Introduction
Gastric cancer is one of the major causes of
cancer-associated mortality (1)
and is the second leading cause of cancer-associated mortality
worldwide (2,3), with an overall five-year survival
rate of only 20–25% (4). To date,
although surgical resection remains the predominant curative
treatment for gastric cancer (5),
chemotherapy remains an essential component of the comprehensive
treatment of gastric cancer, particularly advanced gastric
cancer.
Cisplatin (CDDP), which binds to DNA to generate DNA
adducts (6), is a widely used
chemotherapeutic agent for the treatment of several types of solid
tumour, including gastric cancer. However, its therapeutic efficacy
is usually limited by significant toxicity and the resistance of
gastric cancer cells to CDDP (7–9).
Although the mechanisms underlying the resistance to CDDP are
multifactorial, accumulating evidence suggests an important
association between drug resistance and DNA repair capability
(10,11). As CDDP efficiency is determined by
the balance between DNA damage, DNA synthesis inhibition and DNA
repair capability (12), there is
a requirement for the identification of agents, which can sensitise
gastric cancer cells to CDDP by inhibiting different proteins in
the DNA repair pathways.
Flap endonuclease 1 (FEN1), a multifunctional and
structure-specific nuclease (13),
is a key enzyme, which functions in DNA replication and repair to
avoid genomic instability (14).
It is widely known for its involvement in the penultimate stages of
Okazaki fragment maturation and long-patch-base excision repair
(LP-BER) (14,15). However, FEN1 has been found to be
overexpressed in several types of human cancer (16–19)
and cancer cell lines (20–23),
indicating that it is a promising diagnostic biomarker in breast,
ovarian and gastric cancer (23,24).
Thus, abnormal expression of FEN1 may be associated with cancer
development and disease progression (25), leading to cancer susceptibility
(26). Of note, it has been
reported that FEN1 is a useful target for chemotherapeutic
development (24). Nikolova et
al confirmed that the downregulation of FEN1 in LN308 glioma
cells improved their sensitivity to methylating agents, including
CDDP, to suppress cell proliferation (19). In additional, our previous study
demonstrated that downregulation of the expression of FEN1 inhibits
proliferation and reduces apoptosis (23). These data suggest that FEN1 may
also be an effective therapeutic target in cancer. The efficacy of
the alkylating agents can be improved by inhibiting the DNA repair
pathways (27), as nucleotide
excision repair, in which FEN1 is involved, is pivotal in DNA
repair and is associated with resistance to platinum-based
chemotherapy (11). Thus, taking
into account the fact that FEN1 is a DNA repair protein, the
present study aimed to determine whether CDDP can regulate the
expression of FEN1, and whether changes to the expression of FEN1
may provide a novel target for enhanced chemotherapeutic results in
the treatment of gastric cancer.
To develop a novel chemotherapeutic combination,
which increases the sensitivity of SGC-7901 cells to CDDP, the
present study investigated the functional significance of FEN1 in
CDDP-treated SGC-7901 cells.
Materials and methods
Human gastric cancer cells (SGC-7901) transfected
with FEN1-small interfering (si)RNA (sense 5′-GGA CUU GUA GUC CUG
CGA UTT-3′ and antisense 5′-AUC GCA GGA CUA CAA GUC CTT-3′) and
negative control-siRNA (NC-siRNA) (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) were previously established.
FEN1, Bcl-2-associated X protein (Bax), Bcl-2 and Bcl-extra larger
(xl) antibodies were purchased from Epitomics (Burlingame, CA,
USA). β-actin antibody was purchased from Boster Bioengineering Co.
Ltd. (Wuhan, China). Cisplatin (diluted in RPMI 1640 medium to a
final concentration of 1 mM) was purchased from Sigma-Aldrich (St.
Louis, MO, USA) and then maintained at 4°C in the dark.
Cell culture
The SGC-7901 human gastric cancer cells, obtained
from the Molecular Medicine and Cancer Research Centre of Chongqing
Medical University (Chonqing, China) were routinely cultured in
RPMI-1640 medium (GE Healthcare Life Sciences, Logan, UT, USA)
containing 10% foetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin (GE Healthcare
Life Sciences) at 37°C in a CO2 incubator. The cells
(3×105 per well) were seeded in six-well plates in
growth medium, and were cultured overnight to allow adherence. On
reaching 60–70% confluence, the cells were treated with 10, 20, 30,
40 and 50 µM CDDP (Sigma-Aldrich) for 24 h at 37°C, and with
30 µM CDDP for 24, 48 and 72 h at 37°C. These cells were
used to examine the expression levels of FEN1 induced by CDDP using
western blotting.
Preparation and transfection of cells
with siRNAs
The cells, at a density of 3×105 cells
per well, were cultured on six-well plates in growth medium,
without antibiotics, overnight to allow adherence. On reaching
30–50% confluence, the cells were transfected using 5 µl
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) diluted in 250 µl RPMI-1640
medium, at room temperature. Briefly, 5 µl of the
recombinant lentiviral vector for the FEN1 gene (FEN1-siRNA) and
null vector (NC-siRNA), and the transfection reagent were diluted
in 250 µl RPMI-1640 medium without foetal bovine serum.
Following combining of the mixture of siRNA and
Lipofectamine® 2000 at room temperature for 20 min, the
mixture was added to each well. Following incubation for 6 h, the
transfection complexes were removed and replaced with culture
medium. Following incubation with CDDP (30 µM) in culture
medium, the cells were divided into two groups: siRNA-FEN1+CDDP
group and NC-siRNA+CDDP group. These cells were used for further
experiments.
MTT cell survival assays
The viabilities of the cells were measured using MTT
cell survival assays. Briefly, the cells were seeded at a density
of 5×103 cells per well on 96-well plates and cultured
overnight. At 6 h post-transfection, the cells in the
FEN1-siRNA+CDDP and NC-siRNA+CDDP groups were treated either with
increasing concentrations of CDDP (0, 2.5, 5, 10, 20, 30, 40 and 50
µM) for 48 h, or with 10 µM CDDP for 24, 48 and 72 h)
to assess the viability of the SGC-7901 cells exposed to CDDP.
Subsequently, 20 µl MTT (Sigma-Aldrich), which was diluted
in phosphate-buffered saline (PBS) to obtain a concentration of 5
mg/ml, was added to each well, and the plates were maintained at
37°C for 4 h. The supernatant was then removed, and 150 µl
dimethyl sulfoxide (Sigma-Aldrich) was added to each well to
terminate the reaction. The absorbance was then measured
spectrophotometrically at 450 nm using an El×800 microplate reader
(Bio-Tek Instruments, Inc., Winooski, VT, USA). All the experiments
were performed independently at least three times.
