Introduction
Osteoarthritis (OA) is a chronic age-related
disease, it affects the majority of the adult population, and is
characterized by the slowly progressive destruction of articular
cartilage, and degeneration of ligaments and menisci, as well as
hypertrophy of the joint capsule (1,2). It
is reported that changes in OA include decreased expression of
chondrogenic markers [Aggrecan, collagen type II α1 (COL2A1) and
SOX9], and enhanced expression of certain hypertrophic [matrix
metallopeptidase (MMP13) and alkaline phosphatase] and fibroblastic
[collagen I and collagen II (Col I, II and III, respectively)]
markers (3,4).
Current therapies for OA include a number of
noninvasive (drug treatment and physical therapy) and invasive
therapies (drilling, debridement, osteochondral transplantation,
autologous perichondral and periosteal grafts, and autologous
chondrocyte implantation) to relieve the symptoms (5,6).
Recently, stem cell based cell therapy was observed to provide a
promising approach to OA treatment (7). Bone marrow-derived mesenchymal stem
cells (BMSCs), which can be isolated from the bone marrow aspirate,
and have multipotent differentiation potential (could differentiate
into numerous tissues, such as bone, cartilage and fat),
self-renewal capacity and immunomodulatory properties, has great
potential for use in stem cell-based articular cartilage diseases
(8).
Recent observations have shown that BMSCs also have
shown desirable effects in the treatment of OA, probably via the
secretion of bioactive trophic factors to exert potent
anti-inflammatory, immunomodulatory, and antifibrotic effects
(9,10). Emadedin et al (11) reported that intra-articular
injection of autologous bone marrow mesenchymal stem cells in
patients with OA of the knee did not result in local or systemic
adverse events after a one-year follow-up period. In addition, all
patients were partly satisfied with the results of the study, and
magnetic resonance images (MRI) at baseline and six months
post-stem cell injection displayed an increase in cartilage
thickness, extension of the repair tissue over the subchondral bone
and a considerable decrease in the size of edematous subchondral
patches in three out of six patients. In another case, Buda et
al (12) demonstrated that in
a one-step arthroscopic technique for the treatment of
osteochondral lesions of the knee with bone-marrow-derived cells,
the result of clinical inspection and MRI demonstrated that the
mean international knee documentation committee score prior to
surgery was 29.9±13.2 and was 85.4±4.2 at 29±4.1 months
(P<0.0005), while the knee injury and osteoarthritis outcome
score before surgery was 35.1±11.9 and was 87.3±7.3 at 29±4.1
months (P<0.0005). Control MRI and biopsy samples showed
osteochondral regeneration of the lesion site. Though the desired
result of directed intra-articular injection of bone marrow stem
cells in the treatment of OA diseases was observed, the mechanism
underlying this effect has not been reported. Therefore, in the
current study, the potent anti-inflammatory, immunomodulatory, and
antifibrotic effects of BMSCs on chondrocytes in OA in a coculture
system were explored, as well as the proliferation of chondrocytes
following coculture with BMSCs, in order to evaluate the potential
application of BMSCs in the treatment of OA.
Materials and methods
This study was approved by the ethics committee of
the Southern Medical University (Nanjing, China) and informed
consent was obtained from all patients.
Cell isolation and culture
BMSCs were harvested from patients who underwent
bone marrow aspiration. BMSCs were isolated by density-gradient
centrifugation at 500 × g for 5 min, resuspended and cultured in
Dulbecco's modified Eagle's medium containing 10% (v/v) fetal
bovine serum (Hyclone, Logan, UT, USA) in 100-mm tissue culture
flasks at 37°C in a 5% CO2 humidified incubator. After
24 h, the non-adherent cells were removed. After 10–14 days,
adherent cells were trypsinized and sub-cultured.
Cartilage was harvested from patients with OA who
underwent knee surgery, and chondrocytes were isolated and expanded
as previously described (13).
BMSCs and chondrocytes at passage 1 were used in this study.
Groupings
The chondrocytes were cultured in the six well
plate, and BMSCs were seeded in the Transwell chamber (Corning
Incorporated, Corning, NY, USA), with a 0.4-µm porous
membrane at the bottom to prevent cell migration. Cells were
divided into the following groups: Experimental group, chondrocytes
cocultured with BMSCs; and control group, chondrocytes cultured
alone. In each group 1×104 BMSCs or chondrocytes at
passage 1 were seeded. The culture medium was replaced every 2
days.
Cell proliferation
Chondrocyte proliferation was measured using cell
counting kit-8 (CCK-8, Dojindo Molecular Technologies, Kumamoto,
Japan) as previously described (14). NP cells (103 cells; 100
µl) from the experimental and control groups were seeded
into every well of the 96-well plate. At different time points (1
day, 3 days, 5 days and 7 days), 10 µl CCK-8 solution was
added into each well. After another 2 h, absorbance was measured
spectrophotometrically at 450 nm using a Hitachi F-4500
fluorescence spectrophotometer (Hitachi, Ltd., Tokyo, Japan).
