Introduction
Ganoderma lucidum (Fr.) Karst, of the family
Polyporaceae, has been used as a Traditional medicine for several
thousand years in China, Japan and other countries. Evidence has
accumulated concerning the medicinal use of Ganoderma to
treat various diseases, including cancer and immunological
disorders, and its biotechnological utilization has become popular
(1–5). The mixture of triterpenoids naturally
occurring in G. lucidum inhibits the proliferation of human
and mouse carcinoma cell lines (6). Studies have reported that the
cytotoxicity mediated by triterpene-enriched extracts of G.
tsugae in MCF-7 human breast cancer, prostate cancer and PC-3
cells occurs via apoptosis and cell-cycle arrest (7–9).
Another study demonstrated that apoptosis induced by
triterpene-enriched extracts of G. lucidum occurs through
the suppression of protein kinase C, the activation of
mitogen-activated protein kinases (MAPKs) and G2-phase cell-cycle
arrest (10). Other suggested
mechanisms include a reduction in intracellular calcium levels, the
induction of NAD(P)H: quinone oxido-reductase in cultured hepalcic
7 murine hepatoma cells, activation of MAPKs in rat
pheochromocytoma PC12 cells and stimulation of actin polymerization
in bladder cancer cells in vitro. However, whether the
ingredients in the extract mixtures have antagonistic or
synergistic biological effects is difficult to determine. In
addition, the predominant compound within the extract responsible
for its bioactivity has not been identified, which further
complicates the investigation of the structure-activity
associations. Polyporus umbellatus, also termed Grifola
umbellata is a fungus, which causes white rot in hardwoods. The
sclerotia of P. umbellata, which are bumpy, rugged and dark
brown/black, are used as a diuretic in Chinese medicine. Although
the water extracts of P. umbellata sclerotia have diuretic
effects, its methanol extracts have cytotoxic effects against human
gastric cancer cells, although the active components remain to be
elucidated (11). Khz is an
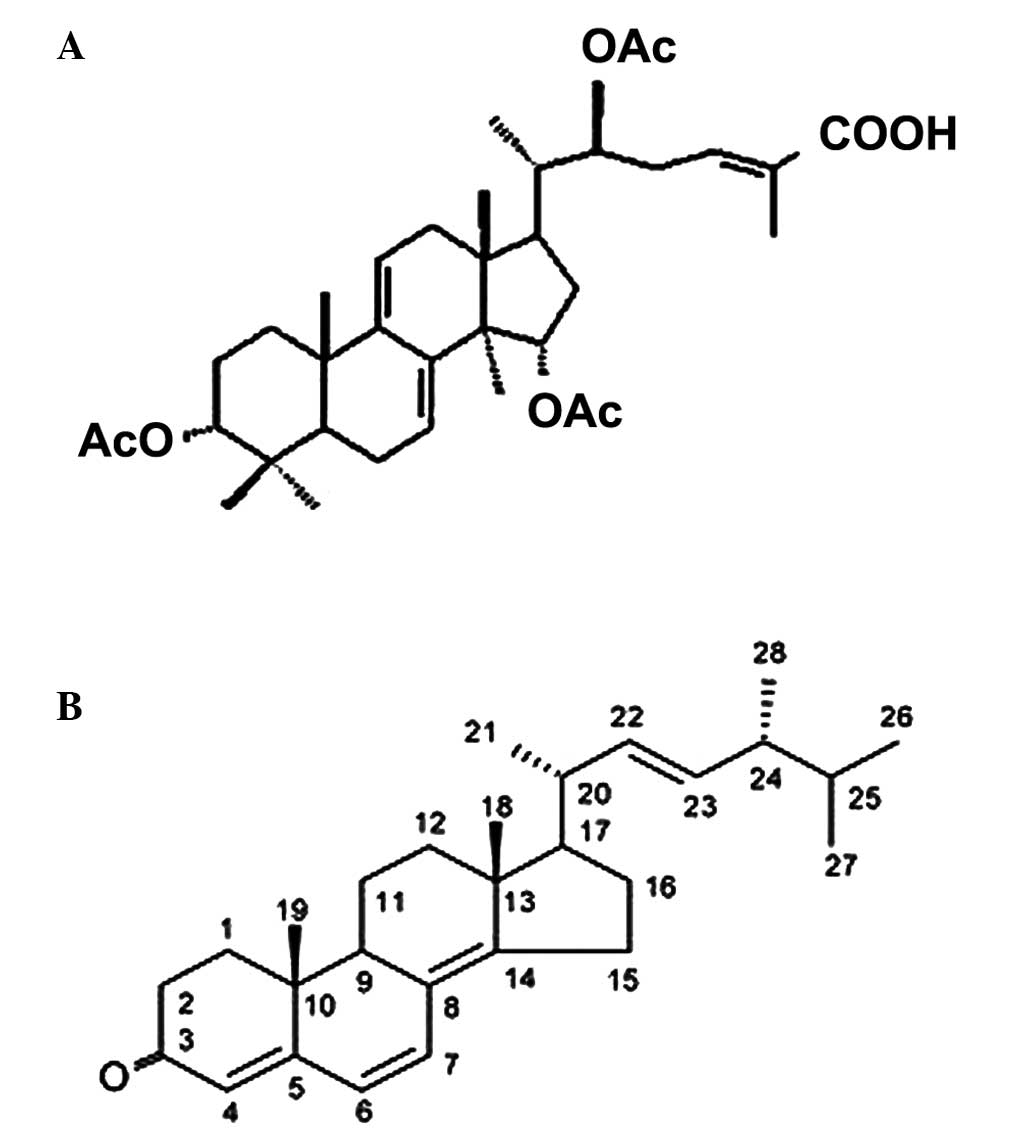
extract mixture, produced from the mycelia of a G. lucidum
(Fig. 1A) (12) and P. umbellatus (Fig. 1B) via nuclear fusion (Fig. 2A). The anticancer effect of the
fusion of G. lucidum and P. umbellatus has been
previously demonstrated (13–15).
