Introduction
Cardiac hypertrophy is an adaptive response of the
heart to numerous neurohormonal stimuli. Physiological cardiac
hypertrophy occurs in response to exercise and results in the
adaptation of contractility to the increase in wall stress
(1,2), whereas pathological hypertrophy
occurs as a response to volume or pressure overload, which is
associated with increased interstitial fibrosis, cell death and
cardiac dysfunction, ultimately leading to heart failure (3). These progressive changes have long
been considered as irreversible (4). Divergent signaling mechanisms may
lead to distinct patterns of hypertrophy, although there is a level
of overlapping between certain signaling pathways. Notably, the Akt
and transforming growth factor β (TGFβ)/small mothers against
decapentaplegic (Smad) signaling pathways (5,6) lead
to cardiomyocyte hypertrophy and cardiac fibrosis, contributing to
pathological hypertrophy and heart failure (HF) (7). However, how to inhibit these
signaling pathways in the heart during increased biomechanical
stress remains to be fully elucidated.
Shensongyangxin (SSYX), is a traditional Chinese
medicine, which was originally developed for the treatment of
cardiac tachyarrhythmias, and is a component of the traditional
Chinese materia medica, consisting of 12 ingredients, including the
plants ginseng, Radix, dogwood, Salvia, Semen
(fried), mistletoe, red peony, Eupolyphaga, nard, berberine,
kadsura and keel (8). Previous
investigations have focussed on the function of SSYX in cardiac
arrhythmia, including tachyarrhythmia, bradycardia, paroxysmal
atrial fibrillation (AF) and premature ventricular contractions
(9–11). In addition, a previous study
demonstrated that, when combined with routine pharmacotherapy, SSYX
significantly normalizes heart rate variability and heart rate
turbulence, and reduces the incidence of ventricular tachycardia
and AF, suggesting that SSYX may provide electrophysiological
benefits to patients with chronic HF (12). In a coronary artery ligation rat
model, SSYX inhibits ventricular remodeling, and improves the
electrophysiological base material of the heart, which may be
associated with affecting the ion channels of myocardial cellular
membranes (13). These previous
findings suggest that SSYX has a potential protective effects on
the cardiovascular system. However, whether SSYX is able to arrest
the development of pressure overload-induced cardiac remodeling and
prevent HF remains to be elucidated. In the present study, mice
were subjected to AB and were treated with SSYX. The current study
investigated whether SSYX attenuated cardiac hypertrophy and
fibrosis, and aimed to provide experimental evidence for the
potential application of SSYX in the treatment of cardiac
remodeling and HF.
Materials and methods
Animals and animal models
All animal procedures were performed in accordance
with the Guide for the Care and Use of Laboratory Animals published
by the US National Institutes of Health, and were approved by the
Animal Care and Use Committee of Renmin Hospital of Wuhan
University (Wuhan, China) (14).
The surgical procedures and subsequent analyses were performed in a
blinded-manner for all groups. Adult male C57/BL6 mice (n=60; 8–10
weeks old; Beijing HFK Bioscience Co., Ltd., Beijing, China) were
used for the present study. Mice were housed with controlled
temperature and humidity under a 12-h light/dark cycle with free
access to food and water in the Cardiovascular Research Institute
of Wuhan University (Wuhan, China). The animals were allowed to
acclimatize to the laboratory environment for a minimum of one
week, then randomly assigned to one of four groups: Vehicle sham
group (n=15); vehicle AB group (n=15); SSYX sham group (n=15); and
the SSYX AB group (n=15). Aortic banding (AB) was performed, as
previously described (15). In
brief, mice were anesthetized with 3% sodium pentobarbital
(Sigma-Aldrich, St. Louis, MO, USA) by intraperitoneal injection.
Mice were then orally intubated in a supine position with a heating
pad. Artificial respiration was maintained using a rodent
ventilator (Somnosuite model; Kent Scientific Corporation,
Torrington, CT, USA). The aortic arch branch was exposed with a
chest expander upon opening of the second and third intercostals by
an incision. The vessel was ligated using a 26G/27G syringe needle
placed parallel above the vessel. Subsequent to rapid withdrawal of
the needle to achieve aortic constriction, the chest was closed in
layers and a total of 0.1 ml 0.5% bupivacaine (Sigma-Aldrich) was
injected subcutaneously close to the edges of the skin incision to
alleviate postoperative pain. After 1 week of either chronic
pressure overload, generated by the AB, or sham surgery (control
group), the mice (15/group) received gavage with 0.3 ml saline
containing SSYX (520 mg/kg; Shijiazhuang Yiling Pharmaceutical Co.,
Ltd., Shijiazhuang, China) or normal saline (vehicle) for 7 weeks.
The mice were subsequently sacrificed by cervical dislocation, and
their hearts and lungs were harvested and weighed in order to
compare the heart weight/body weight (HW/BW; mg/g), lung
weight/body weight (LW/BW; mg/g) and heart weight/tibia length
(HW/TL; mg/ml) ratios in the SSYX-treated and vehicle-treated
mice.
Histological analysis
The hearts were arrested in diastole using 10% KCl
(Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), and were
then weighed, fixed by perfusion with 10% formalin (Sinopharm
Chemical Reagent Co., Ltd.) and embedded in paraffin (Fisher
Scientific UK Ltd., Loughborough, England). The hearts were then
sectioned transversely, close to the apex, in order to visualize
the left and right ventricles. Several sections of each heart (4–5
µm) were prepared, stained with hematoxylin and eosin (Baso
Diagnostics, Inc., Zhuhai, China) for histopathological analysis,
and picrosirius red [PSR; Hyde Venture (Beijing) Biotech Co., Ltd.,
Beijing, China] for collagen deposition, following which the
sections were visualized using light microscopy (Olympus FSX100;
Olympus Corporation, Tokyo, Japan). A single myocyte was measured
using an image quantitative digital analysis system (Image Pro-Plus
6.0; Media Cybernetics, Inc. Rockville, MD, USA). The outline of
100 myocytes were traced in each group.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to detect the mRNA expression
levels of hypertrophic (atrial natriuretic peptide, ANP; brain
natriuretic peptide, BNP; α-myosin heavy chain, α-MHC; and β-MHC)
and fibrotic markers (TGFβ1; connective tissue growth factor, CTGF;
fibronection; collagen I; and collagen III). Total RNA was
extracted from the frozen, pulverized mouse cardiac tissue samples
using TRIzol® reagent (cat. no. 15596-026; Roche
Diagnostics, Basel, Switzerland). The yield and purity levels of
the RNA were spectrophotometrically estimated using A260/A280 and
A230/A260 ratios, obtained via a SmartSpec Plus Spectrophotometer
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The RNA (2 mg of
each sample) was reverse transcribed into cDNA using oligo(dT)
primers (cat. no. 04896866001; Roche Diagnostics) and a
Transcriptor First Strand cDNA Synthesis kit (cat. no. 04896866001;
Roche Diagnostics). The PCR amplifications were quantified using
LightCycler 480 SYBR® Green 1 Master Mix (cat. no.
