patients
exhibiting infection and sepsis (10). However, pitavastatin has not been
considered as an effective tool with therapeutic potential in the
prevention of osteolysis in response to orthopedic wear
particles.
The present study investigated whether treatment
with pitavastatin (a new synthetic statin drug) was able to
alleviate the activation of the monocyte inflammatory response
induced by polymethyl methacrylate (PMMA) particles. The production
of pro-inflammatory cytokines and the intracellular NF-κB signaling
pathway were also examined to identify the role of pitavastatin in
monocyte activation..
Materials and methods
Preparation of PMMA particles and
pitavastatin
Spherical PMMA particles (Polysciences, Inc.,
Warrington, PA, USA) 1–10 µm in diameter (mean diameter, 6.0
µm; 95% <10 µm) were used for all experiments as
previously reported (11–13). The particles were rinsed four times
in 70% ethanol, sterilized in 70% ethanol (Sigma-Aldrich, St.
Louis, MO, USA) overnight, then washed four times in sterile
phosphate-buffered saline (PBS; Wuhan Boster Biological Technology,
Ltd., Wuhan, Hubei, China). Particles were resuspended in
serum-free Minimum Essential Medium (MEM; Life Technologies, Grand
Island, NY, USA) and stored at −20°C. A Limulus Amebocyte Lysate
kit (BioWhittaker, Walkersville, MD, USA) was used to detect
bacterial endotoxins. The optimal PMMA particle concentration for
cell culture experiments was identified to be 2 mg/ml. The
pitavastatin (Sigma-Aldrich) was stored at −20°C until it was
diluted in serum-free MEM immediately prior to use.
Monocyte isolation
Peripheral whole blood was obtained from healthy
volunteers (n=12) into heparinized bottles (Shandong Weigao Group
Medical Polymer Co., Ltd., Weihai, China). The twelve subjects
recruited for the study were healthy male volunteers aged between
23–45 years. All volunteers enrolled in the present study provided
written informed consent. Blood collection was conducted between
January and June 2014 at the Southwest Hospital of the Third
Military Medical University (Chongqing, China). The heparinized
blood was diluted in equal volumes of 10 ml Dulbecco's modified
Eagle's medium (Gibco Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Gibco Life Technologies)
and maintained at 37°C. The 20 ml of diluted blood samples were
then layered onto 10 ml of Ficoll-Paque PLUS (GE Healthcare
Bio-Sciences, Uppsala, Sweden) and centrifuged at 500 × g for 30
min. The buffer mononuclear layer was removed and washed three
times with PBS, and then plated in 12-well plates (Corning
Incorporated, Corning, NY, USA) in 5×105/ml Dulbecco's
modified Eagle's medium supplemented with 10% fetal bovine serum.
All steps were carried out at room temperature. The monocyte
population was allowed to adhere for 120 min at 37°C in an
atmosphere of 5% CO2. Cells (lymphocytes) that had not
adhered were subsequently washed off using PBS and the remaining
monocyte population was used.
Measurement of cytokines
Monocytes were treated with various concentrations
of PIT (0–100 µM) together with 2 mg/ml PMMA for 12 h. The
culture medium was subsequently collected and the concentrations of
TNF-α, IL-1β and IL-6 were measured using a commercially available
ELISA kit (Wuhan Boster Biological Technology, Ltd.) according to
the manufacturer's instructions. The absorbance was measured at 450
nm using a microplate reader (Bio-Tek ELX800; Bio-Tek Instruments,
Inc., Winooski, VT, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total cellular RNA was isolated using TRIzol
(Invitrogen Life Technologies, Camarillo, CA, USA) and cDNA was
synthesized using SuperScript® Reverse Transcriptase
(Invitrogen Life Technologies) according to the manufacturer's
instructions. The primer sequences (Shanghai Shenggong Co., Ltd.,
Shanghai, China) used in the present study were as follows:
Forward, 5′-CGAGTCTGGGCAGGTCTA-3′ and reverse,
5′-CGAAGTGGTGGTCTTGTTG-3′ for TNF-α; forward,
5′-CTTCAGGCAGGCAGTATCACTC-3′ and reverse,
5′-TGCAGTTGTCTAATGGGAACGT-3′ for IL-1β; forward,
5′-TCAATGAGGAGACTTGCCTG-3′ and reverse, 5′-GATGAGTTGTCATGTCCTGC-3′
for IL-6; and forward, 5′-TCACCACCATGGAGAAGGC-3′ and reverse,
5′-GCTAAGCAGTTGGTGGTGCA-3′ for GAPDH. RT-qPCR was performed using a
LightCyclerH 480 Instrument (Roche Diagnostics GmbH, Mannheim,
Germany) and SYBR® Green SuperMix (Sigma-Aldrich). The
amplification program included an initial denaturation step at 95°C
for 5 min and 45 cycles (each consisting of denaturation at 95°C
for 10 sec), annealing at 60°C for 10 sec and extension at 72°C for
10 sec. The comparative cycle threshold (Ct) method
(2−ΔΔCT) was used to calculate relative gene expression.
The results were expressed as the fold change over the control
values.
Western blotting
Cells were collected and lysed in
radioimmunoprecipitation assay buffer (Sigma-Aldrich) supplemented
with a Broad Spectrum Protease Inhibitor Cocktail (BD Pharmingen,
San Diego, CA, USA). Following centrifugation for 10 min at 10,000
× g, the protein content of the supernatant was determined using a
bicinchoninic acid assay kit (Beyotime Institute of Biotechnology,
Shanghai, China). The protein lysates were separated by 10%
SDS-PAGE (Beyotime Institute of Biotechnology) and subsequently
electrotransferred onto a polyvinylidene difluoride membrane (EMD
Millipore, Billerica, MA, USA). The membrane was blocked with 5%
bovine serum albumin (Sigma-Aldrich) for 1 h at room temperature.
