Introduction
Glioblastoma is the most common type of malignant
tumor in the human central nervous system, and the median survival
of patients with glioblastoma is <1 year (1). There are numerous therapeutic options
for the treatment of glioblastoma, including surgery, radiation
therapy and chemotherapy; however, the overall curative effect is
poor (2,3). Therefore, it is critical to explore
the molecular mechanisms of glioblastoma, in order to produce more
optimized and effective treatment strategies (4). Glioblastoma tumors are rich in blood
vessels (5), and vascular
endothelial growth factor (VEGF) has previously been reported to be
overexpressed in glioblastomas (6). In recent years, targeting
angiogenesis has been considered as a novel direction for cancer
treatment. Therefore, research is currently focused on therapies
that target VEGF. Monoclonal antibodies against VEGF and its
receptor (7), ribozymes (8), VEGF antisense therapy (9) and vascular endothelial growth
inhibitors (10), have all been
used to treat glioblastoma, in order to inhibit angiogenesis.
MicroRNAs (miRNAs) are a class of short non-coding
RNAs that control the expression of numerous genes (11). A previous study demonstrated that
miRNAs are involved in tumor initiation and progression, and may
function as tumor suppressors or oncogenes (12). miRNAs have an important role in the
initiation and progression of glioblastoma (13), and regulate the proliferation,
invasion and apoptosis of glioblastoma cells (14,15).
A previous study reported that the expression of miR-566, alongside
four other miRNAs (miR-181d, miR-518b, miR-524-5p and miR-1227), is
correlated with the prognosis of glioblastoma (16). Therefore, it has been suggested
that investigation of miRNAs involved in glioblastoma may aid in
the development of novel treatment strategies. In recent years,
numerous studies regarding miRNAs have been conducted, and miRNAs
have been identified as exhibiting an important role in the
initiation and progression of cancer, and thus have been suggested
as a target for the treatment of glioblastoma (17,18–20).
VHL functions as a tumor suppressor gene and is
located on chromosome 3p25~26, where it encodes a 213 amino acid
protein, known as pVHL. Mutations in VHL have been shown to
contribute to tumorigenesis (21),
and VHL is also involved in the initiation and progression of
glioma (22). Hypoxia-inducible
factor (HIF)-1α is a distinguished target of the E3 ligase activity
of VHL, which targets HIF-1α for degradation (23). In addition, the expression of VEGF
is regulated by HIF-1α, and VEGF is considered the most important
vascular growth factor in the initiation and progression of glioma
(24).
At present, the function of miR-566 in glioma
remains to be elucidated, and further research is required
regarding the molecular mechanism underlying the pathogenesis of
glioma. In the present study, VHL was identified as a functional
target of miR-566, and was shown to regulate the expression of VEGF
by targeting HIF-1α for degradation. In addition, miR-566 was
demonstrated to modulate VEGF by targeting VHL in vitro and
vivo, and the inhibition of miR-566 expression was able to
inhibit the invasion and migration of glioblastoma.
Materials and methods
Cell culture and reagents
The U87 human glioblastoma cell line was obtained
from the Basic Medical Institute, Chinese Academy Of Medical
Sciences (Beijing, China). The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Hyclone, Logan, UT, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Hyclone) at 37°C in an atmosphere containing 5% CO2.
Lentiviral infection and gene
transfection
Lentiviruses including an miR-566 inhibitor segment
(lenti-AS-566) and a negative control segment (lenti-NC) were
obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China). The
U87 glioblastoma cells were infected with lenti-AS-566 or lenti-NC,
or transfected with a VHL negative control (VHL-NC), which was
provided by Professor Jinquan Cheng (H. Lee Moffitt Cancer Center
and Research Institute, Tampa, FL, USA) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. The U87 cells were seeded
in a 6-well plate at 40% confluence with 4.8×105
cells/well. After 24 h, cells were transfected in serum-free medium
(250 µl) with 5 µl virus or 5 µl plasmids
using 5 µl Lipofectamine 2000. After 4–6 h, medium was
replaced with DMEM containing 10% FBS, and cells were incubated at
37°C in 5% CO2.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from the cells using TRIzol
(Invitrogen) 48 h post-infection/transfection. RT-qPCR was used to
detect the expression levels of miR-566 and VEGF mRNA. A one-step
RT-PCR kit (Takara Bio, Inc., Otsu, Japan) was used according to
the manufacturer's protocol. RT-PCR of miRNA-566 was performed with
a 200 ng sample on a thermocycler (PTC-200; Bio-Rad, Hercules, CA,
USA), the reaction conditions were as follows: 16°C for 30 min;
42°C for 30 min; 85°C for 10 min. qPCR of miRNA-566 was performed
with 2 µl miRNA RT product on thermocycler (PTC-200;
Bio-Rad), the reaction conditions were as follows: 95°C for 3 min,
95°C for 12 sec and 62°C for 30 sec, for 40 cycles. RT-PCR of VEGF
was performed with 5 µg sample reaction conditions were as
follows: 37°C for 5 min, 42°C for 60 min, and 70°C for 10 min. qPCR
of VEGF was performed with 5 µl VEGF RT product, reaction
conditions were as follows: 93°C for 1 min, 55°C for 1 min, and
72°C for 1 min, for 40 cycles). The following primers were used and
were obtained from GenePharma Co., Ltd.: Forward:
5′-GGGCGCCUGUGAUCCCAAC-3′ and reverse: 5′-UUCGCAGACGACGGGGUCG-5′
for miRNA-566; and forward: 5′-ATCTTCAAGCCATCCTGTGTGC-3′ and
reverse: 5′-CTTTTAGGGACACCCGGAAC-3′ for VEGF. The expression levels
of mature miR-566 and VEGF were quantified using the
ΔΔCq method. Glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) served as the internal control, and the relative abundance
of miRNA was normalized to U6 small nuclear RNA.