The present study aimed to investigate the mechanism underlying
Khz-induced cell death in breast cancer cells. Whether Khz has the
ability to inhibit cell growth and promote apoptosis in human
breast cancer cells remains to be elucidated; therefore, the
present study examined the effect of Khz on cell viability and on
the expression of apoptosis-associated proteins in MCF-7 human
breast cancer cells.
Materials and methods
Cell lines
The BEAS-2B (normal immortalized), 1799
(non-transformed), 1198 (transformed, non-tumorigenic) and 1170-I
(tumorigenic) cell lines comprise an in vivo lung
carcinogenesis model, which has been previously described (16–18).
The MCF-7 human breast cancer cell line was purchased from American
Type Culture Collection (Manassas, VA, USA) and was maintained in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (GE Healthcare
Life Sciences, Logan, UT, USA), 100 U/ml penicillin G sodium salt
(Sigma-Aldrich, St. Louis, MO, USA), 100 µg/ml streptomycin
sulfate (Sigma-Aldrich) and 0.25 µg/ml amphotericin B
(Sigma-Aldrich). Unless otherwise indicated, the cells were treated
with Khz dose or time dependently.
Khz (fusion of G. lucidum and P.
umbellatus mycelia) extraction method
Khz (Brain Group Co., Ltd., Seoul, Korea) was
extracted first in powder form (1 kg) using clean water (8.5
liters), at 115°C for 60 min extracts at a pressure of 1.8–2 kW,
followed by 60 min maturing at a hydraulic pressure of 5 KW in
order to separate Khz and debris. Subsequently, clean water (7.5
liters) was added to the residual water of the first extraction,
and extraction was performed at 115°C, for 60 min at a pressure of,
followed by 60 min maturation and hydraulic crossroad gathering.
Finally, the extracts from the first extract and the secondary
extraction steps were mixed and boiled at 100°C for 5 min.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
An MTT assay was used to determine the rate of cell
survival. The MCF-7 cells were seeded into 96-well plates at a
density of 2,000 cells per well, and 10/100 µl MTT was added
to each well at a final concentration of 500 µg/ml. The
mixture was incubated for 1 h, following which the liquid in the
wells was removed. Subsequently, 50 µl dimethyl sulfoxide
was added to each well and incubated for 10 min, and the absorbance
was recorded using a UV Max microplate reader (Molecular Devices,
Palo Alto, CA, USA) at 595 nm (19).
Detection of apoptosis
The cells treated with Khz were washed twice in cold
phosphate-buffered saline (PBS) and were stained with annexin
V-fluorescein isothiocyanate (FITC) (cat. no. A13199, Invitrogen
Life Technologies, Carlsbad, CA, USA) and propidium iodide (PI; 5
µg/ml; Sigma-Aldrich), according to the manufacturer's
instructions. Briefly, Annexin V-FITC (5 µl) was added to
the cells and incubated for 1 h at 37°C, and the cells were then
resuspended in 100 µl 1X binding buffer containing 10 mM
HEPES, 140 mM NaCl and 2 mM CaCl2 (pH 7.4;
Sigma-Aldrich). z-VAD-fmk was obtained from R&D Systems
(Minneapolis, MN, USA). The cells were incubated at room
temperature for 15 min, following which PI was added to the cell
suspension prior to flow cytometric analysis (FACSCalibur; BD
Biosciences, San Jose, CA, USA).
Detection of reactive oxygen species
generation
The levels of cytoplasmic reactive oxygen species
(ROS) in the cells were estimated using the oxidation-sensitive
fluorescent dye, 20,70-dichlorodihydrofluorescein diacetate
(H2DCF-DA; Invitrogen Life Technologies). For the DCF staining, the
cells were loaded with H2DCF-DA (100 nM) for 1 h at 37°C, and were
then washed once with PBS, ROS levels were analyzed following Khz
treatment using a flow cytometer (FACSCalibur; BD Biosciences).
N-acetyl cysteine was purchased from Sigma-Aldrich.
Detection of calcium increase
The Ca2+ levels in the MCF-7 cells were
determined by staining with fluo-4 AM. The cells, which had been
treated with Khz for different durations (0, 0.5, 1 and 2 h),
harvested and washed twice with PBS, and then resuspended in fluo-4
AM, followed by incubated incubation at 37°C for 30 min. The
changes in Ca2+ concentration were analyzed using flow
cytometry. Ethylene glycol tetraacetic acid (EGTA) was purchased
from Sigma-Aldrich.
Western blot analysis
The cells were lysed in extraction buffer
(Sigma-Aldrich) containing 31.25 mM Tris-HCl (pH 6.8), 1% sodium
dodecyl sulfate (SDS), 10% glycerol and 2.5% mercaptoethanol, and
the whole-cell lysates were subjected to separation using 10%
SDS-polyacrylamide gel (Sigma-Aldrich) electrophoresis. The
size-fractionated proteins on the gel were then transferred onto a
nitrocellulose membrane (GE Healthcare Life Sciences), and the
membrane was blocked with 5% skimmed milk in Tris-buffered saline
containing 0.05% Tween 20 (Sigma-Aldrich), and was incubated with
the following primary antibodies: Goat polyclonal anti-caspase-7
(cat. no. sc-22179), anti-caspase-8 (cat. no. sc-6136) and
anti-caspase-9 (cat. no. sc-22182), purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Following washing once with
PBS, the membrane was incubated with a horseradish
peroxidase-conjugated mouse anti-goat IgG secondary antibody (cat.
no. sc-2354; Santa Cruz Biotechnology, Inc.). The protein band of
interest was detected using enhanced chemiluminescence reagents (GE
Healthcare Life Sciences, Shanghai, China) (20).