Detection of apoptotic cells using flow
cytometry
The number of apoptotic cells was analysed using
flow cytometry with a fluorescein-isothiocyanate-labelled enhanced
Annexin V/Propidium Iodide (PI) Apoptosis Detection kit
(Invitrogen; Thermo Fisher Scientific, Inc.). According to the
manufacturer's protocol, the adherent and suspended cells were
harvested, centrifuged at 4,800 × g for 5 min, washed twice with
PBS, and resuspended in 15 ml binding buffer in 0.1 M PBS
(Invitrogen; Thermo Fisher Scientific, Inc.). The cells were then
subjected to flow cytometry within 1 h. The apoptotic rates were
quantified using Cell Quest software (version 3.3; BD Biosciences,
San Jose, CA, USA). All the assays were performed three times
independently.
Western blot analysis
Western blot analysis was used to verify the
inhibitory effects of FEN1-siRNA and to analyse the expression
levels of Bax, Bcl-2 and Bcl-xl. Briefly, following the different
treatment procedures, the cells were lysed to extract the total
protein using Radioimmunoprecipitation Assay Lysis Buffer (Beyotime
Institute of Biotechnology, Haimen, China). The concentrations of
the extracted proteins were quantified using a Bicinchoninic Acid
Protein Assay kit (Beyotime Institute of Biotechnology). The
samples were degenerated by boiling in a water bath at 100°C for 5
min until fully denatured. The samples (40 µg protein per
lane) and pre-stained molecular weight markers were separated by
10% SDS-PAGE and transferred electrophoretically onto
polyvinylidene fluoride membranes in a minigel apparatus
(Mini-PROTEAN II; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The membranes were blocked using 5% skimmed-milk powder in
Tris-buffered saline with Tween 20 (TBST; Beyotime Institute of
Biotechnology), containing 10 mM Tris-HCl (pH 7.5), 150 mM NaCl and
0.1% Tween-20 for 1 h at room temperature. The membranes were
washed with three times with TBST for 10 min. The membranes were
then incubated with antibodies against rabbit monoclonal FEN1
(1:1,000; cat. no. ab133311; Epitomics; Abcam, Cambridge, MA, USA),
rabbit monoclonal Bax (1:1,000; cat. no. ab32503; Epitomics;
Abcam), rabbit monoclonal Bcl-2 (1:1,000; cat. no. ab32124;
Epitomics; Abcam), rabbit monoclonal Bcl-xl (1:1,000; cat. no.
ab32370; Epitomics; Abcam), and rabbit polyclonal β-actin (1:1,000;
cat. no. BA2305; Boster Bioengineering Co., Ltd.) overnight at 4°C.
The following day, the membranes were washed three times with TBST
for 30 min and incubated with horseradish peroxidase-conjugated
goat anti-rabbit secondary antibodies (1:1,000; cat. no. BA1055;
Boster Bioengineering Co., Ltd.) at room temperature for 2 h. The
blots were then washed, incubated with Solution A and Solution B
from the BeyoECL Plus western blotting detection reagent kit (cat.
no. P0018; Beyotime Institute of Biotechnology) with a Solution
A:Solution B ratio of 1:1 at room temperature for 10 min, and
measured using a chemiluminescence western blotting detection
system (Bio-Rad Laboratories, Inc.). All the experiments were
performed three times.
Statistical analysis
The quantitative data are reported as the mean ±
standard deviation. All data were analysed using a paired test
using IBM SPSS 19.0 software (IBM SPSS, Armonk, NY, USA). All
P-values were two-sided and P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of FEN1 is upregulated in
CDDP-treated SGC-7901 cells
Our previous study confirmed that FEN1 is
overexpressed in gastric cancer tissues and gastric cell lines,
particularly SGC-7901 cells (23).
Additionally, it has been found that the expression level of FEN1
is increased in 5-FU-R cells, compared with HCT-116 cells (28), which indicates that the effect of
the upregulation of FEN1 may be in response to DNA damage. Thus, in
further investigating whether the expression of FEN1 is induced by
CDDP in SGC-7901 cells, the present study found that the protein
levels of FEN1 were significantly enhanced at a range of CDDP
concentrations (10, 20, 30, 40 and 50 µM CDDP) for 48 h, and
following treatment with 30 µM CDDP for 24, 48 and 72 h
(Fig. 1). These data revealed that
the expression of FEN1 in SGC-7901 cells was also upregulated by
exposure to the chemotherapeutic agent, CDDP. This may be explained
by the fact the enhanced concentration of CDDP or extended
treatment duration resulting in increased DNA damage; however, the
increased expression level of FEN1 may have been in response to
increased DNA damage.
