Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
and highly destructive disease of the joints, characterized by
persistent synovitis, cartilage degradation and bone erosion. At
present, RA remains incurable and patients with RA are often
refractory to a number of drugs (1). Although the exact pathogenesis of RA
remains to be fully elucidated, numerous previous studies have
demonstrated that abnormalities in T and B lymphocytes, and
macrophages are pivotal in its pathogenesis. Genetic and
environmental factors exert orchestrated roles. For example, B
cells aggravate the pathogenic process through autoantibody and
cytokine production. Antigen-activated CD4+ T cells
amplify the immune response. Synovitis is associated with the local
activation of mononuclear cells, formation of new blood vessels and
the release of cytokines, particularly tumor necrosis factor
(TNF)-α, interleukin (IL)-6, IL-1 and IL-17 (2,3). In
parallel, inflammatory responses increase the levels of
procoagulant factors, and the natural anticoagulant pathways and
fibrinolytic activities are inhibited, leading to a thrombotic
tendency. Previous studies identified a previously unrecognized
role for platelets and their activation-induced microparticles in
autoimmune inflammatory arthritis. It appears that there may be
very complex interactions occurring between inflammation and
hemostasis. Coagulation augments inflammation, leading to a
positive feedback mechanism. Inflammation-induced thrombosis
responded to immunosuppressants, statins, anticoagulants and
antiplatelet agents, however, a decreased effectiveness of the
drugs over time and adverse reactions were often observed (4,5). The
initial consensus guidelines for stem cell transplantation in
autoimmune diseases were published in 1997, and the
immunomodulatory potential of stem cells was subsequently confirmed
(6–8). To the best of our knowledge, only a
few previous studies have investigated the role of human umbilical
cord mesenchymal stem cells (hUC-MSCs) in inflammatory thrombosis
in RA (9). In addition, an
important goal in this research is to develop an in vivo,
non-invasive tracking method for determining the retention,
migration and effects of implanted hUC-MSCs. Magnetic
nanoparticles, particularly superparamagnetic iron oxide
nanoparticles (SPIONs), have long been investigated as a contrast
agent for magnetic resonance imaging (MRI) (10). In the present study, the rat model
of collagen type II -induced arthritis (CIA) was established in
order to assess the efficacy of hUC-MSCs in regulating the immune
response and in decreasing inflammatory thrombosis, and to further
investigate the mechanism of repair mediated by hUC-MSCs in CIA
through tracking the cells in vivo and in vitro.
Materials and methods
General experimental process
A total of ninety female Sprague Dawley rats (age,
five weeks; average weight, 175±10 g) were purchased from the
Nanjing University Model Animal Research Center (Nanjing, China).
The rats were housed in clean cages under hygienic conditions and
were allowed to acclimatize for at least 7 days prior to the
experiment. The skin of each rat was shaved and cleaned with iodine
solution (Nanjing Zhonghe Pharmaceuticals, Nanjing, China) prior to
the procedure. A general anesthesia was induced by means of
intramuscular injection of ketamine (30 mg/kg; Heng Rui
Pharmaceuticals, Lianyungang, China) to minimize the suffering of
the rats during the experiments. Following each procedure, the rats
were clinically observed in their cages with respect to food,
water, body weight, temperature and their general behavior. The
protocols were approved by the ethics committee of Northern Jiangsu
People's Hospital (Jiangsu, China). All studies were performed at
the Yangzhou Institute of Hematology (Yangzhou, China) and at the
Animal Experiment Center of Yangzhou and Southeastern
University.
Reagents
Chicken collagen type II (CII), complete Freund's
adjuvant, bromodeoxyuridine (BrdU), mouse anti-rat BrdU monoclonal
antibody (cat. no. B253; 1:500 dilution), goat anti-mouse
fluorescein isothiocyanate (FITC) polyclonal antibody (cat. no.
F5387; 1:64 dilution) and horseradish peroxidase-labeled goat
anti-mouse IgG polyclonal antibody (cat. no. A9917; dilution,
1:150) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
ELISA kits for the detection of IL-1 (cat. no. 24494), IL-17 (cat.
no. 301291), TNF-α (cat. no. 24835), vascular endothelial growth
factor (VEGF; cat. no. 83785), tissue factor (TF; cat. no. 14066),
antithrombin (AT; cat. no. DSPC10) were purchased from R&D
Systems (Minneapolis, MN, USA). FITC mouse anti-rat CD4 monoclonal
antibody (cat. no. 554837; 1:200 dilution), FITC mouse anti-rat
CD11b monoclonal antibody (cat. no. 554982; 1:200 dilution) and
phycoerythrin (PE)-mouse anti-rat CD25 monoclonal antibody (cat.
no. 554866; 1:200 dilution) were purchased from BD Biosciences (San
Jose, CA, USA). SPIONs were prepared at the Chemistry and Chemical
Engineering College of Yangzhou University (Yangzhou, China).
CIA model
The experiments were performed in 5-week-old female
Sprague-Dawley rats (weighing, 175±10 g; Nanjing Medical
University, Nanjing, China). A total of 120 rats were randomly
separated into four groups: Control group (C), the CIA model group
(M), the SPION and BrdU-unlabeled UC-MSCs group (U) and the SPION
and BrdU-labeled UC-MSCs group (L). The CIA model was induced, as
described previously (11). With
the exception of the C group, each rat was intradermally injected
with 0.1 ml (0.25 mg) CII into its right hind-paw on day 1, and an
identical dose of CII was injected into the rat tail and left hind
plantar on day 21 to reinforce the extent of the immune damage.
hUC-MSCs labeling
The solution, containing SPIONs, was added to
2×106/ml hUC-MSCs (obtained from the Human Umbilical
Cord Mesenchymal Stem Cell Bank of Taizhou, Taizhou, China) and
co-cultured for 12 h. The final concentration of SPIONs was 25
µg Fe/ml. An identical dose of hUC-MSCs was added to 15
µM BrdU and co-cultured for 48 h. All experiments were
performed at least in triplicate.
Prussian blue staining
The UC-MSCs were washed three times with
phosphate-buffered saline (PBS) and fixed for 30 min with
glutaraldehyde (Sinopharm Chemical Regent, Shanghai, China) at room
temperature. The cells were washed three times with PBS, prior to
incubation with 20% potassium ferro-cyanide (Sinopharm Chemical
Regent) and 20% hydrochloric acid for 20 min at room temperature.
