Introduction
Epithelial ovarian cancer (EOC) is a gynecological
cancer associated with high mortality worldwide (1). In the USA, the number of newly
diagnosed cases and the cases of EOC-associated mortality were
21,980 and 14,270, respectively, in 2014 (2). Although it has been previously
demonstrated that a variety of non-specific symptoms prior to
diagnosis occur in the majority of patients, early diagnosis
remains a challenge (3).
Furthermore, peritoneal dissemination, which is induced by hypoxic
stress resistance, contributes substantially to the mortality rate
of patients with EOC (4–7). Thus, it is essential to understand
the molecular mechanisms of hypoxia-induced EOC progression.
4.1N, the protein product of the EPB41L1 gene, is a
member of the protein 4.1 family (8). The members of the protein 4.1 family
include 4.1R (9), 4.1B (10), 4.1G (11) and 4.1N (12). Protein 4.1 family members connect
the actin cytoskeleton to various transmembrane proteins (12) and serve important roles in cell
morphogenesis, membrane structure and cell adhesion (13). Recently, the roles of protein 4.1
family members in growth regulation and tumor development have
become increasingly recognized, and a loss of 4.1B and 4.1R
expression has been reported in lung, breast, prostate, ovary and
brain cancer, and meningioma (14–16).
However, the role of 4.1N in cancer remains to be fully elucidated.
It was previously reported that defective expression of 4.1N was
correlated with tumor progression, aggressive behavior and
chemotherapy resistance in EOC (17). Experiments on nude mice
(unpublished data) have demonstrated that 4.1N may significantly
inhibit the ability of peritoneal dissemination of EOC cells.
Hypoxia contributes to enhanced invasiveness,
angiogenesis and distant metastasis in various tumor types
(18). Previous studies have
indicated that hypoxia serves an important role in the initiation
of peritoneal dissemination of EOC cells (8,18,19).
The most important regulator under low levels of oxygen is
hypoxia-inducible factor-1 (HIF-1), which is comprised of the
HIF-1α and HIF-1β subunits (20).
Hypoxic conditions activate HIF-1α during numerous critical
behaviors of cancer progression, including angiogenesis (21,22),
energy metabolism (23),
resistance to radiation therapy and chemotherapy (24,25)
and epithelial-mesenchymal transition (EMT) (26–28).
In cancer, EMT serves an important role in tumor progression and is
marked by a loss of epithelial features, particularly loss of
E-cadherin and an upregulation of mesenchymal properties (29–31).
HIF-1α is an important factor in controlling the expression of
certain EMT regulators, including Snail, Twist and lysyl oxidase
(LOX), all of which are involved in various EMT processes occurring
during embryogenesis and tumorigenesis (7,27,32,33).
The current study hypothesized that 4.1N may
suppress hypoxia-induced EMT in EOC cells via regulating the
expression levels and subcellular localization of HIF-1α.
Materials and methods
Cell culture
A2780 and SKOV-3 cells (American Type Culture
Collection, Manassas, VA, USA) were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.),
respectively with 10% fetal bovine serum (FBS; Yuanheng JInma
Biotechnology Co. Ltd., Beijing, China). Cells were cultured with 2
mg/ml NaHCO3 at 37°C in a humidified chamber with 5%
CO2. To mimic hypoxic conditions, cells were starved
with 0.5% FBS and then were treated with CoCl2 (Aladdin
Industrial, Inc., Shanghai, China) at 250 μmol for different
lengths of time. Cells were treated for a different length of time
to induce HIF-1α expression due to the diverse characteristics of
A2780 (12 and 24 h) and SKOV-3 (48 and 72 h) cells.
Cell transfection and RNA
interference
A2780 cells were transfected with pEGFP-4.1N
[provided by Dr Xiuli An (Red Cell Physiology Laboratory, New York
Blood Center, New York, NY, USA) with sequencing identification
being performed in Gynecological Oncology Laboratory, Department of
Pathology, Peking University, Beijing, China] or pEGFP-3C with
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Human 4.1 short hairpin RNA (shRNA) was obtained from
Shanghai GenePharma Co. Ltd. (Shanghai, China). The target sites
for 4.1N shRNA were 5′-GCA ACA TCA CTC GAA ATAA-3′ (sh4.1N-1) and
5′-ACG GAA ATC CGT TCT CTTT-3′ (sh4.1N-2). For knocking down 4.1N,
two independent experiments with the above two shRNAs were
conducted and the efficiency of interference was confirmed. A
scrambled shRNA 5′-TGT TCG CAT TAT CCG AAC CAT-3′ was used as a
negative control. The cell line (SKOV-3) that endogenously
expressed 4.1N was transfected with different shRNA constructs to
evaluate the effects on tumor cells. Subsequent to incubation at
37°C for 24 h, G418 (800 μg/ml; Invitrogen; Thermo Fisher
Scientific, Inc.) was applied to stably screen and isolate the
resistant colonies.
Cell viability assay
The cells were seeded (3,000 cells/well) into
96-well plates and were incubated at 37°C with 5% carbon dioxide in
the presence of CoCl2 (200, 250, 300 and 0 μmol
cell control) for different lengths of time. The negative control
group underwent the same procedures, however the cells were not
plated. At each end point, a batch of cells was stained with 20
μl sterile 3-(4,5-dimethylthiazol-2-yl)-2,5-
diphenyltetrazolium bromide dye (5 mg/ml; Sigma-Aldrich, St. Louis,
MO, USA) at 37°C for 4 h. The culture medium was then removed and
150 μl dimethyl sulfoxide (Sigma-Aldrich) was added and
thoroughly mixed for 10 min. The cell viability assay was applied
to spectrometric absorbance at 490 nm and was measured using a
microplate reader (Thermo Fisher Scientific, Inc.). The cell
viability rate was calculated as (treatment group-negative control
group)/(cell control group-negative control group). Each group
contained six wells and the experiments were performed in
triplicate.
