Introduction
Skin aging is a complex biological process, which is
affected by a combination of endogenous/intrinsic and extrinsic
factors. Intrinsic aging involves genetics, cellular metabolism,
hormones and metabolic processes, whereas extrinsic aging is
affected by chronic light exposure, pollution, ionizing radiation,
chemicals and toxins (1).
Extrinsic skin aging is characterized by elastosis in the upper
dermis, destruction of fibrillar structure, augmentation of
intercellular substances and moderate infiltration of inflammatory
mediators (2).
Extrinsic aging is primarily associated with
exposure to environmental factors, including ultraviolet (UV)
irradiation. Chronic UV irradiation damages skin proteins and
induces wrinkle formation, dryness, roughness, shallowness and
histological changes in humans and animals (3,4).
Furthermore, chronic UV irradiation can result in edema, erythema,
inflammation, hyperpigmentation, hyperplasia and photo-aging
(5). Certain age-associated skin
lesions have also been associated with UV irradiation, including
actinic keratosis and non-melanoma skin cancer, including basal
cell carcinoma and squamous cell carcinoma (2,6).
These pathogenic changes may be associated with UV
irradiation-induced dermal alterations, including the excessive
secretion of matrix metalloproteinases (MMPs), which degrade
collagen and other extracellular matrix proteins (7).
Collagen is an important extracellular component in
the skin and is comprised of a highly repetitive sequence of
glycines (8). Dermal collagen is a
major component of the skin dermis and is required to maintain skin
structure; with type I collagen being the most abundant subtype of
collagen found in the dermis (8).
UV irradiation-induced abnormalities in the metabolism of skin
collagen are the predominant causes of skin photoaging (9). The UV-induced reduction of type I
collagen in the dermis is widely considered the primary cause of
the wrinkled appearance observed in skin photoaging (9).
MMP-9 is a 92-kDa gelatinase, which degrades
collagen IV, and is one of the primary components of the basement
membrane (10). Mitogen-activated
protein kinases (MAPKs), including extracellular signal-regulated
kinase (ERK), c-Jun N-terminal kinase and p38, are known to be
involved in the regulation of MMP-9 transcription (11). Increased expression of MMP-9,
resulting from the activation of specific transcription factors,
has been shown to degrade collagen and elastin in the skin
(11), potentially contributing to
the effects of UV-induced skin damage and photoaging.
Betaine is a naturally occurring compound, which is
widely distributed in plants, microorganisms, several types of food
and medicinal herbs, including Lycium chinense, which has
been demonstrated to have high levels of betaine (12). Notably, betaine has been reported
to be beneficial for a number of conditions and diseases, including
heart and liver disease (13,14).
In the course of screening photoprotective agents based on
antioxidant activity, our previous investigation evaluated whether
betaine, exhibiting antioxidant properties, may be applied for
photoprotection, regardless of known traditional medicines
(15). However, whether betaine
has protective effects on UV-induced skin damage and photoaging
remains to be fully elucidated.
In the present study, male HR-1 hairless mice were
used to assess the therapeutic effects of betaine on photoaging by
evaluating various parameters of photoaging following exposure to
UVB irradiation. The aim of the investigation was to determine
whether betaine exhibits a protective effect on UVB-induced skin
damage.
Materials and methods
Materials
Betaine was purchased from Wako Pure Chemical
Industries, Ltd. (Tokyo, Japan). A total of 18 HR-1 hairless male
mice (6 weeks-old) were purchased from Japan SLC, Inc. (Shizuoka,
Japan). UVB irradiation was induced using a UVM-225D Mineralight UV
Display Lamp (UVP, LLC, Phoenix, AZ, USA). Replicas of mouse dorsal
skin were obtained using a Repliflo Cartridge kit (CuDerm
Corporation, Dallas, TX, USA). Antibodies targeting MMP-9 (92 kDa),
phosphorylated (p)-MAPK kinase (p-MEK; 45 kDa), MEK (45 kDa), pERK
1/2 (42,44 kDa), and ERK 1/2 (42, 44 kDa) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Secondary antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Experimental animals
HR-1 hairless male mice were allowed to acclimate
for one week prior to study. All experimental protocols were
approved by the Korea Institute of Oriental Medicine Institutional
Animal Care and Use Committee (11-061; Daejeon, Korea). The animals
were housed under constant temperature (24°C) in an atmosphere
containing 50% relative humidity with a 12 h light/dark cycle, and
were provided with access to food and water ad libitum. The
mice were divided into the following three groups: Control (n=5),
UVB-treated vehicle (n=5) and UVB-treated betaine (n=5). The mice
in the UVB-treated vehicle and UVB-treated betaine groups were
exposed to UV irradiation, as described below, whereas the control
animals were not exposed to irradiation. The mice in the
UVB-treated betaine group were administered orally with 0.1 ml
water containing 100 mg betaine/kg body weight/day. As controls,
the mice in the UVB-treated vehicle group were provided with
drinking water only, whereas animals in the control group received
no administration
UVB irradiation
Mice were subjected to UVB irradiation using a
UVM-225D Mineralight UV Display Lamp, which emitted radiation at a
wavelength of 302 nm. The UV strength was measured using an
HD2102-2 UV meter (Delta Ohm, Padova, Italy). For the in
vivo experiments, UVB radiation was applied to the backs of the
mice three times each week (Monday, Wednesday, Friday) for 12
weeks. The level of irradiation was progressively increased between
60 mJ/cm2/exposure at week 1 (one minimal erythematous
dose = 60 mJ/cm2) and 90 mJ/cm2/exposure at
week 7.