They were subsequently washed once in deionized water and were
dehydrated through a graded alcohol series (70, 80, 90 and 95%).
Prussian blue stain (Sinopharm Chemical Regent) was applied to
determine the presence of iron particles in the SPION-labeled
hUC-MSCs under a light microscope (CH30RF200; Olympus, Tokyo,
Japan). The cells positively stained with the Prussian blue stain
were quantified.
Immunocytochemical staining
The hUC-MSCs were transferred to cling film
(Sigma-Alrich), washed three times with PBS and incubated in 3%
H2O2 for 48 h. The cells were subsequently
placed into a 20% HCl solution at room temperature. Mouse anti-BrdU
monoclonal antibody (dilution, 1:500) and subsequently horseradish
peroxidase-labeled goat anti-mouse IgG (dilution, 1:150) were in
turn added to the film. Following washing, the cells with PBS,
positively stained with BrdU stain were counted under a
fluorescence microscope (DX-UCB; Olympus).
Cell transplantation
Aliquots of 1×106 hUC-MSCs (0.5 ml cell
suspension) were injected into the tail vein in the U and L groups
one week following the second CII injection. Alternatively, an
equal volume of saline was injected into the tail vein of the M and
C groups.
MRI examination in vivo
The 7.0 T Micro-MRI system (Bruker Pharma, Siemens
Medical Systems, Erlangen, Germany) was used to detect
SPION-positive cells on days 7, 21 and 35 following the injection
of labeled hUC-MSCs. The hind limbs of the rats in group L were
positioned in complete extension and located in the center of a
standard wrist coil so that the long axis of the joint was parallel
with the MRI unit table. MRI was subsequently performed in the
transverse plane of the joints to ensure anatomic reproducibility
of the image position, and to allow correlations to be made between
the histological slices. The following parameters were used: Three
inches of the coil, T2 × weighted imaging (WI), a thickness of 1 mm
with no interval gap, a repetition time of 200.0 msec, an echo time
of 3.0 msec, a fast low-angle shot, and anisotropy values as
follows: Fractional anisotropy, 30.0 deg.; matrix, 256 × 256; and
field of view, 70.0 mm.
ELISA analysis
Under anesthesia with 3% sodium pento-barbital
(Sigma-Aldrich), the abdominal veins of the rats were perforated
and 2 ml venous blood was obtained. Blood was collected on days 7,
21 and 35 following treatment and centrifuged at 1,400 × g at 4°C
for 10 min. The serum was further extracted in order to measure the
levels of IL-1, IL-17, TNF-α, VEGF, TF and AT using an ELISA kit,
according to the manufacturer's instructions (R&D Systems).
Flow cytometry
On days 7, 21 and 35 following treatment, whole
venous blood of the rats was collected in tubes containing 3.8%
sodium citrate. Blood cells were incubated with FITC-CD4 monoclonal
antibody, FITC-CD11b monoclonal antibody or PE-CD25 monoclonal
antibody in different tubes for 15 min in the dark. Hemolysin (BD
Biosciences) was added to each tube to eliminate the red blood