Western blotting analysis
Cells were lysed in radioimmunoprecipitation assay
lysis buffer supplemented with a protease inhibitor cocktail
(Applygen Technologies, Inc., Beijing, China). The cells were then
centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was
collected, protein (30 μg) was denatured in sodium dodecyl
sulfate (SDS) sample buffer (Applygen Technologies, Inc.) at 100°C
for 5 min and separated by electrophoresis on a 10%
SDS-polyacrylamide gel electrophoresis (Applygen Technologies,
Inc.), then transferred to a nitrocellulose membrane (Applygen
Technologies, Inc.). The membranes were blocked in Tris-buffered
saline with Tween-20 (TBST) with 5% non-fat milk for 1 h at 37°C,
then were incubated with the following primary antibodies: rabbit
monoclonal E-cadherin (cat. no. 1702-1; 1:2,000) and rabbit
polyclonal N-cadherin (cat. no. 21474; 1:500, purchased from
Epitomics, Inc. (Burlingame, CA, USA), rabbit polyclonal HIF-1α
(cat. no. 3716; 1:1,000) from Cell Signaling Technology, Inc.
(Danvers, MA, USA) and rabbit anti-4.1N (donated by Dr Xiuli An;
1:500) in blocking buffer (Applygen Technologies, Inc.) overnight
at 4°C. The membranes were washed three times in TBST and incubated
with horseradish peroxidase-conjugated goat anti-rabbit antibody
(cat. no. ZB-2301; 1:2,000) or anti-mouse (cat. no. ZB-2305;
1:2,000) antibodies (Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China) for 1 h at room temperature and then
visualized by enhanced chemiluminescence (ECL) using a
SuperEnhanced Chemiluminescence detection kit (Applygen
Technologies, Inc.). The Gel Doc™ XR+ imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was used for capturing the
images.
Immunofluorescence
Cells grown on glass cover-slips were fixed with 4%
paraformaldehyde for 10 min at room temperature. Subsequent to
washing in phosphate-buffered saline (PBS; GE Healthcare Life
Sciences, Chalfont, UK), the cells were incubated with the blocking
reagent [1% horse serum albumin (Gibco, Thermo Fisher Scientific,
Inc.) in PBS] for 15 min. Cells were incubated with rabbit
anti-HIF-1α (cat. no. ZA-0552; 1:1,000; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd.) at 4°C overnight. The primary
antibody was omitted for the negative control slides. Subsequent to
washing, samples were incubated with Alexa Fluor 594-conjugated
affinipure goat anti-rabbit IgG (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) for 1 h at 37°C. Subsequent to staining
with 6-diamino-2-phenylindole (Cell Signaling Technology, Inc.),
the cells were examined under a microscope (U-TV0.5XC-3; Olympus,
Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was synthesized
using the FastQuant RT kit [Tiangen Biotech (Beijing) Co., Ltd.,
Beijing, China]. cDNA (10 ng) was then used for the RT-qPCR
reaction using SuperReal PreMix Plus [Tiangen Biotech (Beijing)
Co., Ltd.]. RT-qPCR was performed using an iQ5 Real-Time PCR
Detection System (Bio-Rad Laboratories, Inc.). The results were
calculated by the 2−(ΔΔCt) method. The primer sequences
were as follows: 4.1N, forward 5′-AGG AAA CCA CGC CGA GAC ACA A-3′
and reverse 5′-GGTGGATGAGTTTGCTGT TGGG-3′; Twist, forward 5′-GAC
AGT GAT TCC CAG ACG G-3′ and reverse 5′-GTC CAT AGT GAT GCC TTT
CCT-3′; Snail, forward 5′-TCG GAA GCC TAA CTA CAG CG-3′ and reverse
5′-GAT GAG CAT TGG CAG CGA G-3′; HIF-1α, forward 5′-CTG AGG TTG GTT
ACT GTT GGT ATC-3′ and reverse 5′-AGT GTA CCC TAA CTA GCC GAG
GAA-3′; LOX, forward 5′-ATG GTG CTG CTC AGA TTT CC-3′ and reverse
5′-TGA CAA CTG TGC CAT TCC CA-3′. The cycling conditions were 95°C
for 15 min, followed by 40 cycles at 95°C for 10 sec, 55°C for 20
sec and 72°C for 30 sec.