04707516001; Roche Diagnostics) in the LightCycler® 480
Real-Time Quantitative PCR System (Roche Diagnostics). Briefly,
subsequent to a 5 min initial denaturation at 95°C, a total of 42
primer-extension cycles were conducted. Each cycle consisted of a
10 sec denaturation step at 95°C, a 20 sec annealing step at 60°C
and a 20 sec incubation at 72°C for extension. Then a final
extension step was conducted at 72°C for 10 min. The double
standard curve was used to quantify the PCR results. The results
were normalized to the mRNA expression of
glyceraldehydes-3-phosphate dehydrogenase (GAPDH). The
oligonucleotide primer sequences are listed in Table I.
 | Table IPrimer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
| mRNA | Forward primer | Reverse primer |
|---|
| ANP (mouse) |
5′-ACCTGCTAGACCACCTGGAG-3′ |
5′-CCTTGGCTGTTATCTTCGGTACCGG-3′ |
| BNP (mouse) |
5′-GAGGTCACTCCTATCCTCTGG-3′ |
5′-GCCATTTCCTCCGACTTTTCTC-3′ |
| α-MHC (mouse) |
5′-GTCCAAGTTCCGCAAGGT-3′ |
5′-AGGGTCTGCTGGAGAGGTTA-3′ |
| β-MHC (mouse) |
5′-CCGAGTCCCAGGTCAACAA-3′ |
5′-CTTCACGGGCACCCTTGGA-3′ |
| TGFβ1 (mouse) |
5′-ATCCTGTCCAAACTAAGGCTCG-3′ |
5′-ACCTCTTTAGCATAGTAGTCCGC-3′ |
| CTGF (mouse) |
5′-TGTGTGATGAGCCCAAGGAC-3′ |
5′-AGTTGGCTCGCATCATAGTTG-3′ |
| Fibronectin
(mouse) |
5′-CCGGTGGCTGTCAGTCAGA-3′ |
5′-CCGTTCCCACTGCTGATTTATC-3′ |
| Collagen I
(mouse) |
5′-AGGCTTCAGTGGTTTGGATG-3′ |
5′-CACCAACAGCACCATCGTTA-3′ |
| Collagen III
(mouse) |
5′-CCCAACCCAGAGATCCCATT-3′ |
5′-GAAGCACAGGAGCAGGTGTAGA-3′ |
| GAPDH (mouse) |
5′-ACTCCACTCACGGCAAATTC-3′ |
5′-TCTCCATGGTGGTGAAGACA-3′ |
| ANP (rat) |
5′-AAAGCAAACTGAGGGCTCTGCTCG-3′ |
5′-TTCGGTACCGGAAGCTGTTG CA-3′ |
| BNP (rat) |
5′-CAGCAGCTTCTGCATCGTGGAT-3′ |
5′-TTCCTTAATCTGTCGCCGCTGG-3′ |
| β-MHC (rat) |
5′-TCTGGACAGCTCCCCATTCT-3′ |
5′-CAAGGCTAACCTGGAGAAGATG-3′ |
| GAPDH (rat) |
5′-GACATGCCGCCTGGAGAAAC-3′ |
5′-AGCCCAGGATGCCCTTTAGT-3′ |
Western blotting
The cardiac tissue samples were lysed in
radioimmunoprecipitation assay lysis buffer (Roche Diagnostics),
and protein concentration was measured using a Bicinchoninic Acid
Protein Assay kit (cat. no. 23227; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with a Synergy HT ELISA reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA). The protein lysates (50
µg) were separated on 10% SDS-PAGE (Wuhan Goodbio Technology
Company, Wuhan, China) and transferred onto Immobilon-FL transfer
membranes (cat. no. IPFL00010; Merck Millipore, Darmstadt,
Germany), and were then blocked with 5% non-fat milk for 2 h. The
following primary antibodies (obtained from Cell Signaling
Technology, Inc., Danvers, MA, USA, unless otherwise stated) were
used: Rabbit polyclonal GAPDH (cat. no. sc-25778; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), rabbit monoclonal
phosphorylated (p)-Akt (cat. no. 4691), rabbit monoclonal total
(T)-Akt (cat. no. 4060), rabbit monoclonal p-glycogen synthase
kinase (GSK)3β (cat. no. 9315), rabbit monoclonal T-GSK3β (cat. no.
9322), rabbit polyclonal p-mammalian target of rapamycin (mTOR;
cat. no. 2983), rabbit monoclonal T-mTOR (cat. no. 2971), rabbit
monoclonal p-4E binding protein 1 (4EBP1; cat. no. 2855P), rabbit
monoclonal T-4EBP1 (cat. no. 9644P), rabbit monoclonal p-p70 (cat.
no. 9234P), rabbit monoclonal T-p70 (cat. no. 2708), rabbit
polyclonal p-forkhead box protein (FOXO)1; cat. no. 9461P), rabbit
monoclonal T-FOXO1 (cat. no. 2880P), rabbit polyclonal p-FOXO3a
(cat. no. 9465P), rabbit monoclonal T-FOXO3a (cat. no. 2497P) and
rabbit polyclonal TGFβ (cat. no. ab66043; Abcam, Cambridge, UK),
mouse monoclonal Smad4 (cat. no. sc-7966; Santa Cruz Biotechnology,
Inc.), rabbit monoclonal p-Smad1/5 (cat. no. 9516) and goat
polyclonal T-Smad1/5 (cat. no. sc-6201; Santa Cruz Biotechnology,
Inc.). All primary antibodies from Cell Signaling Technology, Inc.
and Abcam were diluted at 1:1,000, while those from Santa Cruz
Biotechnology, Inc. were diluted at 1:200. Primary antibodies were
incubated overnight with gentle shaking at 4°C. Subsequent to three
washes with phosphate-buffered saline (PBS; Sinopharm Chemical
Reagent Co., Ltd.), the membranes were then incubated with IRdye
800CW-conjugated goat anti-rabbit immunoglobulin (Ig)G (cat. no.