cells. Subsequently, the expression of T-regulatory (Treg) cells
was measured by flow cytometric analysis (FCM). FCM experiments
were performed on 1×105 cells and repeated at least
three times.
Indirect immunofluorescence
On days 7, 21 and 35 following treatment, batches of
rats were anesthetized with 3% sodium pentobarbital and following
collection of venous blood, knee joints, heart, liver, spleen, lung
and kidney were excised and fixed in 10% formaldehyde solution
prior to paraffin embedding. The tissue sections were incubated
with anti-BrdU monoclonal antibody (dilution, 1:500), and
subsequently with goat anti-mouse IgG-FITC (dilution, 1:64) at room
temperature. The cells positively stained with the BrdU were washed
with PBS, and their distribution in tissues was subsequently
assessed by counting under a fluorescence microscope (DX-UCB;
Olympus).
Statistical analysis
The data from all quantitative assays are expressed
as the mean ± standard deviation. Statistical analysis were
performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
Comparisons between groups were performed using one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
General state of the rats and the gross
appearance of the hind legs
All rat models of CIA exhibited a markedly decreased
appetite, reduced activity, listlessness, a slow weight gain and
dull hair. On day 2 following the initial CII injection, skin
petechiae appeared and mild swelling occurred in the homolateral
joint (in the paw, ankle and knees). From day 7 following the CII
injection, the swelling appeared to become progressively more
aggravated, subsequently spreading to the contralateral limbs. The
symptoms exhibited were typically the most severe on day 21,
following the second CII injection (compare the normal appearance
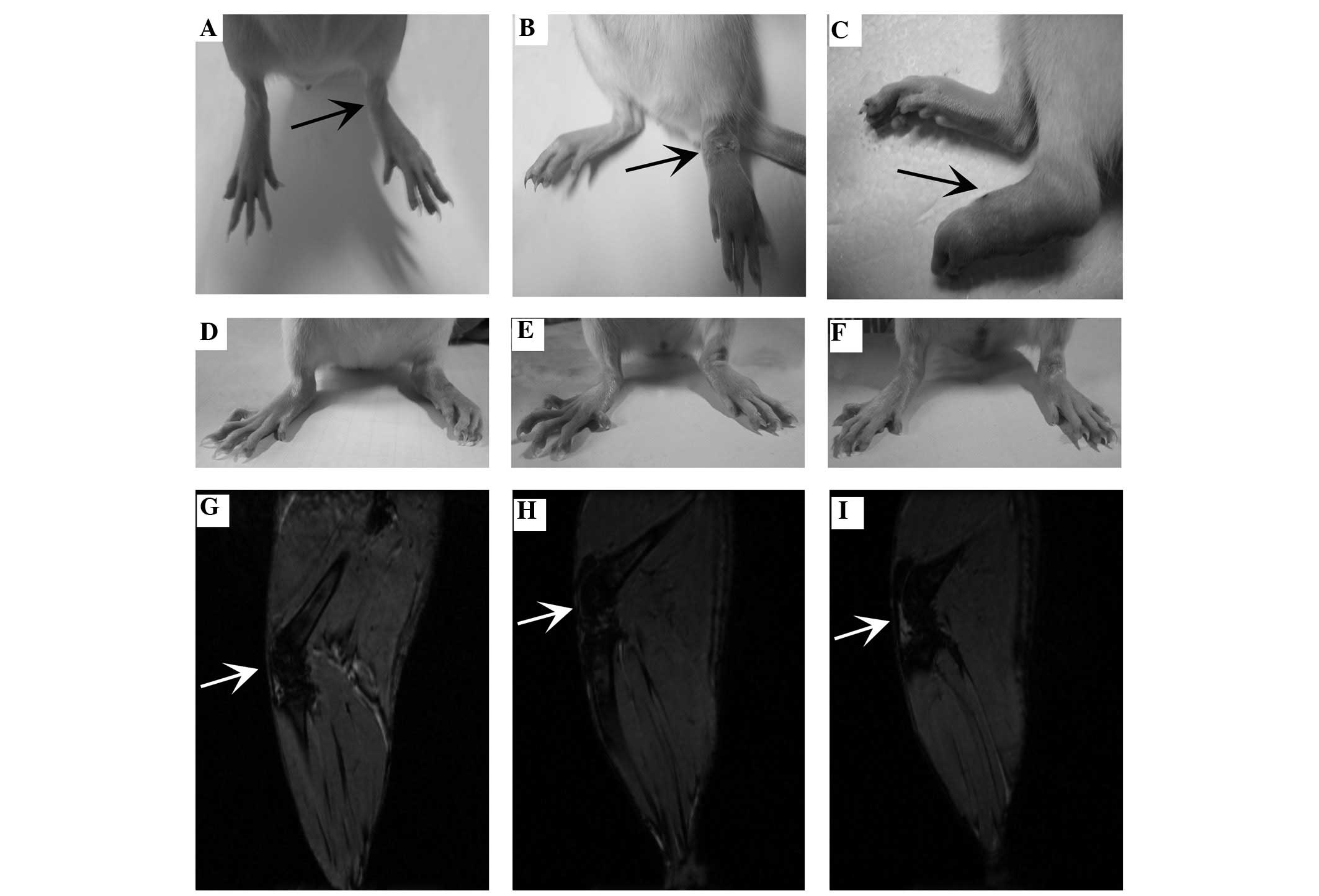
of the rat hind paw in the C group; Fig. 1A–C). Following treatment of the
UC-MSCs with or without SPION labeling, the rats exhibited less
synovial thickening, an increased incidence of cartilage defects
and tissue edema compared with those in group M (Fig. 1A–C).
In vivo MRI tracking of SPION-labeled
hUC-MSCs
SPION-labeled hUC-MSCs, which are illustrated as the
attenuated (dark) signal (Fig.
1D–F), were initially detected in the rat joints by in
vivo MRI on day 2 following the implantation of the hUC-MSCs.
On day 7, the most marked signal attenuation in the knee was
observed in the SPION-labeled group (Fig. 1D), and subsequently the attenuated
signal was gradually reduced up to 35 days (Fig. 1E and F). However, a similar signal
and pathological changes failed to be identified in the heart,
liver, spleen, lung, kidney and other organs of the identical
group.
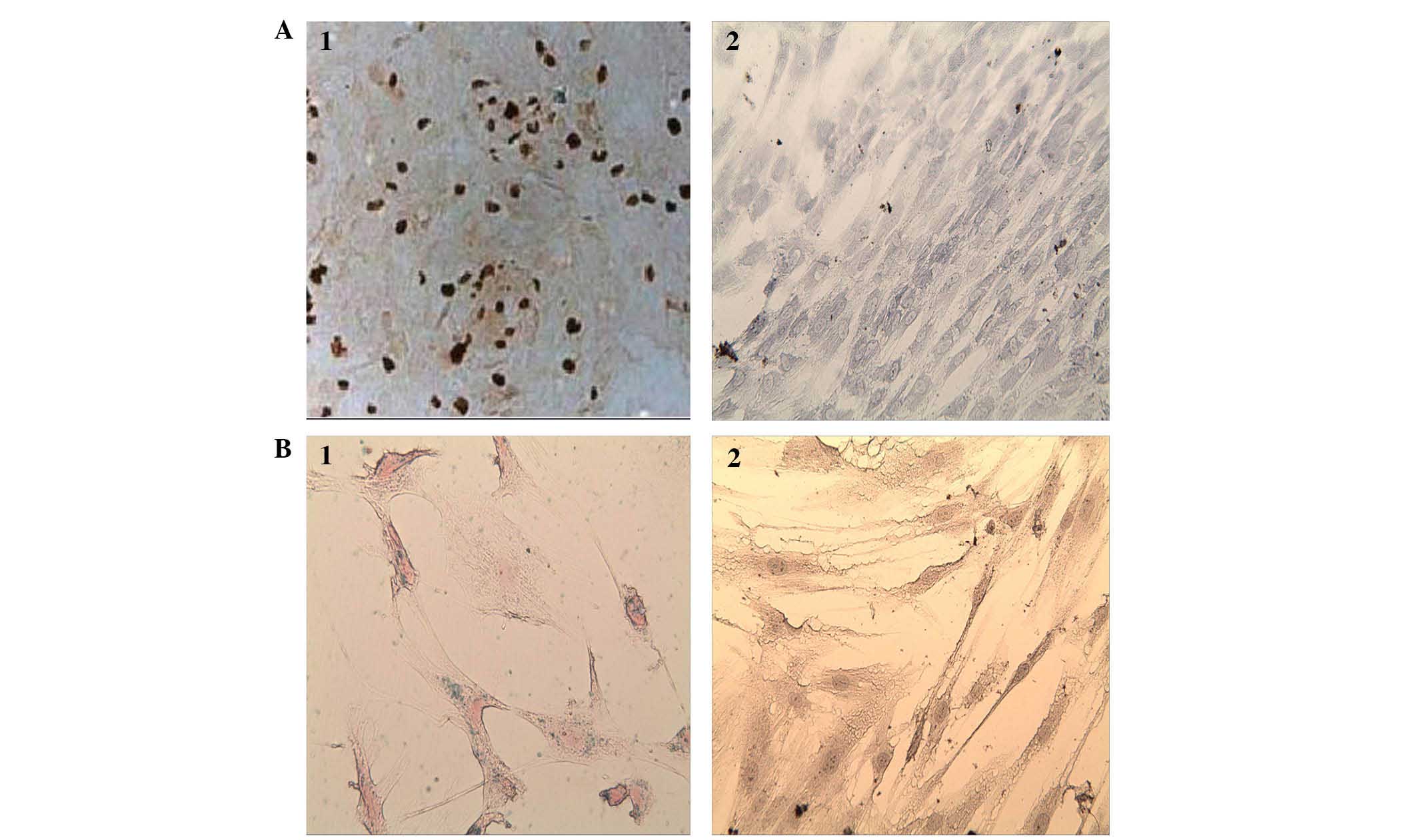
Evaluation of Prussian blue staining of
hUC-MSCs in vitro
Blue iron particles were identified in the cytoplasm
of almost every labeled UC-MSC. Certain labeled hUC-MSCs were
congregated into groups (Fig.