Statistical analysis
All values are presented as the mean ± standard
deviation and are representative of an average of a minimum of
three independent experiments. Student's t-test or analysis of
variance for unpaired data was used to compare the mean values
using GraphPad Prism software, version 5 (GraphPad Software, Inc.,
La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
4.1N inhibits expression and nuclear
accumulation of HIF-1α under hypoxia
A previous study indicated that 4.1N protein was
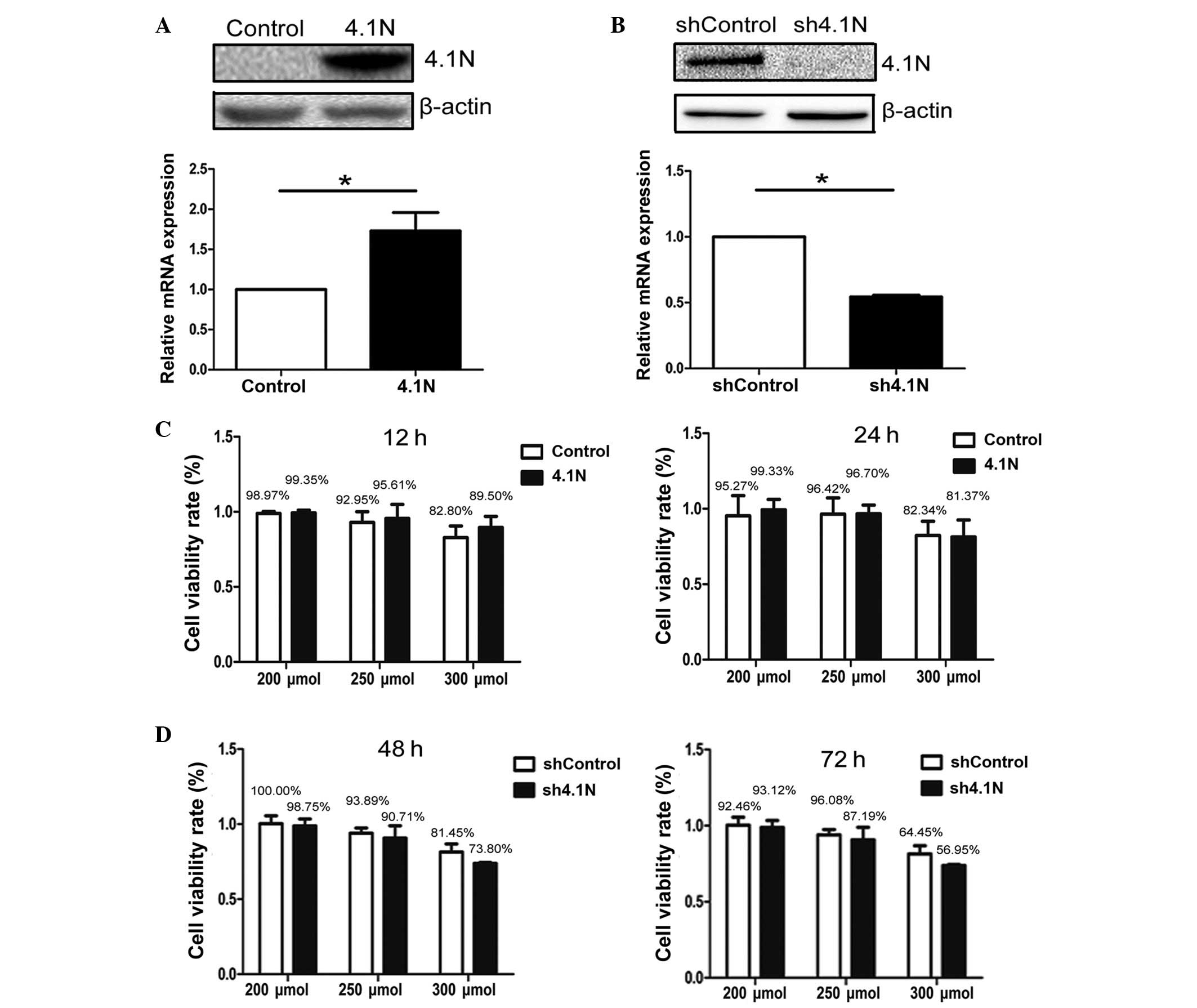
absent in A2780 and present in SKOV-3 cells (17). Overexpression of 4.1N in A2780 and
knockdown of 4.1N in SKOV-3 cells was confirmed by western blotting
and RT-qPCR (Fig. 1A and B),
respectively. Subsequently, the role of 4.1N in the regulation of
HIF-1α expression was investigated under hypoxia. A cell viability
assay was conducted in order to confirm the amount of
CoCl2 that could be tolerated by cells. In A2780 cells,
the cell viability rate was greater than 90% when treated with
CoCl2 at 200 and 250 μmol, however was reduced to
less than 90% at 300 μmol. In SKOV-3 cells, due to the
longer treatment duration (48 and 72 h), the cell viability rate
with CoCl2 at 200 and 250 μmol was lower than
that of A2780 cells, however remained greater than 85%. However,
with treatment with 300 μmol for 48 and 72 h, viability was
reduced to approximately 60% (Fig. 1C
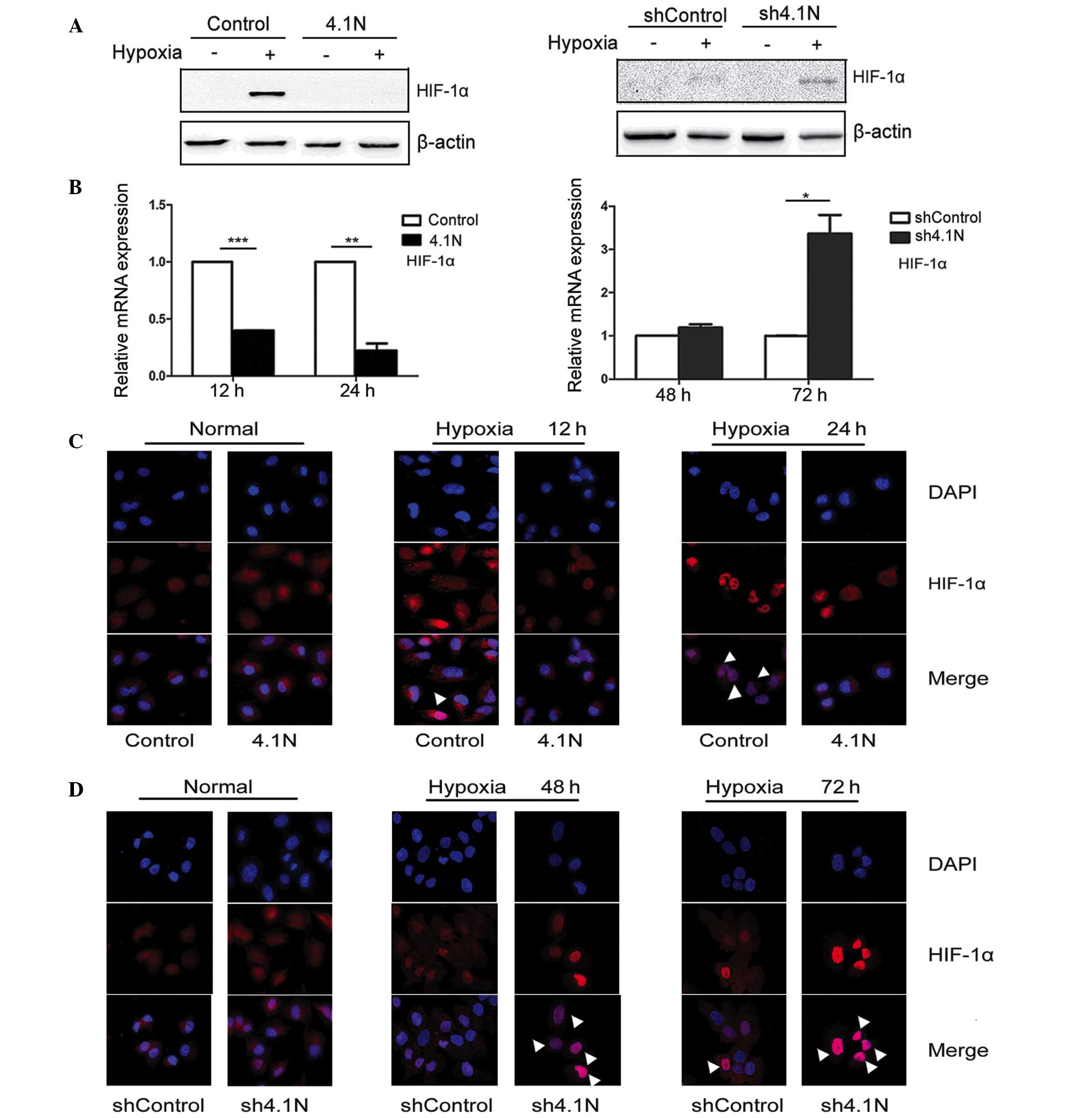
and D). In western blot analysis, proteins were collected
following 24 and 72 h hypoxia treatment for A2780 cells and SKOV-3
cells, respectively. Under normoxic conditions, the expression
levels of HIF-1α were significantly reduced in the presence of
4.1N. By contrast, the amount of HIF-1α protein was markedly
increased with the absence of 4.1N (Fig. 2A). RT-qPCR results are presented in
Fig. 2B. HIF-1α mRNA expression
was significantly downregulated in the presence of 4.1N; whereas,
the stress-response of 4.1N-knockdown cells to hypoxia was
significantly increased at 72 h compared with the control.
Immunofluorescence staining was then conducted in order to confirm
the alterations in HIF-1α subcellular localization under hypoxia.
Nuclear accumulation of HIF-1α in 4.1N-overexpressing A2780 cells
was observed to be markedly reduced compared with that of control