Generation of skin replicas and image
analysis
Replicas of mouse dorsal skin were obtained using a
Repliflo Cartridge kit. Viscous spreadable fluid resin was applied
to the skin surface. After drying and hardening, a solid replica
was obtained from the skin. Wrinkle shadows from the impression
replicas were produced by illuminating the replica on a horizontal
stand with a light source angle of 35°, and images were recorded
and analyzed using Skin Visiometer VL 650 software (Courage +
Khazaka Electronic GmbH, Cologne, Germany). The parameters used for
the assessment of skin wrinkles included the average length, depth
and number of wrinkles.
Histological examination
Mice were anesthetized with intraperitoneal
administration of a diluted solution (1:4 in phosphate-buffered
saline) composed of a 2:1 mixture of 30 mg/kg Zoletil (Virbac,
Carros, France) and 10 mg/kg Rompun (Bayer, Leverkusen, Germany).
Following anesthesia, the middle of the dorsal skin was fixed in
10% neutral buffered formalin (Sigma-Aldrich, St. Louis, MO, USA),
embedded in paraffin (Leica, Vienna, Austria) and sectioned at 5
μm thickness. The sections were stained with hematoxylin and
eosin (H&E; YD Diagnostics Corp. Kyunggi-Do, Korea), and
Masson's trichrome staining (YD Diagnostics Corp.) was used for
collagen fiber analysis. The thickness of the epidermis was
measured under a light microscope using an eyepiece micrometer
(AX-70; Olympus Corporation, Tokyo, Japan).
Western blot analysis
Proteins were extracted from the skin tissue samples
using a Precellys 24 homogenization system (Bertin Technologies,
Montigny-le-Bretonneux, France). Total protein concentration was
determined using a DC™ Protein Assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) with 2 mg/ml bovine serum albumin standard
ampules (Thermo Fisher Scientific, Inc., Loughborough, UK). as a
standard. A total of 20 μg protein from each sample was
electrophoresed on 12% SDS-PAGE (Bio-Rad Laboratories, Inc.). The
proteins were then transferred to polyvinylidene difluoride
membranes (Bio-Rad Laboratories, Inc.), and the membranes were
blocked for 1 h at 25°C in 5% skimmed milk. The blots were then
incubated overnight at 4°C with the appropriate monoclonal
antibodies diluted to 1:1,000: Anti-p-MEK 1/2 (polyclonal; cat. no.
9121), anti-MEK 1/2 (polyclonal; cat. no. 9122), anti-p-ERK 1/2
(polyclonal; cat. no. 9101), anti-ERK 1/2 (polyclonal; cat. no.
9102), anti-MMP-9 (polyclonal; cat. no. 3852) (all from Cell
Signaling Technology, Inc.) and anti-β-actin (polyclonal; cat. no.
sc1616; Santa Cruz Biotechnology, Inc.). The blots were washed
three times for 10 min each with Tris-buffered saline containing 1%
Tween-20 (Bio-Rad Laboratories, Inc.). The membranes were then
incubated for 2 h at room temperature with anti-rabbit and
anti-goat secondary antibodies (Santa Cruz Biotechnology, Inc.).