2A-1). Prussian blue staining was positively taken up by
>98% of the cells.
BrdU labeling of the hUC-MSCs in
vitro
Tan-colored particles were identified in the labeled
hUC-MSC nuclei (Fig. 2B-1, 2). The
uptake of the BrdU stain was >97.5%.
Cell viability of hUC-MSCs as determined
by a trypan blue exclusion assay
The cell viability, as analyzed using the Trypan
blue exclusion assay, was determined to be 99 and 98% in groups U
and L, respectively. No statistical differences in cell viability
were identified between the hUC-MSC-treated groups.
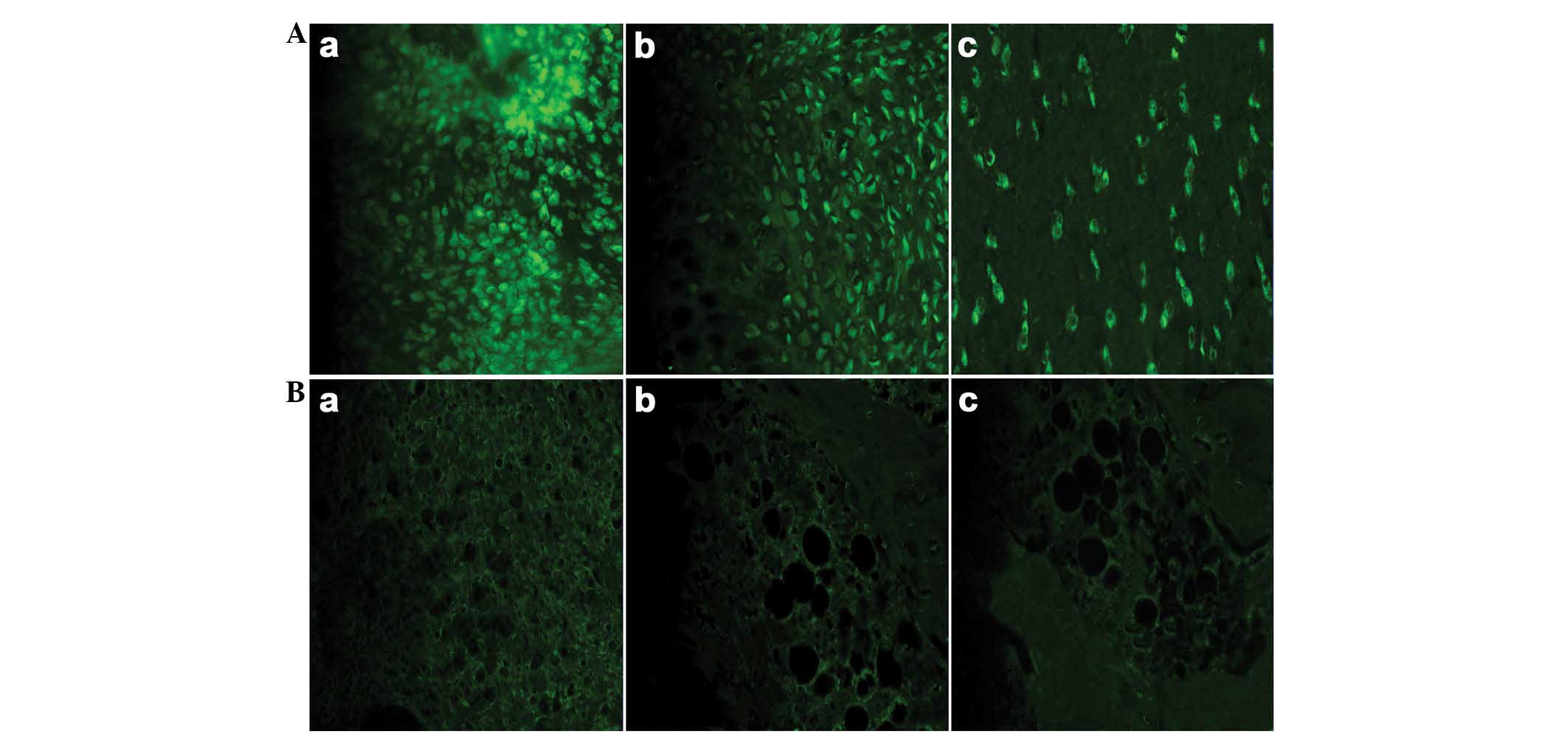
Indirect immunofluorescence analysis of
the tissue samples
The most significant fluorescence intensity of the
inflammatory joints in the BrdU-labeled group was observed on day 7
following the injection of the hUC-MSCs. Subsequently, the
fluorescence intensity was gradually reduced over time. The mean
fluorescence intensity was similar in the two hUC-MSC-treated
groups. With the exception of the inflammatory joints,
immunofluorescence was not detected in other organs, including the
liver, kidney, lung, spleen and heart, which suggested that
hUC-MSCs failed to accumulate in these organs (Fig. 3).
Expression of the Treg cells in the
different groups
The percentage of Treg cells identified in the
lymphocytes of the peripheral blood in group M was significantly
lower compared with that in the C group (P<0.05; Fig. 4A). In groups U and L, the
percentage of Treg cells was higher compared with that in the C
group at 1 and 3 weeks following hUC-MSC transfusion. However, at 5
weeks post-treatment, no differences in the percentages of the Treg
cells were identified. Furthermore, the percentage of Treg cells in
the CIA model group M was lower compared with that in group C and
in the two hUC-MSC-treated groups, U and L, throughout the entire
course of the experiment (Fig.
4B–F).
