Introduction
Diabetic retinopathy (DR), a vascular complication
of diabetes mellitus, is characterized by blood-retinal barrier
(BRB) breakage and subsequent retinal vascular permeability that
represents a clinical hallmark of early DR. The integrity of the
BRB is required for normal vision and its breakage greatly
contributes to the pathology of retinal diseases such as DR
(1). The important mechanisms of
BRB dysfunction are changes in the hyperpermeability
characteristics of retinal endothelial cells (ECs) that are caused
by changes in the internal environment of the retina; these changes
include elevated levels of advanced glycation end products (AGEs),
growth factors and hyperglycemia (2).
AGEs are thought to have a significant role in the
course of DR by inducing BRB dysfunction (3). Although the detailed role of AGEs in
retinal vascular permeability has remained elusive, previous
studies have suggested a central role for vascular endothelial
growth factor (VEGF) in this process (4,5).
Retinal VEGF expression was rapidly increased after a 4 h of
exposure to AGE-modified bovine serum albumin (AGE-BSA) delivered
via intravitreal (i.v.) injection (6). VEGF directly decreases the EC tight
junction (TJ) content or increases their phosphorylation (7), either or the two of these effects may
enhance paracellular permeability. In support of this, a previous
study by our group has demonstrated that methylglyoxal, a potent
precursor of AGEs, increased the expression of VEGF in retinal
tissues, which elicits retinal vascular hyperpermeability (8).
Certain medicinal herbs that have been widely used
for centuries for treating numerous human diseases. (4-hydroxy-3′,
5′-dimetoxy-1,1′-biphenyl)-3-O-β-d-glucoside (OSSC1E-K19) is a
novel biphenyl glycoside compound isolated from Osteomeles
schwerinae C. K. Schneid. (Rosaceae). Osteomeles
schwerinae (O. schwerinae) is a native plant of Asia and
has been used as a Chinese medicinal herb for the treatments of
numerous types of disease, including diarrhea, arthritis,
dysentery, laryngopharyngitis, folliculitis and neuralgia (9). A previous study by our group showed
that an ethanolic extract of this plant inhibited aldose reductase
activity (10). OSSC1E-K19 was
isolated from an ethyl acetate fraction of this plant using
bioactivity-guided isolation. The present study was performed to
investigate whether i.v. injection of OSSC1E-K19 was able to
protect against glycated albumin-induced retinal vascular injury.
In addition, the present study explored the underlying mechanisms
of OSSC1E-K19 in rat eyes which were i.v. injected with glycated
albumin.
Materials and methods
OSSC1E-K19 preparation
The leaves and twigs of O. schwerinae were
collected from Kunming, Yunnan Province, China, in April 2011, and
identified by Professor Joo Hwan Kim (Gachon University, Korea). A
voucher specimen (no. DiAB-141) was deposited in the Herbarium of
the Diabetic Complications Research Team, Korea Institute of
Oriental Medicine, Korea. Air-dried twigs and leaves of O.
schwerinae (4 kg) were extracted with 12 l ethanol (EtOH) three
times by maceration. The combined extracts were filtered and
concentrated using a vacuum evaporator, and 104.16 g of the EtOH
extract was obtained. The EtOH extract was suspended in 500 ml
H2O. This H2O suspension was fractionated by
liquid-liquid partitioning using n-hexane (500 ml), EtOAc (500 ml)
and n-BuOH (500 ml) to yield an n-hexane fraction of 6.30 g, an
EtOAc fraction of 27.0 g and an n-BuOH fraction of 10.08 g. All of
the factions were tested for AGE formation. The EtOAc fraction (27
g), which had the highest inhibitory effect on AGE formation, was
loaded into a column (7×57 cm) that was packed with 70–230 mesh
silica gel. Silica gel (Merck Millipore, Darmstadt, Germany)
chromatography was performed using a mobile phase of
CHCl3 and MeOH (gradient of 40:1-0:1, v/v); ten
fractions were obtained. The fifth fraction (700 mg) was loaded
onto a column (60x2.5 cm) packed with Sephadex LH-20 gel (GE
Healthcare Life Sciences, Chalfont, UK), and column chromatography
was performed using a mobile phase of MeOH and distilled
H2O (gradient of 1:1-1:0, v/v). A total of OSSC1E-K19
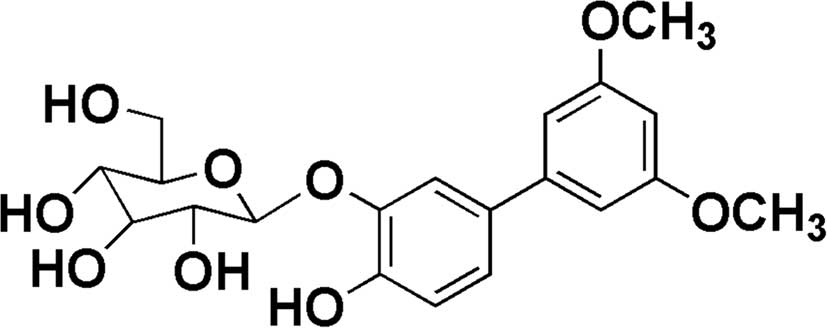
(10 mg; yield, 0.04%) was isolated. The structure of OSSC1E-K19 was
identified as
4-hydroxy-3′,5′-dimethoxy-(1,1′-biphenyl)-3-O-β-D-glucopyranoside
by comparing its nuclear magnetic resonance (NMR) and high
resolution electrospray ionization mass (HRESIMS) data with those
in the literature (11); 1H and
13C-NMR spectra were obtained using a Bruker Avance300
NMR spectrometer (Bruker AXS, Karlsruhe, Germany) with
tetramethylsilane as an internal standard, and HRESIMS was recorded
on a Shimadzu LCMS-IT-TOF spectrometer (Shimadzu Corporation,
Kyoto, Japan). The chemical structure of OSSC1E-K19 is presented in
Fig. 1.