926-32211; LI-COR Biosciences, Lincoln, NE, USA) and IRdye
800CW-conjugated goat anti-mouse IgG (cat. no. 926-32210; LI-COR
Biosciences) secondary antibodies at 1:10,000 for 1 h at 37°C in
Odyssey blocking buffer (LI-COR Biosciences). The blots were
scanned and analyzed using a two-color infrared imaging system
(Odyssey; LI-COR Biosciences).
Cell culture
Rat H9c2 cardiac cells (Cell Bank of the Chinese
Academy of Sciences, Shanghai, China) were cultured in Dulbecco's
modified Eagle's medium (DMEM; cat. no. C11995; Gibco; Thermo
Fisher Scientific, Inc.), supplemented with 10% fetal bovine serum
(cat. no. SV30087.02; GE Healthcare Life Sciences, Logan, UT, USA),
100 U/ml penicillin and 100 mg/ml streptomycin (cat. no. 15140;
Gibco; Thermo Fisher Scientific, Inc.) in an atmosphere containing
5% CO2 at 37°C in an MCO-18 M incubator (Panasonic,
Tokyo, Japan). SSYX was dissolved in ddH2O at a
concentration of 10 mg/ml, prior to being filtered using a 0.2 mm
filter (cat. no. 1140503; Pall Corporation, Port Washington, NY,
USA) for bacteriological sterilization. The cells were divided at
2–3-day intervals once they reached 70–80% confluence. The cells
were subsequently seeded into six-well, 24-well or 96-well culture
plates for 24 h. The culture medium was then replaced with
serum-free DMEM for 12 h prior to experimentation at 37°C.
Subsequently, the cells were incubated with SSYX (0.1, 1, 10, 50
and 100 µg/ml) at 37°C, with or without 1 µM
angiotensin (Ang) II (cat. no. A9525; Sigma-Aldrich), for 24 h. The
cells were seeded at a density of 1×106 cells/well into
six-well culture plates for mRNA extraction, 4×103
cells/well in 96-well plates for cell viability assessment,
5.0×103 cells/well in 24-well plates for cell surface
area examination and 10×106 cells/well into 10 cm
diameter culture plates for protein extraction.
Cell viability assessment
To identify whether SSYX was toxic towards the H9c2
cells, cell viability was assessed using a Cell Couting kit 8
(CCK-8) assay (cat. no. ER612; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). Following treatment, 10 µl CCK-8
solution at a 1/10 dilution was added to each well, and the plate
was incubated at 37°C for 2.5 h. The absorbance was measured at 450
nm using a Bio-Tek plate reader (Synergy2 model; Bio-Tek
Instruments, Inc.). The means of the optical density (OD) of the
five wells were used to measure the percentage of cell viability,
according to the following equation: Cell viability (%) =
(ODtreatment − ODcontrol) × 100%.
Cell surface area
To identify the H9c2 cells and examine the levels of
cardiomyocyte hypertrophy, the cells were characterized using
immunofluorescence staining for cardiaca-actinin. Briefly, the
cells were washed with PBS, fixed with RCL2 (Alphelys, Plaisir,
France), permeabilized in 0.1% Triton X-100 (Amresco LLC, Solon,
OH, USA) in PBS and stained with human monoclonal anti-α-actinin
(cat. no. 05–384; Merck Millipore) at a dilution of 1:100 in 1%
goat serum overnight at 4°C. Subsequent to 5 washes with PBS, the
cells were then incubated with Alexa FluorH 568 goat anti-mouse IgG
monoclonal secondary antibody (cat. no. A11004; Invitrogen; Thermo
Fisher Scientific, Inc.) for 60 min at room temperature. Following
6 washes with PBS, the cells on coverslips were mounted onto glass
slides using SlowFade Gold antifade reagent with DAPI (cat. no.
S36939; Invitrogen; Thermo Fisher Scientific, Inc.). A single cell
was measured using the Image Pro-Plus 6.0 quantitative digital
image analysis system. The outline of 40 cells was traced for each
group.
Statistical analysis
Statistical analysis was conducted using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard error of the mean. Statistical
differences among the treatment groups were determined using
one-way analysis of variance followed by a Tukey's post-hoc test.
Comparisons between two groups were performed using unpaired
Student's t-test. P<0.05 was considered to considered to
indicate a statistically significant difference.
Results
SSYX attenuates pressure overload-induced
cardiac hypertrophy
To investigate whether SSYX has an antagonistic
effect on the hypertrophic response to pressure overload, mice were
subjected to pressure load via AB or sham surgery. SSYX prevented
the development of adverse cardiac remodeling and ventricular
dysfunction, as demonstrated by the HW/BW, HW/TL, LW/BW and LW/TL
ratios (Fig. 1A), and increased
cardiac mass and myocyte cross sectional area (Fig. 1B). The expression levels of ANP,
BNP and β-myosin heavy chain (MHC) hypertrophic markers following
AB surgery were also significantly decreased in the SSYX-treated
mice, whereas the level of α-MHC was significantly increased in the
SSYX-treated mice following AB (Fig.
1C). These results suggested that SSYX negatively regulated the
level of cardiac hypertrophy in response to the pressure
overload.
