Introduction
Gastric cancer (GC) is the fourth most frequently
occurring cancer worldwide and the second leading cause of
cancer-associated mortality (1,2). A
2011 analysis demonstrated that 989,600 new GC cases and 738,000
mortalities were estimated to have occurred in 2008 worldwide
(3). The occurrence of GC varies
with geographical area, with the highest incidence rate of GC in
East Asia, particularly in South Korea, Mongolia, Japan and China
(4). At present, no effective
treatment is available for this disease and identification of early
stage GC is difficult as it is often asymptotic or misdiagnosed. In
addition, the prognosis of patients with advanced GC remains poor
due to its high metastatic recurrence (5) and the complex molecular mechanisms
underlying metastasis are not well characterized (6). Clinical observations reveal that even
following radical surgery for early GC, ~50% of patients suffer
from recurrence and metastasis. Local recurrence and metastatic
disease are also the main causes of mortality of patients with
advanced GC. Therefore, inhibition of GC metastasis is an important
therapeutic strategy. The main obstacle in treating metastatic
disease is that the tumor cells of the primary and metastatic
lesions have biological heterogeneity, which presents differences
in antigenic properties, drug sensitivity, and the ability to
invade and metastasize (7). The
prognosis of ovarian metastases, also known as Krukenberg tumors,
is known to be poor. Krukenberg tumors occur in 0.3–6.7% of GC
patients who undergo surgery and its incidence is significantly
higher in autopsies of GC patients (33–41%) (8). It has been reported that the
incidence of Krukenberg tumors, detected during the follow-up
observation period following radical gastric resection or secondary
surgery, is 3–4% and has a median survival time of 12–17 months
(9). There are relatively few
studies on the incidence, treatment or metastatic mechanisms of
Krukenberg tumors.
Mesenchymal stem cells (MSCs), also termed
mesenchymal stromal cells, belong to a category of clinically
relevant cell types that have the potential to be utilized for
cell-based therapies, as complicated culturing or handling
techniques are not required to yield clinically useful quantities.
Traditionally, MSCs are characterized as having tri-lineage
potential, that is they can be induced to differentiate into three
mesenchymal lineages: Osteoblasts, adipocytes and chondrocytes, but
they may also potentially produce other skeletal tissue cells by
culturing MSCs under defined mechanochemical conditions (10). MSCs are characterized by the
expression of cell surface markers, including CD73, CD90 and CD105,
and the absence of expression of hematopoietic lineage markers
(11). There has been heightened
interest in the capacity of MSCs to home and migrate into tumors
(12). The effects of MSCs within
tumors are varied. The theory that MSCs promote tumor growth and
metastasis has been supported from studies of angiogenesis, tumor
cell survival, the immunosuppressive microenvironment, as well as
maintenance of cancer stem cells (CSCs) and construction of their
mesenchymal niche (13).
Nevertheless, it is important to understand the principles and
mechanisms through which MSCs regulate tumor progression since
these may give insight into the ways in which MSCs may be used to
treat tumors. Tumors are able to disseminate systemically to
initiate metastatic niches in distant target organs. These niches,
composed of bone marrow-derived MSCs, provide permissive conditions
for future metastases (14). Bone
marrow MSCs promote stem cell dormancy in lung cancer (15), breast cancer (16) and prostate cancer (17) cells in a metastatic niche.
MSC-like cells have been isolated from endometrial
cancer (18), glioma (19,20),
bone sarcoma (21) and colorectal
cancer (22). However, MSC-like
cells have not yet been demonstrated in human ovarian metastases of
GC. The present study identified and characterized MSC-like cells
from ovarian metastases of GC.
Materials and methods
Isolation of MSC-like cells from human GC
tissues and corresponding ovarian metastases
The present study was approved by the Institutional
Review Board of Hunan Cancer Hospital and Affiliated Cancer
Hospital of Xiangya Medical School (Changsha, China). All human
materials were obtained with informed consent and approved by the
Ethics Committee of the Hunan Cancer Hospital and Affiliated Cancer
Hospital of Xiangya Medical School. Between January 2002 and
December 2008, patients with ovarian metastasis of GC diagnosed
pathologically at the Hunan Cancer Hospital were recruited. These
human tissues were collected and examined with strict adherence to
the protocol approved by this hospital. A total of 40 fresh GC
tissues and corresponding ovarian metastatic tissue of gastric
origin were collected from 40 female GC patients, with ages ranging
between 43 and 80 years (median, 62.5 years), who had initially
undergone resection at the Hunan Provincial Cancer Hospital
(Changsha, China). The specimens included: Tumor tissue, adjacent
normal tissue (at least 5 cm beyond the primary tumor margin),
distant normal tissue and lymph nodes. The fresh tissue specimens
were collected, washed with phosphate-buffered saline (PBS), cut
into 1-mm3-sized pieces and maintained in Dulbecco's
modified Eagle's medium with low glucose (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS), penicillin (100 U/ml; Beijing Chemeebio Pharma-tech
Co., Ltd., Beijing, China) and streptomycin (100 µg/ml;
Beijing Chemeebio Pharma-tech Co., Ltd.). The tissues were
subsequently incubated at 37°C in humid air with 5% CO2.