The proteins were detected using enhanced chemiluminescence
reagents (Bio-Rad Laboratories, Inc.). Images were captured and
analyzed using ImageQuant LAS 4000 Multi Gauge software (Fuji Photo
Film Co., Ltd., Tokyo, Japan).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean of at least three independent experiments. One-way
analysis of variance and Turkey multiple comparisons test were used
to analyze the results using Prism 5.0 software (GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant result.
Results
Betaine inhibits UVB-induced wrinkle
formation
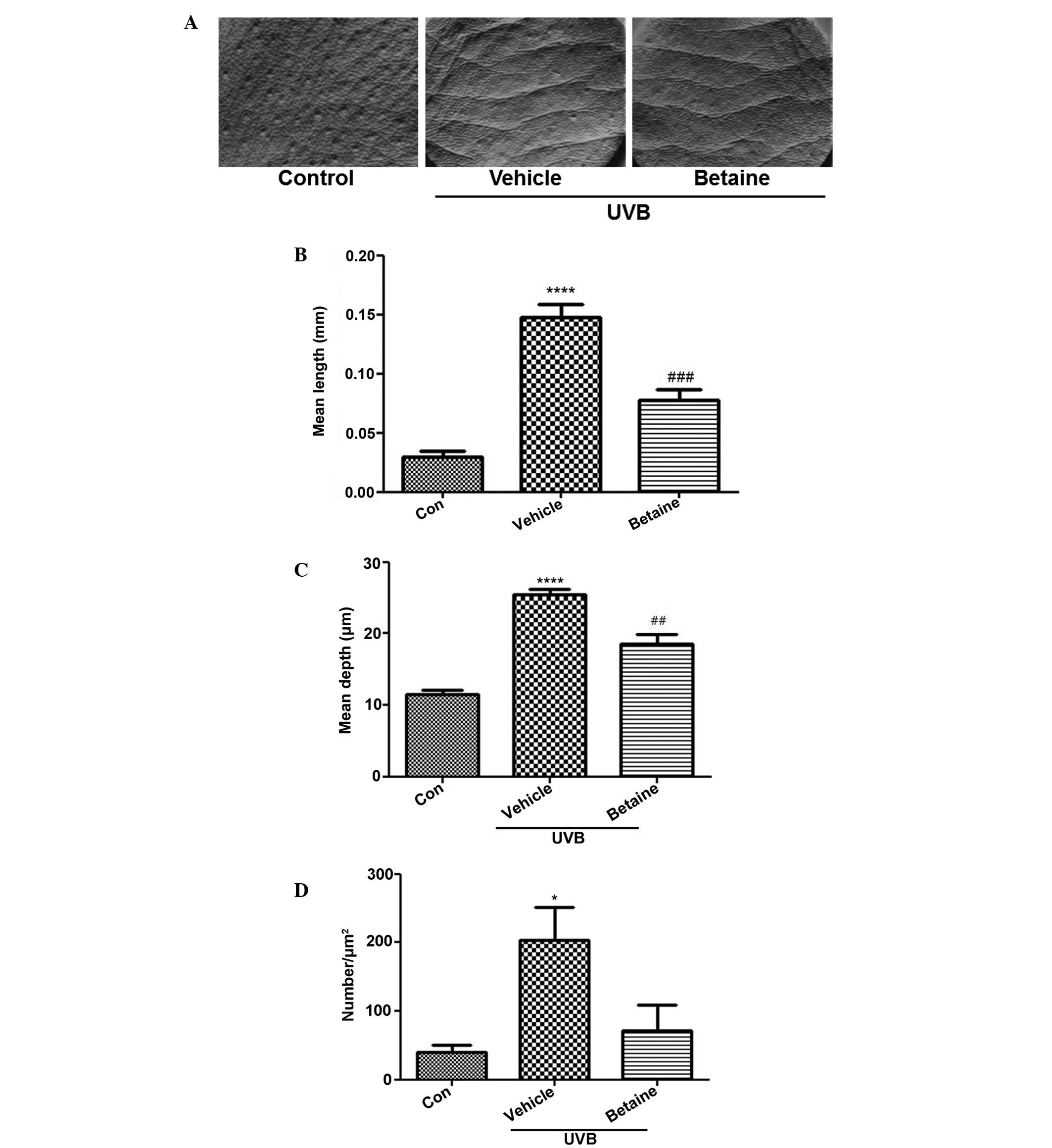
Wrinkle formation was observed in the dorsal region
of hairless mice. UVB exposure induced skin wrinkles, however,
treatment with betaine inhibited wrinkle formation (Fig. 1A). To evaluate the inhibitory
activity of betaine on wrinkles, replicas of the dorsal skin were
analyzed using an image analysis system to quantify the degree of
wrinkle formation. The mean length and depth of the wrinkles in the
UVB-treated groups were significantly increased, compared with
those in the control group (Fig. 1B
and C). Treatment with betaine significantly reduced the mean
length and depth of skin wrinkles. In addition, the number of
wrinkles was also lower in the betaine-treated group, compared with
the vehicle-treated group (Fig.