Protein release in the supernatant
Levels of major inflammatory proteins [interleukin
(IL)-6, IL-8, CCL2/MCP-1, CCL3/MIP-1α, CCL5/RANTES, CXCL1/GROα],
thrombospondin-1 (TSP-1) and tissue inhibitor of
metalloproteinase-1 (TIMP-1) in the supernatant in each group at
day 7 was quantified using ELISA kits (R&D Systems,
Minneapolis, MN, USA) according to the manufacturer's instructions.
The test was performed in triplicate.
Differentiation characteristics of
chondrocytes
Relative expression of cyclooygenase 2 (COX-2),
prostaglandin E2 (PGE2), tumor necrosis factor (TNF)-α, IL-1β,
IL-6, and IL-8 was measured in the two groups at day 7 to evaluate
the anti-inflammatory effects of BMSCs on OA chondrocytes. Relative
expression of type I collagen, type III collagen, MMP13, SOX-9,
Aggrecan and type II collagen was measured in two groups at day 7
to evaluate differentiation characteristics of chondrocytes. RNA
was extracted from chondrocytes in the two groups according to
previously described methods (15), and reverse transcribed into cDNA
according to the manufacturer's instructions. Reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) was
used to evaluate the difference in gene expression in the two
groups.
RT-qPCR
RT-qPCR was performed in a final reaction volume of
20 µl containing 10 µl of 2X SYBR Green PCR Universal
Master mix (Applied Biosystems, Warrington, UK), 300 nM of
resuspended reference gene primer mix, 5 µl of diluted cDNA
and 4 µl of RNase/DNase-free water. The primer sequences are
shown in Table I. The thermal
cycling conditions for RT-qPCR were as follows: 95°C for 10 min
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. All
reactions were performed in duplicate using an ABI
PRISM® 7000 real-time PCR system (Applied
Biosystems).
 | Table IPrimer sequences for real-time
polymerase chain reaction. |
Table I
Primer sequences for real-time
polymerase chain reaction.
| Gene symbol | Primer | Product (bp) |
|---|
| COX-2 | | 382 |
| Forward |
5′-ATAACCCCGCCAAAAGGGG-3′ | |
| Reverse |
5′-AGGAACAGCATGCAGGTAGC-3′ | |
| PGE-2 | | 116 |
| Forward |
5′-GTCGTGTACCTGTCCAAGCA-3′ | |
| Reverse |
5′-GCGCTGGCGATGAACAAC-3′ | |
| IL-1β | | 177 |
| Forward |