The blocked membranes were incubated overnight at 4°C with the
following primary antibodies: Rabbit anti-NF-κB monoclonal antibody
(1:600; cat. no. sc-372), rabbit anti-p-inhibitor of κB (IκB)
monoclonal antibody (1:600; cat. no. sc-101713), rabbit anti-IκB
monoclonal antibody (1:600; cat. no. sc-371), rabbit anti-GADPH
monoclonal antibody (1:1,000; cat. no. sc-25778) and rabbit
anti-TATA box binding protein monoclonal antibody (1:1,000; cat.
no. sc-33736), which were all purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The membranes were then
incubated with horseradish peroxidase-linked goat anti-rabbit
immunoglobulin G (1:10,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA) for 1 h at room temperature. Protein bands were
visualized using a western blotting detection system (ChemiDoc™
XRS+; Bio-Rad Laboratories, Hercules, CA, USA) and analyzed by a
densitometry system (Quantity One v4.6.2; Bio-Rad Laboratories)
according to the manufacturer's instructions.
For extraction of the nucleoprotein, cells were
collected and lysed in the lysis buffer [10 mM Hepes (pH 7.9), 1.5
Mm MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 2% NP-40, 1
mM phenylmethylsulfonyl fluoride] (Beyotime Institute of
Biotechnology) for 20 min, and the lysis buffer was centrifuged at
1,000 × g for 10 min. The protein content of the supernatant was
collected as the cytoplasmic protein. The precipitate was washed
twice and lysed in lysis buffer containing Triton X-100
(Sigma-Aldrich) and collected as the nucleoprotein.
Immunofluorescence
Monocytes were seeded onto 12-well plates, grown in
chamber slides and treated with 2 mg/ml PMMA and/or 100 mM
pitavastatin for 1 h. Cells were fixed with 4% paraformaldehyde
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and
permeabilized with 0.5% Triton X-100. Immunostaining of p65 was
performed using rabbit anti-p65 monoclonal antibody (cat. no.
sc-372; Santa Cruz Biotechnology, Inc.) at a dilution of 1:200,
then with fluorescein isothiocyanate-conjugated goat anti-rabbit
immunoglobulin G secondary antibody (Abcam, Cambridge, MA, USA) at
a dilution of 1:5,000. Finally, the cells were stained with DAPI
(Beyotime Institute of Biotechnology) for 10 min to localize the
nuclei, which served as a reference point. For the negative
controls, the primary antibodies were excluded from the staining
procedure described above. The activation of NF-κB was defined by
its translocation into the nucleus, which was visualized by
fluorescence microscopy (magnification, ×20) using a Nikon Eclipse
E1000 (Nikon Corporation, Tokyo, Japan).
Statistical analysis
All results are expressed as the mean ± standard
deviation. The results were analyzed by analysis of variance,
followed by Student's t-test to determine the significance.
P<0.05 was considered to indicate a statistically significant
difference. All the statistical analyses were performed using the
SPSS 12.0 statistical software (SPSS, Inc., Chicago, IL, USA).
Results
Pitavastatin inhibits PMMA-induced
pro-inflammatory cytokine production
PMMA particles are widely derived from implants used
in TJR, as they result from wear of the material in the prosthetic.
Thus, in the present study, PMMA particles served as a stimulant to
trigger inflammatory monocytes. Varying concentrations of PMMA (1,
2 and 4 mg/ml) were added into the monocyte culture medium 6, 12 or
24 h prior to analysis. It was found that in all of the PMMA
concentration groups, the pro-inflammatory cytokines were increased
in a time-dependent manner and there was no significant differences
noted between the 2 and 4 mg/ml PMMA groups (data not shown).
Therefore, a PMMA particle concentration of 2 mg/ml was selected
and administered 24 h prior to the cell culture experiments.