Statistical analysis
Cell viability was expressed as the mean ± standard
deviation. Statistical comparisons were made between the control
and treatment groups using Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
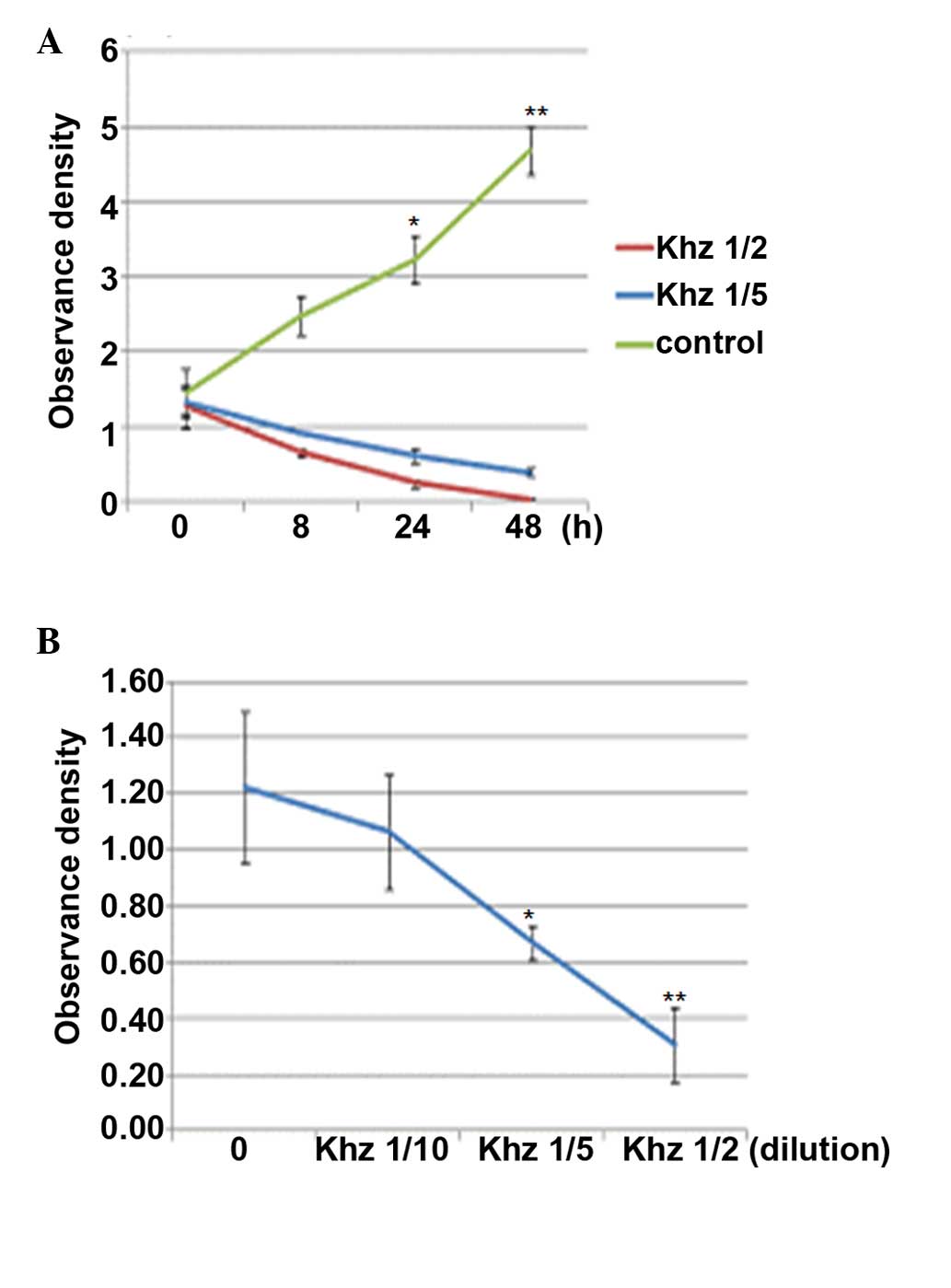
Effects of Khz on cell viability
Khz is the fusion of G. lucidum and P.
umbellatus mycelia, as shown in Fig. 2. The aim of the present study was
to examine whether Khz causes apoptosis in human cancer cells and,
if so, to identify the signaling mechanisms involved. The effects
of Khz on MCF-7 cell growth were determined using an MTT assay. As
shown in Fig. 3, the MCF-7 cells
were treated with different concentrations of Khz for 48 h. Khz
markedly inhibited cell growth in a dose- and time-dependent
manner. Treatment with 1/2 Khz for 24 h resulted in significant
inhibition of cell proliferation.