5′-CTGTCCTGCGTGTTGAAAG-3′ | |
| Reverse |
5′-TGCTTGAGAGGTGCTGATG-3′ | |
| IL-6 | | 184 |
| Forward |
5′-TAGTGAGGAACAAGCCAGAG-3′ | |
| Reverse |
5′-GCGCAGAATGAGATGAGTTG-3′ | |
| IL-8 | | 153 |
| Forward |
5′-CCAAACCTTTCCACCC-3′ | |
| Reverse |
5′-ACTTCTCCACAACCCT-3′ | |
| Sox-9 | | 281 |
| Forward |
5′-AGGTGCTCAAAGGCTACGAC-3′ | |
| Reverse |
5′-GGCATTCCCTGAAGACCTGG-3′ | |
| COL2α1 | | 373 |
| Forward |
5′-CGAAAGGTCAGACGGGTGAA-3′ | |
| Reverse |
5′-GGCATTCCCTGAAGACCTGG-3′ | |
| Aggrecan | | 316 |
| Forward |
5′-ACCTCACCATGCCTTCACTG-3′ | |
| Reverse |
5′-GCTCTCACCTTTCACCACGA-3′ | |
| COL1α2 | | 70 |
| Forward |
5′-TTCTCTAGAACTTTGCTGCTCA-3′ | |
| Reverse |
5′-AAGCATATCATTGGTCCAGGG-3′ | |
| COL3α1 | | 354 |
| Forward |
5′-CGCCCTCCTAATGGTCAAGG-3′ | |
| Reverse |
5′-AGGGCCTGAAGGACCAGCTT-3′ | |
| MMP13 | | 198 |
| Forward |
5′-GACTTCCCAGGAATTGGTGA-3′ | |
| Reverse |
5′-TACCCCAAATGCTCTTCAGG-3′ | |
| GAPDH | | 353 |
| Forward |
5′-CCACATCGCTGAGACACCAT-3′ | |
| Reverse |
5′-AAATGAGCCCCAGCCTTCTC-3′ | |
Statistical analysis
Differences in cell proliferation, gene expression
and protein levels in each group were analyzed by one-way analysis
of variance. Data were analyzed using SPSS version 16.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell proliferation
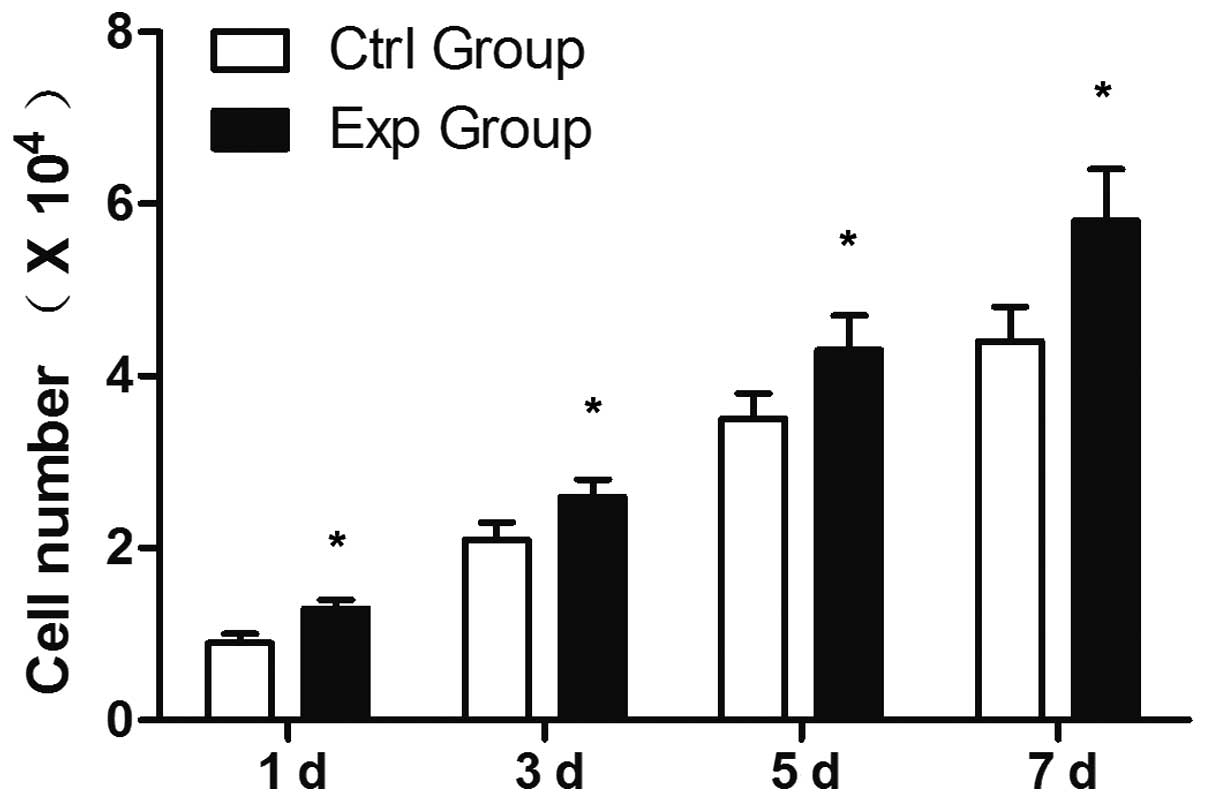
The cell yields in the two groups showed an increase
in cell number with the increase in culture time (Fig. 1). There was a significant
difference between the two groups at different time points, and
greater cell proliferation in the experimental group than that in
the control group (P<0.05).
Release of inflammatory protein in the
supernatant
It has been demonstrated that chondrocytes secrete
various inflammatory proteins in OA (16); thus, the release of inflammatory
protein in the supernatant in two groups was analyzed in the
present study. The coculture system showed the inhibitory effect of
inflammatory activity-related protein secretion. The results of
ELISA demonstrated that the levels of inflammatory protein, such as
IL-6, IL-8, CCL2/MCP-1, CCL3/MIP-1α, CCL5/RANTES and CXCL1/GROα,
decreased in the coculture system, which indicated that BMSCs
exerted anti-inflammatory effects.