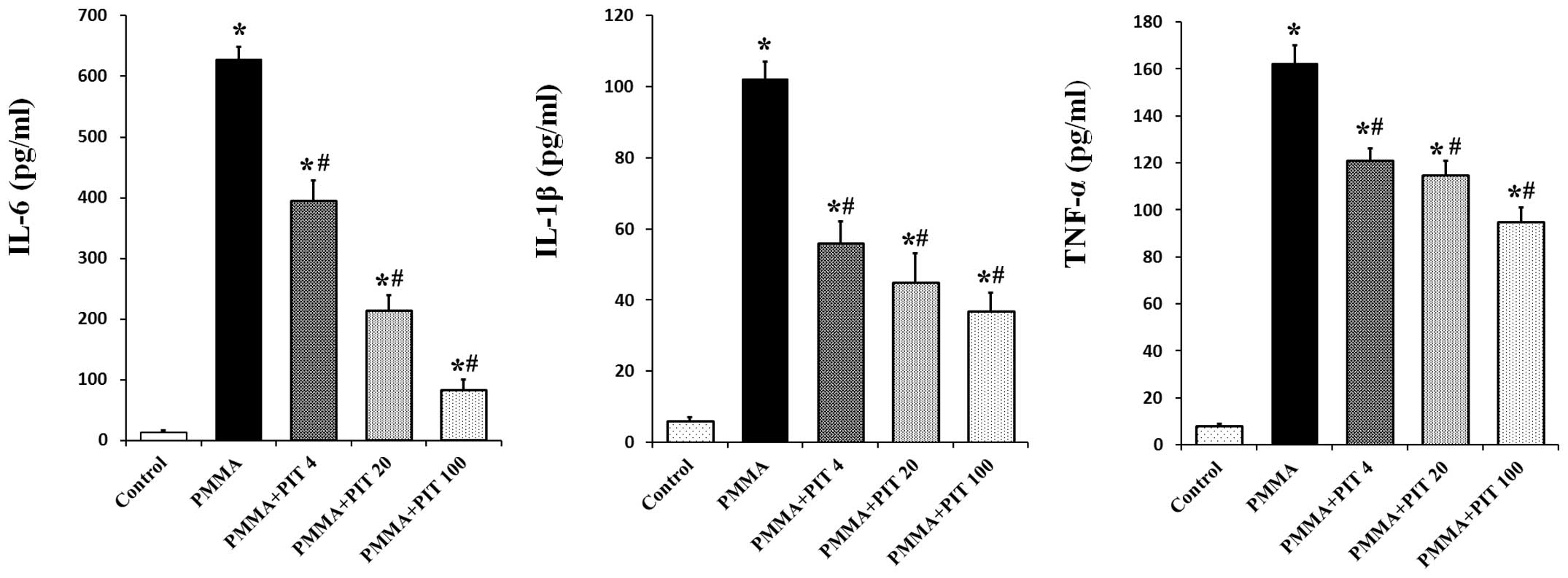
To study the effect of pitavastatin, 2 mg/ml PMMA
was applied to stimulate the monocytes, and 0–100 µM
pitavastatin was simultaneously administrated. After 24 h, as seen
in Fig. 1, 2 mg/ml PMMA induced a significant
production of TNF-α, IL-1β and IL-6 in the monocytes (P<0.05).
However, pitavastatin treatment significantly inhibited the
production of pro-inflammatory cytokines in a dose-dependent manner
(P<0.05).
Pitavastatin inhibits PMMA-induced TNF-α,
IL-1β and IL-6 mRNA expression
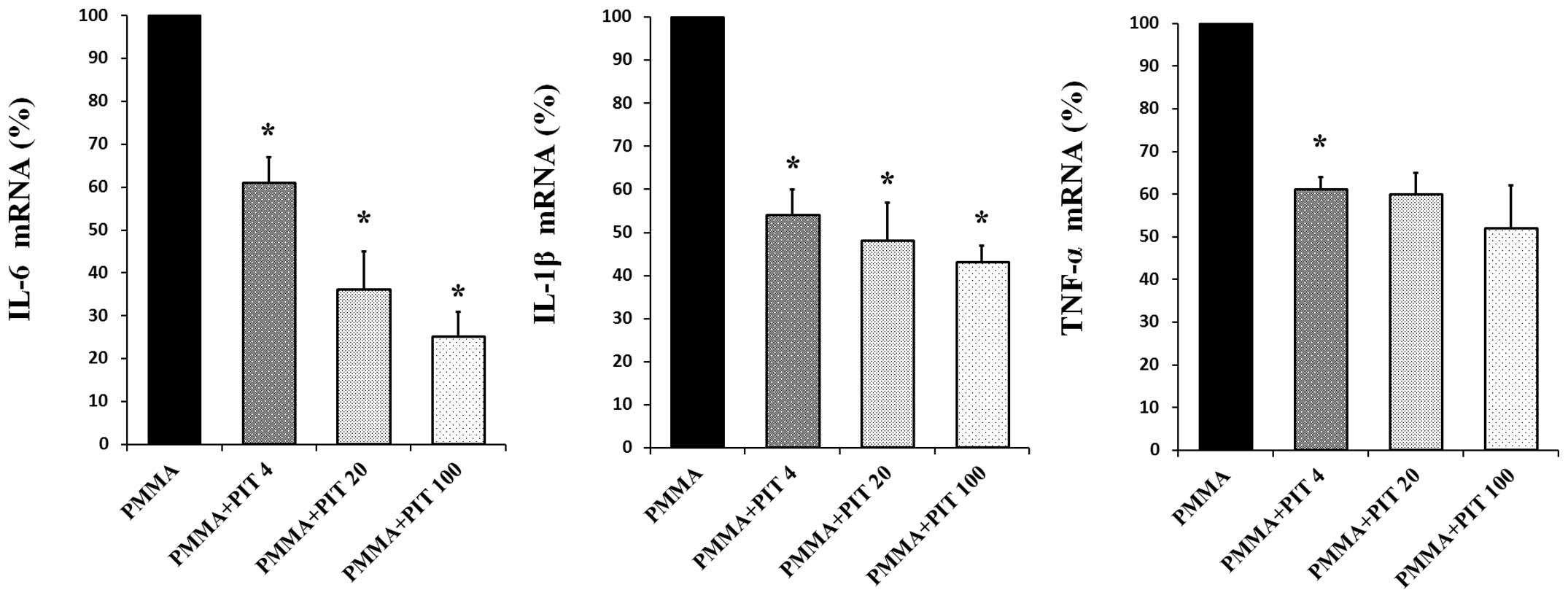
RT-qPCR was conducted to assess whether pitavastatin
inhibits TNF-α, IL-1β and IL-6 mRNA expression. As demonstrated in
Fig. 2, PMMA stimulation of
monocytes resulted in a marked increase in TNF-α, IL-1β and IL-6 at
the transcriptional level. However, treatment with varying
concentrations of pitavastatin (4, 20 and 100 µM)
significantly downregulated PMMA-induced transcription of TNF-α,
IL-1β and IL-6 mRNA in a concentration-dependent manner
(P<0.05).
