Introduction
Mucin 1 (MUC1) is a highly glycosylated
transmembrane protein expressed on the apical surface of epithelial
cells and is aberrantly overexpressed in the majority of malignant
epithelial tumors, including breast, lung, ovarian, prostate and
pancreatic cancer and several types of malignant hematological
tumor (1). Previous studies have
demonstrated that MUC1 is an oncoprotein that is involved in the
regulation of carcinogenesis, tumor progression, invasion and
metastasis of cancer (2). MUC1
consists of a large extracellular N-terminal subunit containing a
variable number of tandem repeats region and a C-terminal subunit
that resides on the cell surface as a heterodimeric complex via a
strong noncovalent interaction. The C-terminal subunit is composed
of a 58 amino acid extracellular domain, a 28 amino acid
transmembrane domain and a 72 amino acid cytoplasmic tail (CT)
(3). MUC1-CT is involved in
numerous signaling pathways, including Wnt/β-catenin (4), c-Src (5), Grb2/Sos (6), glycogen synthase kinase 3 β (4), epidermal growth factor receptor
(7,8) and nuclear factor-κB (9,10),
which regulate the processes of cell survival and proliferation. By
contrast, MUC1 interacts directly with several signaling molecules,
including p53 (11,12), HSP70/90 (13,14)
and caspase-8 (15) to regulate
cell apoptosis.
Hepatocellular carcinoma (HCC) is one of the most
common types of malignant tumor that severely threatens human
health and quality of life. The occurrence of HCC is associated
directly or indirectly with the abnormal expression of multiple
genes, including B lymphoma Moloney murine leukemia virus insertion
region 1 homolog (BMI1) (16),
glypican-3 (17), heat shock
protein 70 (18) and Sal-like
protein 4 (19). Previous studies
have demonstrated that MUC1 is also expressed in HCC cells and
tissues (20–22), however, the mechanisms underlying
the function MUC1 in the development of HCC remain to be
elucidated. In order to determine the oncogenic role of MUC1 in
HCC, MUC1 was knocked down in SMMC-7721 cells, using small
interfering RNA (siRNA), to examine the effects and mechanisms of
MUC1 gene silencing in HCC cells. Our previous study found that
MUC1 gene silencing altered the phenotypic characteristics of the
human hepatic cellular carcinoma cell line SMMC-7721 (23). In addition, cell proliferation was
inhibited significantly in MUC1 gene-silenced SMMC-7721 cells.
Furthermore, no tumor development was observed in mice injected
with MUC1 gene-silenced SMMC-7721 cells. The results indicated that
MUC1 gene silencing can inhibit the growth of SMMC-7721 cells.
The aim of the present study was to determine
whether growth inhibition of MUC1 gene-silenced SMMC-7721 cells is
associated with their apoptotic cell death. Furthermore, the aim
was to elucidate the mechanisms and pathways of this apoptosis,
which could provide a novel therapeutic target for the pathogenesis
and gene therapy of HCC.
Materials and methods
Cell culture
The human HCC cell line, SMMC-7721, was purchased
from the Cell Bank of the Shanghai Institute of Cell Biology,
Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in Iscove's modified Dulbecco's medium (Invitrogen Life
Technologies, Carlsbad, CA, USA) supplemented with heat-inactivated
10% fetal bovine serum (Invitrogen Life Technologies), 100 U/ml
penicillin and 100 µg/ml streptomycin (Invitrogen Life
Technologies), and maintained at 37°C and 5% CO2. MR1-C6
and MR1-D4, two stably transfected SMMC-7721 cell clones, were
established using MUC1 gene specific siRNAs, as previously
described (9). A clone stably
transfected with a scramble siRNA was used as a negative control
(NC). These transfected cells were maintained in the presence of
600 µg/ml G418.
Plate clone formation assay
Approximately 2,000 cells were plated per well in a
6-well plate and each group was analyzed in triplicate. Following
incubation at 37°C and 5% CO2 for 3 weeks, the cells
were gently washed twice in phosphate-buffered saline (PBS) and
fixed in methanol for 15 min. Subsequently, the cell clones were
stained with Giemsa staining solution for 30 min, followed by air
drying. The visualized cell clones were observed and images were
captured using a microscope (IX71; Olympus Corporation, Toyko,
Japan).
Tumor xenograft mouse model and in vivo
imaging
For the tumor xenograft mouse model construction, 15
BALB/c nude mice (4–6 weeks old; 18–20 g) were purchased from
Beijing HFK Bioscience Co., Ltd. (Beijing, China). Animals were
maintained in specific pathogen-free conditions and an environment
with controlled conditions of light and humidity, and free access
to water and a standard laboratory diet. Animal experiments were
performed in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals (Bethesda, MD,
USA). The present study was approved by the ethics committee of the
Scientific Investigation Board of Science and Technology of Jilin
Province (Changchun, China). Mice were randomly divided into four
groups (five animals per group), including the SMMC-7721 group, the
NC group, the MR1-D4 group and the MR1-D9 group. Cells
(2×106) were subcutaneously injected into the right
flank of each mouse. For real-time near-infrared (NIR) imaging, the
mice were anesthetized and imaged on day 21 post-injection, and
in vivo NIR images were obtained using a Xenogen IVIS
Spectrum system (PerkinElmer, Inc., Waltham, MA, USA) using Living
Image software version 3.0 (Caliper Life Sciences, Alameda, CA,
USA). All image analysis and NIR fluorescent signal quantification
was performed using the region of interest (ROI) function of Living
Image software (Caliper Life Sciences).