Protein extraction, western blot analysis
and luciferase reporter assay
Protein samples were extracted using
radioimmunoprecipitation assay buffer (Pierce Biotechnology, Inc.,
Rockford, IL, USA) and the protease inhibitor phenylmethylsulfonyl
fluoride (Sigma-Aldrich, St. Louis, MO, USA) 24 h
post-infection/transfection. The protein from each sample was
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (5% spacer gel and 10% separation gel; Bio-Rad) and
transferred to polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were then washed three times
with Tris-buffered saline containing Tween (Amresco LLC, Solon, OH,
USA), incubated in blocking buffer (Tiangen Biotech, Co., Ltd.,
Beijing, China) for 1 h at 37°C, and incubated with primary
antibodies and homologous secondary antibodies. The antibodies used
were as follows: Mouse anti-GAPDH monoclonal antibody (1:1,000;
TA-08; ZSGB-BIO, Beijing, China); rabbit anti-human VHL polyclonal
antibody (1:100; ab135576; Abcam, Cambridge, UK); rabbit anti-human
polyclonal HIF-1α antibody (1:1,000; 21691-1; SAB, College Park,
MD, USA); rabbit anti-human VEGF polyclonal antibody (1:1,000;
sc-152; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA); mouse
anti-human matrix metalloproteinase (MMP)-2 polyclonal antibody,
(1:1,000; sc-53630; Santa Cruz Biotechnology Inc.); and mouse
anti-human MMP-9 polyclonal antibody (1:1,000; sc-13520; Santa Cruz
Biotechnology Inc.). Horseradish peroxidase-labeled goat
anti-rabbit (ZDR-5306, 1:1,000, ZSGB-BIO) or goat anti-mouse
(ZDR-5307, 1:1,000, ZSGB-BIO) were used as secondary antibodies.
The blots were visualized using enhanced chemiluminescence (W1001,
Promega Corporation, Fitchburg, WI, USA) and a G:BOX F3 system
(Syngene, Cambridge, UK). The bands were quantified using ImageJ
software (1.48V, National Institutes of Health, Bethesda, MD, USA).
For the luciferase reporter assay, the cells were cultured in
96-well plates (10,000 cells/well), infected with 5 µl
lenti-AS-566 or lenti-NC, and incubated with 2 µl
Lipofectamine 2000 and 0.2 µg pVHL luciferase reporter
vectors. Following a 48 h incubation, luciferase activity was
measured using a Dual-Luciferase Reporter system (Promega
Corporation, Madison, WI, USA). Experiments were repeated at least
three times.
Intracranial model
Female nude mice (age, 4–6 weeks; weight, 15–18 g;
n=10/group) were bred at the Institute of Hematology (Tianjin,
China). Mice were maintained in individually ventilated cages under
a 12 h light/dark cycle, at 20–22°C and 40–60% humidity with free
access to food and water. The mice were intracranially implanted
with U87 cells infected with either lenti-AS-566 or lenti-NC under
the direction of a stereotaxic apparatus (25). Briefly, mice were anesthetized by
intraperitoneal injection of 10% chloral hydrate (MB1699, Meilunbio
Company, Dalian, China). The mice were fixed on the brain
stereotactic apparatus, and an incision was made to expose the
brain, 5×105/3 µl cells were injected and the
skin was sutured. A total of 14 days post-cell implantation, the
mice were sacrificed by CO2, and the brain tissue of the
mice was collected, embedded in paraffin and cut into slices. All
animal experiments were approved by the Institutional Animal Care
and Use Committee (Tianjin Medical University Cancer Institute
& Hospital, Tianjin, China).
Fluorescence in situ hybridization and
immunohistochemistry
The brain sections were routinely dewaxed and fixed
in paraformaldehyde (Shanghai Bluestar New Chemical Materials Co.,
Ltd., Shanghai, China), and then digested with pepsin (Amresco
LLC). The samples were subsequently incubated in prehybridization
solution (Amresco LLC) for 4 h at 38°C. Hybridization was performed
in miR-566 hybridization probe liquid overnight at 38°C. The
samples were washed fully using sodium citrate-hydrochloric acid
buffer solution (Leagene, Beijing, China), and incubated with mouse
anti-digoxin antibody (Jackson ImmunoResearch Laboratories, Inc.,
West Grove, PA, USA) at 38°C for 2 h, and Cy3 fluorescent antibody
(Wuhan Boster Biological Technology, Ltd., Wuhan, China) at 38°C
for 30 min. The results were observed under a FV-1000 confocal
laser-scanning microscope (Olympus Corporation, Tokyo, Japan).