Inhibitory activity on AGE formation
Ten milligrams per milliliter bovine serum albumin
(BSA; Roche Diagnostics, Basel, Switzerland) in 50 mM phosphate
buffer (pH 7.4) containing 0.01% sodium azide (to avoid microbial
contamination; cat. no. S-8032, Sigma-Aldrich, St. Louis, MO, USA)
was added to a 0.2 M glucose and fructose solution (Sigma-Aldrich);
this solution was added to the samples (1–150 µM).
Aminoguanidine (AG; cat. no. 396494; Sigma-Aldrich) was used as a
positive inhibitor. After incubation for 14 days at 37°C,
AGEs-specific fluorescence was determined using a
spectrofluorometer (Synergy HT; BIO-TEK, Winooski, VT, USA;
excitation at 370 nm and emission at 440 nm). The concentration
leading to 50% inhibition of AGE formation (IC50) was
determined.
Inhibitory activity on AGE cross-linking
with collagen
AGE-BSA (Wako Pure Chemical Industries, Ltd., Osaka,
Japan) was conjugated with horseradish peroxidase (HRP) using a
peroxidase labeling kit-NH2 (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). HRP-labeled AGE-BSA was incubated with or without
OSSC1E-K19 or AG in collagen-coated microtiter plates. HRP-labeled
AGE-BSA was incubated to react with collagen at 37°C for 4 h. After
washing with a solution of 0.05% Triton-X 100 in phosphate-buffered
saline (PBS), 100 µl 3,3′,5,5′-tetramethylbenzidine
chromogen (cat. no. T4444; Sigma-Aldrich) was added followed by
incubation for 15 min. The absorbance at 450 nm was determined
using an absorbance plate reader (Synergy HT) after the addition of
40 µl 1 N H2SO4.
Preparation of AGE-modified rat serum
albumin (RSA)
AGE-RSA was synthesized as previously described
(12). Non-modified RSA (fraction
V, low-endotoxin; Sigma-Aldrich) was used as the control. The
components of AGE-RSA were determined using a glucose-derived AGE
ELISA kit (Cosmo Bio, Tokyo, Japan). The modified levels of AGE-RSA
were nearly 200-fold higher than those in the non-modified control.
No endotoxins were detected using the E-Toxate kit (cat. no.
ET0200-1KT; Sigma-Aldrich).
Intravitreal injection of AGE-RSA
Injection of AGE-RSA was performed as previously
described (13). A total of 40
male eight week-old Sprague-Dawley rats (Orient Bio, Inc., Sungnam,
Korea) were used in the present study. Rats were housed four per
cage in a sterile humidified environment (50–60%) at 23±1°C, with a
12-h light/dark cycle. Food and water were provided ad
libitum throughout the experiment. The rats were divided into
five groups: 6 µg RSA-injected normal rats; rats injected
with 6 µg AGE-RSA i.v.; and rats injected with AGE-RSA i.v.
and treated with different concentrations of OSSC1E-K19 (50, 100 or
150 µM). For the negative control, 150 µM OSSC1E-K19
was injected. Three days after i.v. injection, the rats were
anesthetized and sacrificed with an overdose of zolazepam (10
mg/kg; Virbac, Carros, France). The present study was approved by
the Institutional Animal Care and Use Committee of the Korea
Institute of Oriental Medicine (protocol no. 12–079; Daejeon,
Korea).
Fluorescein-dextran microscopy
The rats were deeply anesthetized by intraperitoneal
injection of zolazepam (10 mg/kg; Virbac), after which PBS
containing fluoresceindextran (FD40S; Sigma-Aldrich) was injected
into the left ventricle of the heart. The retinal flat was mounted,
and the fluorescent images were captured using an Olympus BX51
microscope with a DP71 digital camera (Olympus, Tokyo, Japan).
Immunofluorescence staining
Retinal sections were de-waxed and re-hydrated by
sequential immersion. The slides were boiled in 10 mM sodium
citrate buffer (pH 6.0) at 121°C for 10 min. The slides were
incubated with a mouse anti-VEGF antibody (1:500; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 2 h. After washing, the
sections were labeled with fluorescein isothiocyanate-conjugated
donkey anti-mouse immunoglobulin G (1:3,000; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Finally, the
sections were mounted in fluorescent mounting medium (Dako North
America, Inc., Carpinteria, CA, USA) containing DAPI counterstain.
The sections were observed and images were captured by fluorescence
microscopy (BX51; Olympus, Tokyo, Japan).