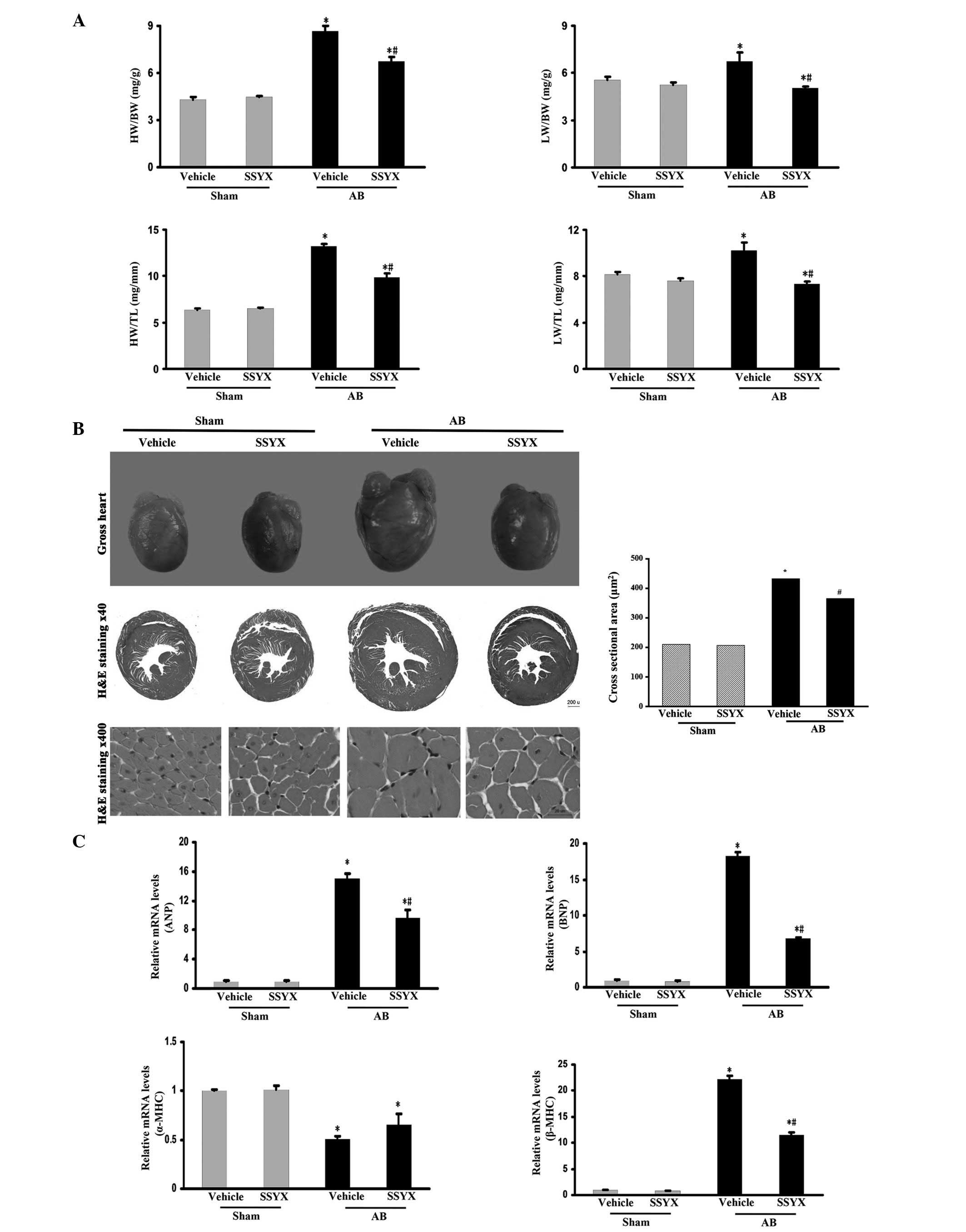 | Figure 1SSYX attenuates cardiac hypertrophy
induced by pressure-overload. (A) HW/BW, LW/BW, HW/TL and LW/TL
ratio of the various treatment groups. (B) Left, representative
histological images of gross heart size (upper) and hematoxylin and
eosin staining (middle, ×40 magnification; bottom, ×400
magnification) of vehicle- and SSYX-treated-mice 8 weeks following
sham or AB surgery; right, statistical results for the cell surface
area (n≥100 cells). The image indicates that SSYX prevented
increased cardiac mass and myocyte cross sectional area induced by
pressure overload. (C) Reverse transcription-quantitative
polymerase chain reaction analyses of the hypertrophic markers ANP,
BNP, β-MHC and α-MHC induced by AB in the mice (n=6). Data are
expressed as the mean ± standard error of the mean.
*P<0.05, vs. vehicle/sham group;
#P<0.05, vs. vehicle/AB group. SSYX, shensongyangxin;
AB, aortic banding; HW, heart weight; BW, body weight; LW, lung
weight; TL, tibial length; ANP, atrial natriuretic peptide; BNP,
brain natriuretic peptide; MHC, myosin heavy chain. |
SSYX attenuates cell hypertrophy in
vitro
To further validate the effect of SSYX on cardiac
hypertrophy, an Ang II (1 µM) in vitro model was used
in cultured H9c2 cells. The cytotoxicity of SSYX was assessed in
the absence of Ang II using a CCK-8 assay. No differences were
observed between the viabilities of the H9c2 cardiomyocytes treated
with different concentrations of SSYX (0.1, 1, 10, 50 and 100
µg/ml) and vehicle control group (Fig. 2A). These results suggested that
SSYX did not exert a cytotoxic effect on the H9c2 cardiomyocytes,
and further experiments were performed. Following stimulation with
Ang II (1 µM), the H9c2 cells exhibited enlarged cell
surface areas, compared with control group, and SSYX (1, 10 and 100
µg/ml) markedly attenuated this enlarged cell surface area
(Fig. 2B). The results of the
RT-qPCR analysis demonstrated that treatment with SSYX (1, 10 and
100 µg/ml) markedly decreased the Ang II (1
µM)-induced mRNA expression levels of ANP, BNP and β-MHC,
particularly following treatment with 10 and 100 µg/ml SSYX
(Fig. 2C). These findings were
concordant with the in vivo results, and suggested that SSYX
attenuated cardiac hypertrophy.