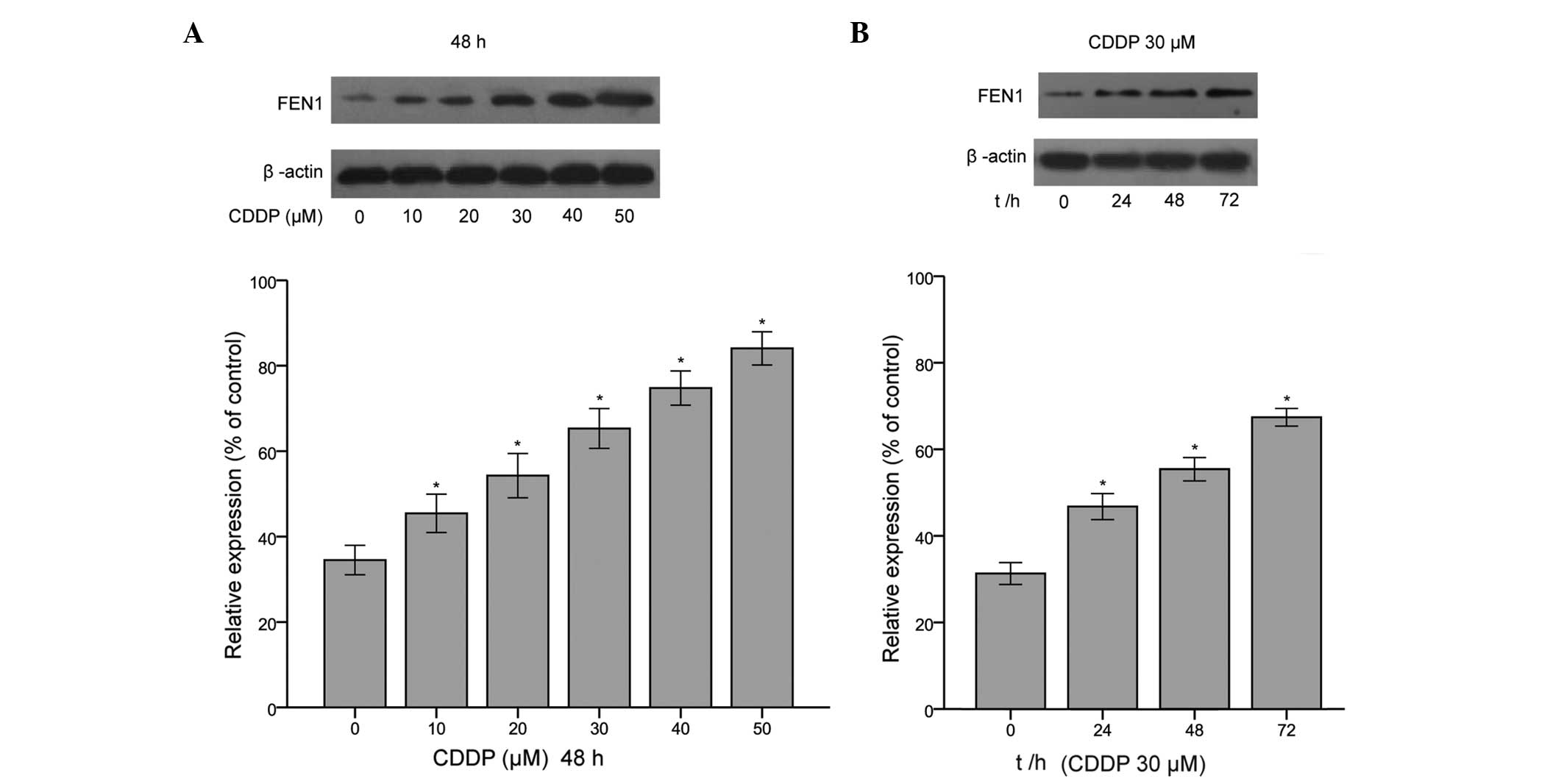 | Figure 1Expression of FEN1 is upregulated in
CDDP-treated SGC-7901 cells. (A) Following treatment with CDDP (10,
20, 30, 40, and 50 mΜ for 48 h and 30 µM CDDP for 24, 48 and
72 h) the expression of FEN1 was significantly induced by CDDP in a
dose- and time-dependent manner, compared with the untreated
control group. The protein levels were determined using western
blotting, and representative images are shown. (B) Protein levels
were quantified using densitometry. The results are presented as
the mean ± standard deviation of three independent experiments,
standardized to β-actin and normalised to 100%. The expression of
FEN1 was significantly increased in the CDDP-treated group,
compared with the control group *P<0.05, vs. control.
CDDP, cisplatin; FEN1, flap endonuclease 1; t/h, time (h). |
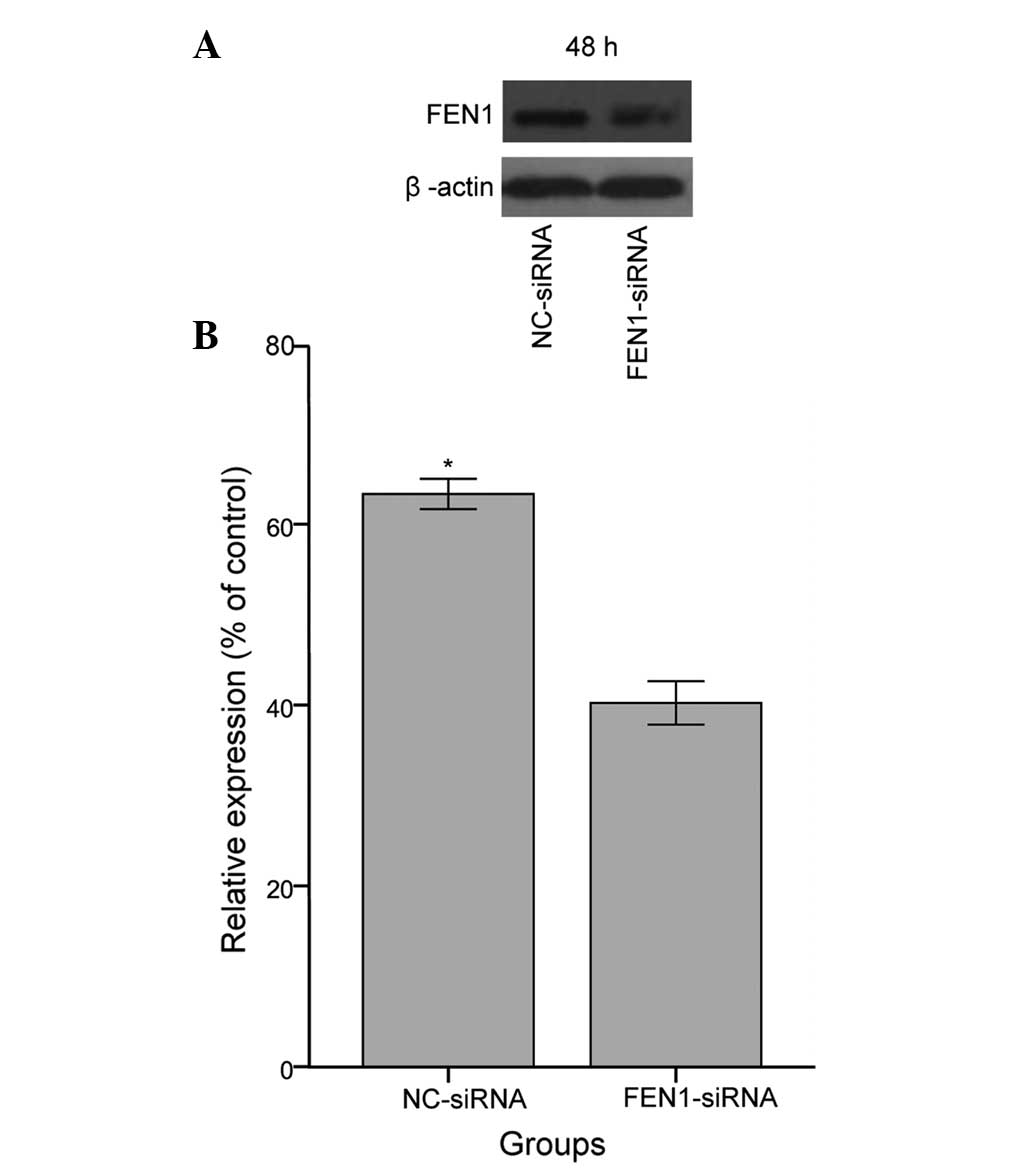
Expression of FEN1 is markedly inhibited
by siRNA in SGC-7901 gastric cancer cells
Several FEN1 inhibitors have been reported to permit
sensitisation to DNA injury agents (29,30).