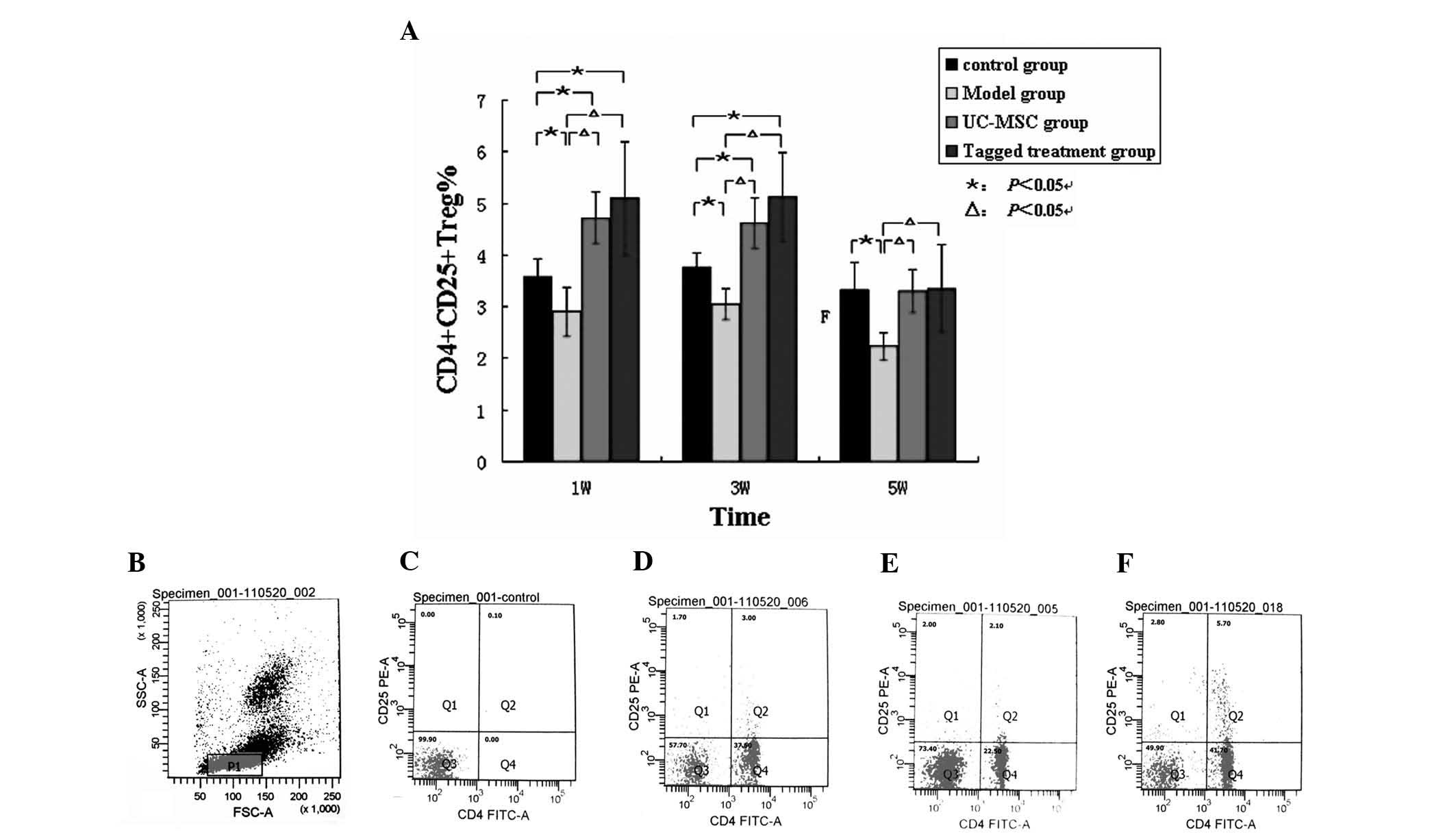 | Figure 4Expression of the Treg cells between
all groups. (A) Flow cytometric analysis demonstrated that the
percentage of Treg cells in the CIA model group M was significantly
lower compared with that in the control group C and the two
hUC-MSC-treated groups, U and L, at the different time points.
ΔP<0.05, compared with the hUC-MSC-treated groups;
*P<0.05, compared with the blank control group.
Representative flow cytometric analysis data at the identical time
points were assessed. (B) The lymphocytes of the frame; the
percentages of Treg cells were measured as (C) 0.1% (the negative
control group); (D) 3.00% (the blank control group C); (E) 2.10%
(group M); and (F) 5.7% (group U). Treg cells,
CD4+CD25+ T cells; W, week, hUC-MSC, human
umbilical cord mesenchymal stem cell; FSC, forward scatter, SSC,
side scatter; FITC, fluorescein isothiocyanate; PE, phycoerythrin;
CIA, collagen type II-induced arthritic; W, weeks. |
Levels of cytokines in the different
groups
The levels of IL-1, IL-17, TNF-α and VEGF in group M
were significantly higher compared with those of the
hUC-MSC-treated groups U and L, and group C, at 1, 3 and 5 weeks
following treatment (P<0.05). The experimental parameters for
group C were lowest during week 1 and were similar to those of the
hUC-MSC-treated group at 5 weeks post-treatment (P<0.05;
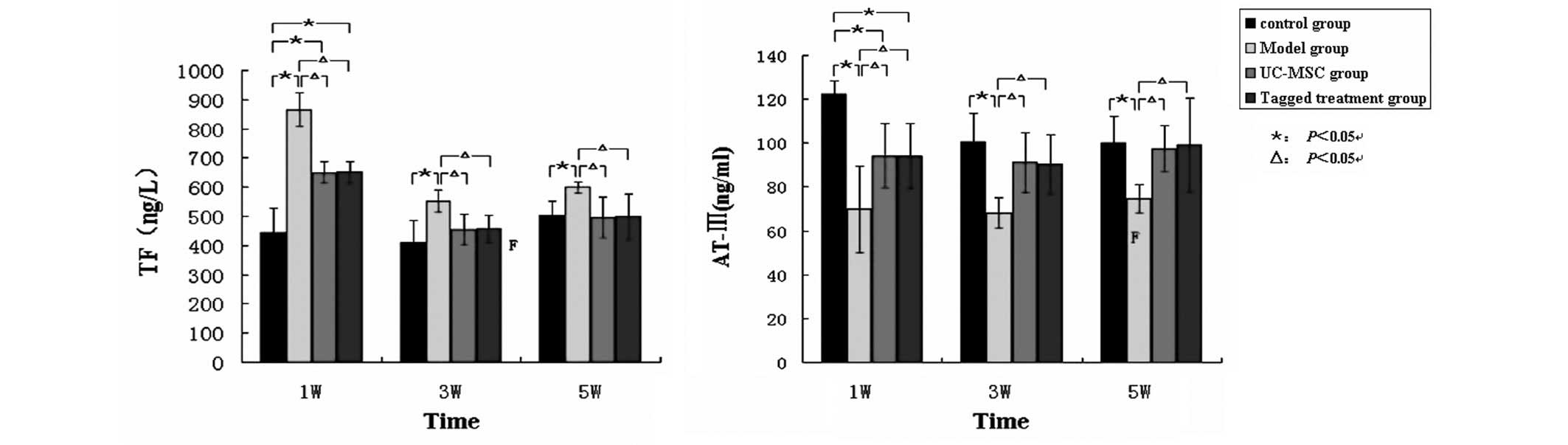
Fig. 5). The level of TF in group
M was significantly higher compared with that in the
hUC-MSC-treated groups at 1, 3 and 5 weeks post-treatment. By
contrast, the level of AT in group M was significantly lower
compared with that of the control group. The levels of AT in the
hUC-MSC-treated groups increased gradually following the treatment
throughout the duration of the experiment, and rose up to a level
similar to that in the control group at 5 weeks post-treatment
(Fig. 6).