cells under hypoxia, particularly following 24 h of hypoxia
(Fig. 2C). In SKOV-3 cells, the
absence of 4.1N led to clear nuclear localization of HIF-1α at 48
and 72 h compared with the controls (Fig. 2D). These results suggest that 4.1N
may suppress the expression and nuclear localization of HIF-1α
under hypoxic conditions.
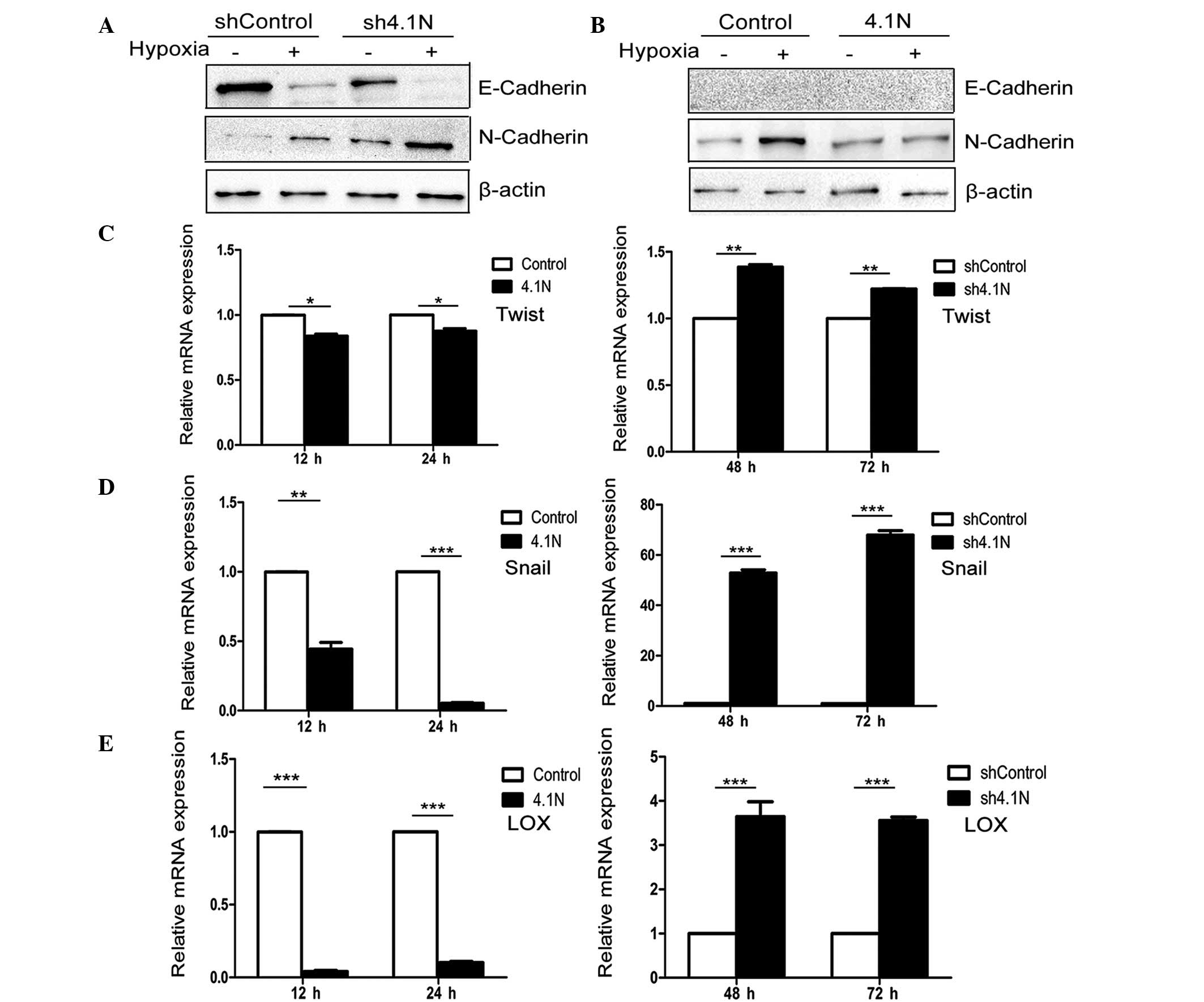
4.1N inhibits hypoxia-induced EMT
Loss expression of epithelial markers such as
E-cadherin and increases in expression of mesenchymal markers such
as N-cadherin are typical events of epithelial cells undergoing
EMT. It was identified that with the absence of 4.1N, the protein
levels of E-cadherin were markedly reduced and the protein levels
of N-cadherin were upregulated under normoxic and hypoxic
conditions, particularly with those with hypoxia (Fig. 3A). When 4.1N was overexpressed,
N-cadherin expression was markedly reduced under conditions of
hypoxia (Fig. 3B). However, due to
the lack of endogenous expression of E-cadherin in A2780 cells, the
effect of hypoxia on E-cadherin of A2780 cells in the 4.1N and
control groups was not detected.
4.1N suppresses the expression of
positive EMT regulators
Hypoxia, an important tumor micro-environmental
factor, induces the expression of numerous EMT regulators,
including Snail, Twist and LOX (7,33,34).
Thus, RT-qPCR was used to observe the transcriptional mRNA levels
of Snail, Twist and LOX. Fig. 3C–E
indicate that overexpression of 4.1N may significantly inhibit the
mRNA expression levels of the three measured EMT regulators under
hypoxic stress, while their mRNA expression levels were
significantly increased in the EOC cells in the absence of 4.1N
under hypoxia.
Discussion
The protein 4.1 family has been observed to serve an
important role in the regulation of growth and tumor progression
(35). A previous study indicated
that 4.1N was a potential tumor suppressor in EOC (17). Previous studies have indicated that
peritoneal dissemination, in which EMT has been reported to be
critical, is one of the key mechanisms in EOC progression (4,6).
Furthermore, the resistance to hypoxic-stress is essential for the
induction of EMT and peritoneal dissemination (7,36).
In the current study, it was demonstrated for the first time, to
the best of our knowledge, that 4.1N may inhibit hypoxia-induced