The medium was replaced every 3 days after the initial plating.
When adherent fibroblast-like cells appeared after 10 days of
culture, the attached cells were trypsinized and passaged (without
dilution) into a new flask for further expansion. The cells of
passage 4 were used for the experiments described. MSCs isolated
from human bone marrow (hBM-MSCs) and the GC cell line BCG-823 from
our institute were selected as controls and used for evaluation of
the experimental results.
Growth curves
The growth curves of human gastric cancer-MSCs
(hGC-MSCs), human gastric cancer ovarian metastatic tissue-MSCs
(hGCOM-MSCs) and hBM-MSCs at passage 4 were seeded in 24-well
plates (8,000 cells/well), followed by counting the number of cells
per well on 14 successive days. The procedure was repeated three
times and their growth curves were compared.
Flow cytometric analysis
hGC-MSCs and hGCOM-MSCs (2.0×106 cells)
were trypsinized, washed twice with PBS and immunostained for 30
min on ice. A minimum of 5×104 cells (in 100 µl
PBS/0.5% BSA/2 mmol/l EDTA) were incubated with the following mouse
anti-human monoclonal antibodies: CD73-FITC (561254), CD90-PE
(555596), CD105-APC (561443), CD34-FITC (561819), CD45-APC-H7
(347463) and HLA-DR-FITC (555558; all from BD Biosciences, Franklin
Lakes, NJ, USA). Labeled cells were analyzed using a flow cytometer
(FACS Calibur; BD Biosciences).
Animal studies
All animal experiments were undertaken in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals (National Institutes of Health, Bethesda, MD,
USA), with the approval of the Hunan Provincial Cancer Hospital
Review Board and Affiliated Cancer Hospital of Xiangya Medical
School and also following Hunan province government Animal Care and
Used Committee-approved protocols. A total of eight, 6-week-old
female nonobese diabetic (NOD)/severe combined immunodeficiency
mice (SCID; Laboratory Animal Center of Shanghai, Academy of
Sciences, Shanghai, China) were housed in individually ventilated
cages and maintained in a standard animal facility under controlled
environmental conditions, at room temperature 26±2°C. BCG-823,
hGC-MSCs or hGCOM-MSCs cells (5×106) in 200 µl
PBS were injected subcutaneously into the lower right flank of
mice. The incidence of tumor formation was observed over 4
weeks.
Osteogenic differentiation in vitro
hGC-MSCs, hGCOM-MSCs or hBM-MSCs were seeded at
5,000 cells/cm2 in 35 mm diameter plates and cultured in
DMEM with 10% FBS, with or without osteogenic supplements (0.1 nM
dexamethasone, 10 mM β-glycerophosphate, 50 mg/l ascorbic acid and
4 µg/ml basic fibroblast growth factor; Sigma-Aldrich, St.
Louis, MO, USA). The medium was changed three times each week and
the cells were induced for 3 weeks. At the end of induction, the
cells were subjected to alkaline phosphatase (ALP) staining
followed by hematoxylin counterstaining (Zhingshan Golden Bridge,
Beijing, China).
Reverse transcription polymerase chain
reaction (RT-PCR)
Total cellular RNA was isolated from hGC-MSCs and
hGCOM-MSCs using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions. A
total of 1.0 µg RNA was processed for cDNA synthesis with
Superscript II reverse transcriptase using Oligo-dT orimers (Toyobo
Co., Ltd., Osaka, Japan). PCR was performed using 1 µg of
the cDNA sample with 0.3 U Taq polymerase (CinnaGen Co., Tehran,
Iran) and 200 µM dNTPs, 10 pM of each primer, reaction
buffer, and MgCl2 (Takara Bio Inc., Otsu, Japan). A
total of 1 µl of this mixture was used as a template for PCR
to assess mRNA expression of ALP, dentin matrix acidic
phosphoprotein 1 (DMP-1), osteocalcin (OSC) and osteopontin (OPN)
using the ABI PRISM 7700 instrument (Applied Biosystems; Thermo
Fisher Scientific, Inc., Foster City, CA, USA) with gene-specific
primers [Table I, and described
previously (23,24)] and the SYBR Green I (Thermo Fisher
Scientific, Inc.) protocol. The PCR amplification was performed for
35 cycles, with the cycling conditions as follows: 94°C for 30 sec,
60°C (primer) for 30 sec, 72°C for 30 sec, with a final extension
at 72°C for 10 min. PCR products were separated on a 1% agarose
gel, stained with ethidium bromide and visualized under UV light.
