Introduction
Total hip replacement improves quality of life, in
terms of its reduction in pain and improved function (1). However, periprosthetic osteolysis is
a major complication following total hip replacement (2,3).
Histopathological studies have shown the infiltration of
macrophages, osteoblasts, osteoclasts and fibroblasts into
peri-prosthetic tissues (4) and
the interstitial membrane (5,6).
Each of these cells are involved in the web of interactions, which
govern periprosthetic bone loss (1).
The receptor of nuclear factor κB (RANK)/RANK ligand
(RANKL)/osteoprotegerin (OPG) axis is at the core of the biological
response of osteolysis (7), and
RANKL activates nuclear factor (NF)κB, which results in
osteoclastgenesis. The RANKL/OPG ratio is a crucial indicator of
bone mass and skeletal integrity (8). OPG is a decoy receptor for RANKL; it
is secreted by osteoblasts and regulates osteoclast activity by
providing an alternative binding site for RANKL, thus inhibiting
the interaction between RANKL and RANK (9).
TNF-α is involved in the development of the
osteolytic response (10) and
controls the release of other proinflammatory factors, including
interleukin (IL)-1β and IL-6 (11,12).
It is also reported that TNF-α inhibits osteoblast differentiation
and promotes osteoblast apoptosis (13,14).
In addition, it has been suggested that TNF-α may act dependently
(15) or independently of RANKL
(10) to induce osteolysis.
TNF-α small interfering (si)RNA has been
demonstrated to be effective in inhibiting wear particle-induced
osteolysis, however certain evidence had shown that the most
sensitive osteolytic response of bone to TNF-α is through the
activation of existing osteoclasts (12). Therefore, osteoclast precursors may
retain the ability to differentiate into osteoclasts through
interaction with RANKL, whose decoy is OPG. OPG protein has been
confirmed to have the ability to prevent periprosthetic osteolysis,
however, due to the short-half life of the biological agent and the
chronic nature of the particle-associated periprosthetic
osteolysis, it is difficult to utilize conventional therapeutic
methods to administer a sufficient quantity of OPG protein to
osteolytic sites around the loosening prosthesis (8).
In previous years, several studies have shown that
gene therapy offers a more efficient, localized, long-term option
(16), compared with drugs, and
have the ability prevent or treat periprosthetic osteolysis.
Therefore, the present study aimed to construct a
lentivirus-mediated siRNA targeting TNF-α in RAW264.7 cells and, at
the same time, induce the overexpres-sion of OPG in MC3T3-E1 cells,
to determine whether the recombined lentivirus, Lenti-siTNFα-OPG,
has the ability to inhibit titanium (Ti) particle-induced
osteolysis.
Materials and methods
Particle preparation
Commercial pure Ti particles (diameter range, 1–10
µm) with a purity of 93% were obtained from Johnson Matthey
Pharma Services (Ward Hill, MA, USA). The particles were incubated
in 75% ethanol for 48 h for sterilization and to remove endotoxin,
which was followed by washing three times in sterile
phosphate-buffered saline (PBS; Wuhan Boster Biological Technology,
Ltd., Wuhan, China). The levels of endotoxin in the particle
solutions was measured using a Limulus Amebocyte Lysate assay
(Xiamen Houshiji, Ltd., Xiamen, China), and the results showed that
the endotoxin level was <0.2 EU/ml. The particles were then
suspended in sterile PBS at 0.1 mg/ml and stored at 4°C until
use.
Lentiviral vector construction and
recombinant lentivirus production
The siRNA target sequences (CCCAAAGGGATGAGAAGTT)
were designed and cloned into a GV118 lentivirus vector (GeneChem
Co., Ltd., Shanghai, China) by restriction endonuclease HpaΙ
and XhoΙ double digestion (GeneChem Co., Ltd.) and T4 DNA
ligase ligation, to construct a pLenti-PU6-siTNF-α-PU6iEGFP
backbone. Based on the mouse OPG gene sequences, PCR primers were
designed (GeneChem Co., Ltd.) to clone the full length of OPG cDNA,
which were cloned into the pLenti-PU6-siTNF-α-PU6iEGFP backbone
using AgeI and BamHI sites to construct the
Lenti-siTNFα-OPG vector. Following construction, the recombined
lentivirus vector and pPACK Packaging Plasmid mix (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) were
cotransfected into 293T cells (GeneChem Co., Ltd.). Three short
hairpin RNAs (shRNAs) were selected based on the sequences of the
mouse TNF-α gene (GenBank: NM_013693; listed in Table I), and a scrambled shRNA served as
a negative control. Preliminary experiments indicated that shRNA2
(sense, 5′-CCAACGGCATGGATCTCAA-3′) downregulated TNF-α mRNA more
markedly than the other tested shRNAs (data not shown). The
pGag/Pol, pRev, pVSV-G, and recombinant lentivirus were packaged
into plasmid vectors, and the recombinant lentivirus was amplified
by transforming 293T cells with the packaging plasmids using
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
pLenti-PU6-siTNF-α-PU6iEGFP was harvested after 48 h. Full-length
OPG cDNA was cloned into the pLenti-PU6-siTNF-α-PU6iEGFP using
AgeI and BamHI sites to construct Lenti-siTNFα-OPG.
The plasmids were then amplified by transfection into 293T cells
and purified with three rounds of density gradient centrifugation
with CsCl. At 48 h post-transfection, the lentiviruses were
harvested and centrifuged at 4,000 × g for 10 min at 4°C to remove
cell debris. Condensation was performed by filtration of the
supernatant into a filtrate collection tube through a filter cup,
followed by centrifugation at 4,000 × g for 13 min. The filter cup
was removed and a sample collection cup was inserted into the
filtrate collection tube, this was then centrifuged at 1,000 × g
for 2 min. Ultimately, a concentrated lentivirus solution was
obtained, with a final titer of 1.5×109 TU/l.
 | Table IPrimers for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primers for reverse
transcription-quantitative polymerase chain reaction analysis.
| Target | Forward primer
(5′→3′) | Reverse primer
(3′→5′) |
|---|
| TNF-α | TCC TCA CCC ACA CCG
TCAG | GCT GAG TTG GTC CCC
CTTC |
| OPG | GAT CCT GGA CAG CTT
CAC AA | AAA CAG CCC AGT GTC
CAT GC |
| RANKL | AGA TTT GCA GGA CTC
GAC TC | CCC ACA ATG TGT TGC
AGT TC |
| IL-1β | TTC TCG CAG CAG CAC
ATC |
CAGCAGGTTATCATCATCATCC |
| IL-6 |
TCCATCCAGTTGCCTTCTTG | TTT CTC ATT TCC ACG
ATT TCC C |
| GAPDH | TGG TGA AGG TCG GTG
TGA AC | GCT CCT GGA AGA TGG
TGA TGG |
Cell culture
RAW264.7 mouse macrophage/monocyte cell line
(American Type Culture Collection, Manassas, VA, USA) was cultured
in α-minimum essential medium (α-MEM; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) containing 10% fetal bovine serum (FBS;
Hyclone; GE Healthcare Life Sciences), 100 U/ml penicillin (Gibco;
Thermo Fisher Scientific, Inc.) and 100 g/ml streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) at 37.6°C under 5% CO2
and 95% humidity. MC3T3-E1 (American Type Culture Collection)
murine osteoblast-like cells were maintained in the same media and
conditions.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The cytotoxicity of the RAW264.7 and MC3T3-E1 cells
transfected with Lenti-siTNFα-OPG was examined using the MTT assay.