Western blot analysis
Retinas were homogenized (Precellys 24; Bertin
Technologies, Saint-Quentin en Yveline, France) in solutions
containing 250 mM sucrose, 1 mM ethylenediaminetetraacetic acid,
0.1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich) and 20 mM
potassium phosphate buffer (Sigma-Aldrich; pH 7.6). Retinal protein
(20 µg) was resolved by 12% SDS-PAGE and transferred onto a
polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and the membranes were incubated with the
following primary monoclonal antibodies overnight at 4°C:
Antibodies against VEGF (1:1,000; cat. no. ab1316; Abcam, San
Francisco, CA, USA) and occludin (1:1,000; cat. no. 711500;
Invitrogen Life Technologies, Inc., Carlsbad, CA, USA) were used.
Monoclonal anti-β-actin antibody (1:3,000; cat. no. A1978) was
purchased from Sigma-Aldrich. Either goat anti-mouse IgG HRP (cat.
no. sc-2005) or anti-rabbit IgG HRP-conjugated antibody (cat. no.
sc-2004; 1:2,000; Santa Cruz Biotechnology, Inc.) was used as a
secondary antibody. Immunoreactive bands were visualized using an
enhanced chemiluminescence western blotting detection system
(Amersham Bioscience, Amersham, NJ, USA). Images were captured and
quantification was performed using a LAS-3000 (Fujifilm, Tokyo,
Japan).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical analysis was performed using one-way analysis
of variance followed by Tukey's multiple comparisons test or an
unpaired Student's t-test. GraphPad Prism 6.0 (GraphPad Inc., La
Jolla, CA, USA) was used for all statistical analyses. P<0.05
was considered to indicate a statistically significant difference
between values.
Results
Inhibitory effect of OSSC1E-K19 on AGEs
formation and its cross-linking with collagen in vitro
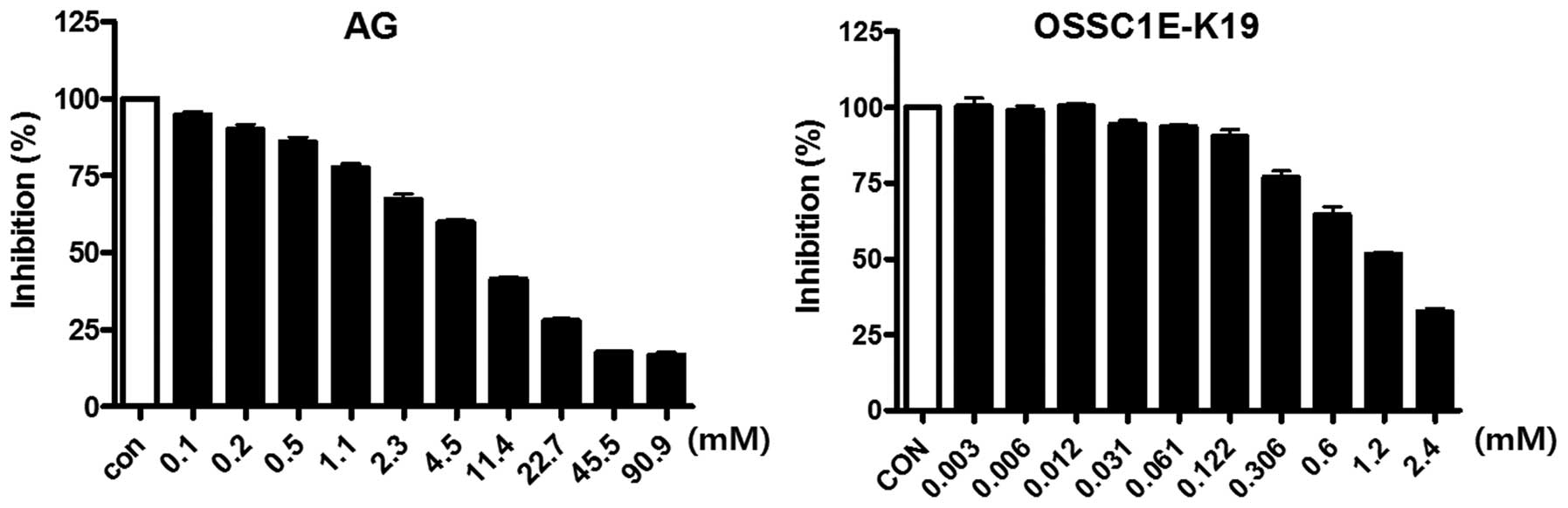
OSSC1E-K19 was examined for its ability to inhibit
AGE formation. As shown in Table
I, OSSC1E-K19 dose-dependently inhibited the formation of
AGE-BSA (IC50, 118.49±4.85 µM). The inhibitory
activity of OSSC1E-K19 was greater than that of AG
(IC50, 1,040.70±44.17 µM). In addition,
OSSC1E-K19 dose-dependently inhibited the cross-linking of AGE-BSA
with collagen, and the IC50 value of OSSC1E-K19 was
8.20±0.60 mM (Fig. 2). AG also
reduced cross-linking between AGE-BSA and collagen
(IC50, 1.39±0.13 mM).
 | Table IInhibitory effects of OSSC1E-K19 and
AG on AGE formation. |
Table I
Inhibitory effects of OSSC1E-K19 and
AG on AGE formation.