Relative expression ratios normalized to that of β-actin (Actb)
were calculated.
 | Table IPrimer sequences for the
amplification of target and control cDNAs. |
Table I
Primer sequences for the
amplification of target and control cDNAs.
| Gene | Forward primer | Reverse primer | Product size
(bp) |
|---|
| ALP |
TAAGGACATCGCCTACCAGCTC |
TCTTCCAGGTGTCAACGAGGT | 170 |
| DMP-1 |
GTGAGTGAGTCCAGGGGAGATAA |
TTTTGAGTGGGAGAGTGTGTGC | 111 |
| OSC |
TGAGAGCCCTCACACTCCTC |
ACCTTTGCTGGACTCTGCAC | 98 |
| OPN |
CAGTTGTCCCCACAGTAGACAC |
GTGATGTCCTCGTCTGTAGCATC | 127 |
| Bmi-1 |
GCTGCCAATGGCTCAATG |
AGGAGACTGCACTGGAGTACTG | 530 |
| ABCG2 |
CGGCTTGCAACAACTATGAC |
ATCCTGCTTGGAAGGCTCTA | 537 |
| Nanog |
ATGCCTCACACGGAGACTG |
CTGCGTCACACCATTGCTA | 369 |
| α-SMA |
CTGACTGAGCGTGGCTATTC |
CCACCGATCCAGACAGAGTA | 452 |
| CD44 |
TCACAGGTGGAAGAAGAGAC |
CATTGCCACTGTTGATCACT | 447 |
| CD73 |
CTCGGCTCTTCACCAAGGTT |
AATTTGGCCTCTTTGAGGAGT | 226 |
| β-actin |
CGTCTGGACCTGGCTGGCCGGGACC |
CTAGAAGCATTTGCGGTGGACGATG | 600 |
Statistical analysis
All results are expressed as the mean ± standard
deviation or median (range). The normality of data distribution was
assessed by the Kolmogorov-Smirnov test. Data analysis was
performed using SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA).
Receiver operating characteristic (ROC) curves were generated to
determine the areas under the ROC curves and their 95% confidence
intervals were calculated. A univariate test was used to examine
the effect of each clinical variable on survival. Student's t-test