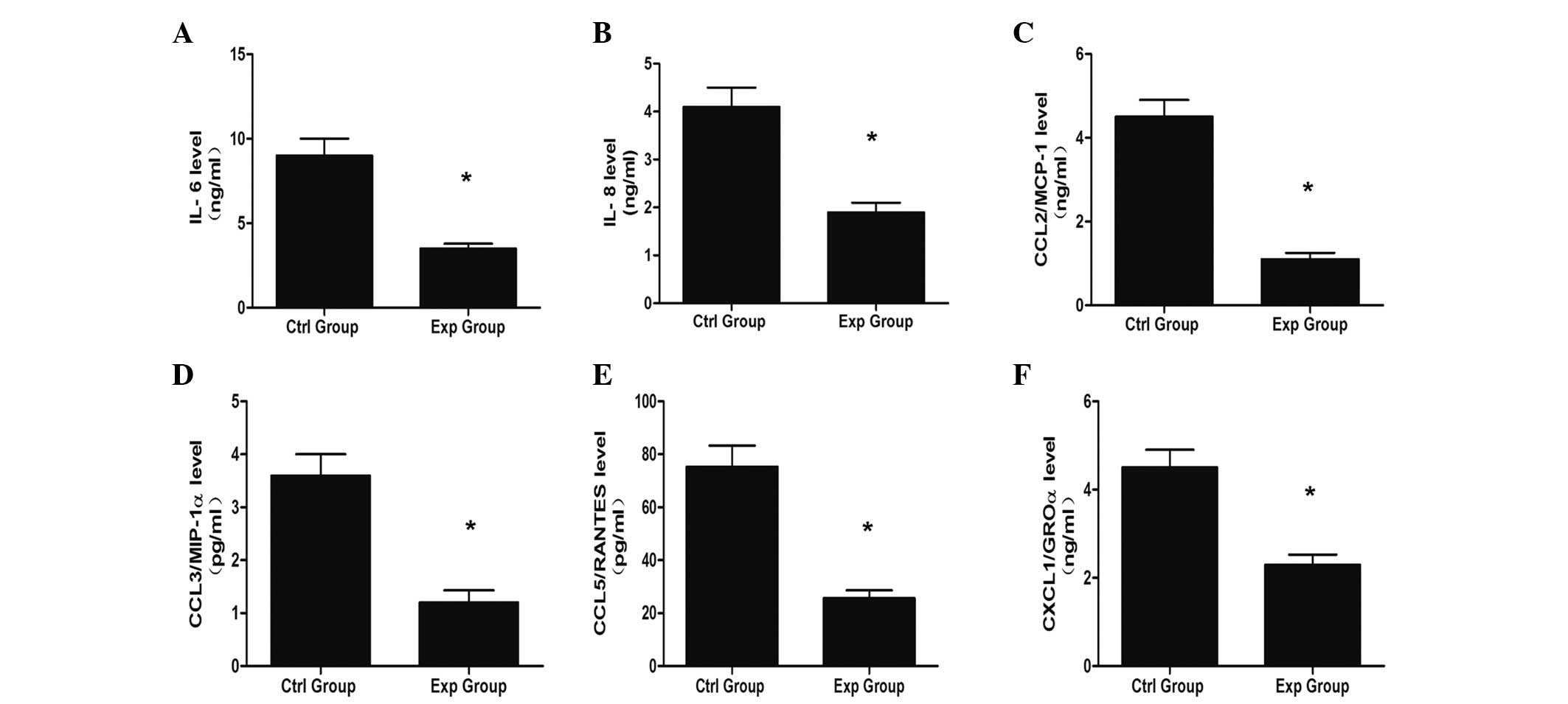
The concentration of IL-6, IL-8, CCL2/MCP-1,
CCL3/MIP-1α, CCL5/RANTES and CXCL1/GROα were measured (Fig. 2A–F). Production of IL-6 (9.00
ng/ml), IL-8 (4.10 ng/ml), CCL2/MCP-1 (4.50 ng/ml), CCL3/MIP-1α
(3.6 ng/ml), CCL5/RANTES (25.60 ng/ml) and CXCL1/GROα (2.30 ng/ml)
in the experimental group were significantly reduced compared with
the production of IL-6 (3.50 ng/ml), IL-8 (1.90 ng/ml), CCL2/MCP-1
(1.10 ng/ml), CCL3/MIP-1α (1.2 ng/ml), CCL5/RANTES (75.30 ng/ml)
and CXCL1/GROα (4.50 ng/ml) in the control group (P<0.05).
Expression of inflammatory genes in OA
chondrocytes
A number of studies have reported that the
expression of inflammatory genes in OA chondrocytes increased
(16–18). The co-culture system notably
resulted in a reduction of COX-2, PEG2, TNF-α, IL-6, IL-8 and IL-1β
mRNA levels compared with the control group (Fig. 3; P<0.05).