The cells (5×103 cells/well) were cultured in 96-well
tissue culture plates for 24 h and incubated with 0.5 mg/ml MTT at
37°C for 4 h. Following the removal of the supernatant, the
insoluble formazan crystals were dissolved in 200 µl
dimethyl sulfoxide, and the absorbance was measured using a Synergy
HT microtiter plate reader (BioTek Instruments, Inc., Winooski,
Vermont, USA) at a wavelength of 570 nm.
Collection of conditioned media (CM)
The RAW264.7 cells were plated in 24-well plates at
a density of 1.0×105 cells in complete α-MEM. Following
24 h attachment at 37°C, the cells were washed with PBS and
stabilized in serum-free Dulbecco's modified Eagle's medium
(Hyclone; GE Healthcare Life Sciences) for 1 h at 37°C.
Subsequently, the cells were subjected to Ti particles (0.1 mg/ml)
with or without Lenti-siTNFa-OPG (5.0×106/ml). Control
groups were treated with equal volumes of PBS and transfection was
conducted by adding 5.0×106/ml Lenti-siTNF-OPG to each
well with 5 µg/ml polybrene and 5 µg/ml Enhanced
Infection Solution for 72 h. Multiplicity of infection (MOI) was
determined by observation of the decrease in TNF-α expression and
overexpression of OPG. Following 24 h of incubation at 37°C, the
cells in the control CM group (Cont CM), CM with Ti particles group
(Ti CM) and CM with Ti particles and Lenti-siTNFa-OPG group
(Ti-lenti CM) were collected, centrifuged at 1,000 × g for 5 min to
remove any cell debris and stored at -20°C until use.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA in the RAW264.7 and MC3T3-E1 cells
following treatment was extracted in 1 ml TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and cDNA was
synthesized from the total RNA. qPCR was used to detect the mRNA
expression levels of TNF-α, OPG and RANKL. The sequences of the PCR
primers are listed in Table I.
Total RNA was extracted from the MC3T3-E1 and RAW264.7 co-cultures
using 1 ml TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. RNA purity was
determined using the 260/280 nm absorbance ratio (NanoDrop; Thermo
Fisher Scientific, Inc., Wilmington, DE, USA). First-strand cDNA
was synthesized with 2 mg total RNA (Fermentas; Thermo Fisher
Scientific, Inc., Pittsburgh, PA, USA), and one-tenth of the total
cDNA was used for each PCR mixture containing 5 µl Express
SYBR Green (Takara Bio, Inc., Otsu, Japan) and 5 µl PCR
Supermix (Fermentas; Thermo Fisher Scientific, Inc.). The PCR
primers (0.5 µl upstream and downstream, respectively) used
to amplify TNF-α, OPG, IL-1β, IL-6 and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) are listed in Table II. The reaction mixture
(20 µl) was subjected to a 45-cycle amplification in a DNA
Thermal Cycler (PerkinElmer, Inc., Waltham, MA, USA) at 95°C for 15
sec and 95°C for 5 sec, followed by 60°C for 30 sec. Relative mRNA
expression levels of the selected genes (TNF-α, OPG and RANKL) were
normalized to GAPDH and quantified using the ΔΔCq method.
Enzyme-linked immunosorbent assay
(ELISA)
The RAW264.7 cells were incubated with/without Ti
particles in the presence or absence of Lenti-siTNFα-OPG for 24 h,
and the cell super-natants were harvested and centrifuged to remove
the cell particles, as described above. Aliquots were stored at
-20°C for TNF-α measurement. A mouse TNF-α ELISA kit (R&D
Systems, Inc. Minneapolis, MN, USA) was used for quantitative
measurement, according to the manufacturer's protocol.
Western blot analysis
The cells were lysed in radioimmuno-precipitation
assay buffer (Beyotime Institute of Biotechnology, Shanghai, China)
with protease inhibitors (Beyotime Institute of Biotechnology).
Subsequently, the protein concentrations were determined using a
Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology). The total protein (22 µg) was
electrophoresed via 10% SDS-polyacrylamide gel electrophoresis,
transferred onto polyvinylidene difluoride membranes (PVDF; EMD
Millipore, Billerica, MA, USA) and blocked in TBS for 1 h. The PVDF
membranes were incubated overnight at 4°C with rabbit anti-mouse
monoclonal TNF-α (1:300; Abcam, Cambridge, MA; cat. no. ab11564)
and rabbit anti-mouse polyclonal OPG (1:200) antibodies (Abcam;
cat. no. ab9986). GAPDH served as a protein loading control.
Following incubation with the primary antibody, the membranes were
washed twice with TBST for 10 min and then washed with TBS for 10
min. Subsequently, the blots were incubated with goat anti-rabbit
monoclonal secondary antibody (1:500; cat. no. 7074P2; Cell
Signaling Technology, Inc., Shanghai, China) at 20°C for 2 h.
Following incubation with the secondary antibody, the membranes
were washed twice in TBST for 10 min and then washed with TBS for
10 min. Following incubation, the proteins were detected by
enhanced chemiluminescence with BeyoECL Plus (Beyotime Institute of
Biotechnology) and scanned using Quantity One analysis software,
version 4.6 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
ALP activity assay
ALP activity was measured using a QuantiChrom™
Alkaline Phosphatase Assay kit (BioAssay Systems, Hayward, CA,
USA). In brief, the culture medium was removed, and following being
washed with PBS, the cells were lysed in 0.5 ml 0.2% Triton X-100
in distilled water with agitation for 20 min at room temperature.
The samples were then incubated with a mixture of assay buffer (pH
10.5), 5 mM Mg acetate (final) and 10 mM pNPP liquid substrate at
room temperature for 10 min. The optical density at 405 nm was
determined (t=0), and this was measured again after 4 min (t=4 min)
on a plate reader (Multiskan Plus; Thermo Fisher Scientific, Inc.).