was applied to determine statistical significance. P<0.05 was
considered to indicate a statistically significant difference.
Results
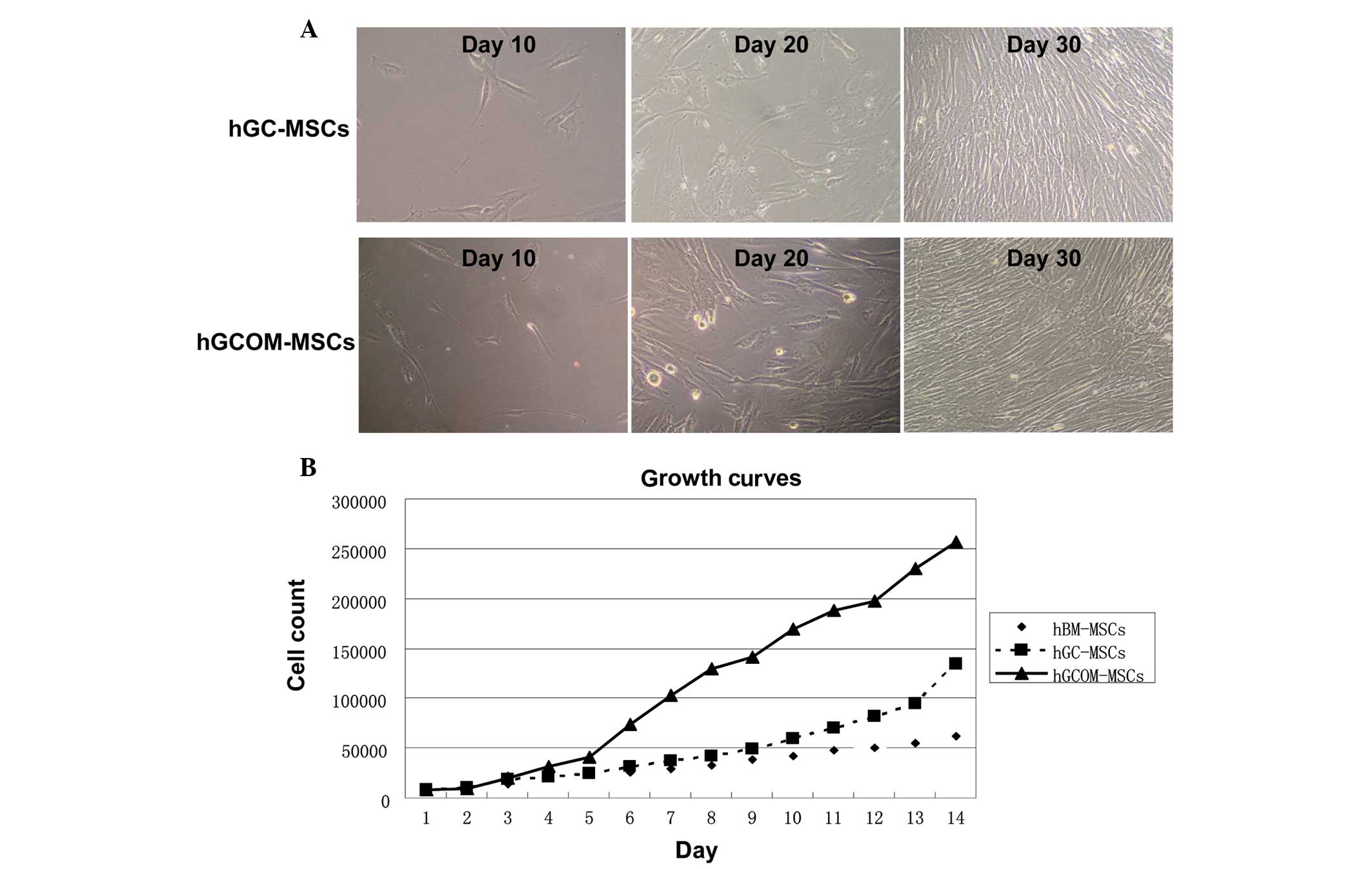
Morphology of hGC-MSCs and
hGCOM-MSCs
The first plastic-adherent cells were detected 1
week after tissue preparation. Primary cell cultures began to form
colonies after 10 days of primary culture and reached a confluence
of 90–95% at day 30 (Fig. 1A).
Cells from human GC tissues and its ovarian metastatic tissues were
seeded at low density on a 6-well plate and allowed to grow until
80% confluence. Cells were passaged at the same cell density.
Growth curves
The growth curves of hGCOM-MSCs, hGC-MSCs and
hBM-MSCs are shown in Fig. 1B.
Within 5 days after plating, the number of hGCOM-MSCs increased by
almost two times that of hGC-MSCs and hBM-MSCs. In addition,
hGCOM-MSCs demonstrated a higher cumulative population doubling
level compared with hGC-MSCs and hBM-MSCs.
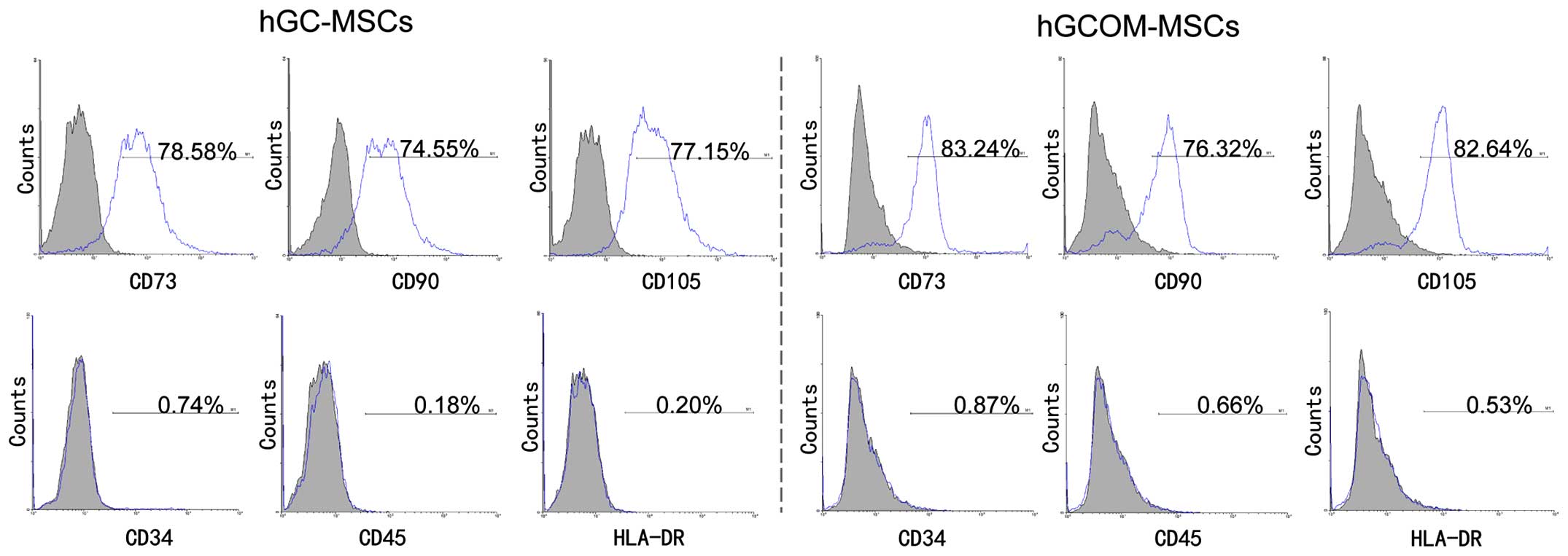
Surface markers and gene expression in
hGC-MSCs and hGCOM-MSCs
Specific cell-surface markers were selected to
evaluate whether the hGC-MSCs and hGCOM-MSCs at passage 4 contained
the MSC population. These experiments were performed with the
objective of isolating a population of pluripotent-like stem cells
derived from human GC and its ovarian metastatic deposits. The
present study revealed that the two types of cells were
CD73+, CD90+, CD105+,
CD34−, CD45− and HLA-DR−, which
indicated characteristics of MSCs (6,25,26).