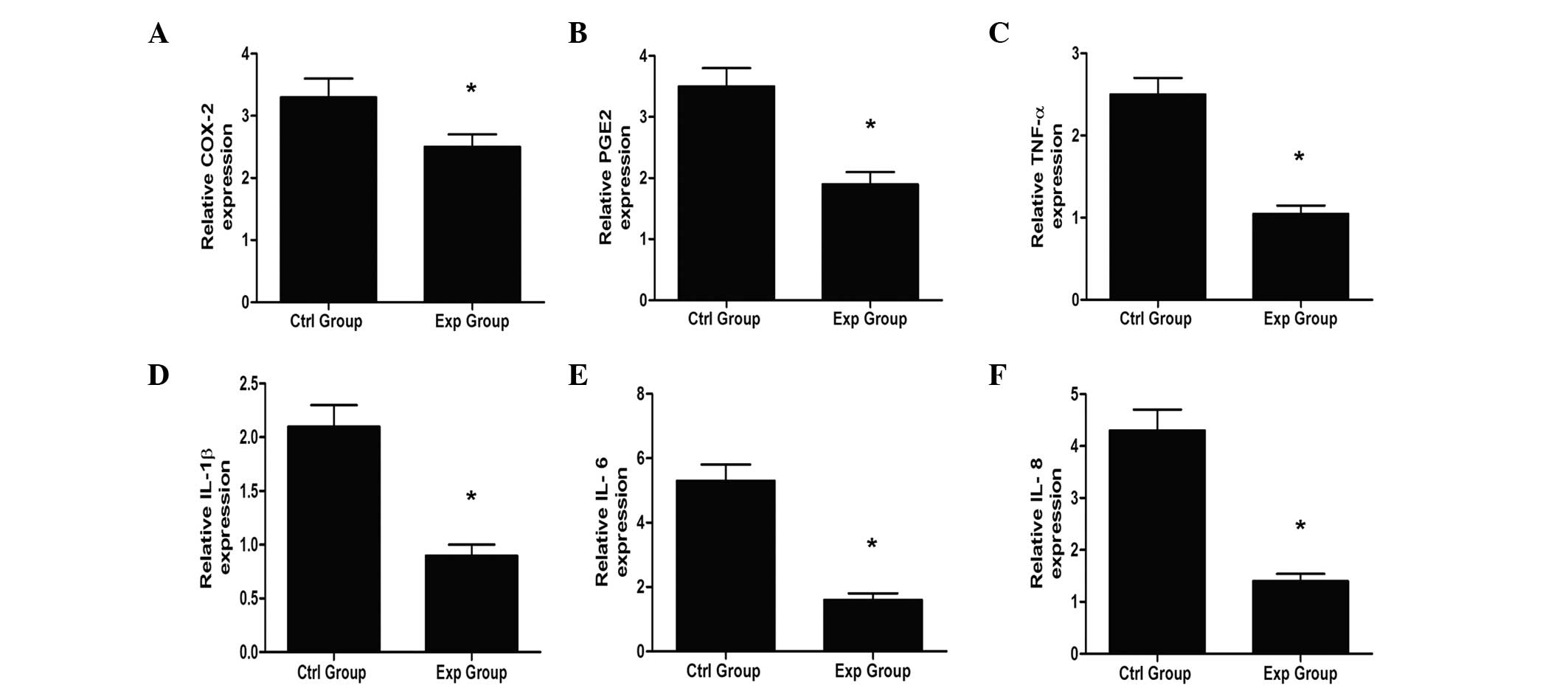 | Figure 3Expression of inflammatory genes. (A)
COX-2, (B) PGE2, (C) TNF-α, (D) IL-1β, (E) IL-6 and (F) IL-8. There
is a significant difference of the expression of inflammatory gene
between 2 groups (*P<0.05), and the expression of
inflammatory gene in the experimental group is less than that in
the control group. COX-2, cyclooxygenase 2, PGE2, prostaglandin E2;
TNF-α, tumor necrosis factor-α; IL, interleukin. |
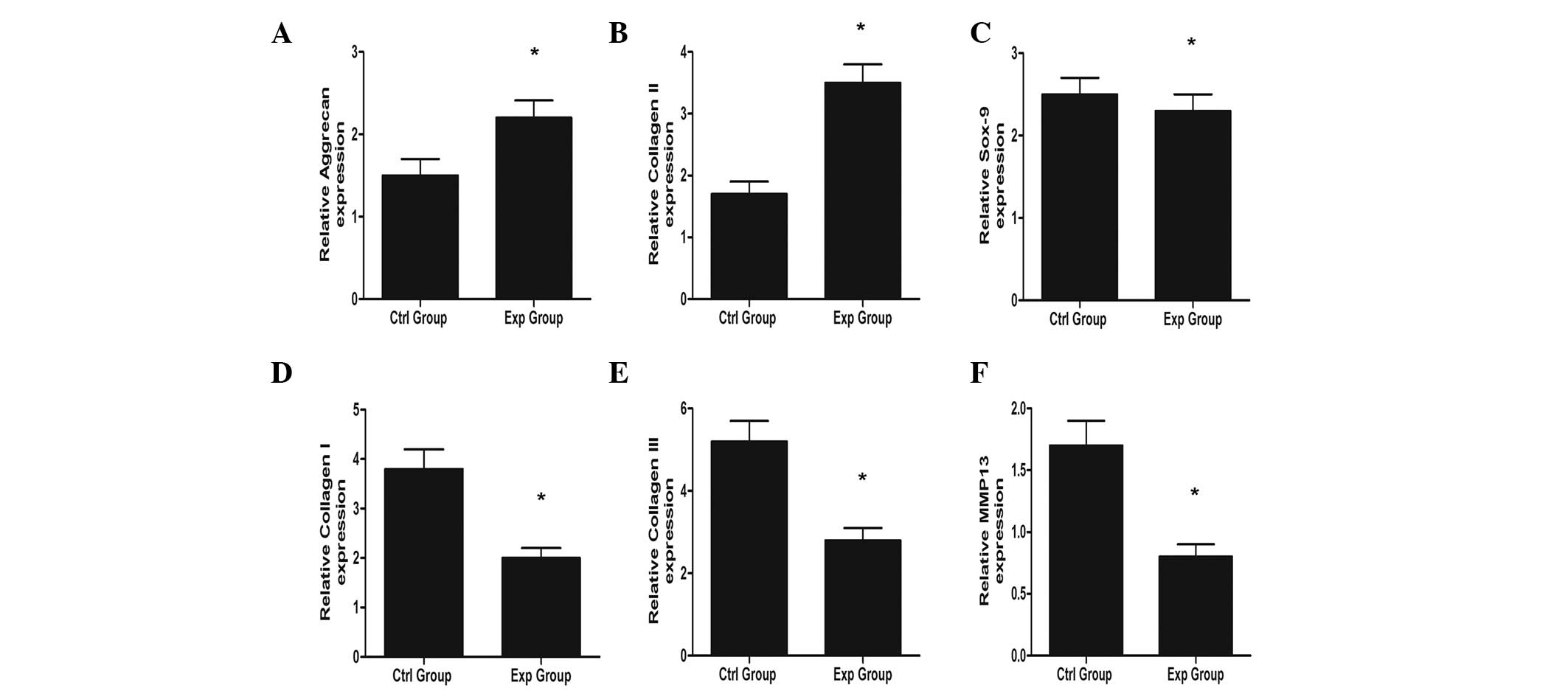
OA chondrocyte differentiation
OA chondrocytes become fibrous and undergo
hypertrophy, which was demonstrated by upregulation of fibrotic
(collagen type I) and hypertrophic (osteopontin, type X collagen
and matrix Gla) genes. However, the expression of fibrotic and
hypertrophic genes in the experimental group decreased after