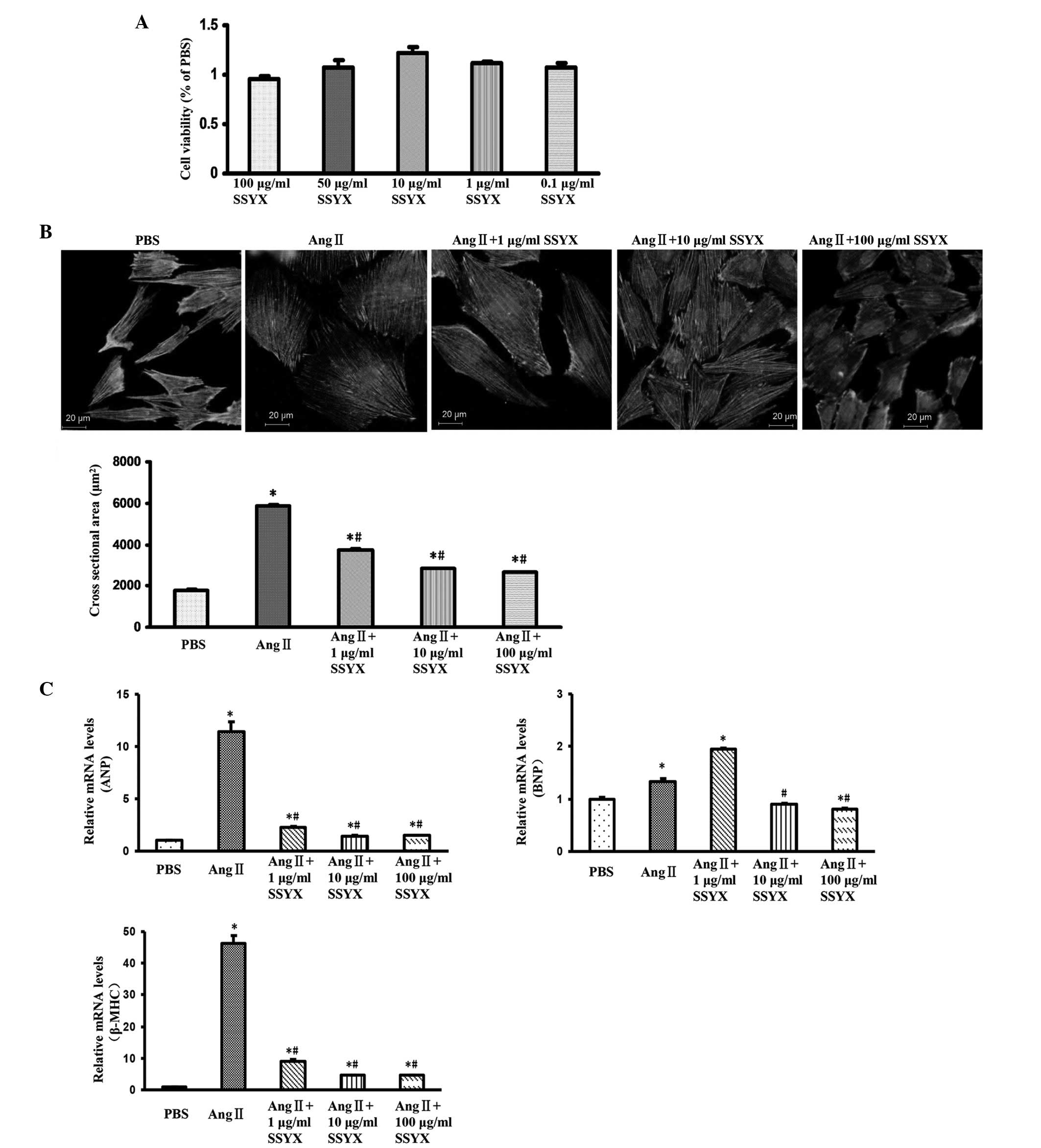 | Figure 2SSYX attenuates cell hypertrophy in
vitro. (A) Cell viability following treatment with various
concentrations of SSYX (0.1, 1, 10, 50 and 100 µg/ml). (B)
Top panel, representative images of cardiomyocytes treated with
various concentrations of SSYX (1, 10 and 100 µg/ml) in
response to Ang II (1 µM). Bottom panel, quantitative
results of the cell surface area (n≥100 cells). The images show
that SSYX (1, 10 and 100 µg/ml) markedly attenuated the
enlarged cell surface area induced by Ang II. (C) Reverse
transcription-quantitative polymerase chain reaction analysis of
the mRNA expression levels of ANP, BNP and β-MHC induced by Ang II
(1 µM) following treatment with various concentrations of
SSYX (1, 10 and 100 µg/ml) for 24 h. Data are expressed as
the mean ± standard error of the mean. *P<0.05, vs.
PBS-treated group; #P<0.05, vs. Ang II group. SSYX,
shensongyangxin; Ang, angiotensin; ANP, atrial natriuretic peptide;
BNP, brain natriuretic peptide; MHC, myosin heavy chain; PBS,
phosphate-buffered saline. |
SSYX inhibits activation of the Akt
signaling pathway following AB
To investigate the molecular mechanisms underlying
the antihypertrophic effects of SSYX, activation of the Akt
signaling pathway was examined. Pressure overload led to increases
in the phosphorylation of Akt, mTOR, GSK3, 4EBP1, p70, FOXO1 and
FOXO3a 8 weeks following surgery. Notably, SSYX significantly
attenuated these increased phosphorylation levels, as shown in
Fig. 3A and 3B. The in vitro data confirmed
that the phosphorylation levels of Akt, mTOR, GSK3, 4EBP1, p70,
FOXO1 and FOXO3a in the H9c2 cells following treatment with SSYX
(10 µM) were lower, thanthose in the cells treated with 1
µM Ang II (Fig. 3C and D).
These results demonstrated that SSYX significantly inhibited
cardiac hypertrophy via inhibition of the Akt signaling
pathway.
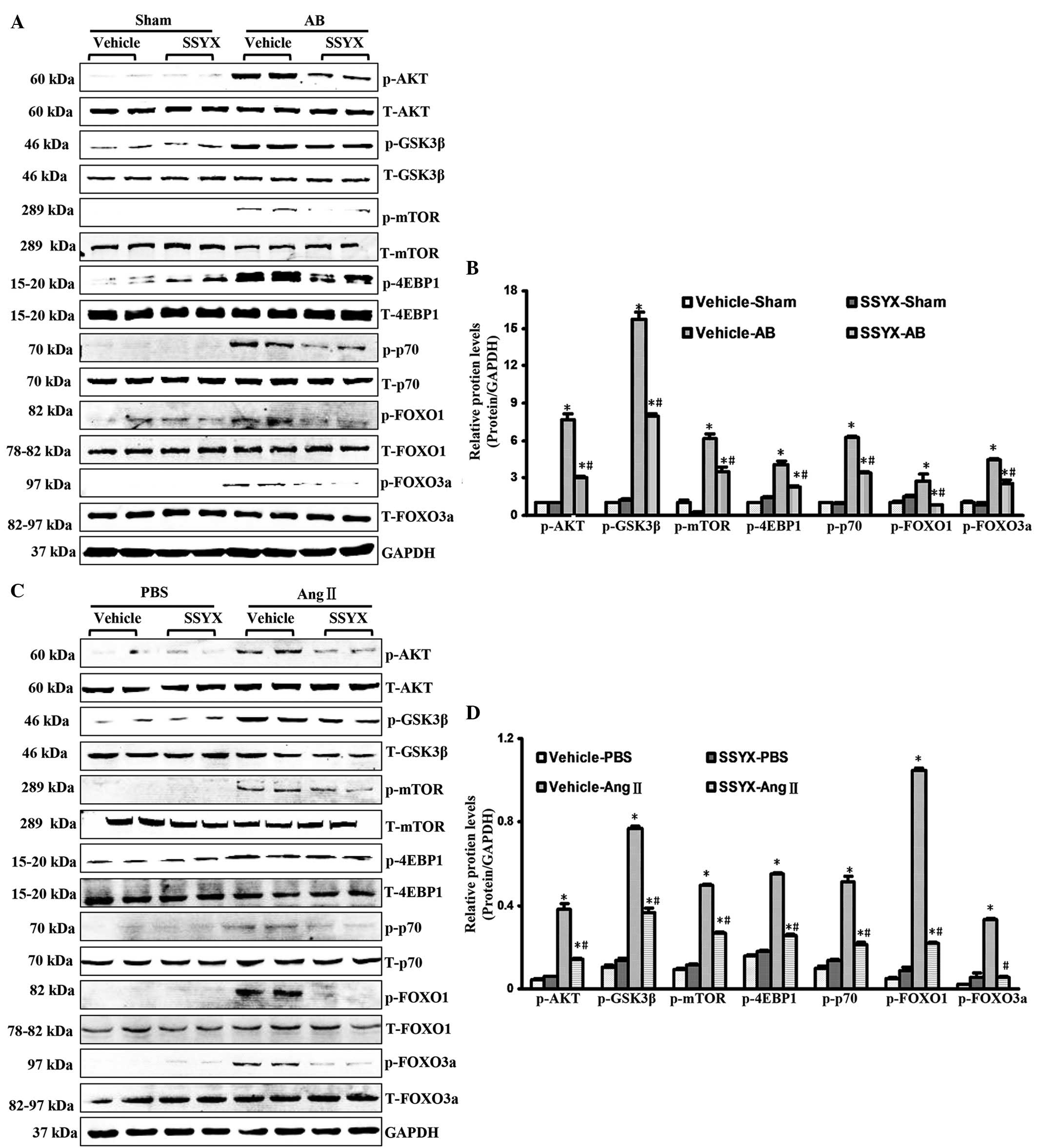 | Figure 3SSYX inhibits the activation of the
phosphoinositide 3-kinase/Akt signaling pathways following AB. (A)
Representative western blots of the phosphorylation and total
protein expression levels of Akt, GSK3β, mTOR, 4EBP1, p70, FOXO1
and FOXO3a in the vehicle- and SSYX-treated-mice 8 weeks following
AB. (B) Quantitative results of the protein expression levels
(n=6). Data are expressed as the mean ± standard error of the mean.