Hoechst 33342 staining
Cells at logarithmic growth stage were cultured in a
96-well plate for 48 h and stained with Hoechst 33342 dye
(Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 0.1
µg/ml at 37°C for 15 min in the dark. Cells were observed
with an inverted fluorescence microscope (IX71; Olympus
Coproration).
Annexin V-PE staining and flow
cytometry
Following culturing the cells for 48 h, cells were
digested with 0.25% trypsin (EDTA free) and washed twice with PBS.
Cells (5×105) were centrifuged (300 x g for 5 min) and
the cell pellets were stained with Annexin V-PE fluorescence
labeling solution (Annexin V-PE apoptosis assay kits purchased from
Nanjing KeyGen Biotech. Co., Ltd., Nanjing, China). Following
incubation in the dark for 5-15 min, flow cytometric analysis was
performed on a FACS Calibur Flow cytometer (BD Biosciences, San
Jose, CA, USA). Data acquisition and analysis were performed using
CellQuest version 4.0 software (BD Biosciences).
DNA ladder assay
Cells were cultured for 48 h, 5×106 cells
were harvested and the DNA was extracted using an Apoptotic DNA
Ladder Extraction kit (Beyotime Institute of Biotechnology, Haimen,
China) according to the manufacturer's instructions.
Electrophoresis of the DNA was performed in a 1% agarose gel.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies) and reverse-transcribed into cDNA
(Takara Bio, Inc., Shiga, Japan), according to the manufacturer's
instructions. RT-qPCR was performed using FastStart Universal
SYBR-Green Master (Rox; Roche Life Science, Indianapolis, IN, USA)
in an Applied Biosystems 7300 system (Applied Biosystems, Foster
City, CA, USA). All the primers that were used for RT-qPCR analysis
were synthesized by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai,
China). The primers used were as follows: MUC1, forward 5′-TGC CGC
CGA AAG AAC TACG-3′ and reverse 5′-TGG GGT ACT CGC TCA TAG GAT-3′;
p53, forward 5′-TTG CAA TAG GTG TGC GTC AGA-3′ and reverse 5′-AGT
GCA GGC CAA CTT GTT CAG-3′; B-cell lymphoma 2 (Bcl-2), forward
5′-TCA GGG ACG GGG TGA ACT-3′ and reverse 5′-CAG GTG CCG GTT CAG
GTA CTC-3′; Bcl-2-associated X protein (Bax), forward 5′-CGC CGT
GGA CAC AGA CTC-3′ and reverse 5′-CCT CCC TTC AAC ACT TCC T-3′;
β-actin, forward 5′-AGT TGC GTT ACA CCC TTTC-3′ and reverse 5′-CCT
TCA CCG TTC CAG TTT-3′. The PCR assays were performed in triplicate
on a step-one RT-qPCR system. Cycle threshold (Ct) values were
normalized to β-actin. The relative level of the mRNAs was
calculated by 2−Δct (Δct=cttarget mRNA −
ctβ-actin).
Western blotting
Western blotting was performed as previously
described (24). Briefly, cells
were lysed with RIPA lysis buffer (Beyotime Institute of
Biotechnology) and the protein concentration in the cell lysates
was measured using the BCA protein assay kit (Beyotime Institute of
Biotechnology). Cytosolic and mitochondrial proteins were extracted
using cytosolic and mitochondrial protein extraction kits (Nanjing
KeyGen Biotech. Co., Ltd.), according to the manufacturer's
instructions. Equivalent quantities of protein from the soluble
fractions of the cell lysates were separated by 10% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes (Millipore,
Billerica, MA, USA). Following blocking with 5% non-fat milk in
Tris-buffered saline containing 0.05% Tween 20 for 1 h, the
membranes were incubated with appropriately diluted primary
antibodies at 4°C overnight. The following primary antibodies were
used: Rabbit anti-human caspase-3 monoclonal antibody (1:1,000;
cat. no. sc-7148; Santa Cruz Biotechnology Inc., Santa Cruz, CA,
USA), rabbit anti-human Bax (cat. no. 50599-2-Ig; Proteintech,
Chicago, IL, USA) and p53 (cat. no. 10442-1-AP; Proteintech)
polyclonal antibodies (1:1,000), rabbit anti-human β-actin
(1:2,000; cat. no. 5779-1), Bcl-2 (cat. no. 1017-1), cytochrome
c (cat. no. 1896-1), Cox IV (cat. no. 7001-1), caspase-8
(cat. no. 1007-1), caspase-9 (cat. no. 1023-1)and poly (ADP-ribose)
polymerase (PARP) (cat. no. 1051-1) monoclonal antibodies (1:1,000;
Epitomics, Burlingame, CA, USA). The blots were then probed with
horseradish peroxidase (HRP)-conjugated secondary antibody
(HRP-conjugated goat anti rabbit IgG antibody; cat. no. RABHRP2;
Sigma-Aldrich) at 1:2,000 for 1 h at room temperature.
Immunoreactive bands were detected by enhanced chemiluminescence
(Thermo Fisher Scientific, Waltham, MA, USA). The intensities of
the bands were quantified by densitometry using ImageJ 1.49 d
software (National Institutes of Health).