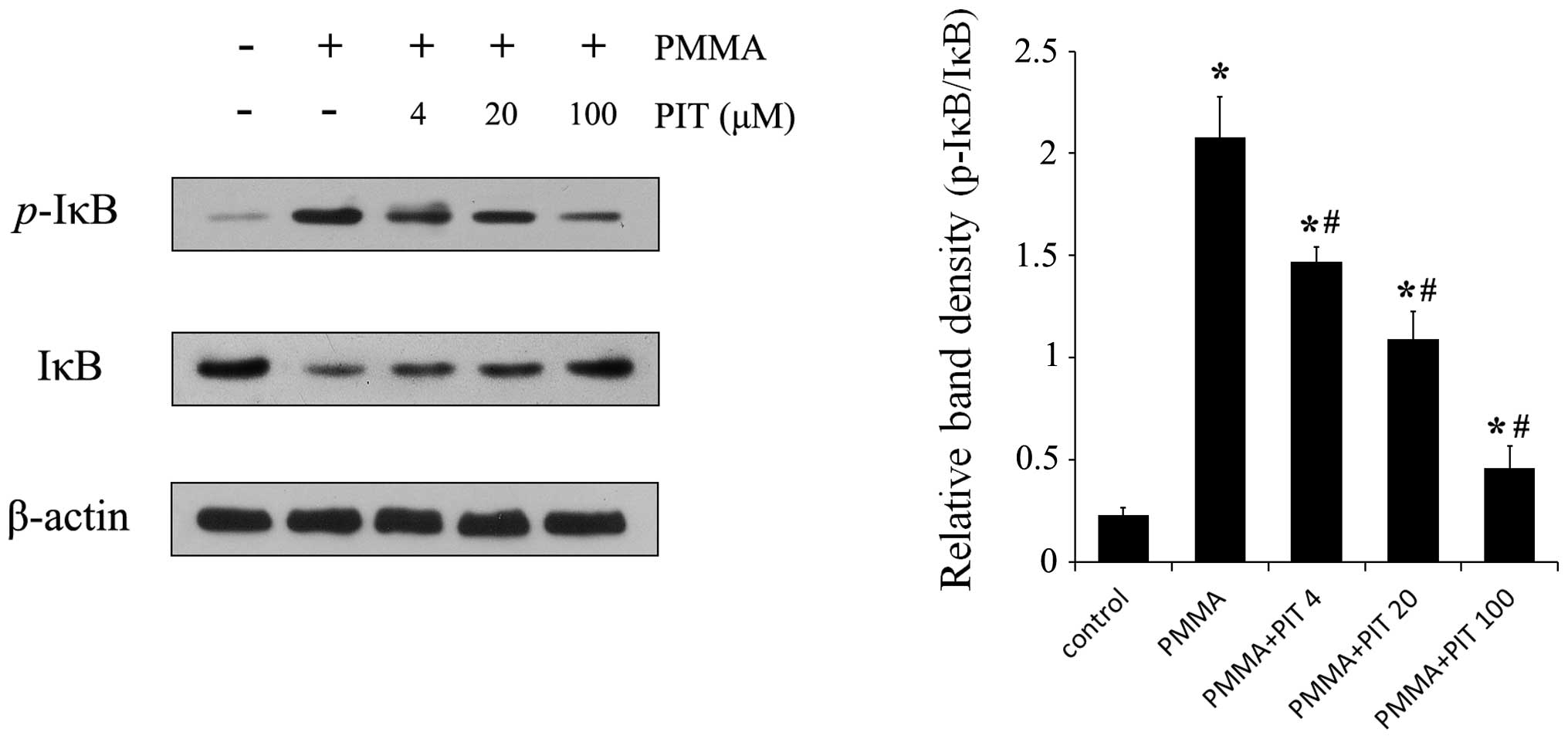
Pitavastatin inhibits PMMA-induced IκB
phosphorylation and degradation
NF-κB is considered to be a master switch in the
regulation of inflammation and immunity (14). As a transcription factor, NF-κB
controls an array of pro-inflammatory genes involved in the
inflammatory signaling cascade, such as TNF-α, IL-1β, and IL-6
(15). Therefore, the effect of
pitavastatin on PMMA-induced NF-κB activation in monocytes were
investigated. As shown in Fig. 3,
monocytes treated with PMMA exhibited significant phosphorylation
of IκB, whereas the various treatment concentrations of
pitavastatin (4, 20 and 100 µM) prevented IκB
phosphorylation. In addition, pitavastatin inhibited degradation of
IκB, which had been induced by PMMA, in a concentration-dependent
manner. These results indicate that pitavastatin significantly
blocks the NF-κB signaling pathway in PMMA-stimulated monocytes by
suppressing IκB phosphorylation and degradation.