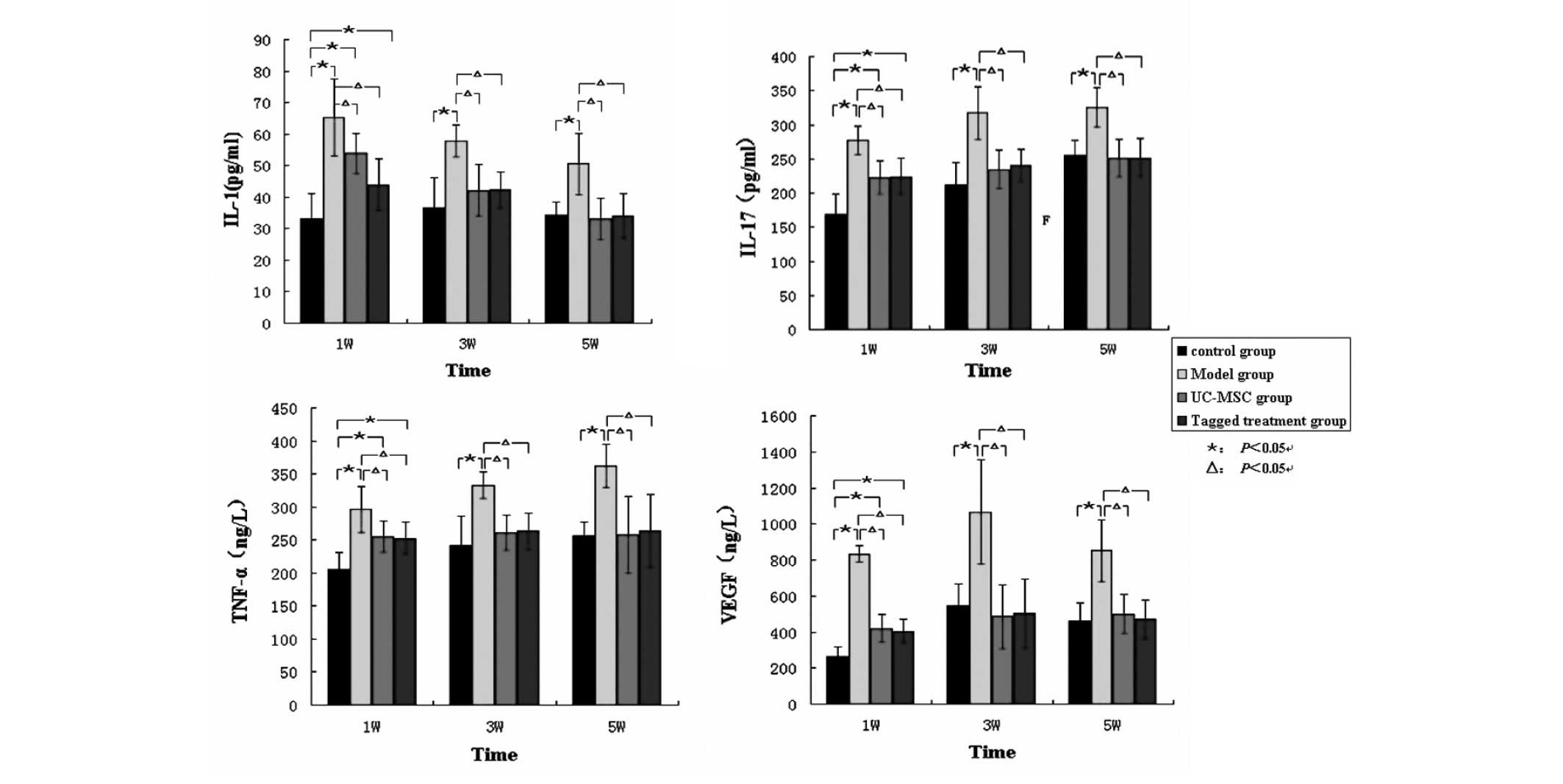 | Figure 5Levels of the serum inflammatory
cytokines in the rats throughout the entire duration of the
experiment. The levels of IL-1, IL-17, TNF-α and VEGF in group M
were significantly higher compared with those in the UC-MSC-treated
groups U and L, and the control group C. However, the levels of
these inflammatory cytokines were similar between the
hUC-MSC-treated groups and the control group.
*P<0.05, compared with the blank control group;
ΔP<0.05, compared with the hUC-MSC-treated groups.
IL, interleukin; TNF-α, tumor necrosis factor-α; VEGF. endothelial
cell growth factor; hUC-MSCs, human umbilical cord mesenchymal stem
cells; W, weeks. |
Discussion
The present study provided direct evidence that
hUC-MSCs injected into rat models of CIA migrated to the
inflammatory joints, and also effectively promoted the recovery
from CII damage, improving the immune inflammatory prothrombotic
status.
The CIA model, similar to RA, exhibits a chronic and
progressive disease course. Although there are different types of
models for RA, the CIA model is the most practical and has been
used extensively in research focusing on immunospecific RA. RA and
CIA are associated with the expression of certain major
histocompatibility class II genes. Furthermore, the CIA model
provides a convenient means to investigate autoantibodies,
including antibodies which are specific for the CII antigen and
rheumatoid factors in the blood. Immunohistopathological
investigations of the affected joints revealed a predominance of
macrophages/fibroblasts, with a marked infiltration of activated T
cells and granulocytes (1,12). In the present study, rat models of
CIA exhibited different degrees of hind limb congestion, edema,
movement disorder, and no obvious self-limiting remission during
the 5 weeks following the establishment of the model. An MRI
revealed a normal signal intensity and significantly thickened
synovia in the CIA group. The levels of IL-1, IL-17, TNF-α and VEGF
in the CIA group were markedly higher compared with those in the
control group at 1, 3 and 5 weeks post-treatment. These results
supported that the CIA model effectively mimicked an RA
manifestation, and therefore is a useful tool for investigating
potential therapeutic strategies against RA. The inflammatory
activity status and joint function of the CIA model rats appeared
to improve 1 week following hUC-MSC treatment, which indicated that
hUC-MSCs may be potentially useful for treating RA.