The quantity of released nitrophenolate was calculated
photometrically, according to the manufacturer's protocol.
Statistical analysis
Data from three independent experiments were
analyzed and are presented as the mean ± standard deviation.
Differences between groups were analyzed using one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Variation in the MOI of Lenti-siTNFα-OPG
transfection has no effect on cell viability
The results of the MTT assay revealed no significant
differences among the RAW264.7 and MC3T3-E1 cells transfected with
different MOIs (30, 50 and 70 MOI) of Lenti-siTNFa-OPG for 48 h
(Fig. 1A and B). As the mRNA
expression levels of TNF-α and OPG were significantly downregulated
at 50 MOI, the present study analyzed the viability of RAW264.7
cells treated with Ti particles and 50 MOI Lenti-siTNFa-OPG at 24,
48 and 72 h, which showed no significant difference, compared with
the untransfected cells (Fig. 1C).
Similarly, no significant difference was observed between the
viability of the MC3T3-E1 cells transfected with 50 MOI
Lenti-siTNFa-OPG (Fig. 1D) at each
time point, compared with the untransfected cells.
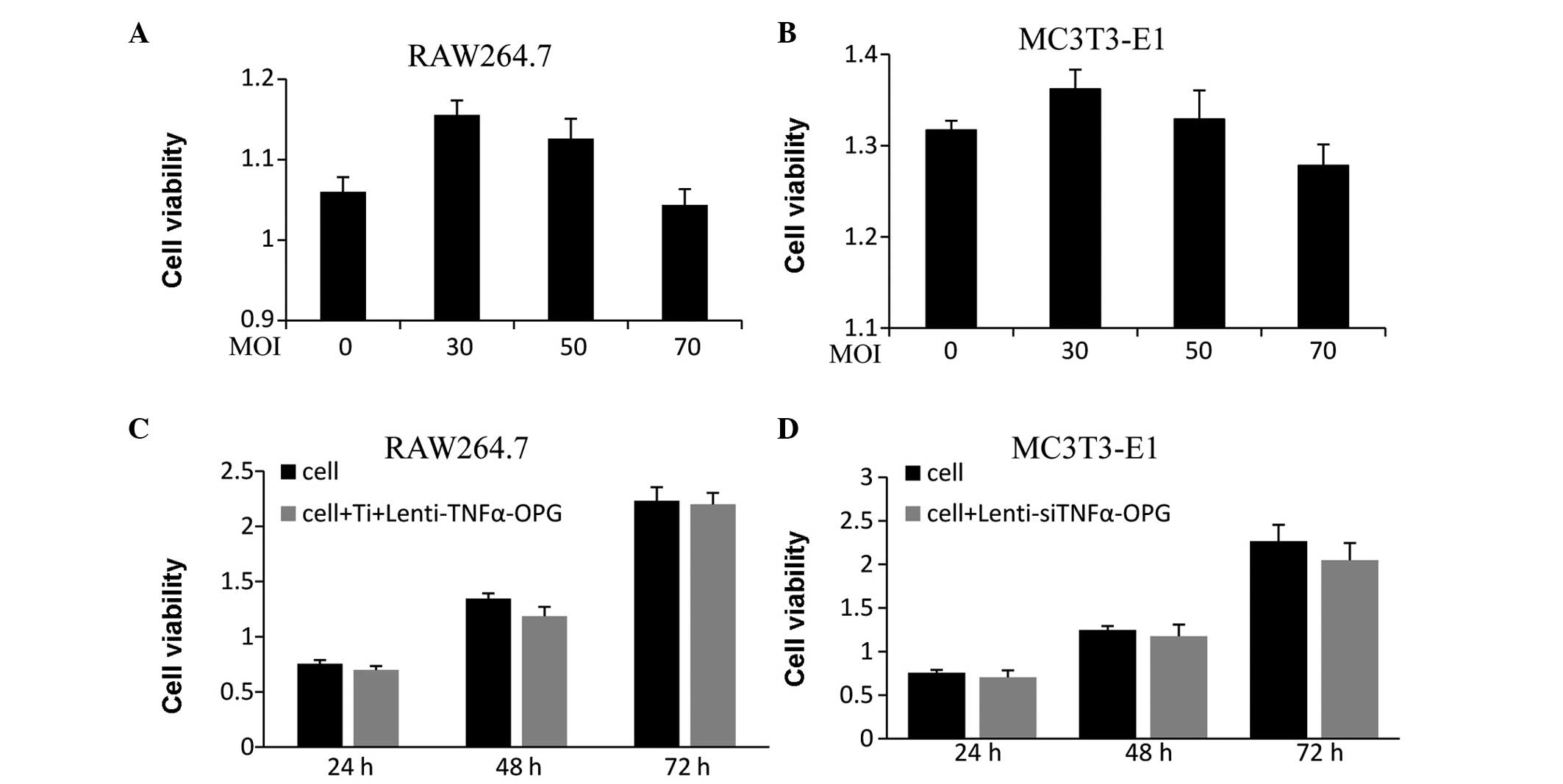 | Figure 1Effects of Lenti-siTNFα-OPG on cell
viability, determined using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
(A and B) RAW264.7 and MC3T3-E1 cells were transfected with
different MOIs of Lenti-siTNFα-OPG for 48 h. (C) RAW264.7 were
treated with Ti and 50 MOI RAW264.7 for 24, 48 and 72 h. (D)
MC3T3-E1 cells were transfected with 50 MOI Lenti-siTNFα-OPG for
24, 48 and 72 h. Data are presented as the mean ± standard
deviation. Ti, titanium; TNF-α, tumor necrosis factor-α; OPG,
osteoprotegerin; si, small interfering; MOI, multiplicity of
infection. |
Lenti-siTNFα-OPG inhibits the expression
of cytokines in RAW264.7 cells
Compared with 30 and 70 MOI, the mRNA expression of
TNF-α in the RAW264.7 cells transfected with 50 MOI
Lenti-siTNFα-OPG was lowest at 48 h (Fig. 2A). The protein expression of TNF-α,
determined using western blot analysis, revealed similar results
(Fig. 2B). These results confirmed
that 50 MOI Lenti-siTNFα-OPG significantly reduced the expression
levels of TNF-α in the RAW264.7 cells treated with 0.1 mg/ml Ti
particles. Following 24 h incubation with a combination of Ti
particles and 50 MOI Lenti-siTNFα-OPG, the protein expression of
TNF-α was inhibited in the RAW264.7 cells, compared with the with
cells in the Ti CM group, as determined using ELISA analysis
(Fig. 2C). To evaluate the
particle-induced inflammatory response, the present study examined
the expression levels of proinflammatory cytokines, including IL-1β
and IL-6. It was found that the downregulation of TNFα by siTNFα
resulted in decreases in the mRNA expression levels of IL-1β
(Fig. 2D) and IL-6 (Fig. 2E), compared with the Ti CM group,
which indicated that TNF-α may control the mRNA expression levels
of IL-6 and IL-1β mRNA. It was also observed that the mRNA
expression of RANKL in the MC3T3-E1 cells decreased markedly when
cultured in lenti-Ti CM, compared with the Ti CM group, which
indicated that TNF-α may have an effect on the expression of RANKL
(Fig. 2F).