coculture with BMSCs, and there were significant differences
between the two groups. The chondrogenic gene (type II collagen and
aggrecan) expression increased in the experimental group, while the
SOX-9 expression decreased after coculture (Fig. 4). The results indicated that BMSCs
showed chondroprotective and antifibrotic effects, as well as
antihypertrophic effects.
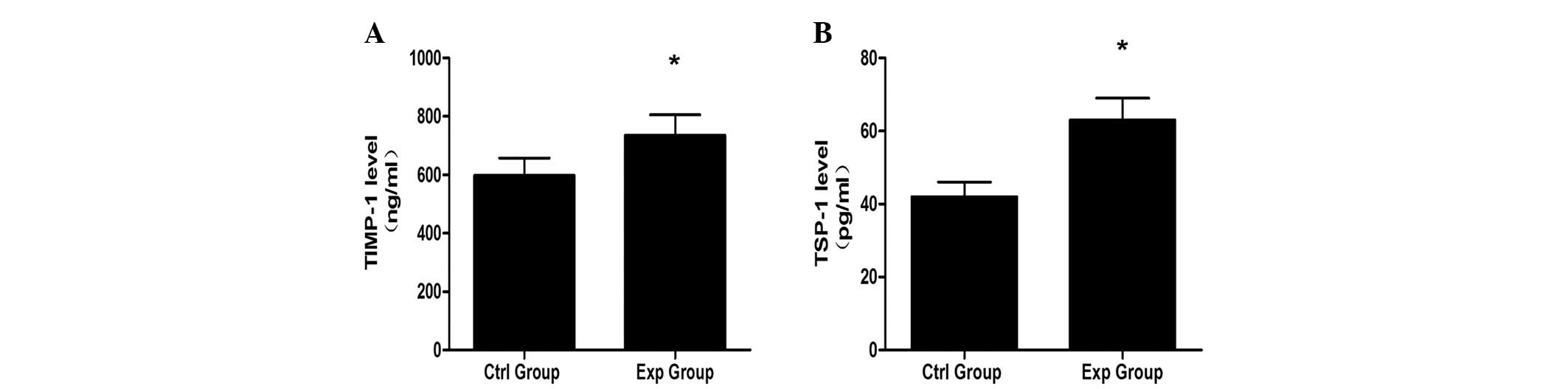
Release of TIMP-1 and TSP-1 in the
supernatant
TSP-1 has been reported to have the ability to
upregulate TIMP-1. TIMP-1 could inhibit the vascularization of
chondrocytes, which suggests that TIMP-1 has chondroprotective
effects. The levels of TSP-1 and TIMP-1 were 63 and 735 pg/ml in
the experimental group, while the level of TSP-1 and TIMP-1 was 42
and 598 pg/ml in the control, respectively (Fig. 5).