Our previous study confirmed that FEN1 is upregulated in SGC-7901
cells, and that the levels of FEN1 can be effectively inhibited by
FEN1-siRNA (22). Thus, to
determine whether FEN1 is involved in sensitivity to CDDP, the
present study knocked down FEN1 via the same specific siRNA in
SGC-7901 cells and used western blot analysis to verify the
inhibitory effects of FEN1-siRNA. As expected, a reduction in the
expression of FEN1 was observed in the SGC-7901 cells transfected
with FEN1-siRNA, compared with the cells transfected with NC-siRNA,
as shown in Fig. 2 (P<0.05). In
the present study, ~ 63% silencing of FEN1 was obtained, relative
to the SGC-7901 cells transfected with NC-siRNA. These observations
confirmed the suppression of FEN1 protein via specific
FEN1-targeted siRNA, indicating that these cells were suitable for
use in further experiments.
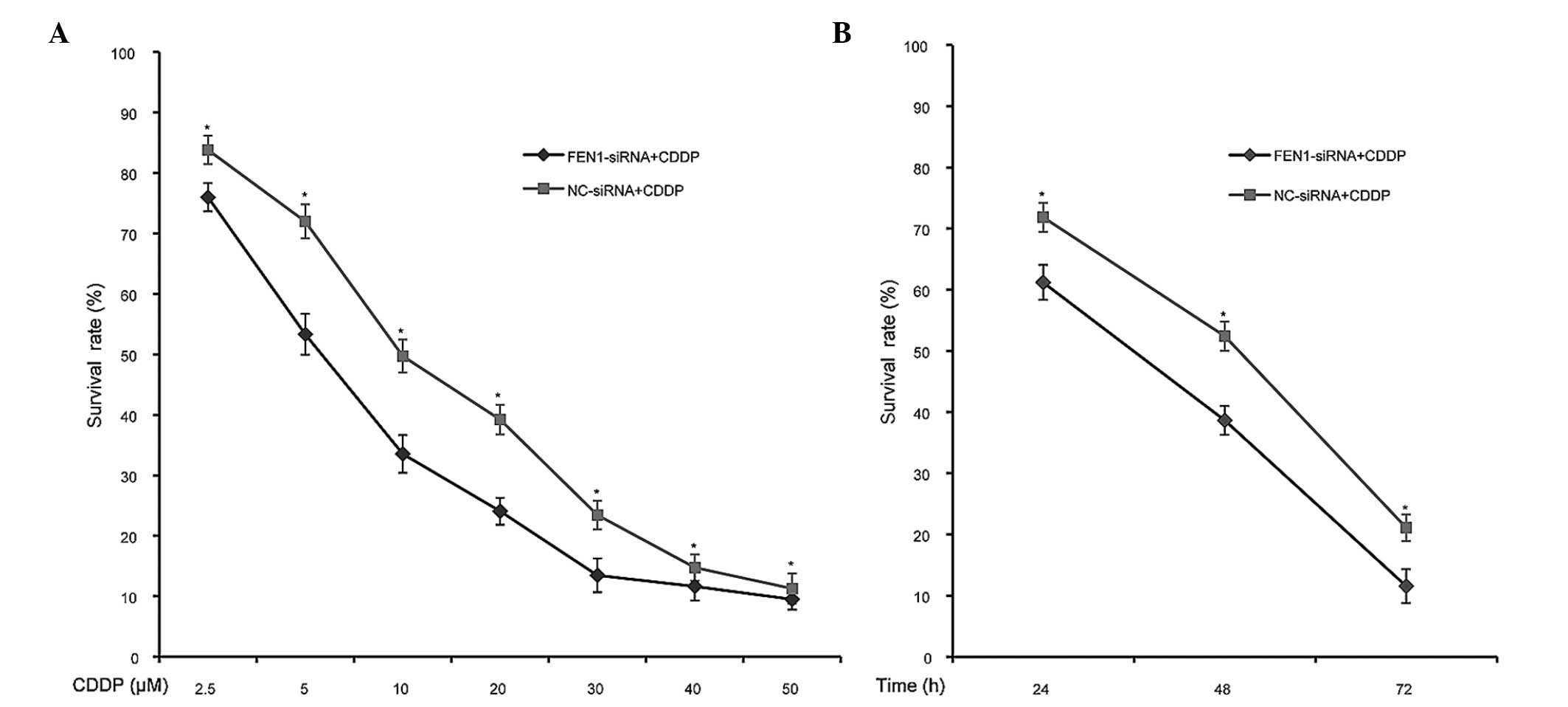
FEN1 silencing decreases the survival
rate of SGC-7901 cells following CDDP treatment
As FEN1 was overexpressed in SGC-7901 cells and was
further upregulated in response to CDDP treatment, the present
study hypothesized that the targeting of FEN1 can sensitise
SGC-7901 cells to CDDP. Thus, to further determine whether the
enhanced sensitivity observed in the FEN1-knockdown SGC-7901 cells
following CDDP treatment is reflected at the level of cell
survival, the present study performed an MTT assay. As expected, a
significantly lower survival rate was observed in the FEN1-siRNA
group, compared with the NC-siRNA group following treatment with
different concentrations of CDDP (0, 2.5, 5, 10, 20, 30, 40 and 50
µM), and following treatment for 24, 48 and 72 h (P<0.05;
Fig. 3). These results confirmed
that the silencing of FEN1 led to reduced survival of the SGC-7901
cells following CDDP treatment, which indicated that FEN1-siRNA
transfection effectively increased the sensitivity of the cells to
CDDP toxicity.