| Compound | Concentration
(µM) | Inhibition (%) | IC50
(µM) |
|---|
| OSSC1E-K19 | 24.5 | 25.53±1.16 | 118.49±4.85 |
| 49 | 37.67±2.40 | |
| 122.5 | 50.86±1.17 | |
| AG | 750 | 39.93±1.74 | 1040.70±44.17 |
| 1000 | 49.97±3.22 | |
| 1250 | 56.62±2.25 | |
Effects of OSSC1E-K19 on BRB breakdown in
AGE-RSA-injected rat eyes
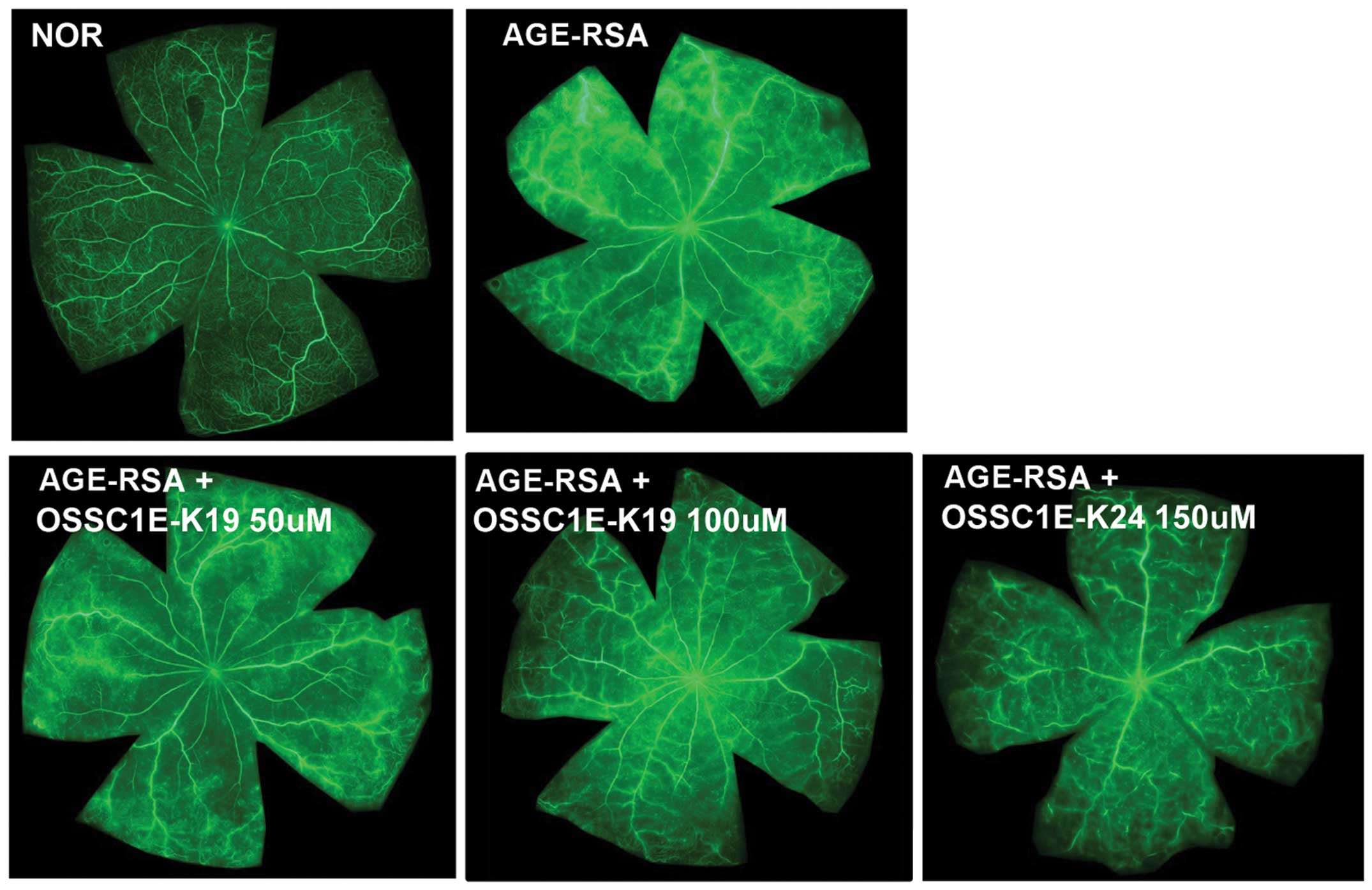
A major clinical hallmark of DR is enhanced vascular
leakage culminating in overt BRB breakage (14). To test the ability of OSSC1E-K19 to
inhibit AGE-induced vascular permeability, the present study
performed fluorescein angiography in rats that were injected with
AGE-RSA i.v.. As shown in Fig. 3,
the fluorescence intensity was enhanced, with dye leakage occurring
throughout the entire retina in the AGE-RSA-injected eyes; however,
these leaks were restricted to the vasculature in the
saline-injected normal rat eyes and in OSSC1E-K19-injected
eyes.