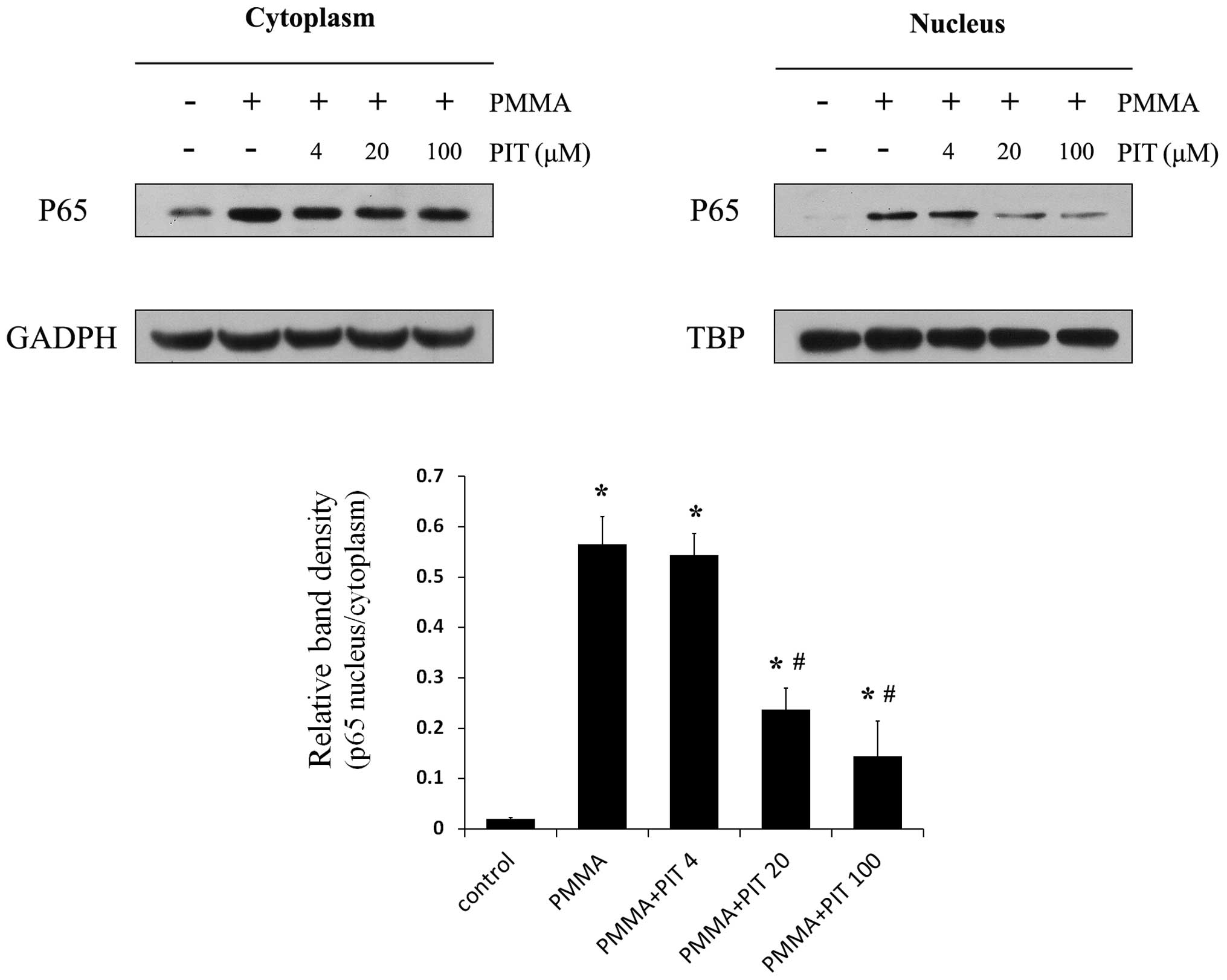
Pitavastatin inhibits PMMA-induced NF-κB
p65 translocation
Having established that pitavastatin inhibits IκB
phosphorylation and degradation, further investigations regarding
its effects on NF-κB signaling were performed. The p65 protein
levels in the cytoplasmic and nuclear fractions of PMMA-induced
monocytes were analyzed. As shown in Fig. 4, PMMA stimulation resulted in
significant nuclear translocation of p65 in the monocytes, whereas
pitavastatin treatment prevented the accumulation of PMMA-induced
nuclear p65 in a concentration-dependent manner. Furthermore,
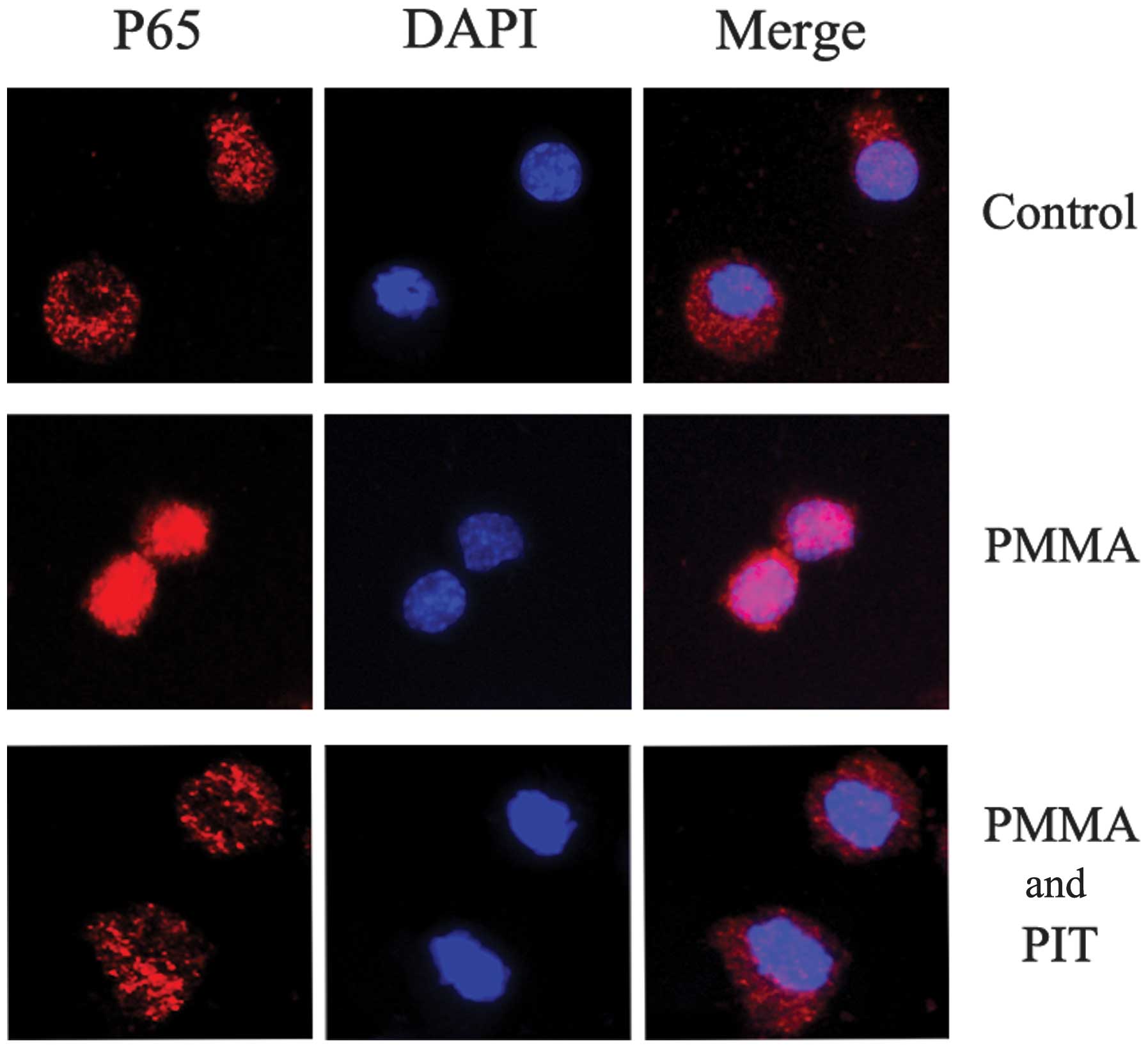
immunocytochemical analysis demonstrated that pitavastatin
treatment exhibited reduced nuclear translocation of endogenous p65
following PMMA stimulation (Fig.
5). These results indicate that pitavastatin significantly
inhibits NF-κB p65 translocation in PMMA-stimulated monocytes.
Discussion
TJR is considered to be the most effective treatment
strategy for end-stage joint diseases, such as osteoarthritis and
rheumatoid arthritis (16).
However, despite the clinical effectiveness of TJR, aseptic
loosening of prostheses continues to present a major problem,
particularly for the long-term success and survival of a prosthesis
(17). There are various causes of
aseptic loosening, however, an inflammatory reaction induced by
excessive production of wear particles from the implant components
and consequent peri-implant osteolysis is regarded as the primary
cause (18).
PMMA particles, a material widely derived from
orthopedic implants, elicit a marked inflammatory response due to
macrophage and enhanced osteoclast formation and activity (19–21).
Upon exposure to PMMA, osteoblasts contribute to periprosthetic
osteolysis by secreting mediators that drive the inflammatory
process (22). These mediators,
which include TNF-α, IL-1β and IL-6, interact with one another
contributing significantly to the inflammatory cascade. TNF-α is
considered to be a key cytokine, which mediates particle-driven
osteoclastogenesis and osteolysis (23). TNF-α increases osteoclast
differentiation by promoting the expression of receptor activation