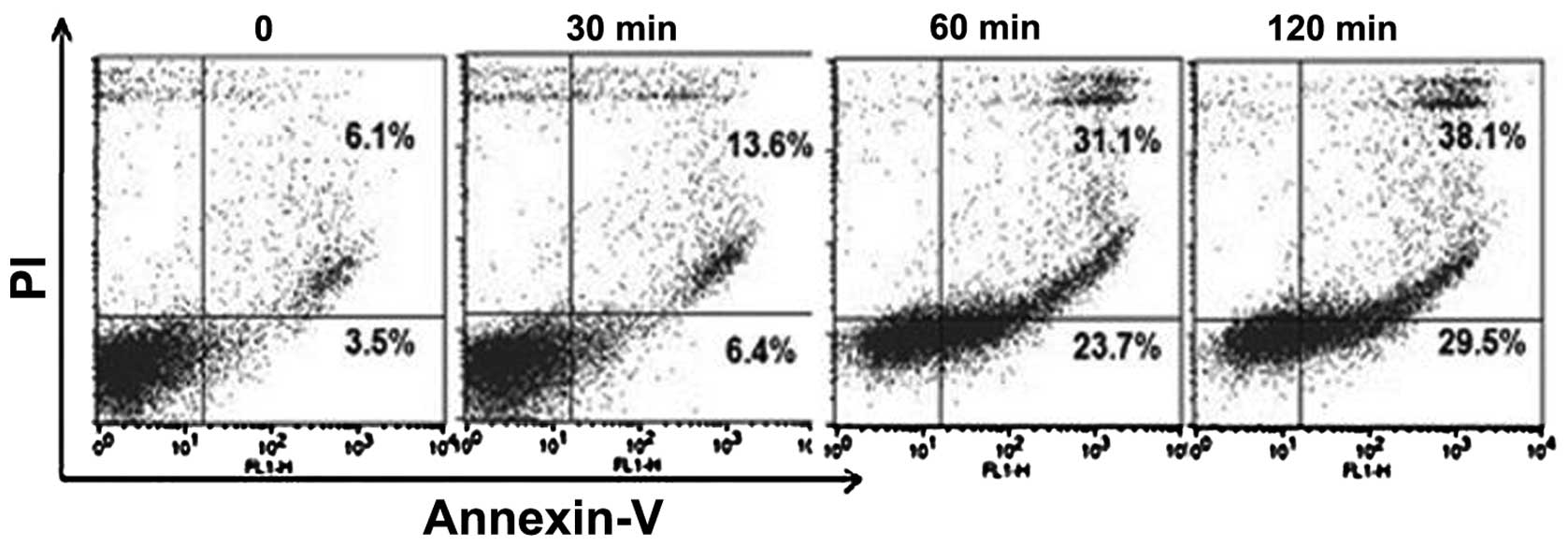
Effects of Khz on apoptosis
To assess whether Khz induces apoptosis, the MCF-7
cells were double stained with annexin V-FITC and PI and analyzed
using flow cytometry, which is the most sensitive and specific
assessment for apoptotic cells in suspension culture. PI was used
to differentiate between the apoptotic cells, which maintained
plasma membrane integrity (annexin V+/PI−)
and those exhibiting loss-of-membrane integrity (annexin
V+/PI+). Following Khz treatment at different
concentrations for 24 h, the majority of the apoptotic cells were
observed in the upper right quadrant (annexin
V+/PI+), indicating that almost all of the
apoptotic cells were in the late stages of apoptosis (data not
shown). Therefore, to detect cells in early apoptosis, the
durations of treatment were reduced. Treatment with 1/2 Khz for 1 h
resulted in the presence of early apoptotic cells in the lower
right quadrant (annexin V+/PI−) (Fig. 4). Following treatment for 2 h, the
cell population was observed to have shifted between viable cells
and those in the early- and late-stages of apoptosis, indicating a
time-dependent increase in the percentage of apoptotic MCF-7
cells.
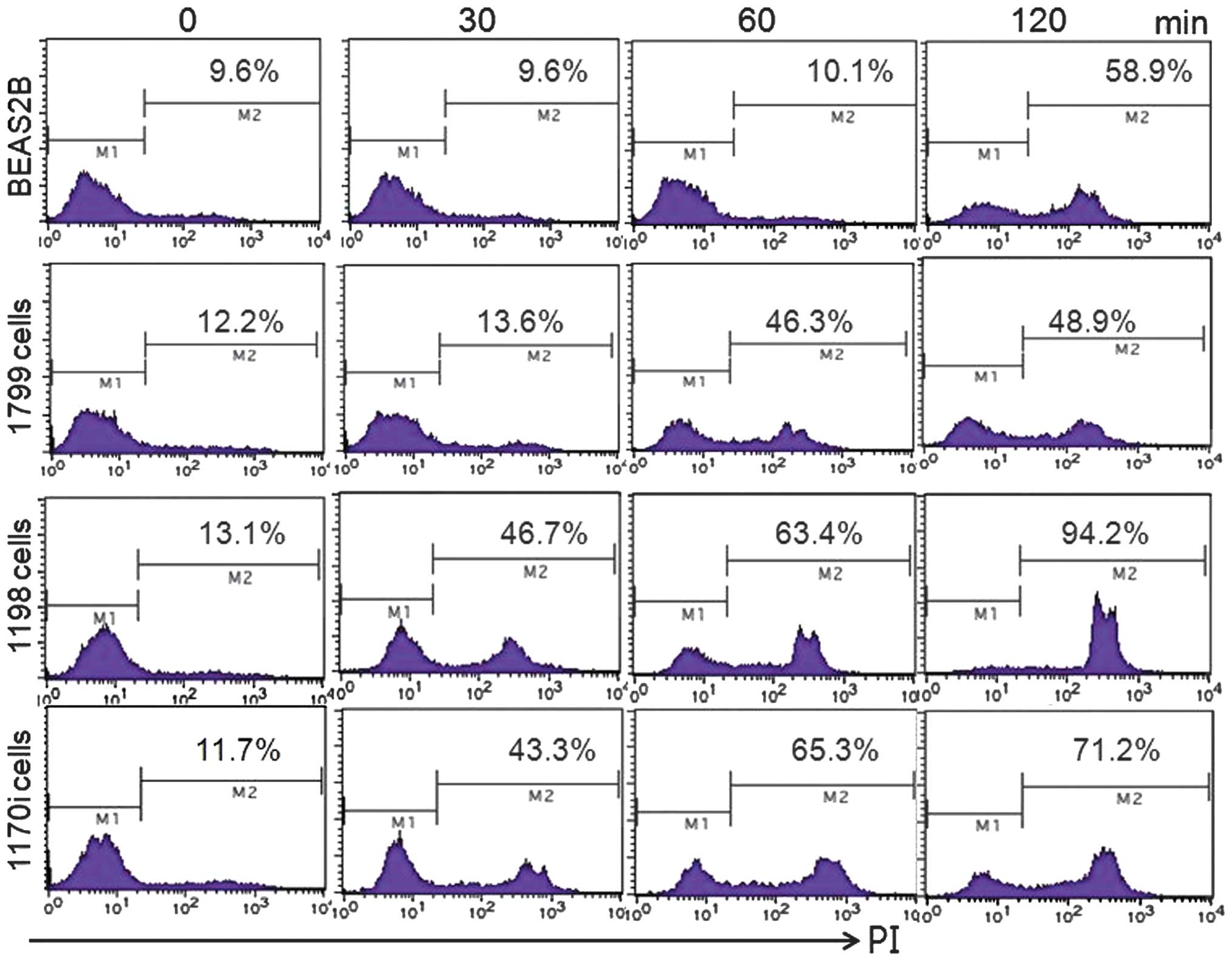
Effect of Khz on transformed cells
An in vitro human lung epithelial
carcinogenesis model comprising several cell lines was used in the
present study to examine whether the induction of apoptosis by Khz
occurred preferentially in transformed cells. BEAS-2B is an
immortalized normal human bronchial epithelial cell line, and 1198
and 1170-I are transformed cell lines derived from BEAS-2B cells