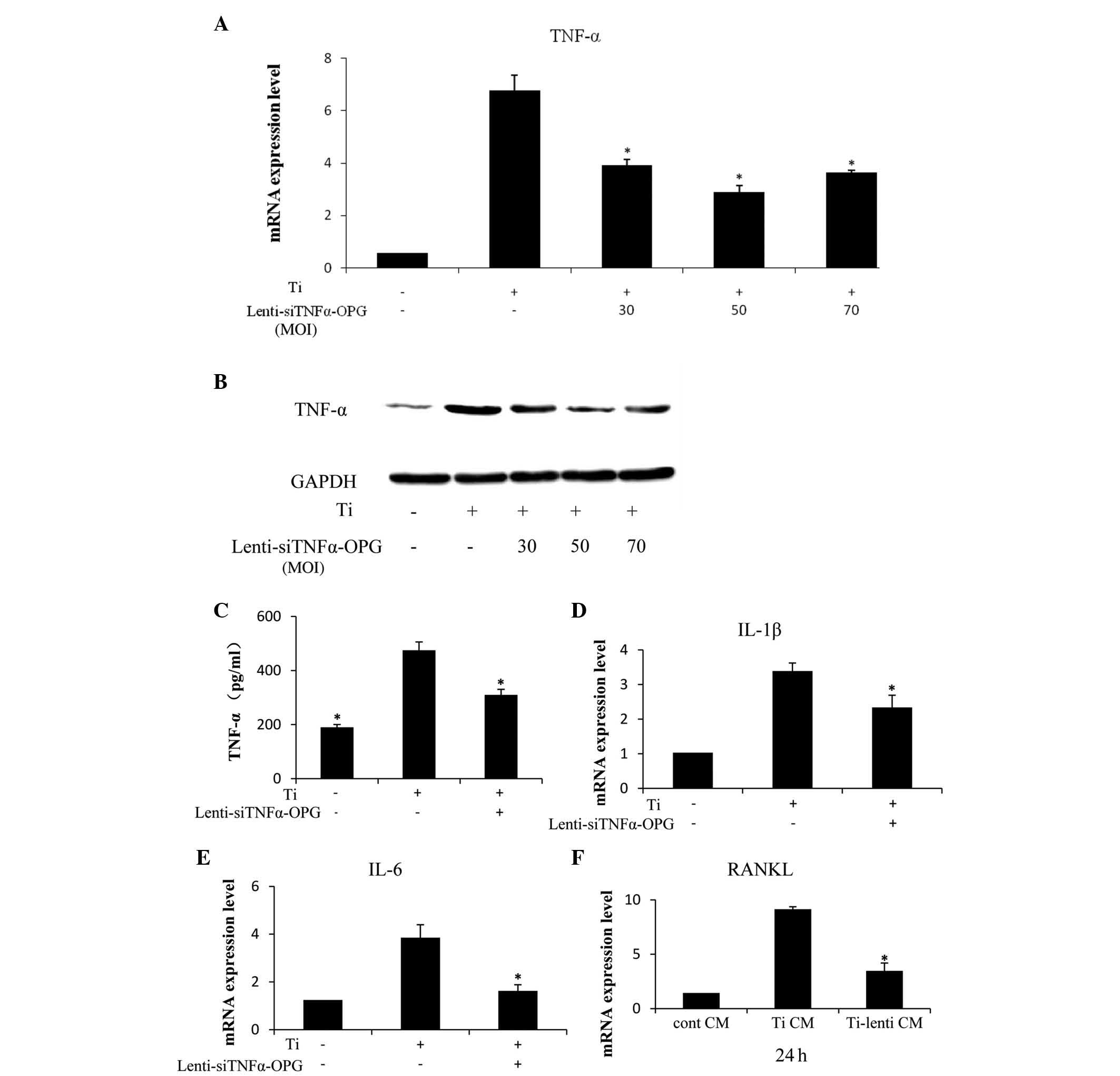 | Figure 2mRNA and protein expression levels of
proinflammatory cytokines in RAW264.7 cells, and the mRNA
expression of RANKL in MC3T3-E1 cells. (A) RAW264.7 cells were
treated with/without Ti particles in the presence or absence of
Lenti-siTNFα-OPG for 48 h. mRNA expression levels of TNF-α were
significantly decreased at 50 MOI. (B) Protein levels of TNF-α were
examined using western blotting after 48 h. (C) Protein levels of
TNF-α were assessed using an enzyme-linked immunosorbent assay
following exposure to Ti particles, or Ti particles and 50 MOI
Lenti-siTNFα-OPG for 24 h. (D and E) mRNA expression levels of IL-6
and IL-1β were significantly downregulated by Lenti-siTNFα-OPG at
50 MOI. (F) mRNA expression levels of RANKL were significantly
decreased in the MC3T3-E1 cells in the Ti-lenti CM group, compared
with the Ti CM group. Data are presented as the mean ± standard
deviation. *P<0.05 vs. Ti CM. Ti, titanium; TNF-α,
tumor necrosis factor-α; IL, interleukin; OPG, osteoprotegerin;
RANKL, receptor of nuclear factor κB ligand; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; MOI, multiplicity of
infection; cont, control; CM, conditioned media; si, small
interfering. |
Lenti-siTNFα-OPG upregulates the
expression of OPG in MC3T3-E1 cells
To assess the mRNA expression levels of OPG, the
MC3T3-E1 cells treated with different MOIs (30, 50 and 70) were
examined using RT-qPCR. The results showed that the expression
level of OPG was highest at an MOI of 50 at 48 h (Fig. 3A). At 48 h post-transfection with
the different MOIs of Lenti-siTNFα-OPG, overexpression of OPG
protein was demonstrated in the MC3T3-E1 cells using western blot
analysis (Fig. 3B). As expected,
the MC3T3-E1 cells transfected with 50 MOI Lenti-siTNFα-OPG
exhibited higher protein expression levels of OPG, compared with
those transfected with 30 and 70 MOI. The present study also
examined the protein expression levels of OPG in the
OPG-overexpressing MC3T3-E1 cells cultured in Cont CM, Ti CM and
Ti-lenti CM using ELISA. The Lenti-siTNFα-OPG and Ti-lenti
CM-treated group exhibited the highest protein expression of OPG,
compared with the Ti CM and Ti-lenti CM-treated groups (Fig. 3C), suggesting that the
overexpression of OPG is more marked when the expression of TNF-α
is decreased.