of NF-κB ligand and macrophage-colony stimulating factor, which are
essential factors involved in the expansion, commitment and
differentiation of osteoclast precursors into mature osteoclasts
(24,25). Fuller et al (26) demonstrated that a low level
concentration of TNF-α stimulation activates osteoclasts and
indicated that this effect cannot be inhibit by osteoprotegerin.
Furthermore, an adenovirus-mediated small interfering RNA targeting
TNF-α was observed to significantly inhibit titanium wear
particle-induced osteoclastogenesis and bone resorption in
macrophages (27). IL-1β is also a
well-established pro-inflammatory cytokine that contributes to
aseptic loosening. As an important downstream molecule in
TNF-α-induced osteoclast differentiation, IL-1β promotes
multinucleation of osteoclast progenitor cells and enhances mature
osteoclast-associated bone resorption (28–30).
Therefore, controlling the synthesis of inflammatory cytokines in
the periprosthetic environment may be a potential target for the
prevention or reduction of wear particle-induced osteolysis. In
addition, the anti-inflammatory effects of statins are well
recognized. Uekawa et al (31) indicated that statin pretreatment
ameliorated early brain injury, following a subarachnoid
hemorrhage, via the attenuation of oxidative stress and
NF-κB-mediated inflammation. Moon et al (32) reported that short-term
administration of statins to patients who had suffered an
atherosclerotic stroke exerts antioxidant effects against lipid
peroxidation via lipid-lowering-dependent and -independent
mechanisms. McGuire et al (33) demonstrated that healthy male
subjects who were administered with statins for three weeks showed
a decline in TNF-α plasma concentrations and toll-like receptor-4
expression in blood monocytes. A meta-analysis of observational
studies demonstrated that, although the use of statins did not
significantly decrease the in-hospital or 28-day mortality, it did
present a survival advantage in patients with infection and sepsis
(10). Thus, it is hypothesized
that statins may inhibit wear particle-induced inflammatory
reactions from implant components.