Tissue sections were routinely deparaffinized and
antigen retrieval was performed in 1 mM EDTA buffer (pH 8.0) for 10
min at 95–100°C. The sections were rinsed three times in
phosphate-buffered saline (PBS) and then blocked in PBS containing
3% normal horse serum for 30 min. Subsequently, the sections were
incubated with anti-VHL, -VEGF, -MMP-2 and -MMP-9 primary
antibodies overnight (dilution, 1:100) at room temperature, rinsed
in PBS and incubated with a goat anti-rabbit (sc-2040, 1:100, Santa
Cruz Biotechnology, Inc.) or goat anti-mouse (sc-2039, 1;100, Santa
Cruz Biotechnology, Inc.) biotinylated secondary antibody at 37°C
for 40 min and ABC-peroxidase reagents (Santa Cruz Biotechnology,
Inc.) at 37°C for 1 h. Immunohistochemical staining was performed
using a DAB horseradish peroxidase color development kit (Beyotime
Institute of Biotechnology, Shanghai, China) and evaluated under a
CX21BIM-SET6 light microscope (Olympus Corporation).
Statistical analysis
Data were statistically analyzed by
χ2-test and one way analysis of variance, using SPSS
11.5 software (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
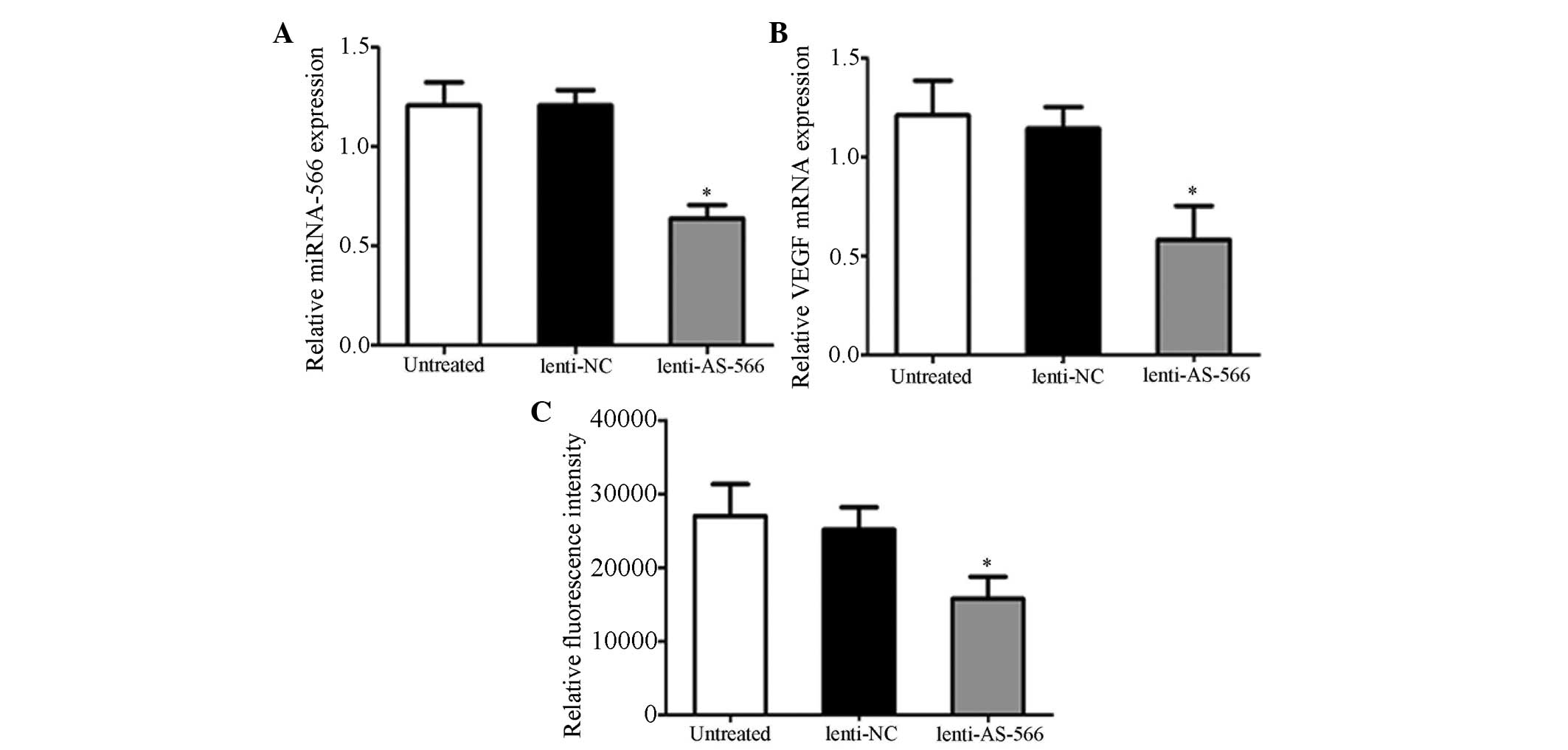
Downregulation of miR-566 inhibits VEGF
and miR-566 targets VHL
To explore the role of miR-566 in glioblastoma, the
expression levels of miR-566 (Fig.
1A) and VEGF mRNA (Fig. 1B)
were evaluated by RT-qPCR in U87 cells post-lenti-AS-566 infection.
The expression levels of miR-566 and VEGF mRNA were significantly
decreased in the lenti-AS-566-infected cells, as compared with the
untreated and lenti-NC-infected cells. These results indicate that
lenti-AS-566 is able to effectively inhibit the expression of
miR-566, and the mRNA expression of VEGF was down-regulated when
the expression of miR-566 was suppressed. Using bioinformatics
(www.targetscan.org) the VHL gene was
predicted to be regulated by miR-566. To determine whether the
prediction was correct, U87 cells were infected with lenti-AS-566,
and transfected with VHL luciferase reporter vectors; the
luciferase activity was measured using a dual-luciferase reporter
system. The luciferase activity was significantly reduced in the
U87 cells infected with lenti-AS-566, as compared with the
untreated cells or those infected with lenti-NC (Fig. 1C). These results indicate that the
VHL gene is a direct target of miR-566.