Co-immunoprecipitation
Cell lysates were first pre-cleaned with protein G
agarose beads (Promega, Madison, WI, USA) for 3 h at 4°C and,
subsequently, equal quantities of sample lysates were incubated
with either 1.0 µg of mouse IgG or anti-MUC1-CT antibody
(hamster anti MUC1-CT monoclonal antibody; Neomarkers Inc.,
Fremont, CA, USA) for 16 h at 4°C, followed by precipitation with
protein G agarose beads. The precipitated proteins from the cell
lysates or the whole cell lysates were subjected to immunoblot
analysis with anti-Bax and anti-caspase-8 antibodies.
Statistical analysis
The data are presented as the mean ± standard
deviation from three independent experiments. Statistical analysis
was performed using SPSS 11.0 (SPSS, Inc., Chicago, IL, USA). The
differences between groups were compared using Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
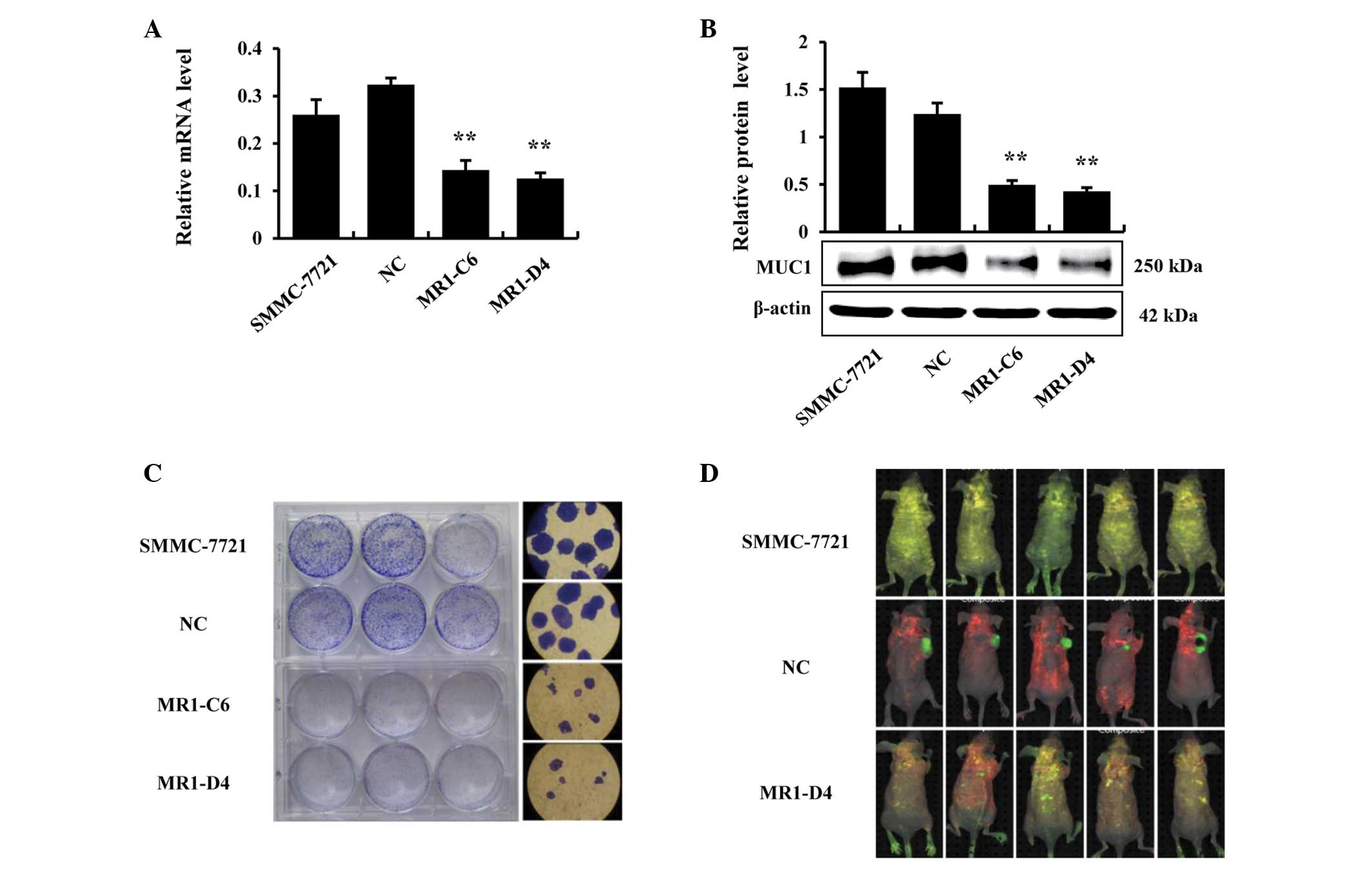
MUC1 gene silencing inhibits the growth
of SMMC-7721 cells in vitro and in vivo
In our previous study, the expression of MUC1 was
knocked down in the human HCC cell line SMMC-7721 by RNA
interference. Two independent MUC1-knockdown clones were
established, designated MR1-C6 and MR1-D4, and one negative control
clone, which was designated NC, was established, as previously
described (23). The effects of
MUC1 gene silencing were validated by RT-qPCR and western blotting,
and the results demonstrated that the expression of MUC1 in MR1-C6
and MR1-D4 cells was significantly reduced, compared with SMMC-7721
or NC cells (P<0.01; Fig. 1A and
B). Subsequently, a clone formation assay was conducted to
confirm that MUC1 gene silencing could inhibit the growth of
SMMC-7721 cells in vitro. The results demonstrated that the
clones in the MR1-C6 and MR1-D4 groups were significantly fewer in
number and smaller in size, compared with those in the SMMC-7721 or
NC groups (Fig. 1C). Furthermore,
SMMC-7721 cells were implanted into BALB/c nude mice to investigate
the inhibitory effects of MUC1 gene silencing on the growth of
SMMC-7721 cells in vivo. By utilizing an in vivo
imaging system, it was observed that the tumors in the MR1-D4 group
were absent, compared with the NC group (Fig. 1D). Taken together, these results
indicate that MUC1 gene silencing significantly inhibits the growth
of SMMC-7721 cells in vitro and in vivo.