Discussion
Osteoarthritis (OA) is also termed chronic
degenerative arthritis, degenerative joint disease or
osteoarthrosis, and is characterized by progressive cartilage
degeneration, subchondral bone impairment, the narrowing of the
joint space, marginal osteophytosis, as well as loss of joint
function (19). The common
symptoms of osteoarthritis included joint pain, stiffness and a
degree of loss of joint motion (20). It is reported that the inflammation
is involved in the occurrence of OA, which promotes the ongoing
joint degeneration (21). Recent
studies have demonstrated that BMSCs have the potential for
application in the treatment of OA as BMSCs have chondrogenic
differentiation potential, and are also involved in the
immunoregulation and tissue repair/regeneration by the secretion of
various soluble factors. Firstly, BMSCs exhibit multilineage
differentiation capacity and can develop into various cell types,
including chondrocytes, osteoblasts and adipocytes (22). Secondly, BMSCs secrete a number of
cytokines, such as hepatocyte growth factor, insulin-like growth
factor-1, epidermal growth factor, keratinocyte growth factor,
angiopoietin-1 and stromal derived factor-1, which possess a wide
range of biological effects in the repair and regeneration of
tissue (23). Therefore, in the
present study the effect of BMSCs on chondrocytes from OA tissues
was investigated, and the results indicated chondroprotective and
antifibrotic effects, as well as antihypertrophic effects on the OA
chondrocytes in a coculture system.
At first, the OA chondrocyte proliferation rate was
analyzed in culture alone or coculture with BMSCs, and the results
indicated that BMSCs significantly improved the cell proliferation
rate, when compared with chondrocyte culture alone. It has been
reported that BMSCs secrete different types of growth factors,
including basic fibroblast growth factor (FGF-2), insulin-like
growth factor 1 (IGF-1) and hepatocyte growth factor (HGF)
(24). Among them, FGF-2 and IGF-1
have the capability to promote cell proliferation via the PI3-K
pathway dependent signal pathway (25). Umeda et al (26) showed that BMSCs are more effective
for increasing the proliferative capacity of nucleus pulposus cells
via activation of rat nucleus pulposus cells by coculture with
BMSCs. In the present study, the results of the CCK-8 assay
confirmed the effects of BMSCs promoting chondrocyte cell
proliferation.
The anti-inflammatory action of BMSCs on OA
chondrocytes was investigated. It is well known that chondrocytes
from patients with OA secrete various inflammatory cytokines and
express inflammation activity-related genes (16). It has also been shown that certain
OA inflammatory factors, such as IL-6, IL-8 and CXCL1/GROα, were
involved in the progression of OA (27). The level of the inflammatory
factors in the chondrocyte culture alone group and the coculture
group were analyzed, a significant decrease in the release of
inflammatory factors in the supernatant was observed after
coculture with BMSCs, which indicated that BMSCs were
anti-inflammatory in OA. BMSCs may inhibit macrophage activity and
thereby suppress the production of catabolic mediators, such as
IL-6, IL-8 and CXCL1/GROα (16,28).
The mRNA expression of the main OA inflammatory factors, such as
COX-2, TGF-α and PEG2, were also measured to evaluate the
anti-inflammatory effect. An increase in the gene expression level
of COX-2, TNF-α and PEG2 were observed during inflammatory and
catabolic processes. The results showed that the mRNA expression of
the predominant OA inflammatory factors in the coculture group was
less than that in the chondrocyte culture alone group.
In addition, the differentiation of chondrocytes was
also investigated in the coculture system. It is reported that
chondrocytes lost their original phenotype during OA progression,
whereby the chondrocytes become ossified and vascularized (29). The results of the present study
showed that the expression of aggrecan and collagen II increased
after coculture with BMSCs, while the expression of SOX-9
decreased. Furthermore, the expression of hypertrophic (MMP13) and
fibroblastic (Collagen I and III) markers of chondrocytes
co-cultured with ASCs decreased, which demonstrated that BMSCs
exerted an antifibrotic and antihypertrophic effect on the OA
chondrocytes. TSP-1 and TIMP-1 may be important in the antifibrotic
and antihypertrophic process, and it was demonstrated that the
secretion of TSP-1 and TIMP-1 increased in the coculture
system.
In conclusion, co-culture with human BMSCs inhibits
inflammatory activity and increases cell proliferation of OA
chondrocytes, as well as exhibiting an antifibrotic and
antihypertrophic effect, which may occur via the secretion of
various growth factors and cytokines from BMSCs.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81000792).
References
|
1
|
Loeser RF: Age-related changes in the
musculoskeletal system and the development of osteoarthritis. Clin
Geriatr Med. 26:371–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moran CJ, Pascual-Garrido C, Chubinskaya
S, et al: Restoration of articular cartilage. J Bone Joint Surg Am.
96:336–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mueller MB and Tuan RS: Functional
characterization of hypertrophy in chondrogenesis of human
mesenchymal stem cells. Arthritis Rheum. 58:1377–1388. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maumus M, Manferdini C, Toupet K, et al:
Adipose mesenchymal stem cells protect chondrocytes from
degeneration associated with osteoarthritis. Stem Cell Res.
11:834–844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falah M, Nierenberg G, Soudry M, Hayden M
and Volpin G: Treatment of articular cartilage lesions of the knee.
Int Orthop. 34:621–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khashan M, Chechik O, Arbel R and Morag G:
The treatment of focal chondral lesions of the knee. Harefuah.