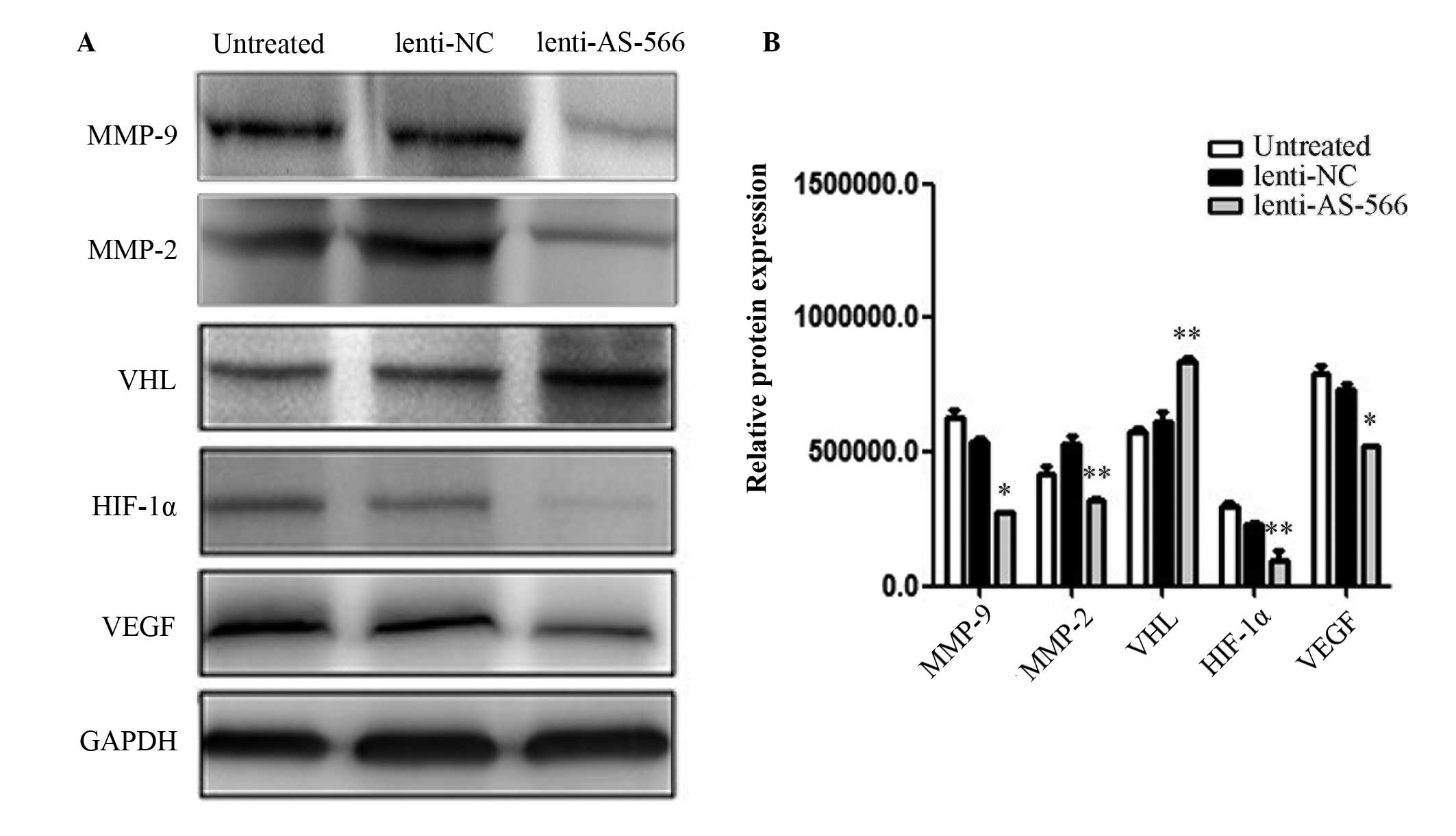
miR-566 regulates VEGF through the
VHL/HIF1-α pathway in vitro
Western blot analysis was conducted to detect
relative protein expression levels in the lenti-AS-566-infected U87
cells (Fig. 2A), and the results
were quantitatively analyzed (Fig.
2B). The protein expression levels of VHL were increased in the
U87 cells infected with lenti-AS-566, as compared with the
untreated cells or those infected with lenti-NC. In addition, the
protein expression levels of HIF-1α, MMP-2, MMP-9 and VEGF were
decreased in the U87 cells infected with lenti-AS-566, as compared
with the untreated cells or those infected with lenti-NC.