MUC1 gene silencing induces phenotypic
alterations of apoptosis in SMMC-7721 cells
Subsequently, whether the growth inhibition of MUC1
gene-silenced SMMC-7721 cells was associated with their apoptotic
death was determined. Using Hoechst 33342 staining, it was
demonstrated that significantly more cells were undergoing
characteristic apoptotic alterations, including nuclear
condensation, nuclear fragmentation and darker dye staining in the
MR1-C6 and MR1-D4 groups, however, these changes were not observed
in the NC group (Fig. 2A). Annexin
V is a calcium-dependent phospholipid binding protein and can bind
to the plasma membrane in the early stages of apoptosis. It is one
of a number of sensitive methods used to detect early apoptosis.
Flow cytometric analysis demonstrated that the percentage of
apoptotic cells in the MUC1 gene-silenced group (MR1-C6, 6.13%;
MR1-D4, 5.29%) was markedly higher than that observed in the
control group (SMMC-7721, 2.42%; NC, 1.5%; Fig. 2B). DNA fragmentation is a
characteristic alteration observed during the late stage of
apoptosis and can be detected using a DNA ladder assay. DNA Ladder
extraction kits were used to extract the genomic DNA of SMMC-7721,
NC, MR1-C6 and MR1-D4 cells. The DNA ladder was observed on 1%
agarose electrophoresis gel in the MR1-C6 and MR1-D4 cells, but not
in the SMMC-7721 or NC cells (Fig.
2C). Furthermore, in order to confirm that MUC1 gene silencing
induced apoptosis of SMMC-7721 cells, the expression and activation
of caspase-3, and its substrate PARP, were detected by western
blotting. The results demonstrated that the cleaved products of
caspase-3 and PARP were expressed in the MR1-C6 and MR1-D4 cells,
but not in the SMMC-7721 or NC cells (Fig. 2D and E). The morphological and
biochemical alterations observed in these cells indicate that MUC1
gene silencing can induce apoptosis of SMMC-7721 cells.
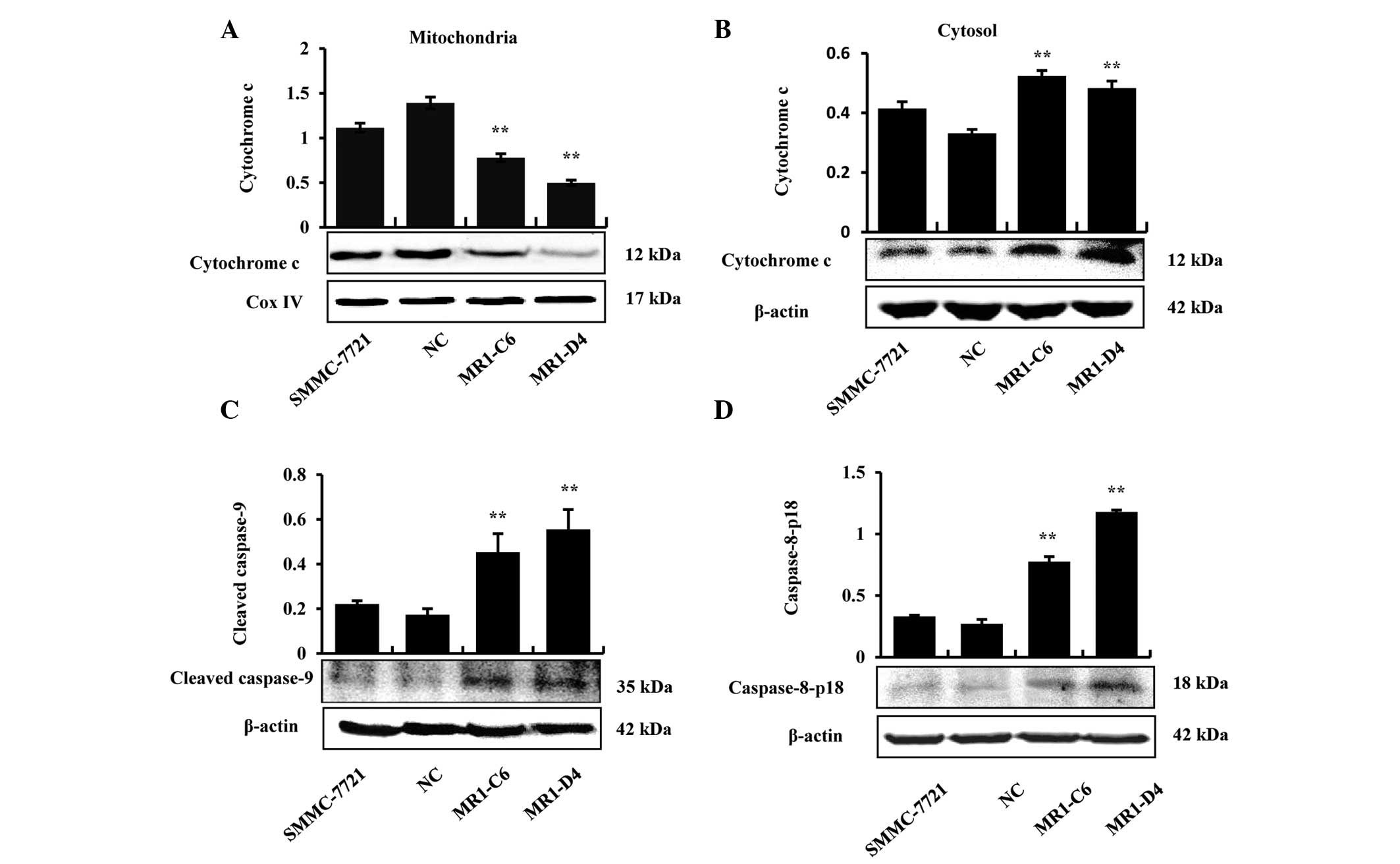
MUC1 gene silencing induces apoptosis of
SMMC-7721 cells through mitochondrial and death receptor apoptotic
pathways
Proteins were extracted from the mitochondria and
cytoplasm to determine whether cytochrome c was released in
MUC1 gene-silenced SMMC-7721 cells, and the results demonstrated