The hGC-MSCs at passage 4 expressed high levels of CD73 (78.58%),
CD90 (74.55%) and CD105 (77.15%). However, they were negative for
the hematopoietic marker CD34 (0.74%), CD45 (0.18%) and HLA-DR
(0.20%). The hGCOM-MSCs at passage 4 also expressed high levels of
CD73 (83.24%), CD90 (76.32%) and CD105 (82.64%), but were negative
for the hematopoietic markers CD34 (0.87%), CD45 (0.66%) and HLA-DR
(0.53%), indicating the mesenchymal lineage of these cells
(Fig. 2). Taken together, these
results demonstrated that cells isolated from human GC tissues and
their ovarian metastatic tissues possess the characteristics of MSC
with regards to the expression of markers. RT-PCR results
demonstrated that stem cell-associated genes, including Snail,
Bmi-1, ABCG2 and Nanog, and mesenchymal lineage-associated genes,
including α-SMA, CD73, CD44 and β-actin, were all expressed in
hBM-MSCs, hGC-MSCs and hGCOM-MSCs (Fig. 3).
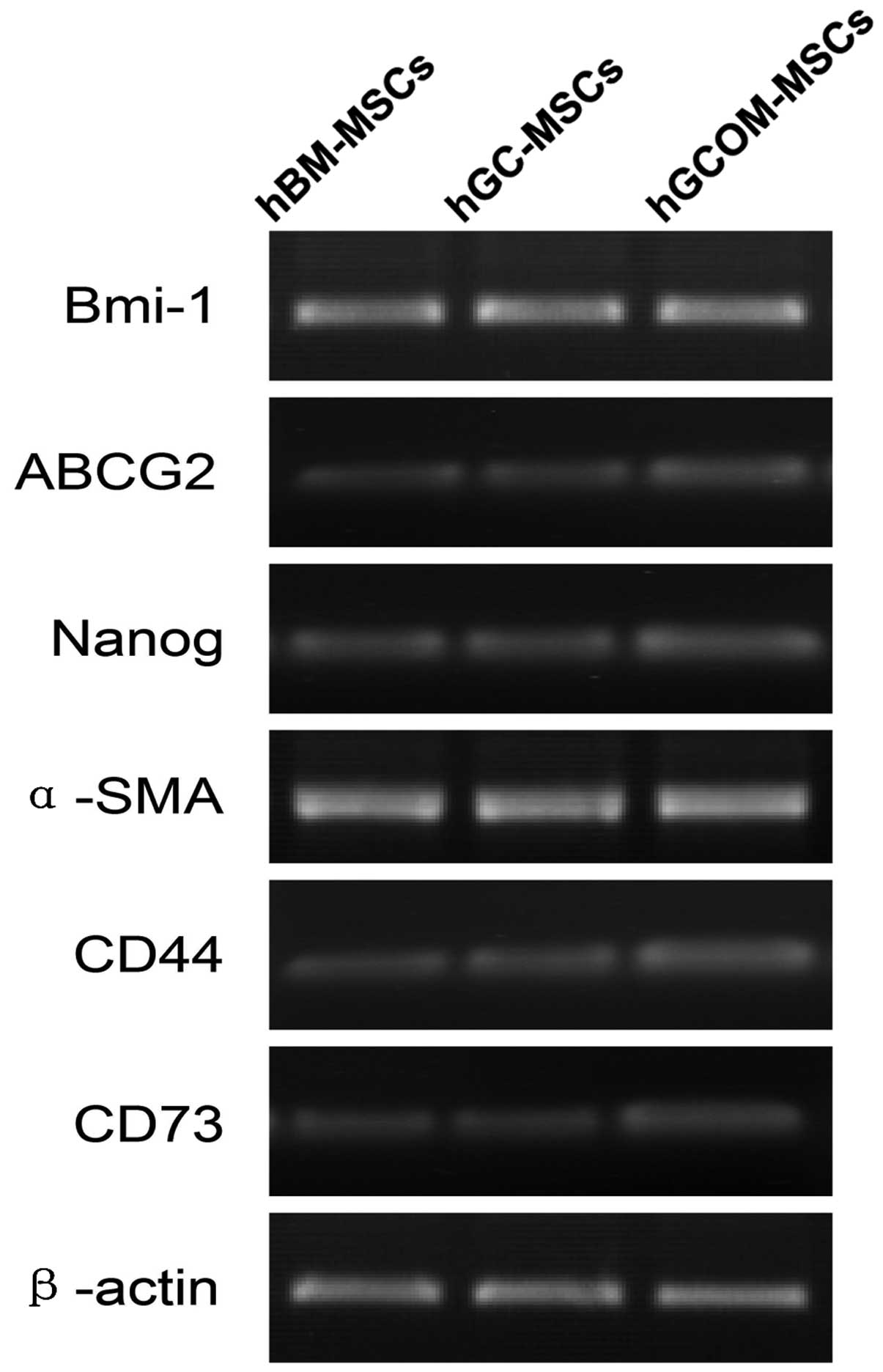 | Figure 3Gene expression of Bmi-1, ABCG2,
Nanog, α-SMA, CD44, CD73 and β-actin in hBM-MSCs, hGC-MSCs and
hGCOM-MSCs. ABCG2, ATP-binding cassette sub-family G member 2;
α-SMA, α-smooth muscle actin; CD, cluster of differentiation; MSC,
mesenchymal stem cells; hGC-MSCs, human gastric cancer-MSCs;
hGCOM-MSCs, human gastric cancer ovarian metastatic tissue-MSCs;
hBM-MSCs, human bone marrow-MSCs. |
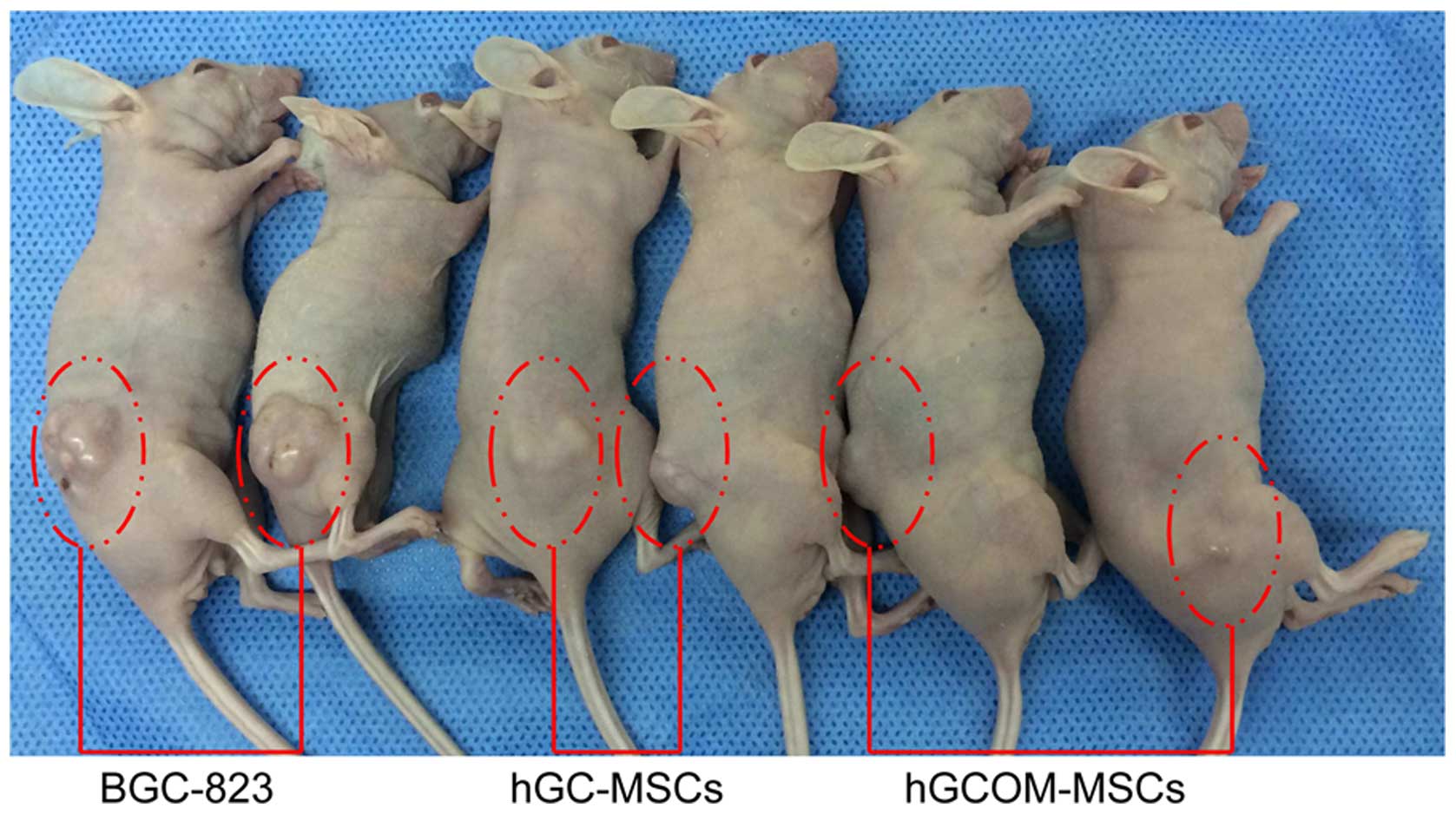
Animal studies
hGC-MSCs and hGCMO-MSCs failed to form tumors in
NOD/SCID mice after >2 weeks (Fig.