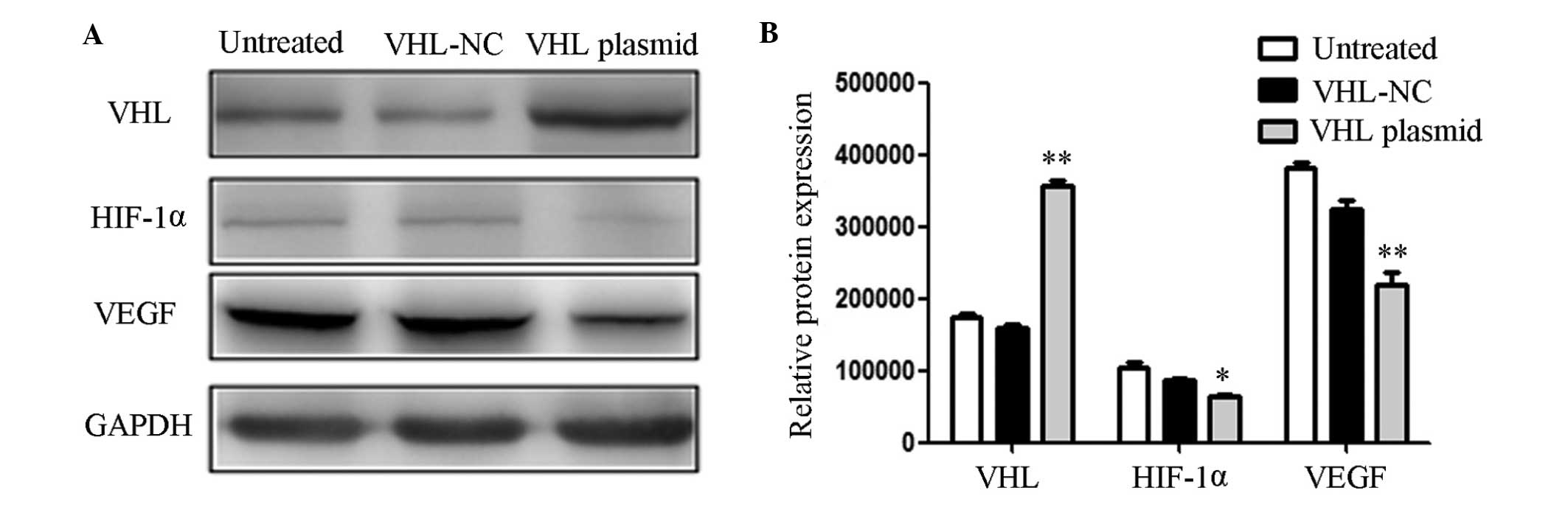
Upregulation of VHL inhibits the
expression of VEGF
The relative protein expression levels were also
measured following transfection of the U87 cells with a VHL
expression plasmid (Fig. 3A), and
the results were quantitatively analyzed (Fig. 3B). The protein expression levels of
VHL were increased in the U87 cells transfected with the VHL
expression plasmid, as compared with the untreated cells and those
transfected with the VHL negative control (VHL-NC). The protein
expression levels of HIF-1α and VEGF were decreased in the U87
cells transfected with the VHL expression plasmid, as compared with
the untreated cells and those transfected with VHL-NC. MMPs are
associated with the invasion and migration of cells, and an
increase in MMP-2 and MMP-9 is able to promote the invasion and
migration of glioblastoma (26).
In the present study, it was suggested that VHL was able to
regulate the expression of VEGF via HIF-1α, and VHL was regulated
by miR-566. Therefore, the inhibition of miR-566 may decrease the
invasive and migratory abilities of the cells. These results
indicate that miR-566 may modulate VEGF via targeting VHL in
vitro.
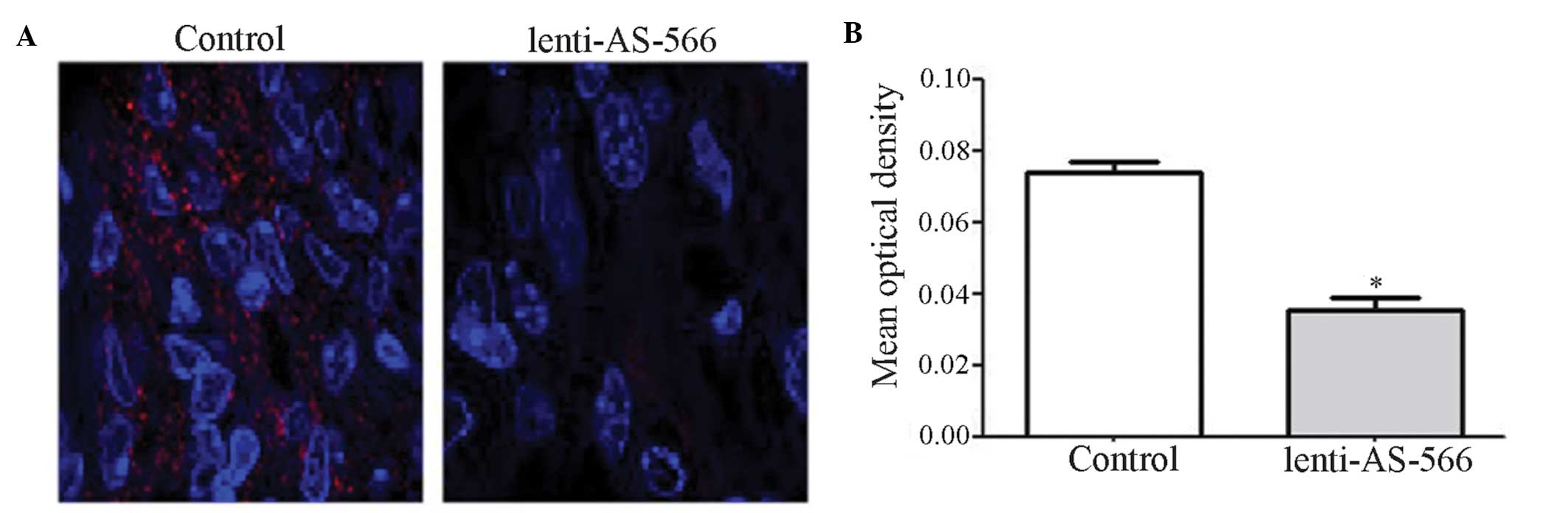
miR-566 is downregulated in vivo
In order to further demonstrate that miR-566 was
able to modulate VEGF by targeting VHL, an intracranial murine
model was generated and brain tissue sections were obtained. The
expression levels of miR-566 were measured in the tissue sections
using fluorescence in situ hybridization (Fig. 4A), and the results were
quantitatively analyzed (Fig. 4B).