*P<0.05, vs. vehicle/sham group;
#P<0.05, vs. vehicle/AB group. (C) Representative
western blots of the phosphorylation and total protein expression
levels of Akt, GSK3β, mTOR, 4EBP1, p70, FOXO1 and FOXo3a in the
vehicle- and SSYX-treated H9c2 cardiomyocytes in response to Ang
II. (D) Quantitative results of the protein expression levels
(n=6). Data are expressed as the mean ± standard error of the mean.
*P<0.05, vs. vehicle/PBS group;
#P<0.05, vs. vehicle/Ang II group. SSYX,
shensongyangxin; AB, aortic banding; PBS, phosphate-buffered
saline; GSK3β, glycogen synthase kinase 3β; mTOR, mammalian target
of rapamycin; 4EBP1, 4E binding protein 1; FOX, forkhead box
protein; T-, total; p-, phosphorylated. |
SSYX inhibits pressure overload-induced
cardiac fibrosis
To evaluate the levels of fibrosis in the heart
following exposure to SSYX, the paraffin-embedded slides were
stained with PSR. Perivascular and interstitial fibrosis were
observed in the vehicle and SSYX-treated mice subjected to AB,
however, the extent of cardiac fibrosis was significantly reduced
in the SSYX-treated mice (Fig. 4A and
B). The expression levels of myocardial pro-fibrotic genes 8
weeks following surgery were also examined. As shown in Fig. 4C, the pressure overload-induced
expression of TGFβ1, CTGF, fibronectin, collagen I and collagen III
were decreased by SSYX. Therefore, the present study investigated
the TGFβ/Smad signaling pathway. The expression levels of TGFβ and
Smad4 were increased by pressure overload, and treatment with SSYX
significantly decreased these expression levels (Fig. 4D). These results indicated that
SSYX significantly attenuated cardiac fibrosis through inhibition
of the TGFβ/Smad signaling pathway.
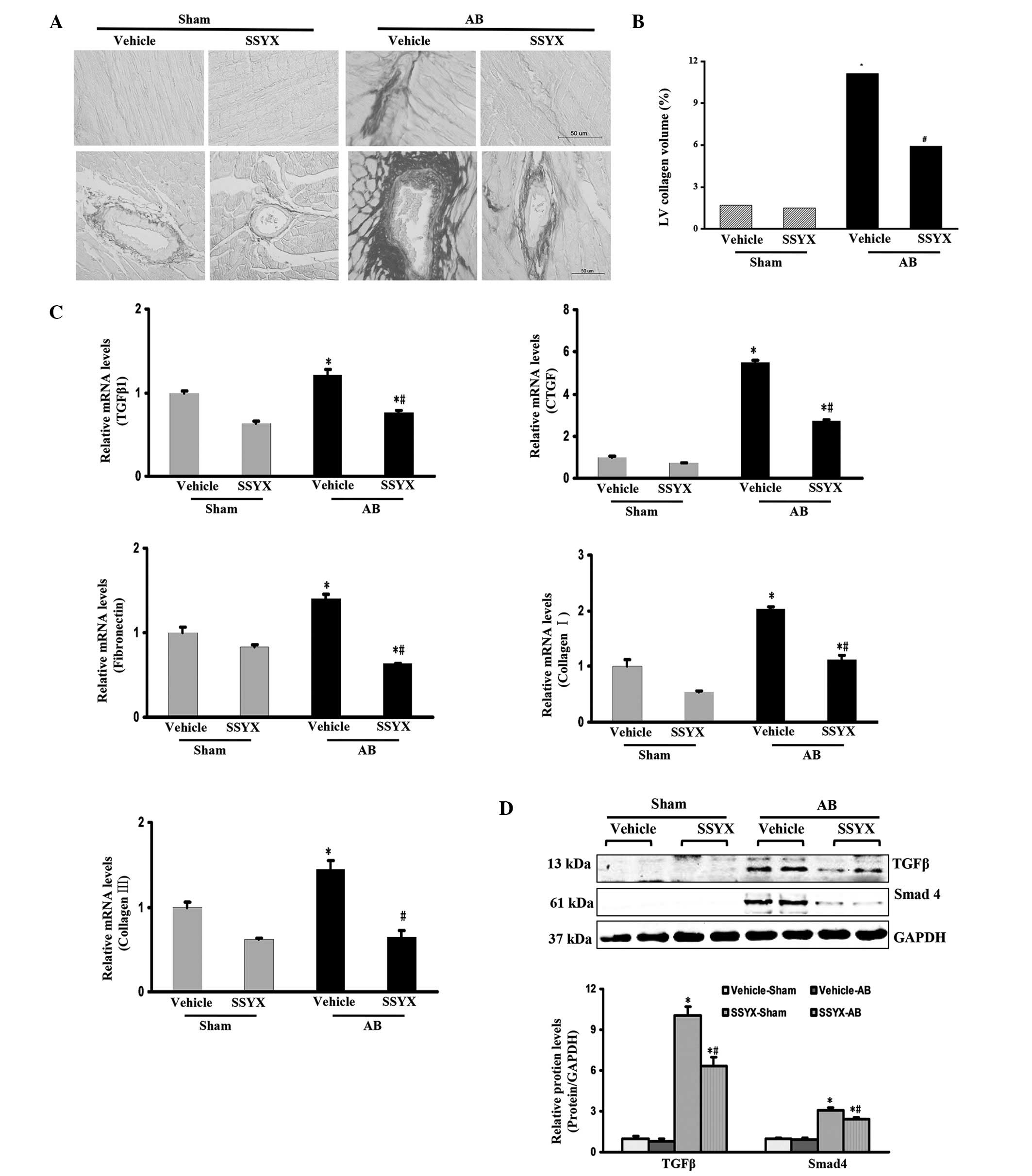 | Figure 4SSYX inhibits cardiac fibrosis induced
by pressure overlord. (A) Picrosirius red staining of the
histological sections of the left ventricule in the various
treatment groups eight weeks following AB (n=6; scale bar, 50
µm). Upper, interstitial fibrosis (magnification, ×400);
bottom, perivascular fibrosis (magnification, ×200). The images
show that the extent of cardiac fibrosis was significantly reduced
in the SSYX-treated mice. (B) Quantitative results of the
expression levels of the proteins. (C) Reverse
transcription-quantitative polymerase chain reaction analysis of
the mRNA expression levels of TGFβ1, CTGF, and fibrotic markers
fibronectin, collagen Iα and collagen III in the mice (n=6). (D)
Representative western blots demonstrating the protein expression
of TGFβ and Smad4. Top panel: Representative western blots; bottom
panel: Quantitative results (n=6). *P<0.05, vs. the
vehicle/sham group; #P<0.05, vs. the vehicle/AB
group. SSYX, shensongyangxin; AB, aortic banding; TGF, transforming
growth factor; CTGF, connective tissue growth factor. |
Discussion
SSYX is a compound of the traditional Chinese
Materia medica, consisting of 12 ingredients, and has attracted
considerable attention due to its anti-arrhythmic properties
(9–11). Previous studies have suggested that
SSYX exhibits protective effects on the cardiovascular system
(12,13). However, the role of SSYX in
pressure overload-induced cardiac remodeling remains to be
elucidated. The present study demonstrated that treatment with SSYX
attenuated the remodeling process of the heart in response to
pressure overload, including cardiac hypertrophy and interstitial
fibrosis. In addition, treatment with SSYX attenuated cardiomyocyte
hypertrophy induced by Ang II in vitro. To the best of our
knowledge, the present study is the first to demonstrate the
important role of SSYX in the regulation of cardiac hypertrophy and
fibrosis.
Cardiac hypertrophy, which is observed in a wide
variety of clinical conditions, is a major pathological process of
heart failure (7). Several
investigations have been performed to elucidate the mechanisms that
stimulate or prevent hypertrophy, identifying a variety of