EMT of EOC cells at least partly via the regulation of HIF-1α.
Previous studies have indicated that the increased
expression and activation of HIF are closely associated with cancer
progression and poor prognosis of patients (37–39).
HIF-1α has been previously reported to be correlated with the
migration and invasion of EOC cells and has been demonstrated to be
an important prognostic marker of patients with EOC (40–42).
The results of the current study indicated that 4.1N may inhibit
the nuclear accumulation of HIF-1α and suppress its expression.
Hypoxia-induced HIF activation is associated with a
concomitant loss of E-cadherin (43), one crucial feature of EMT resulting
in cancer metastasis and drug resistance. Imai et al
(7) reported that
immunolocalization of nuclear HIF-1α was correlated with the loss
of E-cadherin in EOC cells. In the current study, the results
indicate that absence of 4.1N may aggravate hypoxia-induced
E-cadherin loss. It was observed that even under normoxic
conditions, E-cadherin was additionally downregulated due to
knockdown of 4.1N, which implied the potential interaction of 4.1N
and E-cadherin. Additionally, the upregulation of hypoxia-induced
N-cadherin expression was inhibited by 4.1N, which further
suggested the role of 4.1N in impeding hypoxia-induced EMT. The
data of the current study appears to be in support of an
E/N-cadherin switch during EMT (44).
Several transcriptional factors have been
demonstrated to be involved in EMT during tumor development. In the
current study, Snail, Twist and LOX (18) were investigated, which are
important molecules interacting with HIF-1α under hypoxic
conditions (40). Snail and Twist
have been demonstrated to induce EMT by repressing E-cadherin
expression (45,46) through hypoxia signaling and the
classical phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)
pathways (31,47,48).
In addition, the LOX family has multiple roles in tumor progression
including altering extracellular matrix components (49) and regulating Snail (50). In EOC samples, LOX has been
observed to be significantly associated with advanced clinical
stages and metastasis (40). In
the current study, a negative regulation of Twist, Snail and LOX by
4.1N further suggested a role of 4.1N in suppressing
hypoxia-induced EMT.