exposed to beeswax pellets containing cigarette smoke condensate
in vivo. The 1799 cell line is a non-transformed line, which
is derived from BEAS-2B cells exposed to beeswax alone. Khz induced
apoptosis in the transformed 1198 and 1170-I cells, but not in the
non-transformed BEAS-2B or 1799 cells (Fig. 5). These data indicated that Khz
induced apoptosis preferentially in the cancer cells, which
suggested its potential as a cancer therapeutic agent.
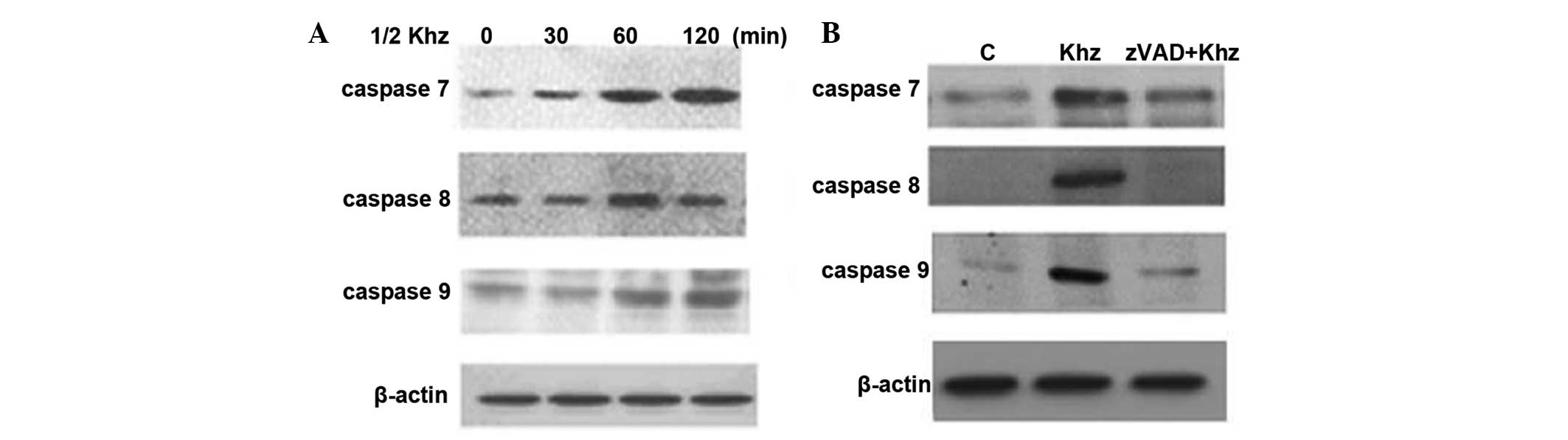
Detection of apoptosis-associated
proteins in Khz-treated MCF-7 cells
Subsequently, the present study analyzed the
activation of caspases following Khz treatment to determine whether
Khz-induced apoptosis was caspase-dependent. The levels of cleaved
caspases 7, 8 and 9 in the MCF-7 cells increased following Khz
treatment (Fig. 6A), indicating
their activation. In addition, pretreatment of these cells with the
pan-caspase inhibitor, z-VAD, resulted in complete inhibition of
Khz-induced apoptosis (Fig. 6B).
These data demonstrated that Khz induced caspase-dependent
apoptosis.
Effects of Khz on ROS production in MCF-7
cells
As oxidative stress is involved in apoptosis induced
by a variety of stresses, the production of ROS following Khz
treatment was analyzed in the present study. As shown in Fig. 7A, ROS levels in the MCF-7 cells
increased by 22.3 and 65.1% in response to Khz treatment for 30 and
60 min, respectively, compared with the untreated cells (7.1%).
Co-treatment with the ROS scavenger, N-acetyl-L-cysteine, prevented
ROS formation (Fig. 7B). These
results suggested that Khz induced apoptosis by generating ROS.