1D).
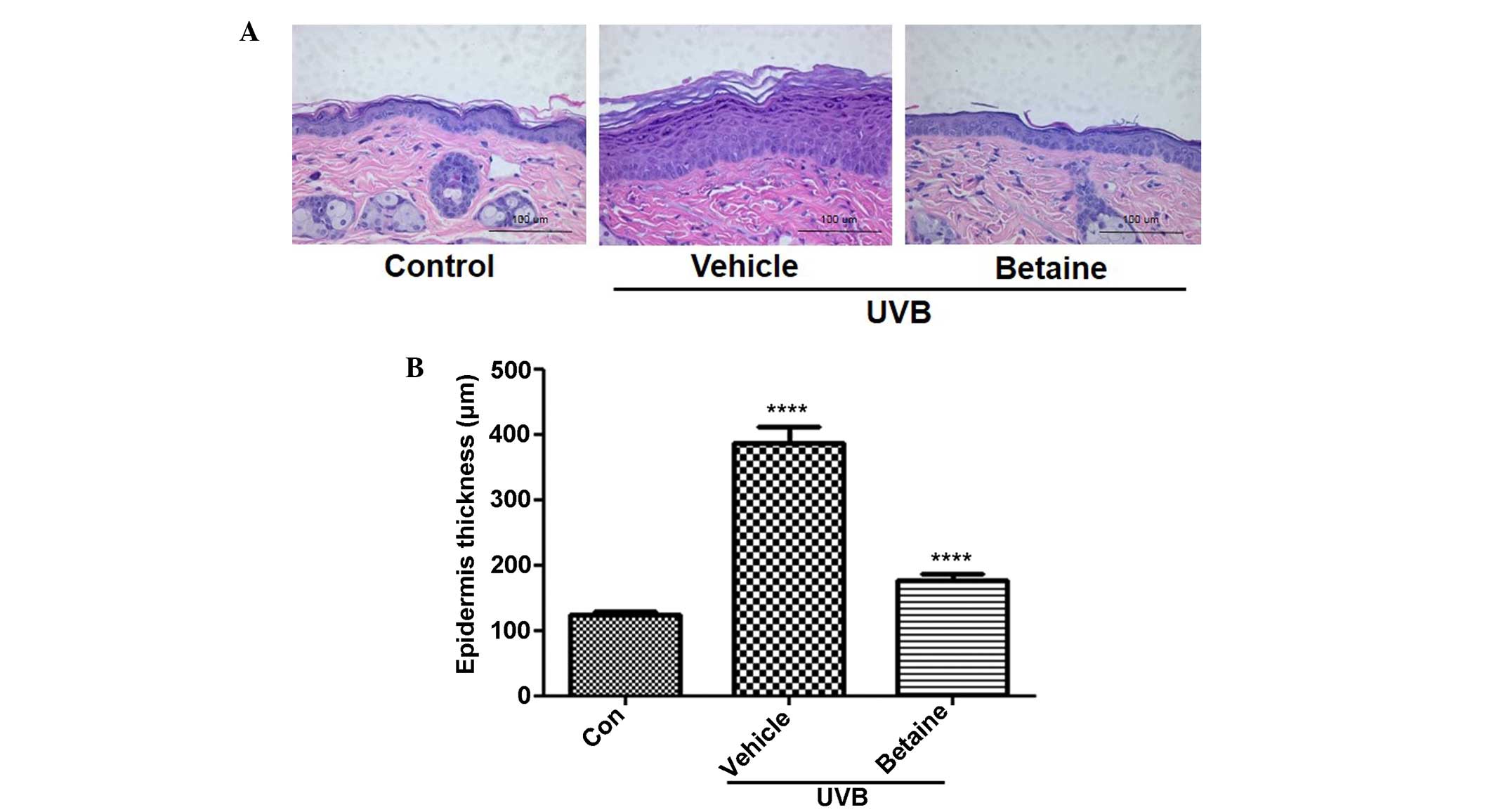
Betaine decreases the thickness of the
epidermis in UVB-induced hairless mice
The effects of betaine on the changes in epidermal
thickness in UVB-irradiated hairless mice were subsequently
examined. Histological observation revealed that the thickness of
the epidermis was significantly increased to 387.64 μm
following UVB irradiation, as shown by H&E staining (Fig. 2A). Compared with the UVB-treated
vehicle group, betaine treatment significantly inhibited the
increase in epidermal thickness (176.94 μm; Fig. 2B).