that cytochrome c was only released from the mitochondria
into the cytoplasm in the MR1-C6 and MR1-D4 groups (Fig. 3A and B). In addition, the results
demonstrated that caspase-9, an initiator caspase, which is
associated with the mitochondrial death pathway, was activated in
the MR1-C6 and MR1-D4 cells (Fig.
3C). The results from western blotting demonstrated that
pro-caspase-8 and cleaved caspase-8 increased in the MR1-C6 and
MR1-D4 groups, compared with the control groups (Fig. 3D). The above results indicate that
MUC1 gene silencing induces apoptosis in SMMC-7721 cells through
the mitochondrial and death receptor apoptotic pathways.
To further analyze the molecular mechanisms of
apoptosis in SMMC-7721, as induced by MUC1 gene silencing, the
expression of the pro-apoptotic protein Bax, the anti-apoptotic
protein Bcl-2 and the tumor suppressor p53 was measured at the
transcriptional and translational levels. RT-qPCR demonstrated that
Bax and p53 significantly increased (P<0.05), while Bcl-2
significantly decreased (P<0.01) in the MR1-C6 and MR1-D4
groups, compared with the NC group (Fig. 4A–C). Western blotting revealed
similar results to RT-qPCR (Fig.
4D). These results suggest that MUC1 gene silencing can
regulate the expression and activation of proteins associated with
the mitochondrial and death receptor apoptotic pathways.
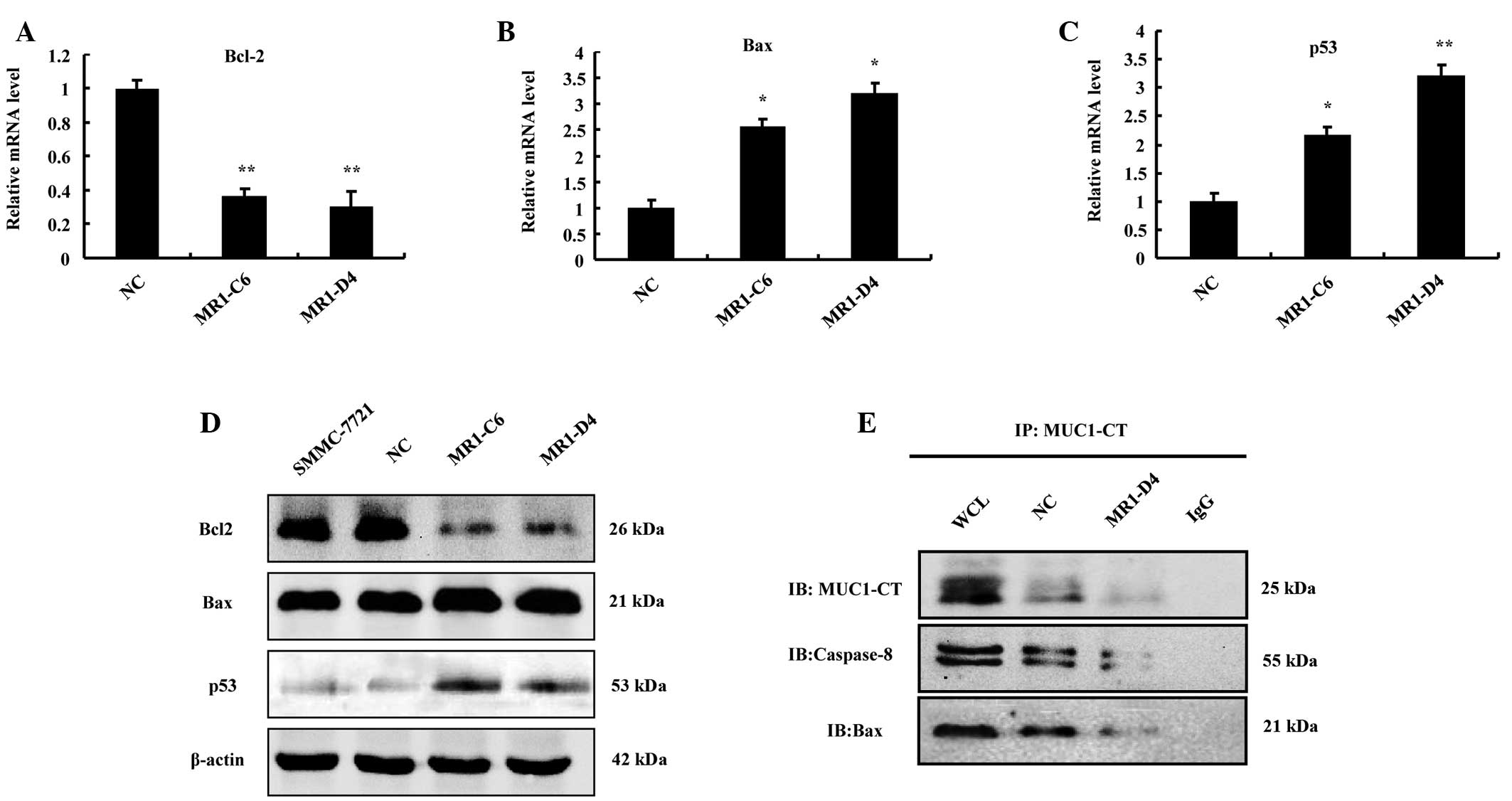 | Figure 4MUC1 gene silencing decreases MUC1-CT
binding with Bax and caspase-8 directly to induce apoptosis of
SMMC-7721 cells. (A-C) Relative mRNA levels of Bcl-2, Bax and p53
(fold change) compared with the NC, *P<0.05,
**P<0.01. (D) Western blotting for the expression of
p53, Bcl-2 and Bax. (E) Cell lysates were subjected to
immunoprecipitation with the anti-MUC1-CT antibody or normal IgG
and then immunoblotted with anti-caspase-8 and anti-Bax antibodies.
Whole cell lysate was not subjected to immunoprecipitation. MUC1,
mucin 1; NC, negative control; Bax, Bcl-2-associated X protein;
Bcl-2, B-cell lymphoma 2; CT, cytoplasmic tail; IgG, immunoglobulin
G. |
MUC1 gene silencing decreases MUC1-CT
binding to Bax and caspase-8
To further investigate the mechanisms of SMMC-7721
cell apoptosis induced by MUC1 knockdown (15), co-immunoprecipitation experiments