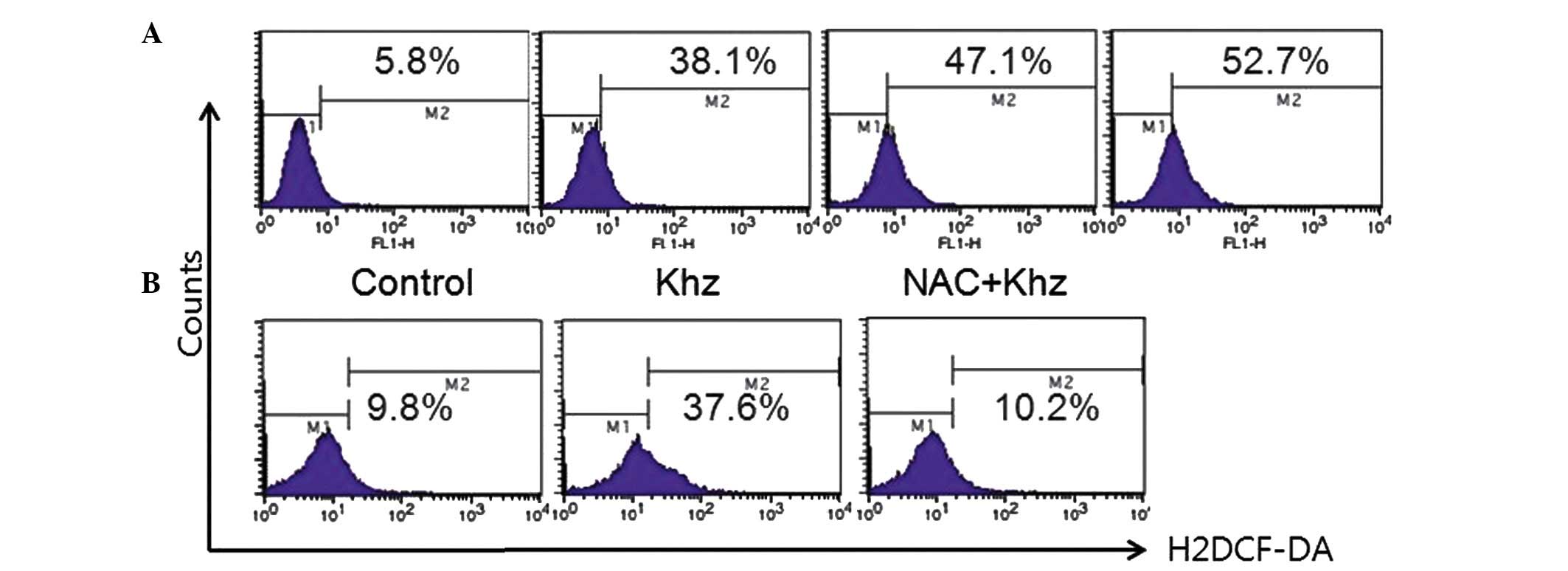 | Figure 7Effect of Khz on the production of ROS
in MCF-7 cells. (A) MCF-7 cells were treated with Khz (1/2) for
different time-periods (0, 0.5, 1 and 2 h) and the production of
ROS was evaluated using flow cytometry. Increasing the duration of
incubation with Khz led to increased levels of ROS in the MCF-7
cells. (B) Cells were pretreated with NAC (1 mM) for 1 h and
treated with 1/2 Khz, following which ROS levels were analyzed. The
histograms reveal two distinct sub-populations, characterized by
low (M1) and high (M2) fluorescence, respectively, corresponding to
cells with low and high ROS staining. ROS, reactive oxygen species;
NAC, N-acetyl-L-cysteine; H2DCF-DA,
20,70-dichlorodihydrofluorescein diacetate. |
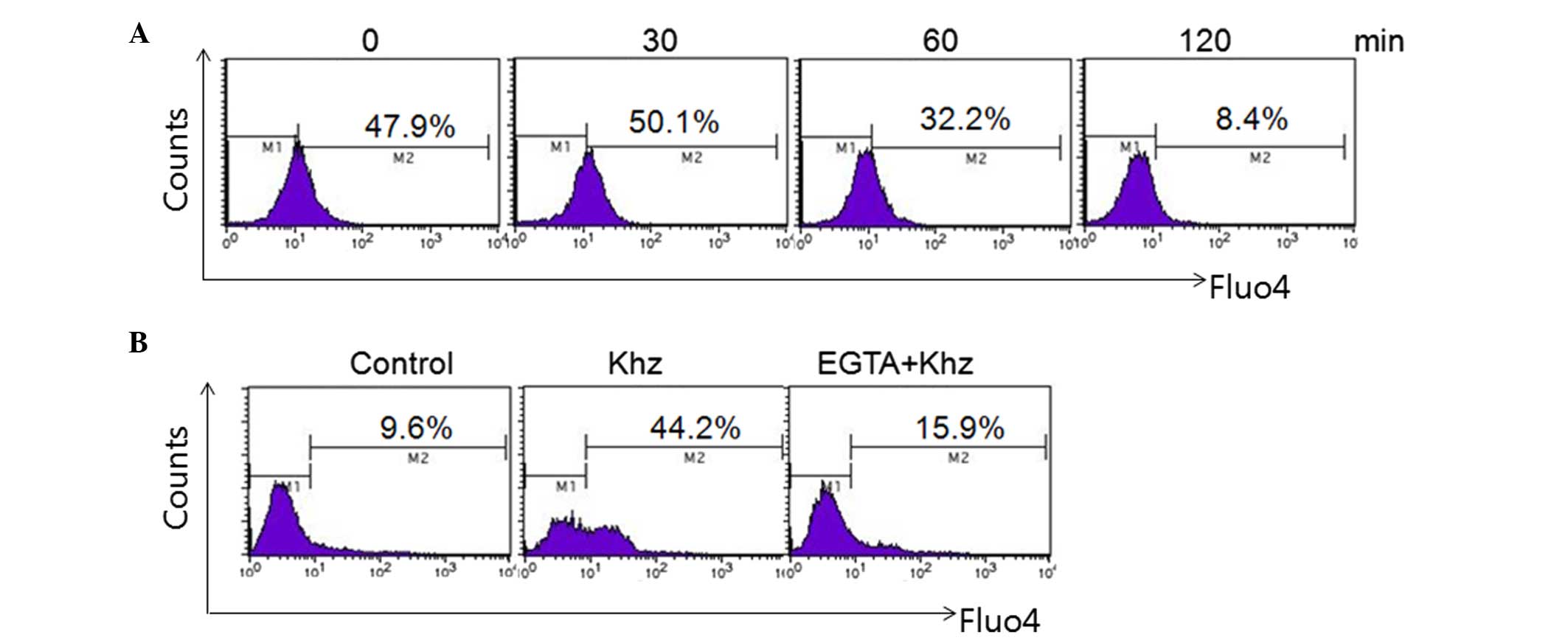
Effects of Khz on
[Ca2+]i production in MCF-7 cells
Assessment of the effect of Khz on intracellular
calcium levels revealed that Khz evoked an increase in
[Ca2+]i in the MCF-7 cells (Fig. 8A). Treatment of the MCF-7 cells
with Khz induced an increase in the percentage of calcium, between
33.6 and 72.2, and 86.2% following treatment for 0.5 and 2 h,
respectively, compared with the untreated cells (7.1%). This
Khz-induced increase in [Ca2+]i was inhibited
by the extracellular Ca2+ chelator, ethylene glycol
tetraacetic acid. These results suggested that Khz triggered
apoptosis by increasing intracellular Ca2+ (Fig. 8B).