The ability of pitavastatin to abrogate
PMMA-mediated monocyte activation was examined in the present
study. The peripheral blood monocyte/macrophage model is a
well-recognized in vitro model for particle stimulation.
Therefore, this was considered to be the most appropriate model for
the established primary instigators of foreign-body inflammatory
response and subsequent osteolysis, as monocytes represent the
circulatory precursors of tissue macrophages. In the current study,
stimulation of monocytes with 2 mg/ml PMMA resulted in a marked
increase in TNF-α, IL-1β and IL-6 at the transcriptional and
translational levels. However, pitavastatin treatment significantly
downregulated the PMMA-induced TNF-α, IL-1β and IL-6 expression at
the transcriptional and translational levels in a
concentration-dependent manner (Figs.
1 and 2).
PMMA particles are potent inducers of the NF-κB
signaling pathway, which is considered to be an important mediator
of inflammatory responses, and essential for osteoclast
differentiation and function (34). In its inactivated state, NF-κB is
located in the cytoplasm as an inactive NF-κB/IκB complex, and its
activity is tightly controlled by the inhibitory protein, IκB
(35). Upon IκB phosphorylation
and subsequent degradation, NF-κB p65 is released and enters the
nucleus to activate specific target gene expression. Therefore, the
activation of NF-κB was assessed in monocytes in the present study
by measuring the quantity of IκB protein expression. Incubation of
monocytes with PMMA caused marked phosphorylation and degradation
of cytosolic IκB, and NF-κB p65 translocation into the nucleus,
whereas pitavastatin treatment significantly inhibited the
phosphorylation and degradation of IκB, as well as NF-κB p65
nuclear translocation in a dose-dependent manner (Figs. 3Figure 4–5). This suggests that pitavastatin may
suppress PMMA-induced activation of the NF-κB signaling pathway,
indicating that the NF-κB pathway may be involved in the
anti-inflammatory effects of pitavastatin.
In conclusion, pitavastatin has been demonstrated to
inhibit PMMA-induced monocyte activation and inflammatory cytokine
release by inhibiting phosphorylation and degradation of IκB, and
subsequent NF-κB p65 translocation. Considering these findings,
pitavastatin may be an efficacious candidate for administration as
a therapeutic agent for periprosthetic osteolysis and aseptic
loosening, which occur following TJR.
References
|
1
|
Snow R, Granata J, Ruhil AV, Vogel K,
McShane M and Wasielewski R: Associations between preoperative
physical therapy and post-acute care utilization patterns and cost
in total joint replacement. J Bone Joint Surg Am. 96:e1652014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gallo J, Goodman SB, Konttinen YT and
Raska M: Particle disease: Biologic mechanisms of periprosthetic
osteolysis in total hip arthroplasty. Innate Immun. 19:213–224.
2013. View Article : Google Scholar :
|
|
3
|
Pearl JI, Ma T, Irani AR, Huang Z,
Robinson WH, Smith RL and Goodman SB: Role of the Toll-like
receptor pathway in the recognition of orthopedic implant
wear-debris particles. Biomaterials. 32:5535–5542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holt G, Murnaghan C, Reilly J and Meek RM:
The biology of aseptic osteolysis. Clin Orthop Relat Res.
460:240–252. 2007.PubMed/NCBI
|
|
5
|
Masana L: Pitavastatin in cardiometabolic
disease: Therapeutic profile. Cardiovasc Diabetol. 12(Suppl 1):
S22013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saito Y: Pitavastatin: An overview.
Atheroscler Suppl. 12:271–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J and Kitajima I: Pitavastatin
inactivates NF-kappaB and decreases IL-6 production through Rho
kinase pathway in MCF-7 cells. Oncol Rep. 17:1149–1154.
2007.PubMed/NCBI
|
|
8
|
Takano K, Yamamoto S, Tomita K, Takashina
M, Yokoo H, Matsuda N, Takano Y and Hattori Y: Successful treatment
of acute lung injury with pitavastatin in septic mice: Potential
role of glucocorticoid receptor expression in alveolar macrophages.
J Pharmacol Exp Ther. 336:381–390. 2011. View Article : Google Scholar
|
|
9
|
Katsuki S, Matoba T, Nakashiro S, Sato K,
Koga J, Nakano K, Nakano Y, Egusa S, Sunagawa K and Egashira K:
Nanoparticle-mediated delivery of pitavastatin inhibits
atherosclerotic plaque destabilization/rupture in mice by
regulating the recruitment of inflammatory monocytes. Circulation.
129:896–906. 2014. View Article : Google Scholar
|
|
10
|
Wan YD, Sun TW, Kan QC, Guan FX and Zhang
SG: Effect of statin therapy on mortality from infection and
sepsis: A meta-analysis of randomized and observational studies.
Crit Care. 18:R712014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clohisy JC, Frazier E, Hirayama T and
Abu-Amer Y: RANKL is an essential cytokine mediator of
polymethylmethacrylate particle-induced osteoclastogenesis. J
Orthop Res. 21:202–212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clohisy JC, Teitelbaum S, Chen S, Erdmann
JM and Abu-Amer Y: Tumor necrosis factor-alpha mediates
polymethylmethacrylate particle-induced NF-kappaB activation in
osteoclast precursor cells. J Orthop Res. 20:174–181. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clohisy JC, Yamanaka Y, Faccio R and
Abu-Amer Y: Inhibition of IKK activation, through sequestering
NEMO, blocks PMMA-induced osteoclastogenesis and calvarial
inflammatory osteolysis. J Orthop Res. 24:1358–1365. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng Q, Liu H, Shi S and Li M: Lycium
ruthenicum polysaccharide attenuates inflammation through
inhibiting TLR4/NF-κB signaling pathway. Int J Biol Macromol.