FEN1 silencing enhances SGC-7901 cell
apoptosis induced by CDDP
To elucidate the mechanism underlying the
sensitivity of FEN1-siRNA cells to CDDP, flow cytometric analysis
was performed with Annexin V/propidium iodide apoptosis detection.
The early apoptotic (Annexin V+/PI−) and late
apoptotic (Annexin V+/PI+) cells were
included. CDDP induced apoptosis in the NC-siRNA+CDDP cells
(49.74±4.68%). However, the apoptotic effect in the FEN1-siRNA+CDDP
cells was more marked, with an apoptotic rate of 73.98±5.19%
(P<0.05, compared with the NC-siRNA+CDDP group), as shown in
Fig. 4. These results indicated
that the silencing of FEN1 led to enhanced levels of apoptosis in
the SGC-7901 cells following CDDP treatment.
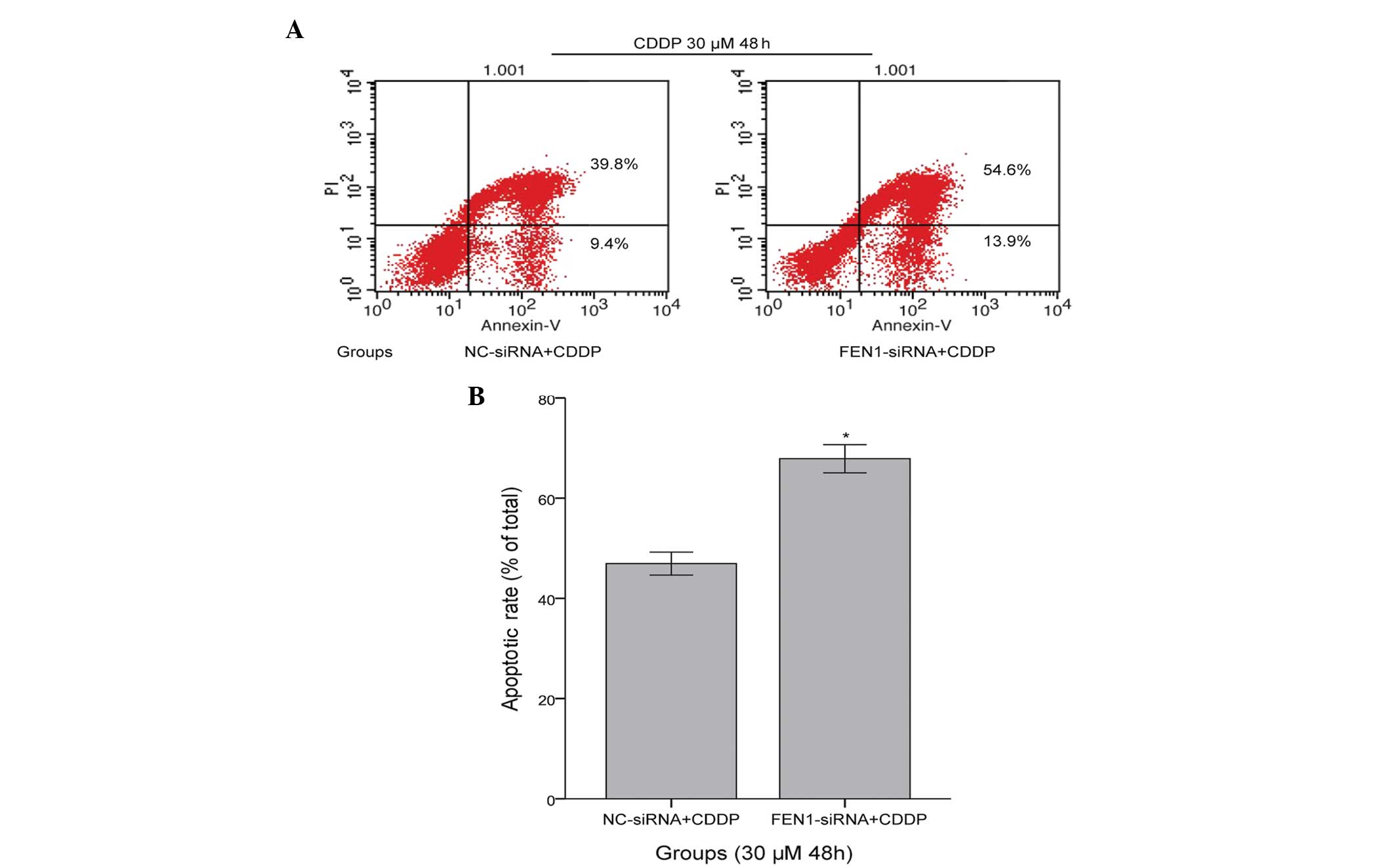 | Figure 4Silencing of FEN1 enhances
CDDP-induced apoptosis of SGC-7901 cells. (A) Apoptosis of the two
groups of SGC-7901 cells. The cells were assessed using flow
cytomettic analysis of Annexin V/propidium iodide. The results
include the viable and non-viable apoptotic cells. The apoptotic
rates of the FEN1-siRNA+CDDP and NC-siRNA+CDDP cells were
quantified using Cell Quest software. Upper left, debris and
damaged cells; lower left, negative control normal cells; upper
right, early apoptotic cells; lower right, late apoptotic and dead
cells. (B) Apoptosis rates of the FEN1-siRNA+CDDP cells were
significantly increased, compared with those of the NC-siRNA+CDDP
cells (*P<0.05). The results are expressed as the
mean ±standard deviation and are representative of the results from
three independent experiments. CDDP, cisplatin; FEN1, flap
endonuclease 1; siRNA, small interfering RNA; NC, normal control;
PI, propidium iodide. |
FEN1 silencing decreases the protein
expression levels of Bcl-2 and Bcl-xl, and increases the protein
expression of Bax
It is well-known that the Bcl-2 family of proteins
are integral in regulating apoptosis. Thus, to determine whether
the knockdown of FEN1 in SGC-7901 cells combined with CDDP
treatment affected the expression of the Bcl-2 family proteins, the
present study examined the anti-apoptotic family members, Bcl-2 and
Bcl-xl, and the pro-apoptotic family member, Bax, via western
blotting. As shown in Fig. 5, the
expression of Bax was elevated in the FEN1-siRNA+CDDP cells,
compared with the NC-siRNA + CDDP cells (P<0.05). By contrast,
the protein expression levels of anti-apoptotic Bcl-2 and Bcl-xl
were significantly decreased in the FEN1-siRNA+CDDP SGC-7901 cells,
compared with the NC-siRNA+CDDP cells (Fig. 5). These relevant apoptotic factors
further suggested that the downregulation of FEN1 may enhance
apoptosis by increasing the sensitivity to CDDP, and this increase
in sensitivity may be associated with the Bcl-2 family proteins, on
which further relevant investigations are required.