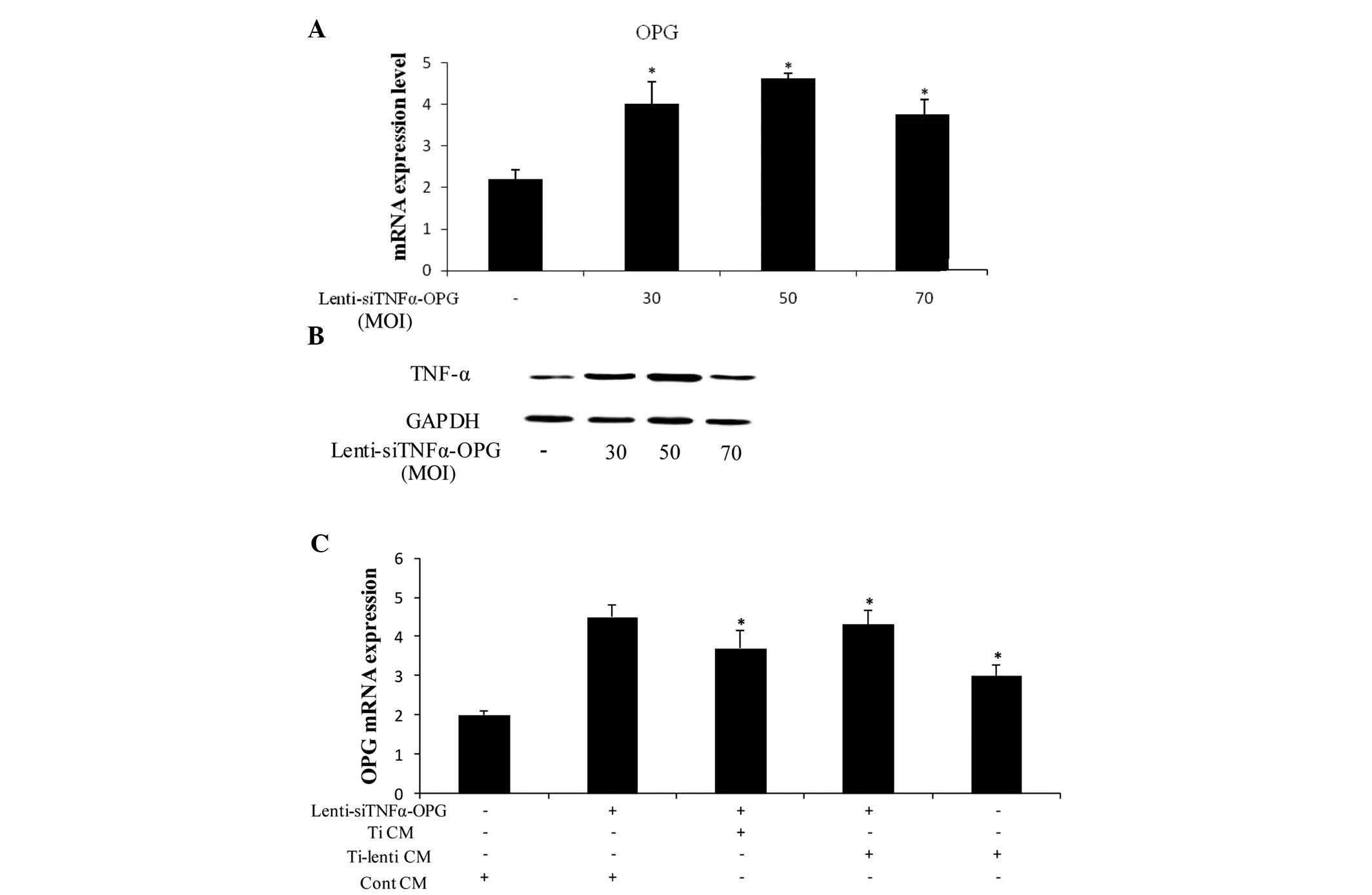 | Figure 3Expression levels of OPG in MC3T3-E1
cells treated with Lenti-siTNFα-OPG and/or different CM. (A)
RAW264.7 were transfected with Lenti-siTNFα-OPG at different MOIs
for 48 h. The mRNA expression of OPG was significantly increased at
50 MOI. *P<0.05 vs. 0 Lenti-siTNFa-OPG. (B) Protein
levels of OPG were determined using western blotting after 48 h.
(C) Expression levels of OPG were significantly increased in the
MC3T3-E1 cells in the Lenti-siTNFα-OPG and Ti-lenti CM-treated
group, compared with the Lenti-siTNFα-OPG and Ti CM-treated and
Ti-lenti-treated groups. Data are presented as the mean ± standard
deviation. *P<0.05 vs. Cont CM + Lenti-siTNFα-OPG.
Ti, titanium; TNF-α, tumor necrosis factor-α; IL, interleukin; OPG,
osteoprotegerin; RANKL, receptor of nuclear factor κB ligand;
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MOI, multiplicity
of infection; Cont, control; CM, conditioned media; si, small
interfering. |
Lenti-siTNFα-OPG promotes osteoblast
differentiation and inhibits osteoclastogenesis in transfected
MC3T3-E1 cells
ALP is a marker of matrix maturation and, during
differentiation from mesenchymal cells to mature osteoblasts, ALP
begins to be expressed in osteoprogenitors and is expressed at high
levels in mature osteoblasts (17). Using an ALP kit, the present study
assessed the activity of ALP in the MC3T3-E1 cells transfected with
or without Lenti-siTNFα-OPG for 48 h. The results revealed that ALP
activity in the MC3T3-E1 cells increased following transfection
with Lenti-siTNFα-OPG (Fig. 4A).
It was also found that ALP activity was significantly higher in the
OPG-overexpressing MC3T3-E1 cells when treated with the different
CM (Fig. 4B). These data indicated
that Lenti-siTNFα-OPG transfection alleviated Ti particle-induced
osteolysis by promoting osteoblast differentiation.