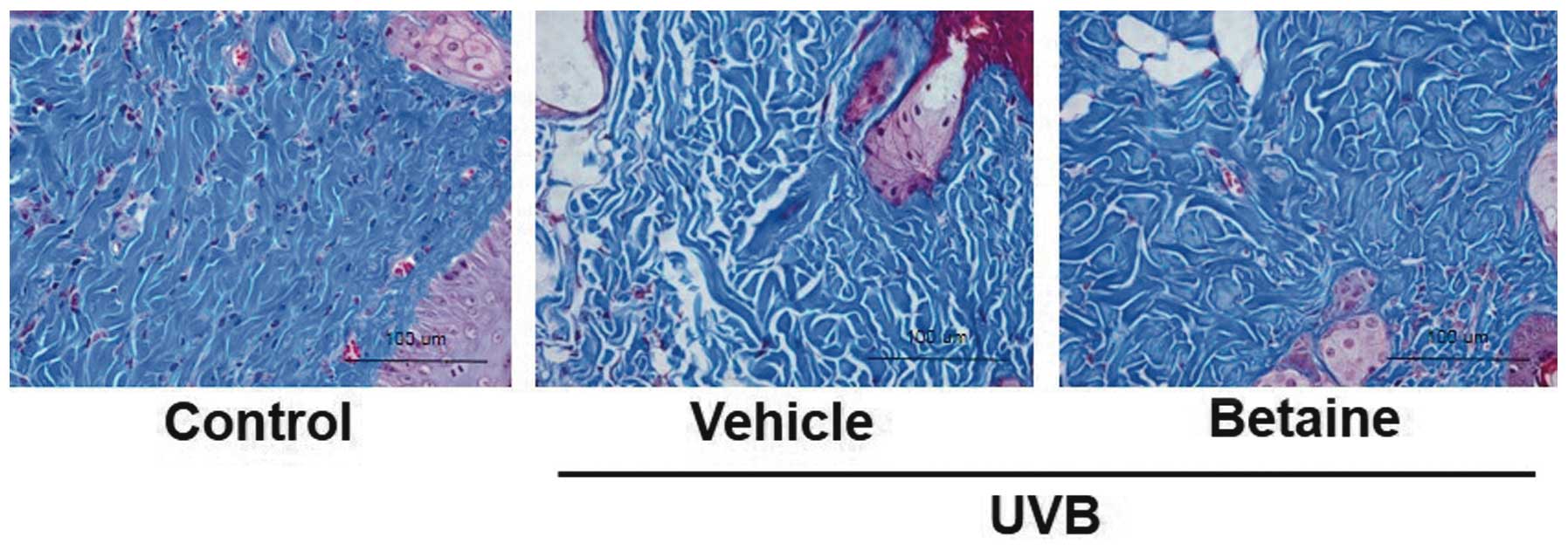
Betaine reduces collagen fiber damage in
UVB-irradiated hairless mice
To visualize changes in collagen fibers in the
dermal areas, histological sections of skin were subjected to
Masson's trichrome staining. The collagen fibers were stained blue
in the dermal areas (Fig. 3).
Compared with the control group, the UVB-irradiated vehicle group
exhibited a decrease in the abundance and density of collagen
fibers. However, the collagen fibers in the betaine-treated group
exhibited less collagen fiber damage, compared with those in the
UVB-irradiated vehicle-treated mice.