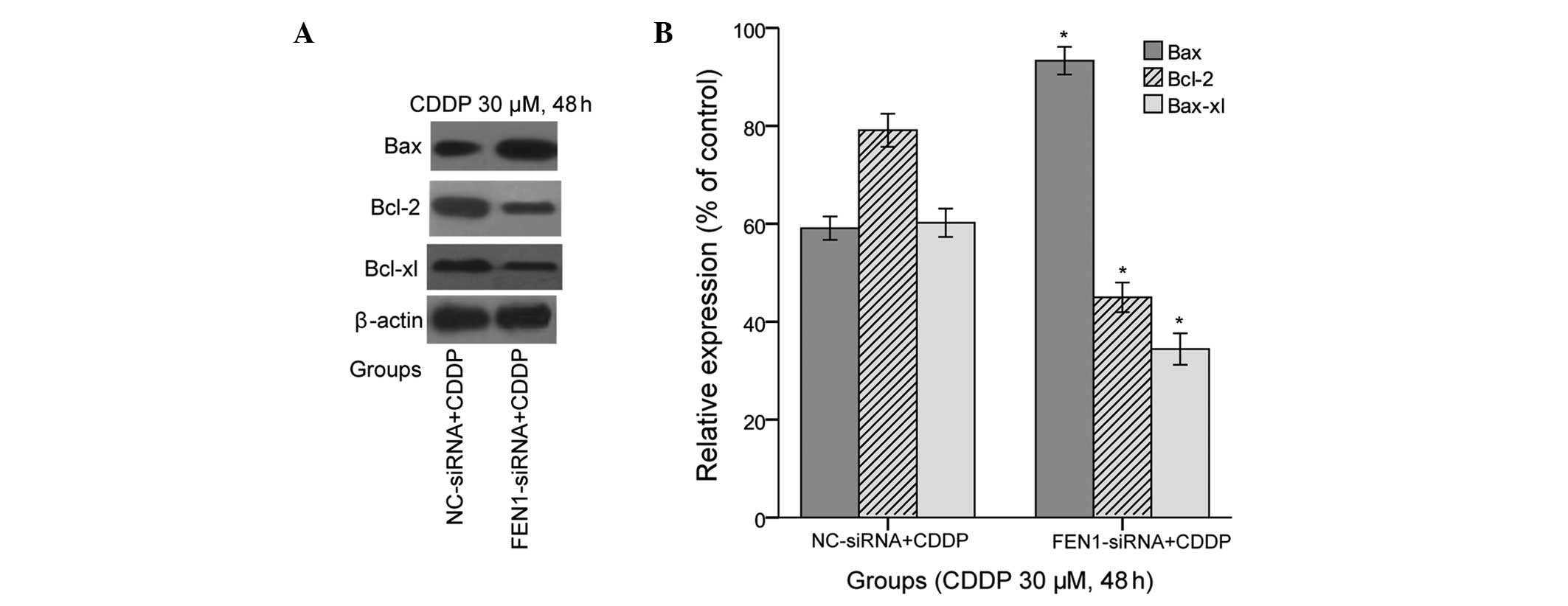 | Figure 5Expression levels of Bcl-2, Bcl-xl and
Bax following FEN1 silencing. (A) Expression levels of Bcl-2,
Bcl-xl and Bax in the FEN1-siRNA+CDDP and NC-siRNA+CDDP cells were
determined using western blotting. Representative images are shown.
(B) Protein levels were quantified using densitometry. The results
are presented as the mean ± standard deviation from three
independent experiments, standardized to β-actin and normalised to
100%. The expression of Bax was significantly increased in the
FEN1-siRNA+CDDP cells, compared with the NC-siRNA+CDDP cells,
whereas the expression levels of Bcl-2 and Bcl-xl were
significantly decreased in the FEN1-siRNA+CDDP cells
(*P<0.05), compared with the NC-siRNA+CDDP cells..
CDDP, cisplatin; FEN1, flap endonuclease 1; siRNA, small
interfering RNA; NC, normal control; Bcl-2, B cell lymphoma-2;
BCL-xl, Bcl-extra large; Bax, Bcl-2-associated X protein. |
Discussion
FEN1, which is involved in RNA primer removal and
LP-BER (14,15), interacts with different proteins to
execute its function in different pathways to maintain genomic
stability (29,31), particularly in DNA replication, DNA
repair, maintenance of telomere stability and apoptotic
fragmentation of DNA (30).
Accumulating evidence has shown that certain FEN1 inhibitors can
permit sensitisation to DNA injury agents (32,33),
although the mechanism remains to be fully elucidated. CDDP, a
well-known DNA-damaging agent, binds to DNA to generate DNA adducts
(6), which are repaired in cells
primarily through the nucleotide excision repair (NER) pathway
(34). It is known that the
resistance of cancer cells to CDDP remains a challenging problem,
and limits its use in clinical treatment (35). Therefore, a novel and promising
concept is the enhancement of the sensitivity of cancer cells to
improve the therapeutic efficacy of CDDP. In addition, further
evidence also indicates that inhibiting the amino acid Asp181 of
FEN1, which affects its endonuclease activity, in combination with
treatment with temozolomide, a DNA-alkylating agent, may be an
effective strategy (24). Thus,
the present study investigated whether alterations in the
expression of FEN1 are associated with CDDP treatment.
The present study demonstrated that the FEN1 protein
was markedly induced by CDDP in a concentration- and time-dependent
manner in SGC-7901 cells, compared with the untreated control
cells, indicating that increased expression of FEN1 may lead to the
increased DNA repair capability of cells in response to DNA damage.