149:542–546. 5492010.In Hebrew.
|
|
7
|
Diekman BO and Guilak F: Stem cell-based
therapies for osteoarthritis: challenges and opportunities. Curr
Opin Rheumatol. 25:119–126. 2013. View Article : Google Scholar :
|
|
8
|
Gupta PK, Das AK, Chullikana A and
Majumdar AS: Mesenchymal stem cells for cartilage repair in
osteoarthritis. Stem Cell Res Ther. 3:252012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baraniak PR and McDevitt TC: Stem cell
paracrine actions and tissue regeneration. Regen Med. 5:121–143.
2010. View Article : Google Scholar :
|
|
10
|
Djouad F, Bouffi C, Ghannam S, Noël D and
Jorgensen C: Mesenchymal stem cells: innovative therapeutic tools
for rheumatic diseases. Nat Rev Rheumatol. 5:392–399. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Emadedin M, Aghdami N, Taghiyar L, et al:
Intra-articular injection of autologous mesenchymal stem cells in
six patients with knee osteoarthritis. Arch Iran Med. 15:422–428.
2012.PubMed/NCBI
|
|
12
|
Buda R, Vannini F, Cavallo M, et al:
One-step arthroscopic technique for the treatment of osteochondral
lesions of the knee with bone-marrow-derived cells: three years
results. Musculoskelet Surg. 97:145–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia Z, Duan X, Murray D, Triffitt JT and
Price AJ: A method of isolating viable chondrocytes with
proliferative capacity from cryopreserved human articular
cartilage. Cell Tissue Bank. 14:267–276. 2013. View Article : Google Scholar
|
|
14
|
Xue K, Qi L, Zhou G and Liu K: A two-step
method of constructing mature cartilage using bone marrow-derived
mesenchymal stem cells. Cells Tissues Organs. 197:484–495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dell'accio F, De Bari C, Eltawil NM,
Vanhummelen P and Pitzalis C: Identification of the molecular
response of articular cartilage to injury, by microarray screening:
Wnt-16 expression and signaling after injury and in osteoarthritis.
Arthritis Rheum. 58:1410–1421. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manferdini C, Maumus M, Gabusi E, et al:
Adipose-derived mesenchymal stem cells exert antiinflammatory
effects on chondrocytes and synoviocytes from osteoarthritis
patients through prostaglandin E2. Arthritis Rheum. 65:1271–1281.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho H, Walker A, Williams J and Hasty KA:
Study of osteoarthritis treatment with anti-inflammatory drugs:
Cyclooxygenase-2 inhibitor and steroids. Biomed Res Int. 2015:2015.
View Article : Google Scholar
|
|
19
|
Frisbie DD, Kisiday JD, Kawcak CE, Werpy
NM and McIlwraith CW: Evaluation of adipose-derived stromal
vascular fraction or bone marrow-derived mesenchymal stem cells for
treatment of osteoarthritis. J Orthop Res. 27:1675–1680. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Strassle BW, Mark L, Leventhal L, et al:
Inhibition of osteoclasts prevents cartilage loss and pain in a rat
model of degenerative joint disease. Osteoarthritis Cartilage.
18:1319–1328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vincent KR, Conrad BP, Fregly BJ and
Vincent HK: The pathophysiology of osteoarthritis: a mechanical
perspective on the knee joint. PM R. 4(Suppl 5): 3–9. 2012.
View Article : Google Scholar
|
|
22
|
Derubeis AR and Cancedda R: Bone marrow
stromal cells (BMSCs) in bone engineering: limitations and recent
advances. Ann Biomed Eng. 32:160–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caplan AI and Dennis JE: Mesenchymal stem
cells as trophic mediators. J Cell Biochem. 98:1076–1084. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boomsma RA and Geenen DL: Mesenchymal stem
cells secrete multiple cytokines that promote angiogenesis and have
contrasting effects on chemotaxis and apoptosis. PLoS One.
7:e356852012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weber GF and Menko AS:
Phosphatidylinositol 3-kinase is necessary for lens fiber cell
differentiation and survival. Invest Ophthalmol Vis Sci.
47:4490–4499. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Umeda M, Kushida T, Sasai K, et al:
Activation of rat nucleus pulposus cells by coculture with whole
bone marrow cells collected by the perfusion method. J Orthop Res.
27:222–228. 2009. View Article : Google Scholar
|
|
27
|
Merz D, Liu R, Johnson K and Terkeltaub R:
IL-8/CXCL8 and growth-related oncogene alpha/CXCL1 induce
chondrocyte hypertrophic differentiation. J Immunol. 171:4406–4415.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen HW, Chen HY, Wang LT, et al:
Mesenchymal stem cells tune the development of monocyte-derived
dendritic cells toward a myeloid-derived suppressive phenotype
through growth-regulated oncogene chemokines. J Immunol.
190:5065–5077. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van der Kraan PM and van den Berg WB:
Chondrocyte hypertrophy and osteoarthritis: role in initiation and
progression of cartilage degeneration? Osteoarthritis Cartilage.
20:223–232. 2012. View Article : Google Scholar
|