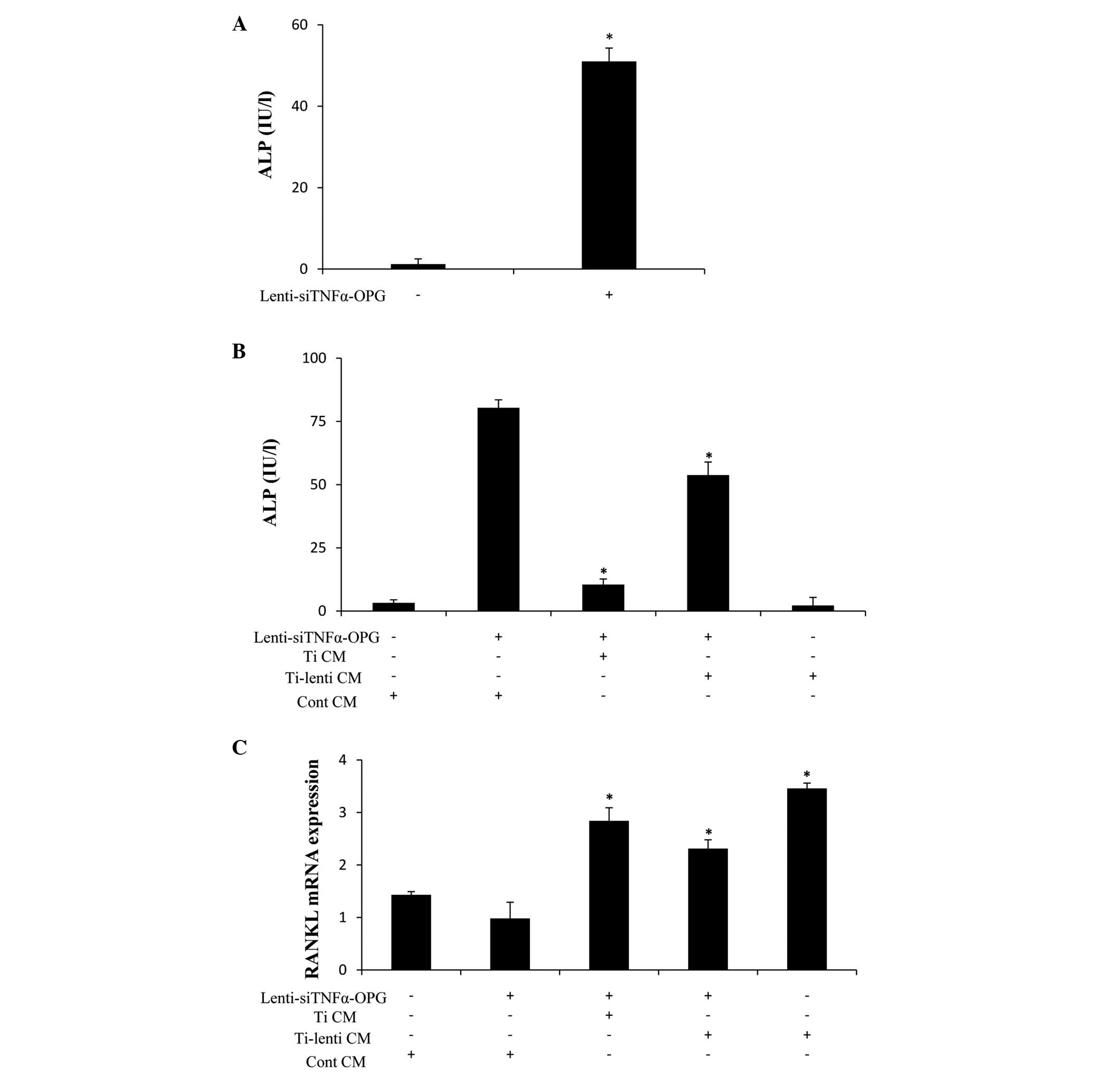 | Figure 4ALP activity and mRNA expression
levels of RANKL in MC3T3-E1 cells. (A) MC3T3-E1 cells transfected
with 50 MOI Lenti-siTNFα-OPG for 48 h. ALP activity was
significantly higher, compared with the control.
*P<0.05 vs. cont. (B) ALP activity was significantly
increased in the MC3T3-E1 cells of the Lenti-siTNFα-OPG and
Ti-lenti CM-treated group, compared with the Lenti-siTNFα-OPG and
Ti CM and Ti-lenti-treated groups. *P<0.05 vs. cont
CM + Lenti-siTNFα-OPG. (C) mRNA expression levels of RANKL were
significantly decreased in the MC3T3-E1 cells of the
Lenti-siTNFα-OPG and Ti-lenti CM-treated group, compared with the
Lenti-siTNFα-OPG and Ti CM and Ti-lenti-treated groups.
*P<0.05 vs. cont CM + Lenti-siTNFα-OPG. Data are
presented as the mean ± standard deviation. Ti, titanium; TNF-α,
tumor necrosis factor-α; IL, interleukin; OPG, osteoprotegerin;
RANKL, receptor of nuclear factor κB ligand; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; MOI, multiplicity of
infection; cont, control; CM, conditioned media; si, small
interfering. |
RANKL has a high level of involvement in
osteoclastogenesis due to its binding to the receptor activator of
RANK. In the present study, RT-qPCR analysis revealed differences
in the mRNA expression levels of RANKL in the OPG-overexpressing
MC3T3-E1 cells cultured in Cont CM, Ti CM and Ti-lenti CM (Fig. 4C). The Lenti-siTNFα-OPG and
Ti-lenti CM-treated group revealed the lowest mRNA expression level
of RANKL, compared with the Ti CM and Ti-lenti CM-treated groups.
These results indicated that the Lenti-siTNFα-OPG may have
suppressed Ti particle-induced osteoclastogenesis.
Discussion
At present, there is no satisfactory treatment
option for peri-prosthetic osteolysis, with the exception of
revision, which requires complicated and expensive surgery, and is
frequently associated with considerable patient morbidity, and even
mortality rates (18). There has
been a focus on nonsurgical methods to prevent aseptic loosening
(19), however, there remains no
satisfactory method for the prevention of aseptic loosening of
joint protheses. To the best of our knowledge, the present study is
the first study to address the effects of the combination of TNF-α
siRNA and overexpression of OPG by construction of a recombined
lentivirus.