In addition, a previous study reported increased expression of FEN1
in 5-FU-R cells, compared with HCT-116 cells (28), which was in accordance with our
findings. As increases in the levels of FEN1 can be induced by
CDDP, the present study subsequently examined whether
downregulation in the expression of FEN1 to decrease the level of
DNA repair enhances the sensitivity to CDDP. Our previous study
confirmed that the expression of FEN1 is upregulated in SGC-7901
cells. Thus, FEN1 was silenced in SGC-7901 cells via specific
FEN1-targeted siRNA to assess the inhibitory effects of FEN1-siRNA,
as shown in Fig. 2. The results
revealed that the siRNA-FEN1 cells treated with different
concentrations of CDDP for different durations presented with
markedly reduced survival rates, compared with the NC-siRNA group,
determined using MTT cell survival assays. This finding is
consistent with the fact that cells exhibiting deficient DNA repair
are markedly more sensitive to CDDP, compared with cells proficient
in repair (36). These data
suggested that FEN1 silencing may enhance the sensitivity of the
cells to CDDP, which may provide a novel and promising strategy for
enhancing the effects of chemotherapy. During the development of
gastric cancer, cells are subjected to clonal proliferation and
apoptosis, and elevated apoptosis provides a potential method for
inhibiting tumour survival (37).
Our previous study confirmed that the knockdown of FEN1 can induce
the apoptosis of SGC-7901 cells (23). In the present study, whether the
silencing of FEN1 promotes apoptosis following treatment with CDDP
was determined. Notably, the results indicated that the reduced
survival observed in the FEN1-siRNA+CDDP group was caused by an
increase in the rate of apoptosis, as determined using flow
cytometry. These results demonstrated that the knockdown of FEN1
significantly sensitised the SGC-7901 cells to CDDP-induced
apoptosis. Taken together, these results indicate that the
downregulation of FEN1 may be a novel target for increasing the
sensitivity of SGC-7901 cells to CDDP to overcome resistance. A
similar finding was obtained in glioblastoma cell lines depleted in
FEN1, which showed increased damage sensitivity to CDDP (19).
It is well known that the use of alkylating agents
as chemotherapeutic drugs is based on their ability to trigger an
apoptotic response (38). In
addition, Larsen et al confirmed that two domain-specific
FEN1 proteins cause early onset lymphoma and extensive embryonic
apoptosis (39). Evidently, a
decrease in FEN1 activity may cause abnormal cell proliferation,
genomic instability and tumourigenesis. However, the pro-apoptotic
mechanism of FEN1 remains to be fully elucidated. In the present
study, to investigate the pro-apoptotic mechanism of FEN1, western
blotting was performed to analyse the expression levels of
anti-apoptotic Bcl-2 and Bcl-xl, and pro-apoptotic Bax. The
pro-apoptotic gene, Bax, was overexpressed in the FEN1-siRNA+CDDP
group, whereas the anti-apoptotic genes, Bcl-2 and Bcl-xl, were
decreased, compared with the levels observed in the NC-siRNA+CDDP
group. To the best of our knowledge, the present study provides the
first demonstration that FEN1 is potentially involved in
CDDP-induced apoptosis, although the detailed mechanism underlying
the action and involvement of other components in the pro-apoptotic
effect of FEN1 remain to be elucidated. The mechanism of FEN1 may
involve the formation of a complex of FEN1 with other proteins to
execute its function through different pathways, and further
investigations to determine the possible apoptotic mechanisms
involved in targeting FEN1 may provide further insights into this
possibility.
In conclusion, the results of the present study
revealed that the silencing of FEN1 in SGC-7901 cells enhanced
their sensitivity to CDDP. These results indicated that FEN1 can be
upregulated by CDDP, and that knockdown of the expression of FEN1
may offer a potential therapeutic approach for enhancing
sensitivity to CDDP-based treatment, via decreased survival rate
and increased apoptosis of cells. Increased apoptosis was further
confirmed by relevant apoptotic factors. These findings not only
increase current knowledge on the biology of FEN1, but also present
a potential novel strategy for enhancing sensitivity to CDDP, via
specific FEN1-targeted siRNA, to inhibit DNA repair.
Acknowledgments
The authors would like to thank the Molecular
Medicine and Cancer Research Center of Chongqing Medical University
for their assistance. This study was supported by the Research Fund
of Chongqing Municipal Health Bureau (grant. no. 2009-2-345).
References
|
1
|
Nagini S: Carcinoma of the stomach: A
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: Estimates of cancer incidence and mortality in Europe in 2008.
Eur J Cancer. 46:765–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meyer HJ and Wilke H: Treatment strategies
in gastric cancer. Dtsch Arztebl Int. 108:698–705. 2011.PubMed/NCBI
|
|
5
|
Chang WJ, Du Y, Zhao X, Ma LY and Cao GW:
Inflammation-related factors predicting prognosis of gastric
cancer. World J Gastroenterol. 20:4586–4596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jamieson ER and Lippard SJ: Structure,
recognition and processing of cisplatin-DNA adducts. Chem Rev.
99:2467–2498. 1999. View Article : Google Scholar
|
|
7
|
Loehrer PJ and Einhorn LH: Drugs five
years later Cisplatin. Ann Intern Med. 100:704–713. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reedijk J: New clues for platinum
antitumor chemistry: Kinetically controlled metal binding to DNA.
Proc Natl Acad Sci USA. 100:3611–3616. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Woźniak K and Błasiak J: Recognition and
repair of DNA-cisplatin adducts. Acta Biochim Pol. 49:583–596.
2002.
|
|
10
|
Olaussen KA, Dunant A, Fouret P, Brambilla
E, André F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH,
et al: DNA repair by ERCC1 in non-small-cell lung cancer and
cisplatin-based adjuvant chemotherapy. N Engl J Med. 355:983–991.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reed E: Platinum-DNA adduct, nucleotide
excision repair and platinum based anti-cancer chemotherapy. Cancer
Treat Rev. 24:331–344. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Wang C and Li Z: A new strategy of
promoting cisplatin chemotherapeutic efficiency by targeting
endoplasmic reticulum stress. Mol Clin Oncol. 2:3–7.
2014.PubMed/NCBI
|
|
13
|
Harrington JJ and Lieber MR: The
characterization of amammalian DNA structure-specific endonuclease.
EMBO J. 13:1235–1246. 1994.PubMed/NCBI
|
|
14
|
Henneke G, Friedrich-Heineken E and
Hübscher U: Flap endo-nuclease 1: A novel tumour suppressor
protein. Trends Biochem Sci. 28:384–390. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Klungland A and Lindahl T: Second pathway
for completion of human DNA base excision-repair: Reconstitution
with purified-proteins and requirement for DNase IV (FEN1). EMBO J.