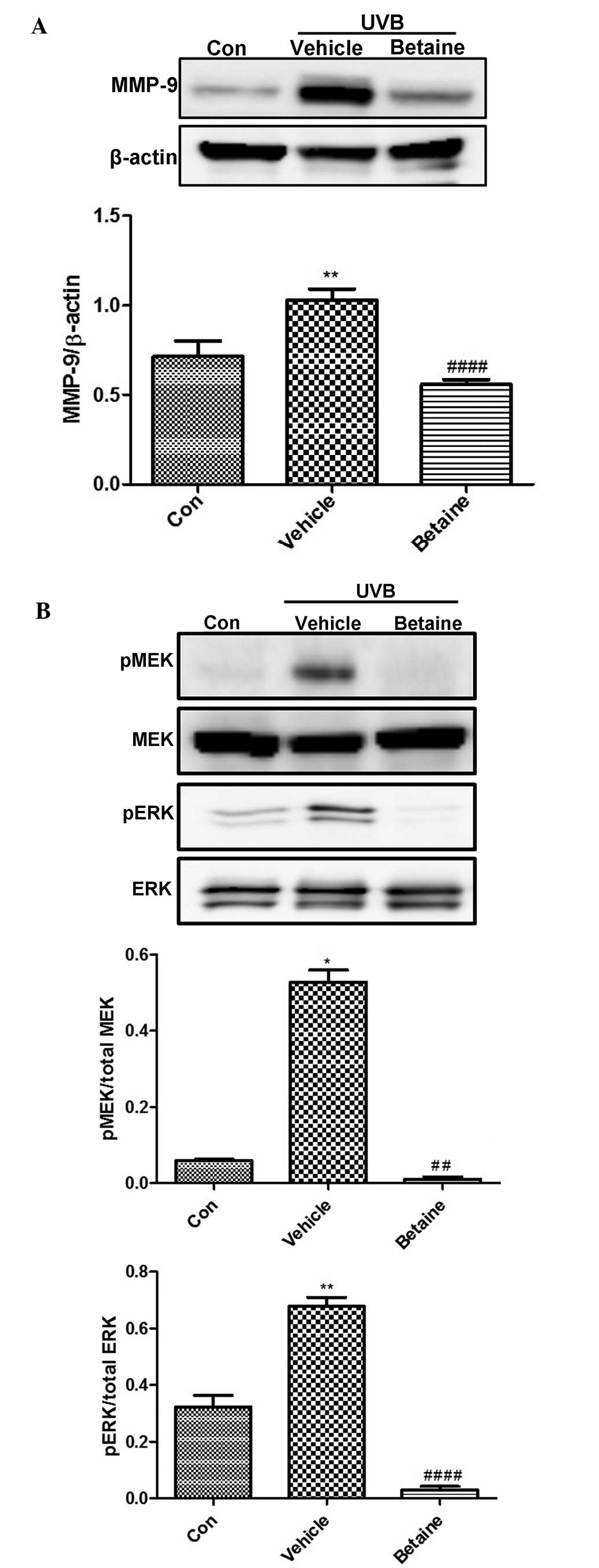
Betaine inhibits the expression of MMP-9
and the phosphory- lation of MEK and ERK in UVB-irradiated hairless
mice
The effects of betaine on the expression and
activity levels of several important modulators of photoaging were
also investigated in the present study. UVB irradiation induced the
expression of MMP-9 (Fig. 4A).
However, betaine protected against UVB-induced photodamage by
suppressing the expression of MMP-9. In addition, betaine inhibited
the UVB-induced increase in the phosphorylation of MEK and ERK
(Fig. 4B). These data indicated
that betaine inhibited UVB-induced MEK and ERK activation.
Discussion
UV irradiation increases collagenase activity and
reduces the production of collagen, resulting in wrinkle formation
through the degradation of collagen in the dermal extracellular
matrix (16). Exposure to UV light
produces free radicals, releasing pro-inflammatory cytokines and
growth factors, which activate proteases that degrade collagen
elastin (17). The present study
investigated the effects of betaine on UV irradiation-induced skin
aging, particularly on the development of skin wrinkles, in a
hairless mouse model.
Collagen and elastin protein fibers, the two main
components of the dermis, act as a structural support system and
provide the skin with strength and resilience (18). Following exposure to UV
irradiation, dermal damage is predominantly manifested
histologically as the disorganization of collagen fibrils and the
accumulation of abnormal elastin-containing material (19). Exposure of the human skin to acute
UV irradiation induces the expression of several MMPs, which
degrade collagen fibrils and other components of the dermal
extracellular matrix (20,21). These pathological changes can lead
to the formation of skin wrinkles (22,23).
In the mouse model used in the present study, UVB-irradiated murine
skin exhibited increased wrinkle formation, and betaine inhibited
this effect, suggesting that betaine may prevent UVB-associated
collagen damage. Reduced damage to collagen fibers in the
betaine-treated mice was observed, supporting this hypothesis.