Effects of OSSC1E-K19 on retinal VEGF
expression in AGE-RSA-injected rat eyes
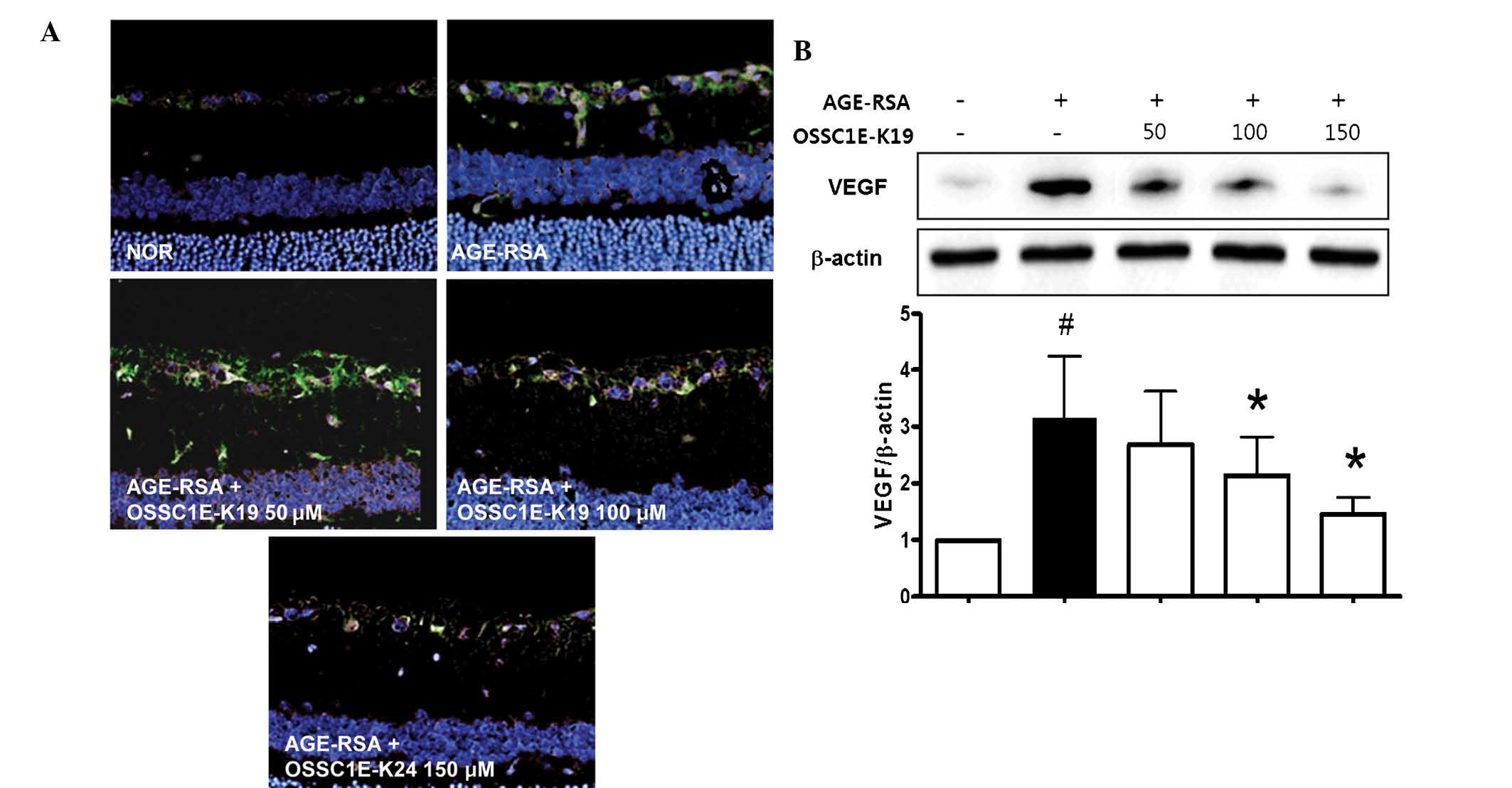
The inhibitory effects of OSSC1E-K19 on retinal VEGF
expression in AGE-RSA-injected eyes were assessed by determining
retinal VEGF expression using immunofluorescence staining and
western blot analysis. In AGE-RSA-injected eyes, immunofluorescence
staining for VEGF revealed that VEGF (green) was present in the
ganglion cell layer, while treatment with OSSC1E-K19 significantly
decreased retinal VEGF expression in AGE-RSA-injected eyes
(Fig. 4A). These
immunofluorescence staining results were confirmed by western blot
analysis. As shown in Fig. 4B,
AGE-RSA treatment led to a significant increase in VEGF expression
compared with that in the saline-injected normal group. However,
treatment with OSSC1E-K19 markedly inhibited the expression of VEGF
in AGE-RSA-injected eyes. Similarly, treatment with OSSC1E-K19
almost completely eliminated the increase in retinal vascular
permeability that was observed in AGE-RSA-injected eyes (Fig. 3). These results strongly suggested
that OSSC1E-K19 inhibits retinal vascular permeability via the
suppression of retinal VEGF expression.