Discussion
In a previous study, it was demonstrated that
tanshinone IIA induces apoptosis in A549 human lung cancer cells
through the induction of ROS and by decreasing mitochondrial
membrane potential (21). In
addition, our previous study demonstrated that Khz induces
apoptosis by increasing intracellular calcium levels and activating
c-Jun N-terminal kinase and NADPH oxidase-dependent generation of
ROS (22). These findings
suggested that Khz induces apoptosis in A549 human lung cancer
cells by generating reactive oxygen species and decreasing the
mitochondrial membrane potential (23). In addition, it has been suggested
that Khz induces apoptosis in human colon carcinoma HCT116 cells,
accompanied by an increase in ROS, the activation of caspase 3 and
increased intracellular Ca2+ (24). In addition, our previous study
demonstrated that crude polysaccharide extract obtained from the
fusion of G. lucidum and P. umbellatus mycelia
induces apoptosis by increasing intracellular Ca2+
levels and activating the P38 and NADPH oxidase-dependent
generation of reactive oxygen species in SNU-1 cells (25). In the present study, fusion of
G. lucidum and P. umbellatus mycelium was perofmeed
and used to treat MCF-7 cells. The results demonstrated that Khz
inhibited cell proliferation and induced apoptosis in the MCF-7
breast cancer cells (Fig. 3). In
addition, the mechanism by which Khz induces apoptosis in cancer
cells was investigated (Fig. 4).
Khz induced apoptosis preferentially in transformed cells, with a
minimal effect on non-transformed cells, suggesting it may offer
potential as a cancer therapeutic agent. Oxidative stress is
associated with apoptotic and non-apoptotic cell death, although
pro-oxidative conditions are not a prerequisite for apoptosis
(Fig. 5). Assessment of the
activation statuses of caspases 7, 8 and 9 revealed that the levels
of cleaved caspases were significantly increased in the cells
treated with Khz (Fig. 6). Taken
together, these results suggested that Khz induced apoptosis by
activating caspases, and that the induction of apoptosis by Khz
required ROS generation (Fig. 7).
It is widely accepted that calcium signaling is important in
apoptosis. The present study demonstrated that there was an
increase in [Ca2+]i in the MCF-7 cells
in response to Khz treatment (Fig.
8).
The pro-apoptotic and cytotoxic effects of Khz
demonstrated in the present study suggest it has potential as a
chemotherapeutic agent for the treatment of human breast cancer.
Further investigations of the effects of Khz, including in
vivo investigations, are necessary to determine its potential
for clinical use.
Acknowledgments
This study was supported by Mr. Young Lye Chae,
chief executive officer, at Brain Group Co., Ltd., Pharmacology and
Drug Development, Korean Institute of Science and Management Career
College (Seoul, Korea; grant no. BRG815-1386).
References
|
1
|
Lin ZB and Zhang HN: Anti-tumor and
immunoregulatory activities of Ganoderma lucidum and its possible
mechanisms. Acta Pharmacol Sin. 25:1387–1395. 2004.PubMed/NCBI
|
|
2
|
Sandodiya BS, Thakur GS, Baqhel RK, Prasad
GB and Bisen PS: Ganoderma lucidum: A potent pharmacological
macrofungus. Curr Pharm Biotechnol. 10:717–742. 2009. View Article : Google Scholar
|
|
3
|
Gao Y, Gao H, Chan E, Tang W, Xu A, Yang
H, Huang M, Lan J, Li X, Duan W, et al: Antitumor activity and
underlying mechanisms of ganopoly, the refined polysaccarides
extracted from Ganoderma lucidum, in mice. Immunol Invest.