MMP-9 is one of the primary enzymes associated with
the degradation of skin collagen and components of the elastic
fibers network. In addition, expression of MMP-9 in the epidermis
has been reported to cause apoptosis, photo-aging and inflammation
by stimulating the expression of inflammatory cytokines, including
as tumor necrosis factor-α and interleukin-1β (24). In the present study, western blot
analysis demonstrated that betaine attenuated UVB-induced
expression of MMP-9 regulated by the MEK/ERK pathway. MAPKs
encompass serine/threonine kinases, which are involved in
regulating several cellular processes, including proliferation,
differentiation, stress adaptation and apoptosis (24). In addition to these functions,
MAPKs are known to regulate the expression of MMP-9 (24). In a previous study, mangiferin
isolated from Anemarrhena asphodeloides was shown to inhibit
UVB-induced wrinkle formation and the expression of MMP-9 (25). Similarly, in the present study,
betaine inhibited UVB-induced epidermal thickening and the protein
expression of MMP-9. Therefore, betaine may exert its protective
effects in a manner similar to that of mangiferin, by inhibiting
the MAPK pathway, inhibiting the expression of MMP-9.
Epidermal thickness is used as a parameter to
reflect quantitative changes in skin photoaging, as epidermal
hypertrophy is considered to cause wrinkle formation (26). Furthermore, an increase in
epidermal thickness occurs following UV exposure and assists in
protecting the skin from further UV damage (27). In the present study, the epidermal
thickness of the dorsal skin was increased by UVB exposure,
however, this effect was significantly inhibited by betaine
administration prior to UVB exposure. These data further supported
that betaine protected the skin against UVB-induced damage.
In conclusion, the present study examined the
anti-photo-aging effects of betaine in a hairless mouse model of
UVB-induced skin damage. The oral administration of betaine reduced
the occurrence of characteristics associated with skin aging.
Furthermore, betaine inhibited UVB-induced increases in skin
thickness, wrinkle formation and collagen fiber loss in the
hairless mice. These data demonstrated that these effects were
mediated through a pathway involving MEK, ERK and MMP-9. Our
results provide a evidence of the photoprotective effect of orally
administered betaine, and suggest it may serve as a photoprotector
against UVB-induced skin damage.
Acknowledgments
The present study was supported by a grant from the
Korea Institute of Oriental Medicine (grant no. K14101).
References
|
1
|
Ganceviciene R, Liakou AI, Theodoridis A,
Makrantonaki E and Zouboulis CC: Skin anti-aging strategies.
Dermatoendocrinol. 4:308–319. 2012. View Article : Google Scholar
|
|
2
|
Tsatsou F, Trakatelli M, Patsatsi A,
Kalokasidis K and Sotiriadis D: Extrinsic aging: UV mediated skin
carcinogenesis. Dermatoendocrinol. 4:285–297. 2012. View Article : Google Scholar
|
|
3
|
Pyun HB, Kim M, Park J, Sakai Y, Numata N,
Shin JY, Shin HJ, Kim DU and Hwang JK: Effects of collagen
tripeptide supplement on photoaging and epidermal skin barrier in
UVB-exposed hairless mice. Prev Nutr Food Sci. 17:245–253. 2012.
View Article : Google Scholar
|
|
4
|
El-Domyati M, Attia S, Saleh F, Brown D,
Birk DE, Gasparro F, Ahmad H and Uitto J: Intrinsic aging vs.
photoaging: A comparative histopathological, immunohistochemical,
and ultra-structural study of skin. Exp Dermatol. 11:398–405. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Afaq F and Mukhtar H: Botanical
antioxidants in the prevention of photocarcinogenesis and
photoaging. Exp Dermatol. 15:678–684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zouboulis CC and Makrantonaki E: Clinical
aspects and molecular diagnostics of skin aging. Clin Dermatol.
29:3–14. 2011. View Article : Google Scholar
|
|
7
|
Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ
and Fisher GJ: Matrix-degrading metalloproteinases in photoaging. J
Investig Dermatol Symp Proc. 14:20–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gelse K, Pöschl E and Aigner T:
Collagens-structure, function and biosynthesis. Adv Drug Deliv Rev.
55:1531–1546. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim MS, Kim YK, Cho KH and Chung JH:
Regulation of type I procollagen and MMP-1 expression after single
or repeated exposure to infrared radiation in human skin. Mech
Ageing Dev. 127:875–882. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holvoet S, Vincent C, Schmitt D and Serres
M: The inhibition of MAPK pathway is correlated with
down-regulation of MMP-9 secretion induced by TNF-alpha in human
keratinocytes. Exp Cell Res. 290:108–119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brenneisen P, Sies H and
Scharffetter-Kochanek K: Ultraviolet-B irradiation and matrix
metalloproteinases: From induction via signaling to initial events.