signaling pathways and transcription factors, however, these
mechanisms remain to be fully elucidated (16,17).
Akt is important in cardiac hypertrophy of the tyrosine kinase
receptor signaling pathways, which regulate cell morphology, cell
survival, angiogenesis and inflammation in cardiomyocytes (5,18).
Cardiac-specific overexpression of a constitutively active form of
Akt leads to significant cardiac hypertrophy, which may be due to
increased cardiomyocyte size (16). GSK3β, which is located downstream
of Akt, is inactivated by the phosphorylation of serine 9 under
hypertrophic conditions, and has been shown to be a negative
regulator of cardiomyocyte hypertrophy (14,19).
Among the downstream effectors of Akt, mTOR is activated by Akt
phosphorylation (16). mTORC1, a
complex form of mTOR, stimulates protein synthesis through the
eukaryotic initiation factor, 4E-BP1, and p70S6 kinase during
protein translation, and the acceleration of protein translation
can enhance cell growth and mass (16,20).
To examine the molecular mechanisms underlying the protective
effects of SSYX against cardiac hypertrophy, the activity of Akt
signaling was examined in the hypertrophic models. The results
demonstrated that Akt signaling activation was inhibited in the
SSYX-treated hearts and H9c2 cells in response to chronic pressure
overload and stimulation with Ang II.
The forkhead box O (FOXO) transcription factor, a
downstream target of the Akt signaling pathway, comprises three
members: FOXO1 (FKHR), FOXO3a (FKHRL-1) and FOXO4 (AFX), all of
which are inactivated by Akt (17). Phosphorylation by Akt leads to
nuclear exclusion and inhibits the forkhead transcriptional program
(17). FOXO transcription factors
regulate key physiological functions, including responses to
stress, cell-cycle progression, protein degradation and apoptosis
(17). A previous study
demonstrated that overexpression of either FOXO1 or FOXO3 decreased
calcineurin phosphatase activity, and hearts from FOXO3-null mice
manifest a hypertrophic phenotype (21). Therefore, the effects of SSYX on
the expression levels of FOXO1 and FOXO3a were further examined
in vivo and in vitro in the present study. The
findings indicated that the inhibitory effects of SSYX on cardiac
hypertrophy were mediated through Akt signaling.
Cardiac fibrosis is an important hallmark of
maladaptive hypertrophy and heart failure, and is characterized by
an increase in the levels of collagen and other extracellular
matrix (ECM) components in the interstitium and perivascular
regions of the myocardium (6).
TGFβ1 is an important regulator of ECM metabolism in various organs
(22). ECM production in the heart
is regulated by TGFβ. Canonical signaling via TGFβ receptors
activates Smad proteins, which phosphorylate Smad2/3 on two serine
residues at their C-terminus and allows binding to Smad4 to form
heteromeric Smad complexes. These enter the nucleus to initiate
gene transcription (23). An
important profibrotic target gene of TGFβ signaling is CTGF, which
is essential for TGFβ-induced collagen synthesis (24). The antifibrotic effects of SSYX
were demonstrated in the present study by the significant decreases
in the mRNA expression levels of TGFβ1, CTGF, and ECM proteins,
including col1agen Ia, col1agen III and fibronectin in the
SSYX-treated mice. The expression levels of TGFβ1 and Smad4 were
also decreased, suggesting that activation of the TGFβ1/Smad
signaling pathway following AB was attenuated by SSYX, which may
mediate the antifibrotic effects of SSYX.
In conclusion, the present study demonstrated that
SSYX exerted protective effects against cardiac hypertrophy and
fibrosis in response to chronic pressure overload and Ang II
stimulation by regulating the Akt and TGFβ1/Smad signaling
pathways. These results provide experimental evidence for the
potential application of SSYX in the treatment of cardiac
remodeling and HF.