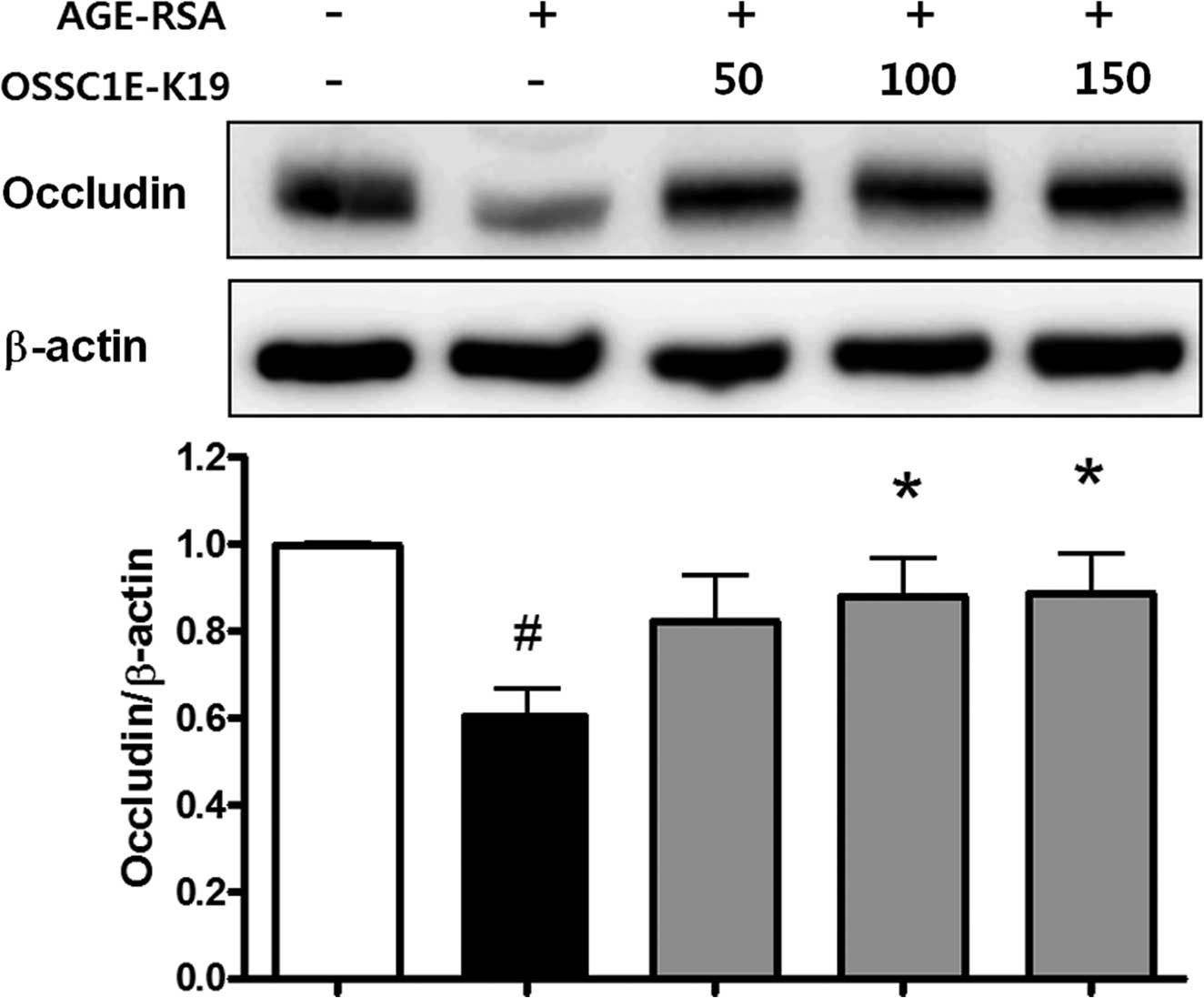
Effects of OSSC1E-K19 on TJ protein loss
in AGE-RSA-injected rat eyes
The loosening of TJs causes BRB breakdown (15). The present study evaluated the
expression of occludin, a well-known TJ protein. AGE-RSA-injected
eyes exhibited significantly lower levels of occludin than those of
saline-injected normal mice. However, the decreased occludin
expression in the AGE-RSA-injected eyes was restored by OSSC1E-K19
treatment (Fig. 5). These findings
suggested that OSSC1E-K19 blocks vascular leakage, possibly by
stabilizing junctional proteins.
Discussion
AGE-induced retinal damage is one of the most
important mechanisms involved in the pathophysiology of DR.
Numerous previous studies have demonstrated that hyperglycemia has
an important role in the pathophysiology of DR by increasing
protein glycation in ocular disease (16,17).
Cross-links between AGEs and long-lived proteins are formed when
glucose binds to target proteins, including collagen, in a process
that is nearly irreversible. Therefore, it is of great interest to
identify novel, therapeutically useful inhibitory agents against
AGE formation or AGE cross-links (18). Various synthetic and natural
products have been tested and proposed as AGE inhibitors (19,20).
AG, as the most well-known AGE inhibitor, attenuated diabetic
angiogenesis, apoptosis and cataract formation in diabetic ocular
disease (17,21). The present study demonstrated that
OSSC1E-K19 has an inhibitory effect on the formation of AGEs and
their cross-linking with collagen in vitro.
In order to evaluate the potential therapeutic
applicability of OSSC1E-K19 as an AGE inhibitor, the present study
evaluated the efficacy of OSSC1E-K19 against BRB breakage induced
by AGE-RSA in rats. AGE levels in the circulation are greatly
enhanced in patients with diabetes (22). Previous studies have proven that
i.v. injection of AGEs into the eyes of normal rats can induce a
diabetic-like vascular injury (23,24).
The administration of exogenous AGEs induces acute vascular leakage
in normoglycemic animals (24),
whereas the inhibition of endogenous AGEs prevents vascular
permeability in diabetic rats (25). Similarly, OSSC1E-K19, a potent AGE
inhibitor, inhibited vascular hyperpermeability in AGE-RSA-injected
eyes, as demonstrated in the present study.
A close correlation has been identified between AGE
formation and VEGF expression in the diabetic retina (4,5).