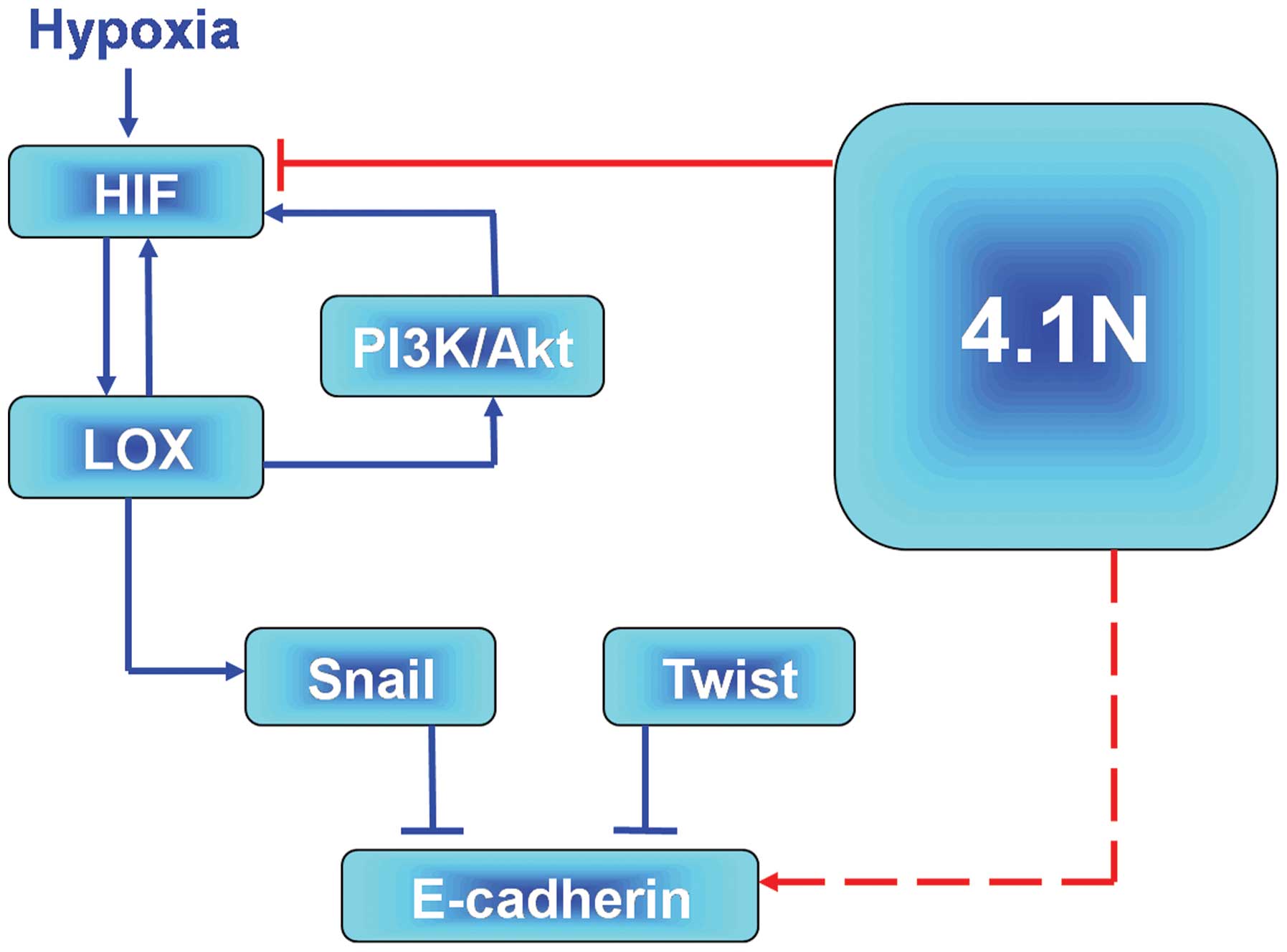
According to the present data, an outline of the
molecular pathway, which 4.1N is involved in during the
hypoxia-induced EOC progression can be provided. On one hand, the
promising hypoxia signalling pathway, HIF-LOX-Snail-E-cadherin
(15) may assist in explaining the
mechanisms by which 4.1N suppresses hypoxia-induced EMT. It was
demonstrated that 4.1N regulated HIF, leading to the low expression
of LOX and Snail and upregulation of E-cadherin. By contrast, it is
also possible that 4.1N may affect the hypoxia-induced expression
of LOX via association with PI3K. LOX-HIF-1α mutual regulation
activated the AKT pathway in epithelial EOC (38) and it was previously reported that
LOX activated the PI3K/Akt signaling pathway to upregulate HIF-1α
protein synthesis (49). In
addition, the association of 4.1N and PI3K was previoulsy
demonstrated. Firstly, 4.1N was demonstrated to regulate PI3K
activity via interactions with the PI3K (50). Secondly, our previous proteomic
analysis indicated that 4.1N may upregu-late the expression of
inositol polyphosphate 5-phosphatase, an enzyme associated with
PI3K. Therefore, 4.1N may deregulate the activity of PI3K and
subsequently inhibit the expression of HIF-1α expression and Akt
activity. LOX-HIF-1α mutual regulation was demonstrated to activate
the Akt pathway in epithelial EOC cells (40). In addition, Pez et al
(51) reported that LOX was able
to activate the PI3K/Akt signaling pathway to upregulate HIF-1α
protein synthesis and Ye et al (52) reported that 4.1N regulated PI3K
activity via interactions with the PI3K enhancer. In addition,
proteome analysis (Zhang et al, unpublished data) indicated
that 4.1N may upregulate the expression of inositol polyphosphate
5-phosphatase, an enzyme associated with PI3K. It is notable that
inositol polyphosphate 5-phosphatases may lead to apoptotic cell
death (53), and share a potential
role in tumor progression. Combining these two hypotheses, the
predictive model presented in Fig.
4 is proposed. However, further studies are required to clarify
the specific signaling networks.
In summary, the results of the present study
indicate that 4.1N is an important regulator for hypoxia-induced
EOC progression. 4.1N may be a potential tumor suppressor and a
therapeutic target for patients with EOC.