Acknowledgments
The current study was supported by the National Key
Basic Research Program of China (973 Program; grant no.
2012CB518606) and grants from the Fundamental Research Funds for
the Central Universities of China (grant no. 2014302020202).
References
|
1
|
Rosca MG, Tandler B and Hoppel CL:
Mitochondria in cardiac hypertrophy and heart failure. J Mol Cell
Cardiol. 55:31–41. 2013. View Article : Google Scholar :
|
|
2
|
Foryst-Ludwig A and Kintscher U: Sex
differences in exercise-induced cardiac hypertrophy. Pflugers Arch.
465:731–737. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abel ED and Doenst T: Mitochondrial
adaptations to physiological vs. pathological cardiac hypertrophy.
Cardiovasc Res. 90:234–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hou J and Kang Y: Regression of
Pathological Cardiac Hypertrophy: Signaling pathways and
therapeutic targets. Pharmacol Ther. 135:337–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pillai VB, Sundaresan N and Gupta MP:
Regulation of Akt signaling by sirtuins: Its implication in cardiac
hypertrophy and aging. Circ Res. 114:368–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Creemers EE and Pinto Y: Molecular
mechanisms that control interstitial fibrosis in the
pressure-overloaded heart. Cardiovasc Res. 89:265–272. 2011.
View Article : Google Scholar
|
|
7
|
Oka T, Akazawa H, Naito AT and Komuro I:
Angiogenesis and cardiac hypertrophy: Maintenance of cardiac
function and causative roles in heart failure. Circ Res.
114:565–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu ZHJ, Hu R, Huo Y, Gong J, Zhang Y, Wei
C and Pu J: Gene expression profile of increased heart rate in
shensongyangxin-treated bradycardia rabbits. Evid Based Complement
Alternat Med. 2014:7159372014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Li N, Jia Z, Lu F and Pu J: Chinese
medicine shensongyangxin is effective for patients with
bradycardia: Results of a randomized, double-blind,
placebo-controlled multicenter trial. Evid Based Complement
Alternat Med. 2014:6057142014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang AH, Pu JL, Qi XY, Miao WL, Hou ZS,
Cong HL, Zhou JZ, Liu XF, Li SM and Han QH: Evaluation of
shensongyangxin capsules in the treatment of paroxysmal atrial
fibrillation: A randomized, double-blind and controlled multicenter
trial. Zhonghua Yi Xue Za Zhi. 91:1677–1681. 2011.In Chinese.
PubMed/NCBI
|
|
11
|
Zou JG, Zhang J, Jia ZH and Cao KJ:
Evaluation of the traditional Chinese Medicine Shensongyangxin
capsule on treating premature ventricular contractions: A
randomized, double-blind, controlled multicenter trial. Chin Med J
(Engl). 124:76–83. 2011.
|
|
12
|
Yang Z, Yu X and Yu ML: Effects of
shensongyangxin capsule on heart rate turbulence and heart rate
variability in chronic heart failure. Chin Med J (Engl).
126:4389–4391. 2013.
|
|
13
|
Chai S, Wang S, Yao L, Wu A, Liu Y and Rao
C: Effect of shensongyangxin capsule on myocardial remodeling and
ventricular fibrillation threshold value in rat with coronary
artery ligation. Zhongguo Zhong Yao Za Zhi. 34:2101–2104. 2009.In
Chinese. PubMed/NCBI
|
|
14
|
Yuan Y, Zong J, Zhou H, Bian ZY, Deng W,
Dai J, Gan HW, Yang Z, Li H and Tang QZ: Puerarin attenuates
pressure overload-induced cardiac hypertrophy. J Cardiol. 63:73–81.
2014. View Article : Google Scholar
|
|
15
|
Yan L, Wei X, Tang QZ, Feng J, Zhang Y,
Liu C, Bian ZY, Zhang LF, Chen M, Bai X, et al: Cardiac-specific
mindin overexpression attenuates cardiac hypertrophy via blocking
AKT/GSK3β and TGF-β1-Smad signalling. Cardiovasc Res. 92:85–94.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aoyagi T and Matsui T: Phosphoinositide-3
kinase signaling in cardiac hypertrophy and heart failure. Curr
Pharm Des. 17:1818–1824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Skurk C, Izymiya Y, Maatz H, Razeghi P,
Shiojima I, Sandri M, Sato K, Zeng L, Schiekofer S, Pimentel D, et
al: The FOXO3a transcription factor regulates cardiac myocyte size
downstream of AKT signaling. J Biol Chem. 280:20814–20823. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu W, Chen C, Fu Y, Wang X and Wang W:
Insulin signaling: A possible pathogenesis of cardiac hypertrophy.
Cardiovasc Ther. 28:101–105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu QQ, Zonj J, Gao L, Dai J, Yang Z, Xu M,
Fang Y, Ma ZG and Tang QZ: Sulforaphane protects H9c2
cardiomyocytes from angiotensin II-induced hypertrophy. Herz.
39:390–396. 2014. View Article : Google Scholar
|
|
20
|
Maillet M, van Berlo JH and Molkentin JD:
Molecular basis of physiological heart growth: Fundamental concepts
and new players. Nat Rev Mol Cell Biol. 14:38–48. 2013. View Article : Google Scholar
|
|
21
|
Ni YG, Berenji K, Wang N, Oh M, Sachan N,
Dey A, Cheng J, Lu G, Morris DJ, Castrillon DH, et al: FOXo
transcription factors blunt cardiac hypertrophy by inhibiting
calcineurin signaling. Circulation. 114:1159–1168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edgley AJ, Krum H and Kelly DJ: Targeting
fibrosis for the treatment of heart failure: A role for
transforming growth factor-β. Cardiovasc Ther. 30:e30–40. 2014.
View Article : Google Scholar
|
|
23
|
Kamato D, Burch M, Piva TJ, Rezaei HB,
Rostam MA, Xu S, Zheng W, Little PJ and Osman N: Transforming
growth factor-β signalling: Role and consequences of Smad linker
region phosphorylation. Cell Signal. 25:2017–2024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szabó Z, Magga J, Alakoski T, Ulvila J,
Piuhola J, Vainio L, Kivirikko KI, Vuolteenaho O, Ruskoaho H,
Lipson KE, et al: Connective tissue growth factor inhibition
attenuates left ventricular remodeling and dysfunction in pressure
overload-induced heart failure. Hypertension. 63:1235–1240. 2014.
View Article : Google Scholar : PubMed/NCBI
|


