AGEs can increase the expression of VEGF in retinal pigment
epithelial cells, Müller cells and smooth muscle cells in
vitro (6). Of note, Lu et
al (6) demonstrated that VEGF
was upregulated in the inner nuclear layer and ganglial cells
following i.v. injection of AGE-BSA into rat retina. Consistent
with these previous studies, the present study showed that i.v.
injection of AGE-RSA stimulated the expression of VEGF in rat
retina. OSSC1E-K19 decreased retinal VEGF expression in the
AGE-RSA-injected eye. These results suggested that OSSC1E-K19 may
prevent AGE-induced BRB breakage by inhibiting VEGF-associated
signaling pathways.
VEGF promotes vascular permeability and alters TJs
in retinal diseases. VEGF is thought to have a role in diabetic
rats (7), and exposure of retinal
ECs and the retina to exogenous VEGF reduced TJ protein contents
and subsequently increased BRB breakage (26). AGE-BSA induces vascular leakage in
a time- and concentration-dependent manner due to suppression of
the expression of TJ proteins, including occludin and ZO-1
(27). TJs are composed of
transmembrane adhesive proteins and scaffolding proteins, as well
as a number of regulatory proteins that also localize to TJs.
Occludin is an important transmembrane protein in TJs that is
responsible for forming the permeability barrier (28). A number of studies have
demonstrated a reduced expression of occludin in retinal ECs in
diabetic animals (29,30). Occludin was originally predicted to
confer barrier properties to TJs (28) and gene deletion of occludin gave
rise to mice with an array of complex phenotypes but with normal TJ
formation and barrier properties in the gut epithelium (31). An inverse correlation between
endothelial permeability and TJ protein content has been indicated.
The present study observed that i.v. injection of OSSC1E-K19
resulted in a stabilization of the occludin content. These results
suggested that OSSC1E-K19 reduced retinal vascular permeability in
AGE-RSA-injected eyes via recovering occludin levels.
In conclusion, the present study demonstrated that
OSSC1E-K19 has an inhibitory effect on the formation of AGEs and
their cross-linking with proteins. In rats that were i.v. injected
with AGE-RSA, treatment with OSSC1E-K19 inhibited AGE-induced
vascular hyperpermeability and resulted in the concomitant
downregulation of VEGF and preservation of TJ integrity.
Acknowledgments
The present study was supported by a grant from the
Korea Institute of Oriental Medicine (KIOM) (no. K14040).
References
|
1
|
Cheung N, Mitchell P and Wong TY: Diabetic
retinopathy. Lancet. 376:124–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klaassen I, Van Noorden CJ and
Schlingemann RO: Molecular basis of the inner blood-retinal barrier
and its breakdown in diabetic macular edema and other pathological
conditions. Prog Retin Eye Res. 34:19–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leto G, Pricci F, Amadio L, Iacobini C,
Cordone S, Diaz-Horta O, Romeo G, Barsotti P, Rotella CM, di Mario
U and Pugliese G: Increased retinal endothelial cell monolayer
permeability induced by the diabetic milieu: Role of advanced
non-enzymatic glycation and polyol pathway activation. Diabetes
Metab Res Rev. 17:448–458. 2001. View
Article : Google Scholar
|
|
4
|
Segawa Y, Shirao Y, Yamagishi S, Higashide
T, Kobayashi M, Katsuno K, Iyobe A, Harada H, Sato F, Miyata H, et
al: Upregulation of retinal vascular endothelial growth factor
mRNAs in spontaneously diabetic rats without ophthalmoscopic
retinopathy. A possible participation of advanced glycation end
products in the development of the early phase of diabetic
retinopathy. Ophthalmic Res. 30:333–339. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murata T, Nagai R, Ishibashi T, Inomuta H,
Ikeda K and Horiuchi S: The relationship between accumulation of
advanced glycation end products and expression of vascular
endothelial growth factor in human diabetic retinas. Diabetologia.
40:764–769. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu M, Kuroki M, Amano S, Tolentino M,
Keough K, Kim I, Bucala R and Adamis AP: Advanced glycation end
products increase retinal vascular endothelial growth factor
expression. J Clin Invest. 101:1219–1224. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Antonetti DA, Barber AJ, Khin S, Lieth E,
Tarbell JM and Gardner TW: Vascular permeability in experimental
diabetes is associated with reduced endothelial occludin content:
Vascular endothelial growth factor decreases occludin in retinal
endothelial cells. Penn State Retina Research Group. Diabetes.
47:1953–1959. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim J, Lee YM, Kim CS, Sohn E, Jo K, Shin
SD and Kim JS: Ethyl pyruvate prevents methyglyoxal-induced retinal
vascular injury in rats. J Diabetes Res. 2013:4608202013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsieh CF and Chaw SM: Osteomeles
schwerinae C. K. Schneid. (Rosaceae): A new record for the flora of
Taiwan. Bot Bull Acad Sin. 37:281–285. 1996.