were conducted to determine the interaction between MUC1-CT and Bax
or caspase-8 in SMMC-7721 cells. The results demonstrated that
MUC1-CT can bind directly to Bax or caspase-8 and the interaction
was reduced markedly in the MUC1 gene-silenced group (Fig. 4E). Taken together, these results
suggest that MUC1 gene silencing can reduce the interaction of
MUC1-CT with Bax or caspase-8, therefore resulting in activation of
the mitochondrial and death receptor apoptotic pathways.
Discussion
Our previous study found that the knockdown of MUC1
in SMMC-7721 cells can significantly inhibit cell proliferation and
induce cell cycle arrest (23). In
the present study, a clone formation assay in vitro and a
tumor xenograft mouse model with an in vivo imaging system
were utilized and the growth inhibitory effects of MUC1 silencing
in SMMC-7721 cells were further confirmed. Since MUC1 is an
oncoprotein, it is not only involved in the regulation of
carcinogenesis and tumor progression, invasion and metastasis
(2), but it is also important in
the inhibition of cell apoptosis (25-27).
Consequently, the present study examined in depth whether the
growth inhibition of MUC1 gene-silenced cells is associated with
apoptotic cell death. Hoechst 33342 staining, flow cytometry with
Annexin V-PE staining and a DNA Ladder assay were performed, and
all results consistently demonstrated that MUC1 gene silencing
induces cells to undergo apoptosis. These results are similar to
results from a previous study, in which a MUC1-downregulated
gastric carcinoma cell line was found to be more apoptotic compared
with the controls (28). Since
caspase activation has a key role in the process of apoptosis,
caspase-3, in particular, is activated during early apoptosis. Once
activated, caspase-3 can increase its substrate, PARP, which is an
indicator of apoptosis. The expression of caspase-3 and PARP was
detected by western blotting and the results demonstrated that
cleaved caspase-3 and PARP were present, suggesting that caspase-3
was activated in MUC1 gene-silenced cells. Taken together, these
results indicate that MUC1 gene silencing can not only inhibit the
growth of SMMC-7721 cells but can also induce apoptosis of these
cells.