A lack of understanding of how stem cells exert
their therapeutic effects is a major obstacle in regenerative
medicine. At present, nanoparticles are used for various biomedical
applications in which they facilitate laboratory diagnostics and
therapeutics (13). By contrast
with conventional MRI, which is performed using extracellular
contrast agents, SPION-enhanced macrophage MRI, which is based on
the non-invasive, in vivo assessment of macrophage
infiltration in infected synovia, may accurately monitor the
progression of joint inflammation during treatment (14). SPIONs elicit marked T2 × WI
measurements in the scanned images, and T2 × WI relaxation effects
and a homogenous magnetic field lead to signal attenuation. The
extent of the reduction in the signal is usually associated with
the quantity of the labeled stem cells (15). In the present study, SPIONs
combined with 7.0T MRI were successfully used for tracking the
labeled hUC-MSCs. Furthermore, previous studies revealed that the
optimal concentration of the SPION-labeled stem cells was ~25
µg/ml, since a low concentration of Feridex was insufficient
for allowing efficient labeling, whereas a high concentration (44.8
µg Fe/ml) was toxic to the cells (16). Based on these results, a
concentration of Feridex (25 µg/ml) was used in the present
study, which was low in toxicity, while allowing for efficient
labeling. The trypan blue staining revealed that the viability of
the UC-MSCs in the SPION-labeled group was >90%, which was
sufficient for the treatment. Therefore, the use of MRI in
vivo to track the hUC-MSCs with SPION proved to be successful,
in view of the high sensitivity and specificity of the method in
the assessment of joint disorders.
Immunofluorescence analysis revealed that the
results of the BrdU-tagging experiment in vitro were
consistent with those of the in vivo MRI tracking analysis
of the hUC-MSCs. The fluorescence intensity of the inflammatory
hind limbs reached a maximum on day 7 following the injection of
the cells, which demonstrated that a large number of the UC-MSCs
had migrated to the hind limbs. Furthermore, the fluorescence
intensity was decreased over time, indicating that the accumulation
of the hUC-MSCs into the inflammatory joints was reduced. By
contrast, no immunofluorescence was detected in the liver, kidney,
lung, spleen or heart, demonstrating that these tissues failed to
exhibit any retention of the hUC-MSCs. A previous study confirmed
that Treg cells exert an important role in peripheral immune
tolerance and in the prevention of pathogenic autoimmunity in RA
(17). In the present study, the
quantity of the Treg cells in the CIA model group was lower
compared with that of the hUC-MSC-treated groups over the period of
5 weeks. These results suggested that treatment with hUC-MSCs may
lead to an increase in the number of Treg cells.
Previous studies suggested that IL-1 exerts an
important role in the processes of bone erosion and cartilage
destruction associated with RA, by regulating the expression of
other inflammatory mediators. IL-1-induced destruction of the
extracellular matrix accelerated RA joint destruction and promoted
coagulation by downregulating the expression of thrombomodulin and
the endothelial cell protein C receptor (18). In addition, IL-1 also promoted
fibrinolysis by increasing the production of the plasminogen
activator inhibitor and decreasing the production of tissue-type
plasminogen activator (19). TNF-α
is considered to regulate a wide range of biologically active
polypeptides involved in synovial inflammation reaction, and it
also has a direct effect on vascular endothelial cells.
Furthermore, the complete IL-17 cytokine profile includes the
variants IL-17A and B, the former making an essential contribution
towards the pathogenesis of RA, whereas the latter enhances the
effects of TNF-α on the production of cytokines and chemokines
(20). VEGF activates the
chemotaxis of monocytes and lymphocytes, and promotes the
proliferation of fibroblasts and synovial cells in addition to
releasing inflammatory mediators, thereby aggravating the severity
of synovitis. VEGF also increases the permeability of veins and
promotes the exudation of intra-vascular substances. Therefore,
VEGF is considered to be the most important of the vascular
permeability factors (21). Taken
together, the pathogenic inflammatory cytokines stimulate arthritic
processes, including the destruction of the synovium, cartilage and
bone, while also damaging the vascular endothelial cells and
promoting the synthesis of TF. Furthermore, these inflammatory
cytokines also trigger the coagulation pathway, promote blood
coagulation, and enhance the activity of the fibrinolytic system.
When plasminogen is degraded and AT is depleted, the body enters
into a hypercoagulable state (22). The present study revealed that
IL-1, IL-17, TNF-α, VEGF and TF were markedly increased, whereas AT
was reduced in the untreated rat model of CIA. Notably, all
inflammatory cytokines, and also TF and AT, gradually returned to
almost normal levels on day 35 after UC-MSC infusion. Therefore,
hUC-MSCs proved capable of reducing the adverse effects of the CIA
model rat immune disorders, in addition to reducing the release of
inflammatory cytokines and restoring the equilibrium of the
coagulation-fibrinolysis system. The possible mechanisms, which may
be involved are follows: i) hUC-MSCs regulate the immune function
of the CIA model rats by upregulating the Treg cells and
downregulating the expression of the Th17 cells and neutrophils,
subsequently restoring the balance of the immune cells by inducing
immunotolerance, promoting immune modulation and reducing the
release of inflammatory cytokines. ii) hUC-MSCs may migrate to the
sites of inflammation by local inflammatory signals during the
acute phase of inflammation and tissue damage. hUC-MSCs are
subsequently able to repair the damaged joints and immune
vasculitis by making cell-cell contacts (23). iii) hUC-MSCs may also produce a
variety of cytokines via the paracrine pathway to improve the local
microenvironment and to promote lesion recovery (24).
Taken together, the results of the present study
revealed that hUC-MSCs may migrate to the sites of inflammation in
the CIA rats and effectively improve the immune-associated
inflammatory and prothrombotic state.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 81270590) and the Health
Department of Jiangsu Province (no. H201048).