|
|
10
|
Lee J, Jang DS, Yoo NH, Lee YM, Kim JH and
Kim JS: Single-step separation of bioactive flavonol glucosides
from Osteomeles schwerinae by high-speed counter-current
chromatography. J Sep Sci. 33:582–586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JS, Kim J, Kim CS, Kim YS, Shon E,
Jung D, Lee YM, Jung SH and Lee YR: Phenyl derivatives or
pharmaceutically acceptable salts thereof, method for preparing
same, and composition comprising same as active ingredients for
preventing, improving or treating diseases related to vascular
endothelial cells or diabetes. Patents. 2013
|
|
12
|
Vlassara H, Striker LJ, Teichberg S, Fuh
H, Li YM and Steffes M: Advanced glycation end products induce
glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad
Sci USA. 91:11704–11708. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim J, Kim KM, Kim CS, Sohn E, Lee YM, Jo
K and Kim JS: Puerarin inhibits the retinal pericyte apoptosis
induced by advanced glycation end products in vitro and in vivo by
inhibiting NADPH oxidase-related oxidative stress. Free Radic Biol
Med. 53:357–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sander B, Larsen M, Engler C,
Lund-Andersen H and Parving HH: Early changes in diabetic
retinopathy: Capillary loss and blood-retina barrier permeability
in relation to metabolic control. Acta Ophthalmol (Copenh).
72:553–559. 1994. View Article : Google Scholar
|
|
15
|
Giebel SJ, Menicucci G, McGuire PG and Das
A: Matrix metal-loproteinases in early diabetic retinopathy and
their role in alteration of the blood-retinal barrier. Lab Invest.
85:597–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stitt AW: Advanced glycation: An important
pathological event in diabetic and age related ocular disease. Br J
Ophthalmol. 85:746–753. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim J, Kim CS, Sohn E, Lee YM, Jo K, Shin
SD and Kim JS: Aminoguanidine protects against apoptosis of retinal
ganglion cells in Zucker diabetic fatty rats. Eur Rev Med Pharmacol
Sci. 18:1573–1578. 2014.PubMed/NCBI
|
|
18
|
Thornalley PJ: Use of aminoguanidine
(Pimagedine) to prevent the formation of advanced glycation
endproducts. Arch Biochem Biophys. 419:31–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoon J, Lee H, Chang HB, Choi H, Kim YS,
Rho YK, Seong S, Choi DH, Park D and Ku B: DW1029 M, a novel
botanical drug candidate, inhibits advanced glycation end-product
formation, rat lens aldose reductase activity and TGF-β 1
signaling. Am J Physiol Renal Physiol. 306:F1161–F1170. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim J, Jeong IH, Kim CS, Lee YM, Kim JM
and Kim JS: Chlorogenic acid inhibits the formation of advanced
glycation end products and associated protein cross-linking. Arch
Pharm Res. 34:495–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan H, Guo Y, Zhang J, Ding Z, Ha W and
Harding JJ: Effect of carnosine, aminoguanidine and aspirin drops
on the prevention of cataracts in diabetic rats. Mol Vis.
14:2282–2291. 2008.
|
|
22
|
Makita Z, Vlassara H, Cerami A and Bucala
R: Immunochemical detection of advanced glycosylation end products
in vivo. J Biol Chem. 267:5133–5138. 1992.PubMed/NCBI
|
|
23
|
Stitt AW, Bhaduri T, McMullen CB, Gardiner
TA and Archer DB: Advanced glycation end products induce
blood-retinal barrier dysfunction in normoglycemic rats. Mol Cell
Biol Res Commun. 3:380–388. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vlassara H, Fuh H, Makita Z, Krungkrai S,
Cerami A and Bucala R: Exogenous advanced glycosylation end
products induce complex vascular dysfunction in normal animals: A
model for diabetic and aging complications. Proc Natl Acad Sci USA.
89:12043–12047. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wautier JL, Zoukourian C, Chappey O,
Wautier MP, Guillausseau PJ, Cao R, Hori O, Stern D and Schmidt AM:
Receptor-mediated endothelial cell dysfunction in diabetic
vasculopathy. Soluble receptor for advanced glycation end products
blocks hyperpermeability in diabetic rats. J Clin Invest.
97:238–243. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu HZ and Le YZ: Significance of outer
blood-retina barrier breakdown in diabetes and ischemia. Invest
Ophthalmol Vis Sci. 52:2160–2164. 2011. View Article : Google Scholar :
|
|
27
|
Sheikpranbabu S, Kalishwaralal K, Lee KJ,
Vaidyanathan R, Eom SH and Gurunathan S: The inhibition of advanced
glycation end-products-induced retinal vascular permeability by
silver nanoparticles. Biomaterials. 31:2260–2271. 2010. View Article : Google Scholar
|
|
28
|
Furuse M, Hirase T, Itoh M, Nagafuchi A,
Yonemura S and Tsukita S and Tsukita S: Occludin: A novel integral
membrane protein localizing at tight junctions. J Cell Biol.
123:1777–1788. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bucolo C, Ward KW, Mazzon E, Cuzzocrea S
and Drago F: Protective effects of a coumarin derivative in
diabetic rats. Invest Ophthalmol Vis Sci. 50:3846–3852. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim J, Kim CS, Lee IS, Lee YM, Sohn E, Jo
K, Kim JH and Kim JS: Extract of Litsea japonica ameliorates
blood-retinal barrier breakdown in db/db mice. Endocrine.
46:462–469. 2014. View Article : Google Scholar
|
|
31
|
Schulzke JD, Gitter AH, Mankertz J,
Spiegel S, Seidler U, Amasheh S, Saitou M, Tsukita S and Fromm M:
Epithelial transport and barrier function in occludin-deficient
mice. Biochim Biophys Acta. 1669:34–42. 2005. View Article : Google Scholar : PubMed/NCBI
|