Generally, cell apoptosis involves two major
signaling pathways, including the mitochondrial apoptotic pathway
and the death receptor apoptotic pathway (29). The intrinsic mitochondria apoptotic
pathway is often characterized by the release of cytochrome
c from the mitochondrial intermembrane space into the
cytoplasm and activation of caspase-9. Bcl-2 is a major
anti-apoptotic protein and Bax is a pro-apoptotic protein. These
two proteins have been identified as major regulators in the
mitochondrial apoptotic pathway. The tumor suppressor p53 can
directly activate Bax and downregulate Bcl-2, in the absence of
other proteins, to permeabilize mitochondria and engage the
apoptotic program (30,31). In the present study, it was
demonstrated that MUC1 gene silencing in SMMC-7721 cells could
induce apoptosis, and further experiments revealed that MUC1 gene
silencing induced cytochrome c release from the mitochondria
to the cytoplasm and that caspase-9 was also activated. In
parallel, the expression of Bax and p53 were also significantly
increased, while the expression of Bcl-2 was markedly reduced, as
compared with the control group, suggesting that the intrinsic
mitochondrial apoptotic pathway was activated in MUC1 gene-silenced
cell lines. It is well established that caspase-8, an initiator
caspase, is required to activate the membrane bound
receptor-mediated extrinsic apoptotic signaling pathway. To
investigate whether the receptor-mediated apoptotic pathway is
simultaneously activated with the mitochondrial apoptotic pathway
when cell apoptosis occurs, the expression of caspase-8 was
examined. The results demonstrated that caspase-8 was activated in
MUC1 gene-silenced cell lines, suggesting that the receptor
apoptotic pathway is also involved in MUC1 gene silencing-induced
cell apoptosis. Ahmad et al reported that MUC1-CT could bind
directly to Bax or caspase-8 in HCT116 cells and in breast cancer
MCF7 cells to prevent Bax from localizing to the mitochondria
(32) or inhibit the
FAS-associated death domain protein from recruiting caspase-8
(15), thus suppressing the
activation of proteins that are associated with the mitochondrial
or receptor apoptotic pathways. In the present study,
co-immunoprecipitation was also conducted to determine whether
MUC1-CT could bind to Bax or caspase-8 directly. The results were
positive and consistent with previous results, demonstrating that
MUC1 gene silencing may reduce the interaction of MUC1-CT with Bax
or caspase-8, and thus lead to activation of the mitochondrial or
death receptor apoptotic pathway, ultimately resulting in SMMC-7721
cell apoptosis.
In conclusion, the present study demonstrated that
MUC1 gene silencing induces the growth inhibition of SMMC-7721
cells via the mitochondrial and death receptor apoptotic pathways,
and combined with our previous results, further suggests that MUC1
is important in the progression of HCC development, and thus MUC1
is a potential target for liver cancer therapy.
Acknowledgments
The authors would like to thank Dr O.J. Finn for the
pcDNA3-MUC1 plasmid, which was used to transfect the SMMC-7721 cell
line. This study was supported by grants from the Double Tenth
Engineering of Major Research Project of Jilin Provincial Science
and Technology Department (grant no. 20140201012YY) and the Major
Development Programs for New Drugs of the Chinese Academy of
Sciences during the 12th Five-Year Plan Period (grant no.
2011ZX09102-001-36).
References
|
1
|
Kufe DW: Functional targeting of the MUC1
oncogene in human cancers. Cancer Biol Ther. 8:1197–1203. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nath S and Mukherjee P: MUC1: A
multifaceted oncoprotein with a key role in cancer progression.
Trends Mol Med. 20:332–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kufe DW: Targeting the human MUC1
oncoprotein: A tale of two proteins. Cancer Biol Ther. 7:81–84.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang L, Chen D, Liu D, Yin L, Kharbanda S
and Kufe D: MUC1 oncoprotein blocks glycogen synthase kinase
3beta-mediated phosphorylation and degradation of beta-catenin.
Cancer Res. 65:10413–10422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Kuwahara H, Ren J, Wen G and Kufe D:
The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1
carcinoma-associated antigen with GSK3 beta and beta-catenin. J
Biol Chem. 276:6061–6064. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pandey P, Kharbanda S and Kufe D:
Association of the DF3/MUC1 breast cancer antigen with Grb2 and the
Sos/Ras exchange protein. Cancer Res. 55:4000–4003. 1995.PubMed/NCBI
|
|
7
|
Lau SK, Shields DJ, Murphy EA,
Desgrosellier JS, Anand S, Huang M, Kato S, Lim ST, Weis SM,
Stupack DG, et al: EGFR-mediated carcinoma cell metastasis mediated
by integrin αvβ5 depends on activation of c-Src and cleavage of
MUC1. PLoS One. 7:e367532012. View Article : Google Scholar
|
|
8
|
Schroeder JA, Thompson MC, Gardner MM and
Gendler SJ: Transgenic MUC1 interacts with epidermal growth factor
receptor and correlates with mitogen-activated protein kinase
activation in the mouse mammary gland. J Biol Chem.
276:13057–13064. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahmad R, Raina D, Trivedi V, Ren J, Rajabi
H, Kharbanda S and Kufe D: MUC1 oncoprotein activates the IkappaB
kinase beta complex and constitutive NF-kappaB signalling. Nat Cell
Biol. 9:1419–1427. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmad R, Raina D, Joshi MD, Kawano T, Ren
J, Kharbanda S and Kufe D: MUC1-C oncoprotein functions as a direct
activator of the nuclear factor-kappaB p65 transcription factor.