Acknowledgments
The current study was supported by the National
Natural Science Foundation of China (grant no. 81472430).
References
|
1
|
Hess LM and Stehman FB: State of the
science in ovarian cancer quality of life research: A systematic
review. Int J Gynecol Cancer. 22:1273–1280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rooth C: Ovarian cancer: Risk factors,
treatment and management. Br J Nurs. 22(Suppl): S23–S30. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hua KT, Wang MY, Chen MW, Wei LH, Chen CK,
Ko CH, Jeng YM, Sung PL, Jan YH, Hsiao M, et al: The H3K9
meth-yltransferase G9a is a marker of aggressive ovarian cancer
that promotes peritoneal metastasis. Mol Cancer. 13:1892014.
View Article : Google Scholar
|
|
5
|
Naora H and Montell DJ: Ovarian cancer
metastasis: Integrating insights from disparate model organisms.
Nat Rev Cancer. 5:355–366. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Auersperg N, Wong AS, Choi KC, Kang SK and
Leung PC: Ovarian surface epithelium: Biology, endocrinology and
pathology. Endocr Rev. 22:255–288. 2001.PubMed/NCBI
|
|
7
|
Imai T, Horiuchi A, Wang C, Oka K, Ohira
S, Nikaido T and Konishi I: Hypoxia attenuates the expression of
E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells.
Am J Pathol. 163:1437–1447. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peters LL, Weier HU, Walensky LD, Snyder
SH, Parra M, Mohandas N and Conboy JG: Four paralogous protein 4.1
genes map to distinct chromosomes in mouse and human. Genomics.
54:348–350. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Conboy J, Kan YW, Shohet SB and Mohandas
N: Molecular cloning of protein 4.1, a major structural element of
the human erythrocyte membrane skeleton. Proc Natl Acad Sci USA.
83:9512–9516. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parra M, Gascard P, Walensky LD, Gimm JA,
Blackshaw S, Chan N, Takakuwa Y, Berger T, Lee G, Chasis JA, et al:
Molecular and functional characterization of protein 4.1B, a novel
member of the protein 4.1 family with high level, focal expression
in brain. J Biol Chem. 275:3247–3255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Walensky LD, Blackshaw S, Liao D, Watkins
CC, Weier HU, Parra M, Huganir RL, Conboy JG, Mohandas N and Snyder
SH: A novel neuron-enriched homolog of the erythrocyte membrane
cytoskeletal protein 4.1. J Neurosci. 19:6457–6467. 1999.PubMed/NCBI
|
|
12
|
Wang H, Liu C, Debnath G, Baines AJ,
Conboy JG, Mohandas N and An X: Comprehensive characterization of
expression patterns of protein 4.1 family members in mouse adrenal
gland: Implications for functions. Histochem Cell Biol.
134:411–420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun CX, Robb VA and Gutmann DH: Protein
4.1 tumor suppressors: Getting a FERM grip on growth regulation. J
Cell Sci. 115:3991–4000. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dafou D, Grun B, Sinclair J, Lawrenson K,
Benjamin EC, Hogdall E, Kruger-Kjaer S, Christensen L, Sowter HM,
Al-Attar A, et al: Microcell-mediated chromosome transfer
identifies EPB41L3 as a functional suppressor of epithelial ovarian
cancers. Neoplasia. 12:579–589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong SY, Haack H, Kissil JL, Barry M,
Bronson RT, Shen SS, Whittaker CA, Crowley D and Hynes RO: Protein
4.1B suppresses prostate cancer progression and metastasis. Proc
Natl Acad Sci U S A. 104:12784–12789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robb VA, Li W, Gascard P, Perry A,
Mohandas N and Gutmann DH: Identification of a third protein 4.1
tumor suppressor, protein 4.1R, in meningioma pathogenesis.
Neurobiol Dis. 13:191–202. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xi C, Ren C, Hu A, Lin J, Yao Q, Wang Y,
Gao Z, An X and Liu C: Defective expression of protein 4.1N is
correlated to tumor progression, aggressive behaviors and
chemotherapy resistance in epithelial ovarian cancer. Gynecol
Oncol. 131:764–771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pouysségur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakayama K, Kanzaki A, Hata K, Katabuchi
H, Okamura H, Miyazaki K, Fukumoto M and Takebayashi Y:
Hypoxia-inducible factor 1 alpha (HIF-1 alpha) gene expression in
human ovarian carcinoma. Cancer Lett. 176:215–223. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Semenza GL: Oxygen sensing, homeostasis
and disease. N Engl J Med. 365:537–547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liao D and Johnson RS: Hypoxia: A key
regulator of angiogenesis in cancer. Cancer Metastasis Rev.
26:281–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee K, Zhang H, Qian DZ, Rey S, Liu JO and
Semenza GL: Acriflavine inhibits HIF-1 dimerization, tumor growth
and vascularization. Proc Natl Acad Sci U S A. 106:17910–17915.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo W, Hu H, Chang R, Zhong J, Knabel M,
O'Meally R, Cole RN, Pandey A and Semenza GL: Pyruvate kinase M2 is
a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell.