The expression levels of miR-566 were significantly decreased in
the lenti-AS-566 group, as compared with the control group. These
results indicate that lenti-AS-566 functioned effectively in the
intracranial model, and was able to inhibit the expression of
miR-566.
miR-566 modulates VEGF via targeting VHL
in vivo
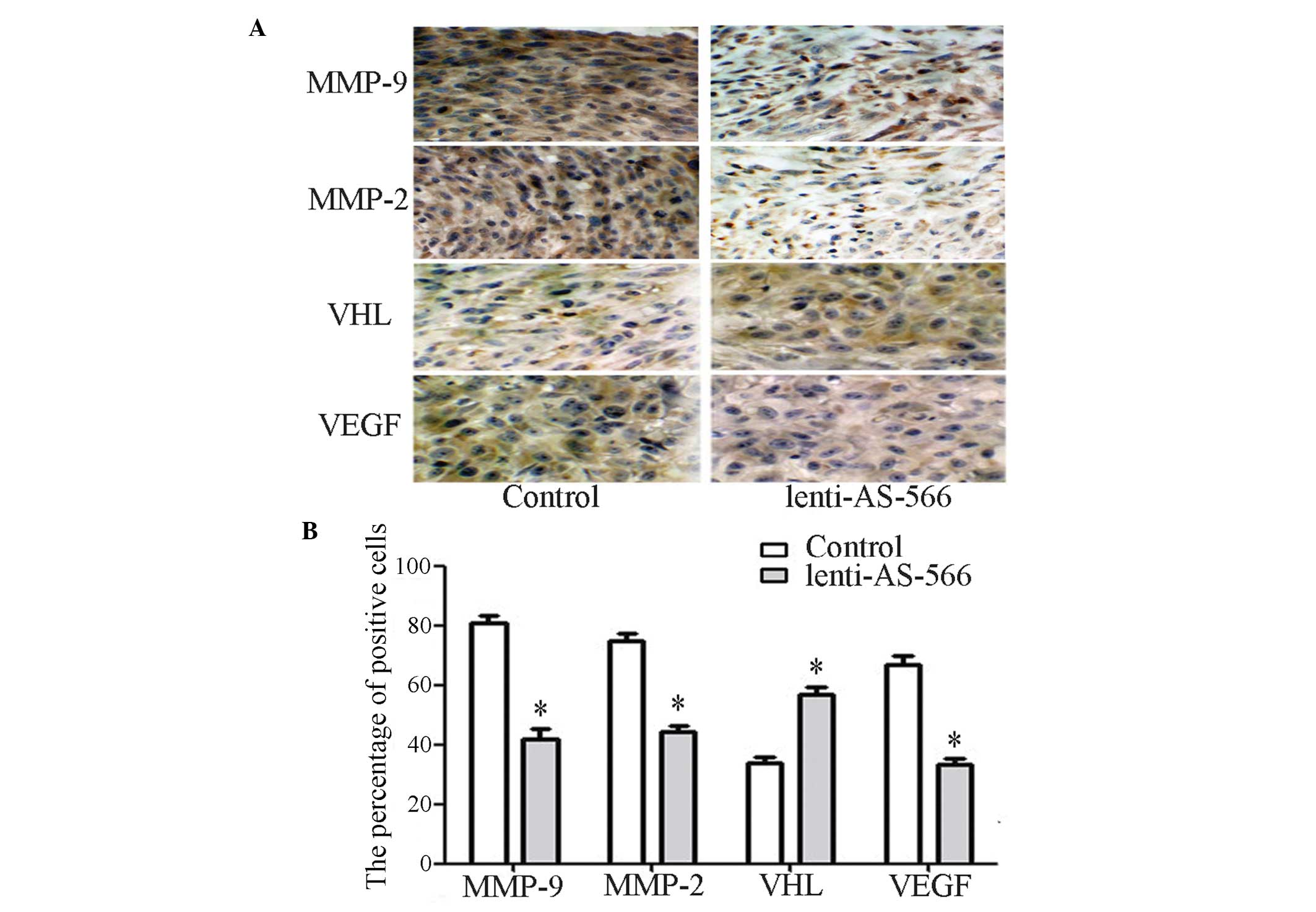
The expression levels of VHL and VEGF were detected
in the brain tissue sections using immunohistochemistry (Fig. 5A), and the results were
quantitatively analyzed (Fig. 5B).
The expression levels of VHL in the tissue sections of the
lenti-AS-566 group were increased, as compared with the control
group. In addition, the expression levels of VEGF, MMP-2 and MMP-9
were decreased, as compared with the control group. These results
indicate that VHL may regulate the expression of VEGF, and VHL is
regulated by miR-566. Furthermore, the invasive and migratory
abilities of the cells may be suppressed following the inhibition
of miR-566, suggesting that miR-566 is able to modulate VEGF by
targeting VHL in vivo.
Discussion
In recent years, numerous studies regarding miRNAs
have been conducted, and miRNAs have been identified as having an
important role in the initiation and progression of cancer, and
have been suggested as a target for the treatment of glioblastoma.