67:330–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Q and Verma IM: NF-kappaB regulation in
the immune system. Nat Rev Immunol. 2:725–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grunfeld R, Aydogan U and Juliano P: Ankle
arthritis: Review of diagnosis and operative management. Med Clin
North Am. 98:267–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gallo J, Goodman SB, Konttinen YT, Wimmer
MA and Holinka M: Osteolysis around total knee arthroplasty: A
review of pathogenetic mechanisms. Acta Biomater. 9:8046–8058.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Qu X, Wu C, Zhai Z, Tian B, Li H,
Ouyang Z, Xu X, Wang W, Fan Q, et al: The effect of enoxacin on
osteoclastogenesis and reduction of titanium particle-induced
osteolysis via suppression of JNK signaling pathway. Biomaterials.
35:5721–5730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sabokbar A, Fujikawa Y, Murray DW and
Athanasou NA: Bisphosphonates in bone cement inhibit PMMA particle
induced bone resorption. Ann Rheum Dis. 57:614–618. 1998.
View Article : Google Scholar
|
|
20
|
Lohmann CH, Dean DD, Küster G, Casasola D,
Buchhorn GH, Fink U, Schwartz Z and Boyan BD: Ceramic and PMMA
particles differentially affect osteoblast phenotype. Biomaterials.
23:1855–1863. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramachandran R, Goodman SB and Smith RL:
The effects of titanium and polymethylmethacrylate particles on
osteoblast phenotypic stability. J Biomed Mater Res A. 77:512–517.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zambonin G, Colucci S, Cantatore F and
Grano M: Response of human osteoblasts to polymethylmetacrylate in
vitro. Calcif Tissue Int. 62:362–365. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu FX, Wu CL, Zhu ZA, Li MQ, Mao YQ, Liu
M, Wang XQ, Yu DG and Tang TT: Calcineurin/NFAT pathway mediates
wear particle-induced TNF-α release and osteoclastogenesis from
mice bone marrow macrophages in vitro. Acta Pharmacol Sin.
34:1457–1466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Crotti TN, Smith MD, Findlay DM, Zreiqat
H, Ahern MJ, Weedon H, Hatzinikolous G, Capone M, Holding C and
Haynes D: Factors regulating osteoclast formation in human tissues
adjacent to peri-implant bone loss: Expression of receptor
activator NFkappaB, RANK ligand and osteoprotegerin. Biomaterials.
25:565–573. 2004. View Article : Google Scholar
|
|
25
|
Jiang Y, Jia T, Gong W, Wooley PH and Yang
SY: Effects of Ti, PMMA, UHMWPE and Co-Cr wear particles on
differentiation and functions of bone marrow stromal cells. J
Biomed Mater Res A. 101:2817–2825. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fuller K, Murphy C, Kirstein B, Fox SW and
Chambers TJ: TNFalpha potently activates osteoclasts, through a
direct action independent of and strongly synergistic with RANKL.
Endocrinology. 143:1108–1118. 2002.PubMed/NCBI
|
|
27
|
Guo H, Zhang J, Hao S and Jin Q:
Adenovirus-mediated small interfering RNA targeting tumor necrosis
factor-α inhibits titanium particle-induced osteoclastogenesis and
bone resorption. Int J Mol Med. 32:296–306. 2013.PubMed/NCBI
|
|
28
|
Quinn JM, Horwood NJ, Elliott J, Gillespie
MT and Martin TJ: Fibroblastic stromal cells express receptor
activator of NF-kappaB ligand and support osteoclast
differentiation. J Bone Miner Res. 15:1459–1466. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
García-López S, Villanueva R and Meikle
MC: Alterations in the synthesis of IL-1β, TNF-α, IL-6 and their
downstream targets RANKL and OPG by mouse calvarial osteoblasts in
vitro: Inhibition of bone resorption by cyclic mechanical strain.
Front Endocrinol (Lausanne). 4:1602013.
|
|
30
|
Simsa-Maziel S, Zaretsky J, Reich A, Koren
Y, Shahar R and Monsonego-Ornan E: IL-1RI participates in normal
growth plate development and bone modeling. Am J Physiol Endocrinol
Metab. 305:E15–E21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Uekawa K, Hasegawa Y, Ma M, Nakagawa T,
Katayama T, Sueta D, Toyama K, Kataoka K, Koibuchi N, Kawano T, et
al: Rosuvastatin ameliorates early brain injury after subarachnoid
hemorrhage via suppression of superoxide formation and nuclear
factor-kappaB activation in rats. J Stroke Cerebrovasc Dis.
23:1429–1439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moon GJ, Kim SJ, Cho YH, Ryoo S and Bang
OY: Antioxidant effects of statins in patients with atherosclerotic
cerebrovascular disease. J Clin Neurol. 10:140–147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McGuire TR, Kalil AC, Dobesh PP, Klepser
DG and Olsen KM: Anti-inflammatory effects of rosuvastatin in
healthy subjects: A prospective longitudinal study. Curr Pharm Des.
20:1156–1160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamanaka Y, Karuppaiah K and Abu-Amer Y:
Polyubiquitination events mediate polymethylmethacrylate (PMMA)
particle activation of NF-kappaB pathway. J Biol Chem.
286:23735–23741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hayden MS and Ghosh S: NF-κB in
immunobiology. Cell Res. 21:223–244. 2011. View Article : Google Scholar : PubMed/NCBI
|