16:3341–3348. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lam JS, Seligson DB, Yu H, Li A, Eeva M,
Pantuck AJ, Zeng G, Horvath S and Belldegrun AS: Flap endonuclease
1 is overex-pressed in prostate cancer and is associated with a
high Gleason score. BJU Int. 98:445–451. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh P, Yang M, Dai H, Yu D, Huang Q, Tan
W, Kernstine KH, Lin D and Shen B: Overexpression and
hypomethylation of flap endonuclease 1 gene in breast and other
cancers. Mol Cancer Res. 6:1710–1717. 2008.PubMed/NCBI
|
|
18
|
Iacobuzio-Donahue CA, Maitra A, Olsen M,
Lowe AW, van Heek NT, Rosty C, Walter K, Sato N, Parker A, Ashfaq
R, et al: Exploration of global gene expression patterns in
pancreatic adenocarcinoma using cDNA microarrays. Am J Pathol.
162:1151–1162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nikolova T, Christmann M and Kaina B: FEN1
is overexpressed in testis, lung and brain tumors. Anticancer Res.
29:2453–2459. 2009.PubMed/NCBI
|
|
20
|
Sato M, Girard L, Sekine I, Sunaga N,
Ramirez RD, Kamibayashi C and Minna JD: Increased expression and no
mutation of the Flap endonuclease (FEN1) gene in human lung cancer.
Oncogene. 22:7243–7246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
LaTulippe E, Satagopan J, Smith A, Scher
H, Scardino P, Reuter V and Gerald WL: Comprehensive gene
expression analysis of prostate cancer reveals distinct
transcriptional programs associated with metastatic disease. Cancer
Res. 62:4499–4506. 2002.PubMed/NCBI
|
|
22
|
Wang K, Xie C and Chen D: Flap
endonuclease 1 is a promising candidate biomarker in gastric cancer
and is involved in cell proliferation and apoptosis. Int J Mol Med.
33:1268–1274. 2014.PubMed/NCBI
|
|
23
|
Abdel-Fatah TM, Russell R, Albarakati N,
Maloney DJ, Dorjsuren D, Rueda OM, Moseley P, Mohan V, Sun H,
Abbotts R, et al: Genomic and protein expression analysis reveals
flap endnuclease 1 (FEN1) as a key biomarker in breast and ovarian
cancer. Mol Oncol. 87:1326–1338. 2014. View Article : Google Scholar
|
|
24
|
Panda H, Jaiswal AS, Corsino PE, Armas ML,
Law BK and Narayan S: Amino acid Asp181 of 5′-flap endonuclease 1
is a useful target for chemotherapeutic development. Biochemistry.
48:9952–9958. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng L, Dai H, Zhou M, Li M, Singh P, Qiu
J, Tsark W, Huang Q, Kernstine K, Zhang X, et al: FEN1 mutations
result in autoimmunity, chronic inflammation and cancers. Nat Med.
13:812–819. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu L, Zhou C, Zhou L, Peng L, Li D, Zhang
X, Zhou M, Kuang P, Yuan Q, Song X and Yang M: Functional FEN1
genetic variants contribute to risk of hepatocellular carcinoma,
esophageal cancer, gastric cancer and colorectal cancer.
Carcinogenesis. 33:119–123. 2012. View Article : Google Scholar
|
|
27
|
Middleton MR and Margison GP: Improvement
of chemotherapy efficacy by inactivation of a DNA-repair pathway.
Lancet Oncol. 4:37–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Das D, Preet R, Mohapatra P, Satapathy SR
and Kundu CN: 1,3-Bis (2-chloroethyl)-1-nitrosourea enhances the
inhibitory effect of resveratrol on 5-fluorouracil
sensitive/resistant colon cancer cells. World J Gastroenterol.
19:7374–7388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dianova II, Bohr VA and Dianov GL:
Interaction of human AP endonuclease 1 with flap endonuclease 1 and
proliferating cell nuclear antigen involved in long-patch base
excision repair. Biochemistry. 40:12639–12644. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saharia A, Guittat L, Crocker S, Lim A,
Steffen M, Kulkarni S and Stewart SA: Flap endonuclease 1
contributes to telomere stability. Curr Biol. 18:496–500. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Parrish JZ, Yang C, Shen B and Xue D:
CRN-1, a Caenorhabditis elegans FEN1 homologue, cooperates with
CPS-6/EndoG to promote apoptotic DNA degradation. EMBO J.
22:3451–3460. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tumey LN, Huck B, Gleason E, Wang J,
Silver D, Brunden K, Boozer S, Rundlett S, Sherf B, Murphy S, et
al: The identification and optimization of 2,4-diketobutyric acids
as flap endonuclease 1 inhibitors. Bioorg Med Chem Lett.
14:4915–4918. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tumey LN, Bom D, Huck B, Gleason E, Wang
J, Silver D, Brunden K, Boozer S, Rundlett S, Sherf B, et al: The
identification and optimization of a N-hydroxy urea series of flap
endonuclease 1 inhibitors. Bioorg Med Chem Lett. 15:277–281. 2005.
View Article : Google Scholar
|
|
34
|
Chu G: Cellular responses to cisplatin.
The roles of DNA-binding proteins and DNA repair. J Biol Chem.
269:787–790. 1994.PubMed/NCBI
|
|
35
|
Galluzzi L, Vitale I, Michels J, Brenner
C, Szabadkai G, Harel-Bellan A, Castedo M and Kroemer G: Systems
biology of cisplatin resistance: Past, present and future. Cell
Death Dis. 5:e12572014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Popoff SC, Beck DJ and Rupp WD: Repair of
plasmid DNA damaged in vitro with cis- or
trans-diamminedichloroplatinum(II) in Escherichia coli. Mutat Res.
183:129–137. 1987.PubMed/NCBI
|
|
37
|
Fuertes MA, Alonso C and Pérez JM:
Biochemical modulation of cisplatin mechanisms of action:
Enhancement of antitumor activity and circumvention of drug
resistance. Chem Rev. 103:645–662. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sawyers C: Targeted cancer therapy.
Nature. 432:294–297. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Larsen E, Kleppa L, Meza TJ, Meza-Zepeda
LA, Rada C, Castellanos CG, Lien GF, Nesse GJ, Neuberger MS,
Laerdahl JK, et al: Early-onset lymphoma and extensive embryonic
apoptosis in two domain-specific Fen1 mice mutants. Cancer Res.
68:4571–4579. 2008. View Article : Google Scholar : PubMed/NCBI
|