4). However, GC cells (BGC-823) used as xenograft controls were
able to form tumors 2 weeks after transplantation. Tumor formation
was followed for 4 weeks.
Differentiation potential of hGC-MSCs and
hGCOM-MSCs
In addition to their colony-forming ability and the
expression of specific antigens on their cell surfaces, the
capacity for tri-lineage differentiation is a key property of MSCs
(27). The differentiation
potential was evaluated by culturing the cells in osteogenic media.
Long-term cultures (3 weeks) of cells grown in the presence of
osteogenic media demonstrated the capacity to form Alizarin
Red-positive condensed nodules with high levels of calcium covering
the entire wells. The deposits were sparsely scattered throughout
the adherent layer as single mineralized zones (Fig. 5A).
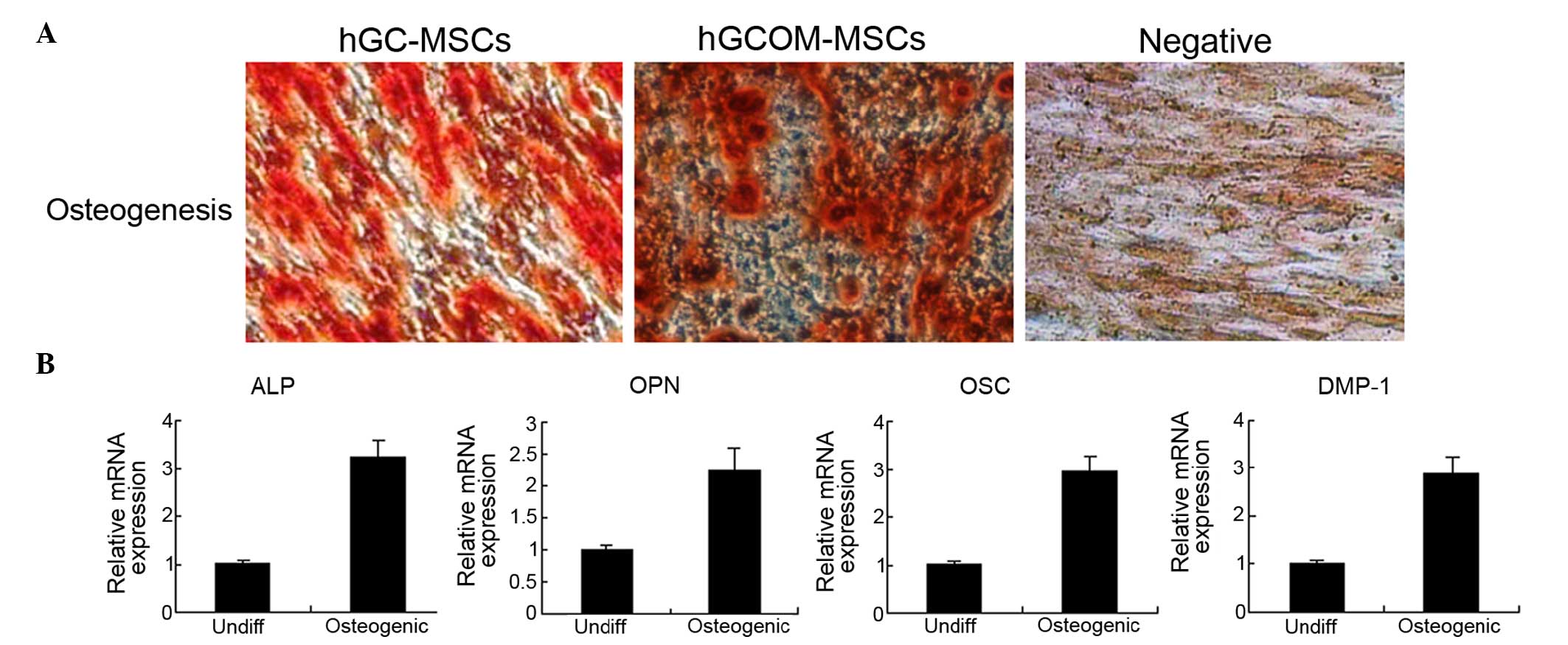 | Figure 5Osteogenic differentiation in
hGC-MSCs and hGCOM-MSCs. (A) Mineralized deposit identified by
Alizarin Red staining in cells grown in osteogenic medium for 3
weeks. Alizarin Red staining of hGC-MSCs and hGCOM-MSCs as viewed
under the light microscope (magnification, ×100). (B) Reverse
transcription-polymerase chain reaction was used to determine the
difference in gene expression profiles of osteoblastic-specific
markers (ALP, OPN, OSC and DMP-1) between 1 month
osteoblastic-differentiated cells and undifferentiated cells
(Undiff). MSCs, mesenchymal stem cells; hGC-MSCs, human gastric
cancer-MSCs; hGCOM-MSCs, human gastric cancer ovarian metastatic
tissue-MSCs; ALP, alkaline phophatase; DMP-1, dentin matrix acidic
phosphoprotein 1; OSC, osteocalcin; OPN, osteopontin. |
In addition, the expression levels of ALP, OPN, OSC
and DMP-1 genes, considered to be osteo-specific genes, were
analyzed at the mRNA level after 3 weeks of osteoinduction. The
expression of these mRNAs (relative to Actb) in differentiated
hGC-MSCs and hGCOM-MSCs was higher than that in undifferentiated
hGC-MSCs and hGCOM-MSCs (Fig. 5B).
These results indicate that hGC-MSCs and hGCOM-MSCs cultured in
osteogenic media can differentiate into osteoblast-like cells.
Discussion
MSCs are a subset of non-hematopoietic adult stem
cells that originate from the mesoderm. They possess self-renewal
ability and exhibit multilineage differentiation into not only
mesoderm-lineages, including chondrocytes, osteocytes and
adipocytes, but also ectodermal cells and endodermal cells
(28). MSCs exist in almost all
tissues. They can be easily isolated from the bone marrow, adipose
tissue, umbilical cord, fetal liver, muscle and lung, and can be
successfully expanded in vitro (29). Due to a lack of specific markers to
define MSCs, their identification depends on their ability to
adhere rapidly to tissue culture plastic, a panel of surface
markers, including CD31, CD34, CD45, CD29, CD90 and CD105, as well