34:171–198. 2005. View Article : Google Scholar
|
|
4
|
Yue GG, Fung KP, Tse GM, Leung PC and Lau
CB: Comparative studies of various Ganoderma species and their
different parts with regard to antitumor and immunomodulating
activities in vitro. J Altern Complement Med. 12:777–789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
IIIana-Esteban C: The fungus maitake
(Grifola frondosa) and its therapeutic potential. Rev Iberoam
Micol. 25:141–144. 2008.In Spanish. View Article : Google Scholar
|
|
6
|
Jang KJ, Han MH, Lee BH, Kim BW, Kim CH,
Yoon HM and Choi YH: Induction of apoptosis by ethanol extracts of
Ganoderma lucidum in human gastric carcinoma cells. J Acupunct
Meridian Stud. 3:24–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yue QX, Song XY, Ma C, Feng LX, Guan SH,
Wu WY, Yang M, Jiang BH, Liu X, Cui YJ and Guo DA: Effects of
triterpenes from Ganoderma lucidum on protein expression profile of
HeLa cells. Phytomedicine. 17:606–613. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao S, Ye G, Fu G, Cheng JX, Yang BB and
Peng C: Ganoderma lucidum exerts anti-tumor effects on ovarian
cancer cells and enhances their sensitivity to cisplatin. Int J
Oncol. 38:1319–1327. 2011.PubMed/NCBI
|
|
9
|
Liu RM and Zhong JJ: Ganoderic acid Mf and
S induce mitochondria mediated apoptosis in human cervical
carcinoma HeLa cells. Phytomedicine. 15:349–355. 2011. View Article : Google Scholar
|
|
10
|
Li L, Li T, Wang XJ, Xu JP and Wang SG:
Effects of Ganoderma lucidum spores on HepG2 cells proliferation
and growth cycle. Zhong Yao Cai. 31:1514–1518. 2008.In Chinese.
|
|
11
|
Zhao YY, Chao X, Zhang Y, Lin RC and Sun
WJ: Cytotoxic steroids from Polyporus umbellatus. Planta Med.
76:1755–1758. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang W, Liu JW, Zhao WM, Wei DZ and Zhong
JJ: Ganoderic acid T from Ganoderma lucidum mycelia induces
mitochondria mediated apoptosis in lung cancer cells. Life Sci.
80:205–211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang ZE, Yi YJ, Guo YT, Wang RC, Hu QL
and Xiong XY: Inhibition of migration and induction of apoptosis in
LoVo human colon cancer cells by polysaccharides from Ganoderma
lucidum. Mol Med Rep. 12:7629–7636. 2015.PubMed/NCBI
|
|
14
|
Ruan W, Wei Y and Popovich DG: Distinct
responses of cytotoxic Ganoderma lucidum triterpenoids in human
carcinoma cells. Phytother Res. 29:1744–1752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Z, Huang K, Fu X, Zhou Z, Cui Y and Li
H: A chemically sulfated polysaccharide derived from Ganoderma
lucidum induces mitochondrial-mediated apoptosis in human
osteosarcoma MG63 cells. Tumour Biol. 35:9919–9926. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JE, Koo KH, Kim YH, Sohn J and Park
YG: Identification of potential lung cancer biomarkers using an in
vitro carcinogenesis model. Exp Mol Med. 40:709–720. 2008.
View Article : Google Scholar
|
|
17
|
Palombo JD, Ganguly A, Bistrian BR and
Menard MP: The anti-proliferative effects of biologically active
isomers of conjugated linoleic acid on human colorectal and
prostatic cancer cells. Cancer Lett. 177:163–172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klein-Szanto AJ, Iizasa T, Momiki S,
Garcia-Palazzo I, Caamano J, Metcalf R, Welsh J and Harris CC: A
tobacco-specific N-nitrosamine or cigarette smoke condensate causes
neoplastic transformation of xenotransplanted human bronchial
epithelial cells. Proc Natl Acad Sci USA. 89:6693–6697. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang KS, Wang P, Yamabe N, Fukui M, Jay T
and Zhu BT: Docosahexaenoic acid induces apoptosis in MCF-7 cells
in vitro and in vivo via reactive oxygen species formation and
caspase 8 activation. PLoS One. 5:e102962010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu SJ, Kim HS, Cho SW and Sohn J: IL-4
inhibits proliferation of renal carcinoma cells by increasing the
expression of p21WAF1 and IRF-1. Exp Mol Med. 36:372–379. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan Z, Yang R, Jiang Y, Yang Z, Yang J,
Zhao Q and Lu Y: Induction of apoptosis in human promyelocytic
leukemia HL60 cells by panaxynol and panaxydol. Molecules.
16:5561–5573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim TH, Kim JS, Kim ZH, Huang RB and Wang
RS: Khz (fusion of Ganoderma lucidum and Polyporus umbellatus
mycelia) induces apoptosis by increasing intracellular calcium
levels and activating JNK and NADPH oxidase-dependent generation of
reactive oxygen species. PLoS One. 7:e462082012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim TH, Kim JS, Kim ZH, Huang RB and Wang
RS: Khz (Fusion of Ganoderma lucidum and Polyporus umbellatus
Mycelia) induces apoptosis in A549 human lung cancer cells by
generating reactive oxygen species and decreasing the Mitochondrial
membrane potential. Food Sci Biotechnol. 23:859–864. 2014.
View Article : Google Scholar
|
|
24
|
Kim TH, Kim JS, Kim ZH, Huang RB, Chae YL
and Wang RS: Khz (Fusion product of Ganoderma lucidum and Polyporus
umbellatus mycelia) induces apoptosis in human colon carcinoma
HCT116 cells, accompanied by an increase in reactive oxygen
species, activation of caspase 3 and increased intracellular
Ca2+. J Med Food. 18:332–336. 2015. View Article : Google Scholar
|
|
25
|
Kim TH, Kim JS, Kim ZH, Huang RB, Chae YL
and Wang RS: Khz-cp (crude polysaccharide extract obtained from the
fusion of Ganoderma lucidum and Polyporus umbellatus mycelia)
induces apoptosis by increasing intracellular calcium levels and
activating P38 and NADPH oxidase-dependent generation of reactive
oxygen species in SNU-1 cells. BMC Complement Altern Med.
14:2362014. View Article : Google Scholar
|