Kefas et al (10)
transfected glioma cells with a miR-7 plasmid, which inhibited the
activity of the epidermal growth factor receptor (EGFR)
3′-untranslated region (3′-UTR) by 83% and effectively reduced the
invasive ability of the cells. Gabriely et al (27) specifically inhibited miR-21 in
glioma, which reduced the invasiveness and malignancy of the cancer
cells. In addition, Zhang et al (16) demonstrated that miR-566 was
overexpressed in glioma cell lines, and inhibition of miR-566 was
able to suppress the proliferative and invasive behavior of glioma
cells via the EGFR/Akt pathway. Thus, miR-566 may function as an
oncogene. The present study aimed to determine the role of miR-566
in glioblastoma, and to investigate the association between
miR-566, VHL and VEGF.
The VHL gene is known to act as a tumor suppressor,
and is also the causal gene of VHL disease (28). Furthermore, VHL is involved in the
initiation and progression of numerous types of human cancer,
particularly clear-cell renal cell carcinoma (29,30).
Chen et al transfected renal carcinoma cells that did not
express pVHL with the normal VHL gene, and detected a marked
inhibition in the growth of the tumor cells (31). In addition, a previous study
demonstrated that VHL is able to target HIF-1α for degradation by
ubiquitin E3 ligase (23). It is
well known that hypoxia is an important factor in the induction of
VEGF, and under hypoxic conditions VEGF gene expression is
upregulated due to induction of the HIF-1α pathway (32). In the present study, U87 cells were
infected with lenti-AS-566 and then incubated with VHL luciferase
reporter vectors; VHL was identified as a direct target of miR-566.
miR-566 may bind to the complimentary binding sites in the 3′-UTR
of VHL (16). In addition,
following inhibition of miR-566 by lenti-AS-566, western blotting
revealed that VHL was upregulated, and HIF-1α, MMP-2, MMP-9 and
VEGF were downregulated. Similar results were also obtained when
the cells were transfected with a VHL expression plasmid. These
results indicated that miR-566 was able to modulate VEGF by
targeting VHL in vitro. In order to further demonstrate this
regulatory relationship, an intracranial murine model was
generated, and immunohistochemical staining demonstrated that VHL
was upregulated, and MMP-2, MMP-9 and VEGF were downregulated when
miR-566 was inhibited. These results suggested that miR-566 was
able to modulate VEGF by targeting VHL in vivo, and the
downregulation of MMP-2 and MMP-9 indicated that the invasive and
migratory abilities of the cells were inhibited.
In conclusion, the present study demonstrated that
miR-566 was able to modulate VEGF by targeting VHL in vitro
and in vivo. In addition, the inhibition of miR-566 may
suppress the invasion and migration of glioma; therefore, miR-566
may be considered a novel therapeutic target of glioblastoma.
However, the mechanism underlying the effects of miR-566 in
glioblastoma remains to be elucidated, and more in-depth research
is required.
Acknowledgments
The present study was supported by the China
National Natural Scientific Fund (grant no. 81101916).
References
|
1
|
Krex D, Klink B, Hartmann C, von Deimling
A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger
G, Weller M and Schackert G; German Glioma Network: Long-term
survival with glioblastoma multiforme. Brain. 130:2596–2606. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trial Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gabayan AJ, Green SB, Sanan A, Jenrette J,
Schultz C, Papagikos M, Tatter SP, Patel A, Amin P, Lustig R, et
al: GliaSite brachytherapy for treatment of recurrent malignant
gliomas: A retrospective multi-institutional analysis.
Neurosurgery. 58:701–709. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giunti L, Pantaleo M, Sardi I, Provenzano
A, Magi A, Cardellicchio S, Castiglione F, Tattini L, Novara F,
Buccoliero AM, et al: Genome-wide copy number analysis in pediatric
glioblastoma multiforme. Am J Cancer Res. 4:293–303.
2014.PubMed/NCBI
|
|
5
|
Kirsch M, Schackert G and Black PM:
Angiogenesis, metastasis, and endogenous inhibition. J Neurooncol.
50:173–180. 2000. View Article : Google Scholar
|
|
6
|
Carroll RS, Zhang J, Bello L, Melnick MB,
Maruyama T and McL Black P: KDR activation in astrocytic neoplasms.
Cancer. 86:1335–1341. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oshika Y, Nakamura M, Tokunaga T, Ohnishi
Y, Abe Y, Tsuchida T, Tomii Y, Kijima H, Yamazaki H, Ozeki Y, et
al: Ribozyme approach to downregulate vascular endothelial growth
factor (VEGF) 189 expression in non-small cell lung cancer (NSCLC).
Eur J Cancer. 36:2390–2396. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Wu J and Gao D: Experimental
research of gene therapy for human gliomas with vascular
endothelial growth factor(165) antisense RNA. Zhonghua Yi Xue Za
Zhi. 80:386–388. 2000.In Chinese.
|
|
9
|
Drake CJ, LaRue A, Ferrara N and Little
CD: VEGF regulates cell behavior during vasculogenesis. Dev Biol.