Ann NY Acad Sci. 973:31–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shin YG, Cho KH, Kim JM, Park MK and Park
JH: Determination of betaine in lycium chinense fruits by liquid
chromatography-electrospray ionization mass spectrometry. J
Chromatogr A. 857:331–335. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cave M, Deaciuc I, Mendez C, Song Z,
Joshi-Barve S, Barve S and McClain C: Nonalcoholic fatty liver
disease: Predisposing factors and the role of nutrition. J Nutr
Biochem. 18:184–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bidulescu A, Chambless LE, Siega-Riz AM,
Zeisel SH and Heiss G: Usual choline and betaine dietary intake and
incident coronary heart disease: The atherosclerosis risk in
communities (ARIC) study. BMC Cardiovasc Disord. 7:202007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Im AR, Kim YH, Uddin MR, Chae S, Lee HW,
Kim YH, Kim YS and Lee MY: Betaine protects against
rotenone-induced neuro-toxicity in PC12 cells. Cell Mol Neurobiol.
33:625–635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiang HM, Chen HC, Chiu HH, Chen CW, Wang
SM and Wen KC: Neonauclea reticulata (Havil.) merr stimulates skin
regeneration after UVB exposure via ROS scavenging and modulation
of the MAPK/MMPs/collagen pathway. Evid Based Complement Alternat
Med. 2013:3248642013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Afaq F, Adhami VM and Mukhtar H:
Photochemoprevention of ultraviolet B signaling and
photocarcinogenesis. Mutat Res. 571:153–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou H, Li B, Zhang Z, Xue C, Yu G, Wang J,
Bao Y, Bu L, Sun J, Peng Z and Su S: Moisture absorption and
retention properties and activity in alleviating skin photodamage
of collagen polypeptide from marine fish skin. Food Chem.
135:1432–1439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vicentini FT, Fonseca YM, Pitol DL,
Iyomasa MM, Bentley MV and Fonseca MJ: Evaluation of protective
effect of a water-in-oil microemulsion incorporating quercetin
against UVB-induced damage in hairless mice skin. J Pharm Pharm
Sci. 13:274–285. 2010.PubMed/NCBI
|
|
20
|
Vayalil PK, Mittal A, Hara Y, Elmets CA
and Katiyar SK: Green tea polyphenols prevent ultraviolet
light-induced oxidative damage and matrix metalloproteinases
expression in mouse skin. J Invest Dermatol. 122:1480–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ryu J, Park SJ, Kim IH, Choi YH and Nam
TJ: Progective effect of porphyra-334 on UVA-induced photoaging in
human skin fibroblasts. Int J Mol Med. 34:796–803. 2014.PubMed/NCBI
|
|
22
|
Bosset S, Barré P, Chalon A, Kurfurst R,
Bonté F, André P, Perrier P, Disant F, Le Varlet B and Nicolas JF:
Skin ageing: Clinical and histopathologic study of permanent and
reducible wrinkles. Eur J Dermatol. 12:247–252. 2002.PubMed/NCBI
|
|
23
|
Chauhan P and Shakya M: Modeling signaling
pathways leading to wrinkle formation: Identification of the skin
aging target. Indian J Dermatol Venereol Leprol. 75:463–468. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Onoue S, Kobayashi T, Takemoto Y, Sasaki I
and Shinkai H: Induction of matrix metalloproteinase 9 secretion
from human keratinocytes in culture by ultraviolet B irradiation. J
Dermatol Sci. 33:105–111. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim HS, Song JH, Youn UJ, Hyun JW, Jeong
WS, Lee MY, Choi HJ, Lee HK and Chae S: Inhibition of UVB-induced
wrinkle formation and MMP-9 expression by mangiferin isolated from
anemarrhena asphodeloids. Eur J Pharmacol. 689:38–44. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Urikura I, Sugawara T and Hirata T:
Protective effect of fuco-xanthin against UVB-induced skin
photoaging in hairless mice. Biosci Biotechnol Biochem. 75:757–760.
2011. View Article : Google Scholar
|
|
27
|
Rabe JH, Mamelak AJ, McElgunn PJ, Morison
WL and Sauder DN: Photoaging: Mechanisms and repair. J Am Acad
Dermatol. 55:1–19. 2006. View Article : Google Scholar : PubMed/NCBI
|