Cancer Res. 69:7013–7021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh PK, Behrens ME, Eggers JP, Cerny RL,
Bailey JM, Shanmugam K, Gendler S, Bennett EP and Hollingsworth MA:
Phosphorylation of MUC1 by Met modulates interaction with p53 and
MMP1 expression. J Biol Chem. 283:26985–26995. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei X, Xu H and Kufe D: Human mucin 1
oncoprotein represses transcription of the p53 tumor suppressor
gene. Cancer Res. 67:1853–1858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Banerjee S, Mujumdar N, Dudeja V,
Mackenzie T, Krosch TK, Sangwan V, Vickers SM and Saluja AK: MUC1c
regulates cell survival in pancreatic cancer by preventing
lysosomal permeabilization. PLoS One. 7:e430202012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren J, Bharti A, Raina D, Chen W, Ahmad R
and Kufe D: MUC1 oncoprotein is targeted to mitochondria by
heregulin-induced activation of c-Src and the molecular chaperone
HSP90. Oncogene. 25:20–31. 2006.
|
|
15
|
Agata N, Ahmad R, Kawano T, Raina D,
Kharbanda S and Kufe D: MUC1 oncoprotein blocks death
receptor-mediated apoptosis by inhibiting recruitment of caspase-8.
Cancer Res. 68:6136–6144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yonemitsu Y, Imazeki F, Chiba T, Fukai K,
Nagai Y, Miyagi S, Arai M, Aoki R, Miyazaki M, Nakatani Y, Iwama A
and Yokosuka O: Distinct expression of polycomb group proteins EZH2
and BMI1 in hepatocellular carcinoma. Hum Pathol. 40:1304–1311.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Capurro M, Wanless IR, Sherman M, Deboer
G, Shi W, Miyoshi E and Filmus J: Glypican-3: a novel serum and
histochemical marker for hepatocellular carcinoma.
Gastroenterology. 125:89–97. 2013. View Article : Google Scholar
|
|
18
|
Jego G, Hazoumé A, Seigneuric R and
Garrido C: Targeting heat shock proteins in cancer. Cancer Lett.
332:275–285. 2013. View Article : Google Scholar
|
|
19
|
Oikawa T, Kamiya A, Zeniya M, Chikada H,
Hyuck AD, Yamazaki Y, Wauthier E, Tajiri H, Miller LD, Wang XW,
Reid LM and Nakauchi H: Sal-like protein 4 (SALL4), a stem cell
biomarker in liver cancers. Hepatology. 57:1469–1483. 2013.
View Article : Google Scholar
|
|
20
|
Bozkaya G, Korhan P, Cokaklı M, Erdal E,
Sağol O, Karademir S, Korch C and Atabey N: Cooperative interaction
of MUC1 with the HGF/c-Met pathway during hepatocarcinogenesis. Mol
Cancer. 11:642012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan SF, Li KZ, Wang L, Dou KF, Yan Z, Han
W and Zhang YQ: Expression of MUC1 and its significance in
hepatocellular and cholangiocarcinoma tissue. World J
Gastroenterol. 11:4661–4666. 2005.PubMed/NCBI
|
|
22
|
Gad A, Tanaka E, Matsumoto A, Wahab MA,
Serwah Ael-H, Attia F, Ali K, Hassouba H, el-Deeb Ael-R, Ichijyo T,
et al: Assessment of KL-6 as a tumor marker in patients with
hepato-cellular carcinoma. World J Gastroenterol. 11:6607–6612.
2005. View Article : Google Scholar
|
|
23
|
Li QS, Wang FL, Liu GM, Yuan HY, Chen TX,
Wang J, Xie F, Zhai RP, Wang F, Guo YY, et al: Impact of Mucin1
knockdown on the phenotypic characteristics of the human
hepatocellular carcinoma cell line SMMC-7721. Oncol Rep.
31:2811–2819. 2014.PubMed/NCBI
|
|
24
|
Wang FL, Li QS, Ni WH, Fang F, Sun XX, Xie
F, Wang J, Wang F, Gao SJ and Tai GX: Expression of human
full-length MUC1 inhibits the proliferation and migration of a B16
mouse melanoma cell line. Oncol Rep. 30:260–268. 2013.PubMed/NCBI
|
|
25
|
Castorina A and Giunta S: Mucin 1 (MUC1)
signalling contributes to increase the resistance to cell death in
human bronchial epithelial cells exposed to nickel acetate.
Biometals. 27:1149–1158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin L, Kufe T, Avigan D and Kufe D:
Targeting MUC1-C is synergistic with bortezomib in downregulating
TIGAR and inducing ROS-mediated myeloma cell death. Blood.
123:2997–3006. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Q, Li D, Ren J, Li C and Xiao Z: MUC1
activates JNK1 and inhibits apoptosis under genotoxic stress.
Biochem Biophys Res Commun. 440:179–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Costa NR, Paulo P, Caffrey T,
Hollingsworth MA and Santos-Silva F: Impact of MUC1 mucin
downregulation in the phenotypic characteristics of MKN45 gastric
carcinoma cell line. PloS One. 6:e269702011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zimmermann KC and Green DR: How cells die:
Apoptosis pathways. J Allergy Clin Immunol. 108(4 Suppl): S99–S103.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
Bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Srinivas G, Kusumakumary P, Nair MK,
Panicker KR and Pillai MR: Mutant p53 protein, Bcl-2/Bax ratios and
apoptosis in paediatric acute lymphoblastic leukaemia. J Cancer Res
Clin Oncol. 126:62–67. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahmad R, Alam M, Rajabi H and Kufe D: The
MUC1-C oncoprotein binds to the BH3 domain of the pro-apoptotic BAX
protein and blocks BAX function. J Biol Chem. 287:20866–20875.
2012. View Article : Google Scholar : PubMed/NCBI
|