145:732–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moeller BJ, Richardson RA and Dewhirst MW:
Hypoxia and radiotherapy: Opportunities for improved outcomes in
cancer treatment. Cancer Metastasis Rev. 26:241–248. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rohwer N and Cramer T: Hypoxia-mediated
drug resistance: Novel insights on the functional interaction of
HIFs and cell death pathways. Drug Resist Updat. 14:191–201. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Esteban MA, Tran MG, Harten SK, Hill P,
Castellanos MC, Chandra A, Raval R, O'brien TS and Maxwell PH:
Regulation of E-cadherin expression by VHL and hypoxia-inducible
factor. Cancer Res. 66:3567–3575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krishnamachary B, Zagzag D, Nagasawa H,
Rainey K, Okuyama H, Baek JH and Semenza GL: Hypoxia-inducible
factor-1-dependent repression of E-cadherin in von hippel-lindau
tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A
and ZFHX1B. Cancer Res. 66:2725–2731. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mak P, Leav I, Pursell B, Bae D, Yang X,
Taglienti CA, Gouvin LM, Sharma VM and Mercurio AM: ERbeta impedes
prostate cancer EMT by destabilizing HIF-1alpha and inhibiting
VEGF-mediated snail nuclear localization: Implications for gleason
grading. Cancer Cell. 17:319–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nieto MA: The ins and outs of the
epithelial to mesenchymal transition in health and disease. Ann Rev
Cell Dev Biol. 27:347–376. 2011. View Article : Google Scholar
|
|
30
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Evans AJ, Russell RC, Roche O, Burry TN,
Fish JE, Chow VW, Kim WY, Saravanan A, Maynard MA, Gervais ML, et
al: VHL promotes E2 box-dependent E-cadherin transcription by
HIF-mediated regulation of SIP1 and snail. Mol Cell Biol.
27:157–169. 2007. View Article : Google Scholar :
|
|
33
|
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang
SY, Liu CJ, Teng SC and Wu KJ: Direct regulation of TWIST by
HIF-1alpha promotes metastasis. Nat Cell Biol. 10:295–305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pouyssegur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bernkopf DB and Williams ED: Potential
role of EPB41L3 (protein 4.1B/Dal-1) as a target for treatment of
advanced prostate cancer. Expert Opin Ther Targets. 12:845–853.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Joshi HP, Subramanian IV, Schnettler EK,
Ghosh G, Rupaimoole R, Evans C, Saluja M, Jing Y, Cristina I, Roy
S, Zeng Y, Shah VH, Sood AK and Ramakrishnan S: Dynamin 2 along
with microRNA-199a reciprocally regulate hypoxia-inducible factors
and ovarian cancer metastasis. Proc Natl Acad Sci USA.
111:5331–5336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Milosevic M, Warde P, Ménard C, Chung P,
Toi A, Ishkanian A, McLean M, Pintilie M, Sykes J, Gospodarowicz M,
et al: Tumor hypoxia predicts biochemical failure following
radiotherapy for clinically localized prostate cancer. Clin Cancer
Res. 18:2108–2114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cangul H, Salnikow K, Yee H, Zagzag D,
Commes T and Costa M: Enhanced overexpression of an
HIF-1/hypoxia-related protein in cancer cells. Environ Health
Perspect. 110(Suppl 5): S783–S788. 2002. View Article : Google Scholar
|
|
39
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
40
|
Ji F, Wang Y, Qiu L, Li S, Zhu J, Liang Z,
Wan Y and Di W: Hypoxia inducible factor 1α-mediated LOX expression
correlates with migration and invasion in epithelial ovarian
cancer. Int J Oncol. 42:1578–1588. 2013.PubMed/NCBI
|
|
41
|
Wong C, Wellman TL and Lounsbury KM: VEGF
and HIF-1alpha expression are increased in advanced stages of
epithelial ovarian cancer. Gynecol Oncol. 91:513–517. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Osada R, Horiuchi A, Kikuchi N, Yoshida J,
Hayashi A, Ota M, Katsuyama Y, Melillo G and Konishi I: Expression
of hypoxia-inducible factor 1alpha, hypoxia-inducible factor 2alpha
and von hippel-lindau protein in epithelial ovarian neoplasms and
allelic loss of von hippel-lindau gene: Nuclear expression of
hypoxia-inducible factor 1alpha is an independent prognostic factor
in ovarian carcinoma. Hum Pathol. 38:1310–1320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Beavon IR: Regulation of E-cadherin: Does
hypoxia initiate the metastatic cascade? Mol Pathol. 52:179–188.
1999. View Article : Google Scholar
|
|
44
|
Tomita K, van Bokhoven A, van Leenders GJ,
Ruijter ET, Jansen CF, Bussemakers MJ and Schalken JA: Cadherin
switching in human prostate cancer progression. Cancer Res.
60:3650–3654. 2000.PubMed/NCBI
|
|
45
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thomassin L, Werneck CC, Broekelmann TJ,
Gleyzal C, Hornstra IK, Mecham RP and Sommer P: The pro-regions of
lysyl oxidase and lysyl oxidase-like 1 are required for deposition
onto elastic fibers. J Biol Chem. 280:42848–42855. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Peinado H, Del Carmen Iglesias-de la Cruz
M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A and
Portillo F: A molecular role for lysyl oxidase-like 2 enzyme in
snail regulation and tumor progression. EMBO J. 24:3446–3458. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pez F, Dayan F, Durivault J, Kaniewski B,
Aimond G, Le Provost GS, Deux B, Clézardin P, Sommer P, Pouysségur
J and Reynaud C: The HIF-1-inducible lysyl oxidase activates HIF-1
via the Akt pathway in a positive regulation loop and synergizes
with HIF-1 in promoting tumor cell growth. Cancer Res.
71:1647–1657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ye K, Hurt KJ, Wu FY, Fang M, Luo HR, Hong
JJ, Blackshaw S, Ferris CD and Snyder SH: Pike. A nuclear gtpase
that enhances PI3kinase activity and is regulated by protein 41N.
Cell. 103:919–930. 2000. View Article : Google Scholar
|
|
53
|
Kisseleva MV, Cao L and Majerus PW:
Phosphoinositide-specific inositol polyphosphate 5-phosphatase IV
inhibits Akt/protein kinase B phosphorylation and leads to
apoptotic cell death. J Biol Chem. 277:6266–6272. 2002. View Article : Google Scholar
|