RANKL is essential for the promotion of
osteoclastogenesis. It binds to its signaling receptor, RANK, on
the membranes of macrophages and osteoclast precursors, thereby
providing signals required for their survival, maturation and
activation (20). Mature
osteoclasts secret large quantities of inflammatory factors,
including TNF-α, IL-1β and IL-6, which in turn activate osteoclasts
and prompt an inflammatory response (10). Among these inflammatory factors,
TNF-α acts as a link between inflammatory processes and
osteoclastogen-esis. It is involved in the osteolytic response
predominantly by two mechanisms: An indirect mechanism, in which
TNF-α enhances the inflammatory response by promoting the
expression levels of RANKL, IL-6 and IL-1β (21) and a direct mechanism, in which
TNF-α synergizes with RANKL to enhance osteoclast formation of bone
erosions (22). In the present
study, Lenti-siTNFα-OPG transfection inhibited osteolysis by the
two mechanisms. It decreased the level of TNF-α secreted by
osteoclasts and subsequently suppressed the expression levels of
IL-1β and IL-6 stimulated by the Ti particles. It also decreased
the expression of TNF-α, which ma have led to the reduction in the
expression of RANKL, controlling osteoclast maturation and function
(23). As shown in the data of the
present study, Lenti-siTNFα-OPG transfection effectively reduced
the expression of RANKL in the MC3T3-E1 cells by interfering with
the levels of the proinflammatory cytokines secreted from the
RAW264.7 cells induced by Ti particles. In addition, TNF-α and
RANKL support osteoclast survival, therefore, downregulation in the
expression of TNF-α reduces the numbers of osteoclasts (24). Therefore, Lenti-siTNFα-OPG
transfection may prevent the inflammatory response, which is
important in periprosthetic osteolysis.
ALP is a vital early marker of matrix maturation,
which is expressed in preosteoblasts during osteoblast
differentiation and exhibits upregulated expression levels in
mature osteoblasts and downregulated expression levels in
osteocytes (25). In the present
study, Lenti-siTNFα-OPG transfection promoted osteoblast
differentiation, accompanied by the expression of ALP. Furthermore,
the transfection of Lenti-siTNFα-OPG resulted in suppression of the
expression of RANKL and the inhibition of osteoclastogenesis
(26). Compared with
preosteo-blasts, the ratio of RANKL to OPG is markedly higher in
mature osteoblasts (17). In
addition, in the presence of TNF-α, the level of apoptosis in
mature osteoblasts is higher than in preosteoblasts (27). Therefore, Lenti-siTNFα-OPG
transfection may induce osteoblast maturation and inhibit
osteoclast differentiation.
OPG has been identified as a negative regulator of
the RANKL/RANK/OPG axis (28).
Overexpression of OPG upregulates the OPG/RANKL ratio, inhibiting
the interaction of RANKL and RANK, leading to suppression of the
inflammatory response and osteoclastogenesis. The results of the
present study clearly revealed that Lenti-siTNFα-OPG transfection
increase the expression of OPG in the MC3T3-E1 cells. In addition,
comparison of the mRNA expression levels of RANKL among the
MC3T3-E1 cells of the Lenti-siTNFα-OPG and Ti-lenti CM-treated,
Lenti-siTNFα-OPG and Ti CM-treated and Ti-lenti CM-treated groups,
the Lenti-siTNFα-OPG and Ti-lenti CM-treated MC3T3-E1 cells
exhibited the lowest mRNA expression level of RANKL. This indicated
that the combination of the decreased expression of TNF-α in
RAW264.7 cells with increased the expression of OPG in MC3T3-E1
cells downregulated the mRNA expression levels of RANKL more
effectively. As RANKL is critical for osteoclastogenesis, it was
hypothesized that Lenti-siTNFα-OPG transfection may be more
effective in inhibiting osteoclastogenesis, compared with siRNA
that target only the downregulation of TNF-α or upregulation of
OPG.
Gene therapy is an attractive option for treatment
of osteolysis. Major problems in the approaches to treat localized
chronic inflammatory/osteolytic disorders, including aseptic
loosening, include the lack of adequate suppressive agents and
effective specific therapeutic delivery systems (18). Although the conventional system of
administering biological drugs, including bisphosphonates, relies
on vascular perfusion to the local sites of loosening, viral vector
mediated gene therapy provides a novel means of delivering
therapeutic genes to the site of disease to express gene products
in a persistent and localized manner (18). In addition, investigations have
been performed on TNF-α siRNA or OPG cDNA gene delivery
(29,30), and they have been demonstrated to
be more potent, efficacious and cost-effective in inhibiting wear
particle-induced inflammatory response and osteoclastogenesis. As a
promising vector of siRNA, the lentivirus compares favorably with
other transgenic methods for transducing genes in vivo.
Transfection with a lentivirus is more efficient than other methods
due to its stable expression of siRNA in mammalian cells (31). Overall, Lenti-siTNFα-OPG appears to
be an effective mechanism to prevent Ti particle-induced
osteolysis.
For investigations aim to examine the effect of
Lenti-siTNFα-OPG on periprosthetic osteolysis in vitro, and
to fully understand the molecular mechanisms underlying the
therapeutic effects, as well as safety concerns.
In conclusion, the present study demonstrated that
the inhibition of TNF-α and overexpression of OPG by recombined
lentivirus transfection effectively alleviated the Ti
particle-induced inflammatory response and osteoclastogenesis in
vitro, and indicated that Lenti-siTNFα-OPG may be a potential
therapeutic method for the prevention of Ti particle-induced
osteolysis.
Acknowledgments
The study was supported by funding from the National
Natural Science Foundation of China (grant no. 81170386). All
experiments were performed in the Laboratory of Cardiology of the
First Affiliated Hospital of Harbin Medical University (Harbin,
China). The authors would like to thank the managers and staff for
their hospitability, time and opinions.
References
|
1
|
Ollivere B, Wimhurst JA, Clark IM and
Donell ST: Current concepts in osteolysis. J Bone Joint Surg Br.
94:10–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gallo J, Kamínek P, Tichá V, Riháková P
and Ditmar R: Particle disease. A comprehensive theory of
periprosthetic osteolysis: A review. Biomed Pap Med Fac Univ
Palacky Olomouc Czech Repub. 146:21–28. 2002. View Article : Google Scholar
|
|
3
|
Harris WH: Wear and periprosthetic
osteolysis: The problem. Clin Orthop Relat Res. 66–70. 2001.
View Article : Google Scholar
|
|
4
|
Schmalzried TP, Jasty M and Harris WH:
Periprosthetic bone loss in total hip arthroplasty. Polyethylene
wear debris and the concept of the effective joint space. J Bone
Joint Surg Am. 74:849–863. 1992.PubMed/NCBI
|
|
5
|
Agarwal S: Osteolysis-basic science,
incidence and diagnosis. Curr Orthopaed. 18:220–231. 2004.