224:178–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kefas B, Godlewski J, Comeau L, Li Y,
Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, Lawler S and
Purow B: microRNA-7 inhibits the epidermal growth factor receptor
and the Akt pathway and is down-regulated in glioblastoma. Cancer
Res. 68:3566–3572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Dutta A and Abounader R: The role
of microRNAs in glioma initiation and progression. Front Biosci
(Landmark Ed). 17:700–712. 2012. View
Article : Google Scholar
|
|
12
|
Haapa-Paananen S, Chen P, Hellström K,
Kohonen P, Hautaniemi S, Kallioniemi O and Perälä M: Functional
profiling of precursor MicroRNAs identifies MicroRNAs essential for
glioma proliferation. PLoS One. 8:e609302013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiong J, Bing Z, Su Y, Deng D and Peng X:
An integrated mRNA and microRNA expression signature for
glioblastoma multiforme prognosis. PLoS One. 9:e984192014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen L, Li H, Han L, Zhang K, Wang G, Wang
Y, Liu Y, Zheng Y, Jiang T, Pu P, et al: Expression and function of
miR-27b in human glioma. Oncol Rep. 26:1617–1621. 2011.PubMed/NCBI
|
|
15
|
Chen L, Zhang W, Yan W, Han L, Zhang K,
Shi Z, Zhang J, Wang Y, Li Y, Yu S, et al: The putative tumor
suppressor miR-524-5p directly targets Jagged-1 and Hes-1 in
glioma. Carcinogenesis. 33:2276–2282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang KL, Zhou X, Han L, Chen LY, Chen LC,
Shi ZD, Yang M, Ren Y, Yang JX, Frank TS, et al: MicroRNA-566
activates EGFR signaling and its inhibition sensitizes glioblastoma
cells to nimotuzumab. Mol Cancer. 13:632014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanase CP, Enciu AM, Mihai S, Neagu AI,
Calenic B and Cruceru ML: Anti-cancer therapies in high grade
gliomas. Curr Proteomics. 10:246–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Odjélé A, Charest D and Morin P Jr: miRNAs
as important drivers of glioblastomas: a no-brainer? Cancer
Biomark. 11:245–252. 2012.PubMed/NCBI
|
|
19
|
Gabriely G, Yi M, Narayan RS, Niers JM,
Wurdinger T, Imitola J, Ligon KL, Kesari S, Esau C, Stephens RM,
Tannous BA and Krichevsky AM: Human glioma growth is controlled by
microRNA-10b. Cancer Res. 71:3563–3572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gaur AB, Holbeck SL, Colburn NH and Israel
MA: Downregulation of Pdcd4 by mir-21 facilitates glioblastoma
proliferation in vivo. Neuro Oncol. 13:580–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zinnamosca L, Laudisi A, Petramala L,
Marinelli C, Roselli M, Vitolo D, Montesani C and Letizia C: von
Hippel Lindau disease with colon adenocarcinoma, renal cell
carcinoma and adrenal pheochromocytoma. Intern Med. 52:1599–1603.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, Han L, Zhang K, Shi Z, Zhang J,
Zhang A, Wang Y, Song Y, Li Y, Jiang T, et al: VHL regulates the
effects of miR-23b on glioma survival and invasion via suppression
of HIF-1α/VEGF and β-catenin/Tcf-4 signaling. Neuro Oncol.
14:1026–1036. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo Y, Meng X, Ma J, Zheng Y, Wang Q, Wang
Y and Shang H: Human papillomavirus 16 E6 contributes HIF-1α
induced warburg effect by attenuating the VHL-HIF-1α interaction.
Int J Mol Sci. 15:7974–7986. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang CS, Pu PY, Li YH, Zhang ZY, Qiu MZ,
Huang Q and Wang GX: An in vitro study on the suppressive effect of
glioma cell growth induced by plasmid-based small interference RNA
(siRNA) targeting human epidermal growth factor receptor. J
Neurooncol. 74:267–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim JY, Grunke SD, Levites Y, Golde TE and
Jankowsky JL: Intracerebroventricular viral injection of the
neonatal mouse brain for persistent and widespread neuronal
transduction. J Vis Exp. 91:518632014.PubMed/NCBI
|
|
26
|
Könnecke H and Bechmann I: The role of
microglia and matrix metalloproteinases involvement in
neuroinflammation and gliomas. Clin Dev Immunol. 2013:9141042013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gabriely G, Wurdinger T, Kesari S, Esau
CC, Burchard J, Linsley PS and Krichevsky AM: MicroRNA 21 promotes
glioma invasion by targeting matrix metalloproteinase regulators.
Mol Cell Biol. 28:5369–5380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ferrara N: Vascular endothelial growth
factor: Molecular and biological aspects. Curr Top Microbiol
Immunol. 237:1–30. 1999.PubMed/NCBI
|
|
29
|
Baldewijns MM, van Vlodrop IJ, Vermeulen
PB, Soetekouw PM, van Engeland M and de Bruïne AP: VHL and HIF
signalling in renal cell carcinogenesis. J Pathol. 221:125–138.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Linehan WM, Rubin JS and Bottaro DP: VHL
loss of function and its impact on oncogenic signaling networks in
clear cell renal cell carcinoma. Int J Biochem Cell Biol.
41:753–756. 2009. View Article : Google Scholar :
|
|
31
|
Chen F, Kishida T, Duh FM, Renbaum P,
Orcutt ML, Schmidt L and Zbar B: Suppression of growth of renal
carcinoma cells by the von Hippel-Lindau tumor suppressor gene.
Cancer Res. 55:4804–4807. 1995.PubMed/NCBI
|
|
32
|
Fang Y, Yu S, Ma Y, Sun P, Ma D, Ji C and
Kong B: Association of Dll4/notch and HIF-1a-VEGF signaling in the
angiogenesis of missed abortion. PloS One. 8:e706672013. View Article : Google Scholar
|