References
|
1
|
Lee DM and Weinblatt ME: Rheumatoid
arthritis. Lancet. 358:903–911. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choy E: Understanding the dynamics:
Pathways involved in the pathogenesis of rheumatoid arthritis.
Rheumatology (Oxford). 51(Suppl 5): v3–v11. 2012. View Article : Google Scholar
|
|
3
|
Rampersad RR, Tarrant TK, Vallanat CT,
Quintero-Matthews T, Weeks MF, Esserman DA, Clark J, Di Padova F,
Patel DD, Fong AM and Liu P: Enhanced Th17-cell responses render
CCR2-deficient mice more susceptible for autoimmune arthritis. PLoS
One. 6:e258332011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aksu K, Donmez A and Keser G:
Inflammation-induced thrombosis: Mechanisms, disease associations
and management. Curr Pharm Des. 18:1478–1493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boilard E, Nigrovic PA, Larabee K, Watts
GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E,
Farndale RW, Ware J and Lee DM: Platelets amplify inflammation in
arthritis via collagen-dependent microparticle production. Science.
327:580–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Snowden JA, Saccardi R, Allez M, Ardizzone
S, Arnold R, Cervera R, Denton C, Hawkey C, Labopin M, Mancardi G,
et al: Haematopoietic SCT in severe autoimmune diseases: Updated
guidelines of the European group for blood and marrow
transplantation. Bone Marrow Transplant. 47:770–790. 2012.
View Article : Google Scholar :
|
|
7
|
Liu R, Zhang Z, Lu Z, Borlongan C, Pan J,
Chen J, Qian L, Liu Z, Zhu L, Zhang J and Xu Y: Human umbilical
cord stem cells ameliorate experimental autoimmune
encephalomyelitis by regulating immunoinflammation and
remyelination. Stem Cells Dev. 22:1053–1062. 2013. View Article : Google Scholar
|
|
8
|
Chao KC, Chao KF, Fu YS and Liu SH:
Islet-like clusters derived from mesenchymal stem cells in
Wharton's Jelly of the human umbilical cord for transplantation to
control type 1 diabetes. PLoS One. 3:e14512008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van de Putte LB, Tyndall A, van den Hoogen
FH and Smolen JS: Hematopoietic stem cell transplants for
autoimmune disease: Role of EULAR. European League Against
Rheumatism. J Rheumatol Suppl. 48:98–99. 1997.PubMed/NCBI
|
|
10
|
Xie J, Liu G, Eden HS, Ai H and Chen X:
Surface-engineered magnetic nanoparticle platforms for cancer
imaging and therapy. Acc Chem Res. 44:883–892. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin CM, Gu J, Zhang Y, Shen LJ, Ma L, Ni
J, Wang ZQ and Wu W: Effect of UC-MSCs on inflammation and
thrombosis of the rats with collagen type II induced arthritis.
Zhonghua Xue Ye Xue Za Zhi. 33:215–219. 2012.In Chinese. PubMed/NCBI
|
|
12
|
Lange F, Bajtner E, Rintisch C, Nandakumar
KS, Sack U and Holmdahl R: Methotrexate ameliorates T cell
dependent autoimmune arthritis and encephalomyelitis but not
antibody induced or fibroblast induced arthritis. Ann Rheum Dis.
64:599–605. 2005. View Article : Google Scholar
|
|
13
|
Mahmoudi M, Sant S, Wang B, Laurent S and
Sen T: Superparamagnetic iron oxide nanoparticles (SPIONs):
Development, surface modification and applications in chemotherapy.
Adv Drug Deliv Rev. 63:24–46. 2011. View Article : Google Scholar
|
|
14
|
Lefevre S, Ruimy D, Jehl F, Neuville A,
Robert P, Sordet C, Ehlinger M, Dietemann JL and Bierry G: Septic
arthritis: Monitoring with USPIO enhanced macrophage MR imaging.
Radiology. 258:722–728. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu SL, Zhang JQ, Hu X, Hu R, Luo HS, Li F,
Xia YZ, Li JT, Lin JK, Zhu G and Feng H: In vitro labeling of human
umbilical cord mesenchymal stem cells with superparamagnetic iron
oxide nanoparticles. J Cell Biochem. 108:529–535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Janic B, Rad AM, Jordan EK, Iskander AS,
Ali MM, Varma NR, Frank JA and Arbab AS: Optimization and
validation of FePro cell labeling method. PLoS One. 4:e58732009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Almeida DE, Ling S, Pi X,
Hartmann-Scruggs AM, Pumpens P and Holoshitz J: Immune
dysregulation by the rheumatoid arthritis shared epitope. J
Immunol. 185:1927–1934. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Ye F, Xiong H, Hu DN, et al: IL-1β
induces IL-6 production in retinal Müller cells predominantly
through the activation of p38 MAPK/NF-κB signaling pathway. Exp
Cell Res. 331:223–231. 2015. View Article : Google Scholar
|
|
19
|
Joseph L, Fink LM and Hauer-Jensen M:
Cytokines in coagulation and thrombosis: A preclinical and clinical
review. Blood Coagul Fibrinolysis. 13:105–116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kouri VP, Olkkonen J, Ainola M, Li TF,
Björkman L, Konttinen YT and Mandelin J: Neutrophils produce
interleukin-17B in rheumatoid synovial tissue. Rheumatology
(Oxford). 53:39–47. 2014. View Article : Google Scholar
|
|
21
|
Noma H, Mimura T and Eguchi S: Association
of inflammatory factors with macular edema in branch retinal vein
occlusion. JAMA Ophthalmol. 131:160–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Undas A, Gissel M, Kwasny-Krochin B,
Gluszko P, Mann KG and Brummel-Ziedins KE: Thrombin generation in
rheumatoid arthritis: Dependence on plasma factor composition.
Thromb Haemost. 104:224–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun L, Akiyama K, Zhang H, Hou Y, Zhao S,
Xu T, Le A and Shi S: Mesenchymal stem cell transplantation
reverses multiorgan dysfunction in systemic lupus erythematosus
mice and humans. Stem Cells. 27:1421–1432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gu ZF, Akiyama K, Ma X, Zhang H, Feng X,
Yao G, Hou Y, Lu L, Gilkeson GS, Silver RM, et al: Transplantation
of umbilical cord mesenchymal stem cells alleviates lupus nephritis
in MRL/lpr mice. Lupus. 19:1502–1514. 2010. View Article : Google Scholar : PubMed/NCBI
|