View Article : Google Scholar
|
|
6
|
Purdue PE, Koulouvaris P, Nestor BJ and
Sculco TP: The central role of wear debris in periprosthetic
osteolysis. HSS J. 2:102–113. 2006. View Article : Google Scholar
|
|
7
|
Abu-Amer Y, Darwech I and Clohisy JC:
Aseptic loosening of total joint replacements: Mechanisms
underlying osteolysis and potential therapies. Arthritis Res Ther.
9(Suppl 1): S62007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boyce BF and Xing L: Biology of RANK,
RANKL and osteoprotegerin. Arthritis Res Ther. 9(Suppl 1): S12007.
View Article : Google Scholar :
|
|
9
|
Takahashi N, Maeda K, Ishihara A, Uehara S
and Kobayashi Y: Regulatory mechanism of osteoclastogenesis by
RANKL and Wnt signals. Front Biosci (Landmark Ed). 16:21–30. 2011.
View Article : Google Scholar
|
|
10
|
Kwan Tat S, Padrines M, Théoleyre S,
Heymann D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: Interrelations
in bone resorption pathophysiology. Cytokine Growth Factor Rev.
15:49–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akisue T, Bauer TW, Farver CF and Mochida
Y: The effect of particle wear debris on NFkappaB activation and
pro-inflammatory cytokine release in differentiated THP-1 cells. J
Biomed Mater Res. 59:507–515. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greenfield EM and Bechtold J; Implant Wear
Symposium 2007 Biologic Work Group: What other biologic and
mechanical factors might contribute to osteolysis? J Am Acad Orthop
Sur. 16(Suppl): S56–S62. 2008.
|
|
13
|
Abbas S, Zhang YH, Clohisy JC and Abu-Amer
Y: Tumor necrosis factor-alpha inhibits pre-osteoblast
differentiation through its type-1 receptor. Cytokine. 22:33–41.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu J, Wu HF, Ang ES, Yip K, Woloszyn M,
Zheng MH and Tan RX: NF-kappaB modulators in osteolytic bone
diseases. Cytokine Growth Factor Rev. 20:7–17. 2009. View Article : Google Scholar
|
|
15
|
Yoshino T, Yamaguchi M, Shimizu M, Yamada
K and Kasai K: TNF-α aggravates the progression of
orthodontically-induced inflammatory root resorption in the
presence of RANKL. J Hard Tissue Biol. 23:155–162. 2014. View Article : Google Scholar
|
|
16
|
Froelich S, Tai A and Wang P: Lentiviral
vectors for immune cells targeting. Immunopharmacol Immunotoxicol.
32:208–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rucci N, Rufo A, Alamanou M and Teti A:
Modeled microgravity stimulates osteoclastogenesis and bone
resorption by increasing osteoblast RANKL/OPG ratio. J Cell
Biochem. 100:464–473. 2007. View Article : Google Scholar
|
|
18
|
Philpott A, Weston-Simons JS,
Grammatopoulos G, Bejon P, Gill HS, McLardy-Smith P, Gundle R,
Murray DW and Pandit H: Predictive outcomes of revision total hip
replacement-A consecutive series of 1176 patients with a minimum
10-year follow-up. Maturitas. 77:185–190. 2014. View Article : Google Scholar
|
|
19
|
Goodman SB, Trindade M, Ma T, Genovese M
and Smith RL: Pharmacologic modulation of periprosthetic
osteolysis. Clin Orthop Relat Res. 39–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang CT, Lin YT, Chiang BL, Lee SS and Hou
SM: Over-expression of receptor activator of nuclear factor-kappaB
ligand (RANKL), inflammatory cytokines and chemokines in
periprosthetic oste-olysis of loosened total hip arthroplasty.
Biomaterials. 31:77–82. 2010. View Article : Google Scholar
|
|
21
|
Teitelbaum SL: Osteoclasts; culprits in
inflammatory osteolysis. Arthritis Res Ther. 8(201)2006.
|
|
22
|
Ritchlin CT, Haas-Smith SA, Li P, Hicks DG
and Schwarz EM: Mechanisms of TNF-alpha and RANKL mediated
osteoclasto-genesis and bone resorption in psoriatic arthritis. J
Clin Invest. 111:821–831. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He X, Andersson G, Lindgren U and Li Y:
Resveratrol prevents RANKL-induced osteoclast differentiation of
murine osteoclast progenitor RAW264.7 cells through inhibition of
ROS production. Biochem Biophys Res Commun. 401:356–362. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adapala NS, Barbe MF, Langdon WY, Nakamura
MC, Tsygankov AY and Sanjay A: The loss of Cbl-phosphatidylinositol
3-kinase interaction perturbs RANKL-mediated signaling, inhibiting
bone resorption and promoting osteoclast survival. J Biol Chem.
285:36745–36758. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inose H, Ochi H, Kimura A, Fujita K, Xu R,
Sato S, Iwasaki M, Sunamura S, Takeuchi Y, Fukumoto S, et al: A
microRNA regulatory mechanism of osteoblast differentiation. Proc
Natl Acad Sci USA. 106:20794–20799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bonnelye E, Chabadel A, Saltel F and
Jurdic P: Dual effect of strontium ranelate: Stimulation of
osteoblast differentiation and inhibition of osteoclast formation
and resorption in vitro. Bone. 42:129–138. 2008. View Article : Google Scholar
|
|
27
|
Almeida M, Han L, Ambrogini E, Weinstein
RS and Manolagas SC: Glucocorticoids and tumor necrosis factor α
increase oxidative stress and suppress Wnt protein signaling in
osteoblasts. J Biol Chem. 286:44326–44335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pivonka P, Zimak J, Smith DW, Gardiner BS,
Dunstan CR, Sims NA, Martin TJ and Mundy GR: Theoretical
investigation of the role of the RANK-RANKL-OPG system in bone
remodeling. J Theor Biol. 262:306–316. 2010. View Article : Google Scholar
|
|
29
|
Dong L, Wang R, Zhu YA, Wang C, Diao H,
Zhang C, Zhao J and Zhang J: Antisense oligonucleotide targeting
TNF-alpha can suppress Co-Cr-Mo particle-induced osteolysis. J
Orthop Res. 26:1114–1120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goater JJ, O'Keefe RJ, Rosier RN, Puzas JE
and Schwarz EM: Efficacy of ex vivo OPG gene therapy in preventing
wear debris induced osteolysis. J Orthop Res. 20:169–173. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kay MA, Glorioso JC and Naldini L: Viral
vectors for gene therapy: The art of turning infectious agents into
vehicles of therapeutics. Nat Med. 7:33–40. 2001. View Article : Google Scholar : PubMed/NCBI
|


