as showing multilineage differentiation potential. The complexity
of MSC interaction with the tumor microenvironment means that tumor
growth and tumor metastasis can be affected by MSCs, directly or
indirectly (30).
MSCs also exist in almost all tissues [needs
citation]. Although it is hypothesized that bone marrow functions
as a reservoir for disseminated tumor cells and that metastasis to
secondary organs occurs due to the recirculation of disseminated
tumor cells from bone marrow, the precise mechanisms remain to be
elucidated. It was reported that in brain tumors affecting adult
patients, tumor cells with stem-like characteristics have only been
isolated from high-grade gliomas (31). By contrast, data from the present
study demonstrated that tumor cells with stem cell-like can be
isolated from GC and its ovarian metastatic tissues in the same
patients (6/40). Further analysis of hGC-MSCs and hGCOM-MSCs
cultures demonstrated that the isolated cells expressed a complex
molecular profile that combined mesenchymal and gastric elements,
suggesting a high potential of plasticity. Assessing in vivo
tumorigenicity, it was verified that non self-renewing cells did
not form tumors when engrafted into immunodeficient mice, whereas
the three long-term self-renewing ones assessed generated tumors
similar to the histological and molecular profile of the original
human lesion.
Our success in derivation of self-renewing
oncospheres from ovarian metastases of GC is comparable with or
above the 50% average success rate. Cao et al (24) initially reported the existence of
MSC-like cells that can be isolated in vitro from GC
tissues. In contrast to GC cells, these cells share most of the
biochemical characteristics of hBM-MSCs, but also display unique
characteristics, attributable to their unique locations. Our
observation suggests that the ability to derive cells with
properties akin to gastric progenitors or gastric stem cells from
ovarian metastases of GC could be more dependent on the absolute
numbers of cells endowed with progenitor or stem properties within
the cultured tumor sample, than on the optimization of culture
conditions for each histological tumor sub-type. Further
experiments are required to address this issue.
In order to further investigate the tumorigenicity
of the isolated human gastric MSCs and ovarian metastatic MSCs,
these cells were injected into nude mice and the results revealed
that human gastric MSCs and ovarian metastatic MSCs were not able
to form tumors even 1 month after initial injection, suggesting
that human GC tissue MSCs and ovarian metastatic MSCs have not
undergone transformation and are MSC-like cells but not CSCs. The
long prevailing model of metastasis recognizes the importance of
'seed' and 'soil' for metastatic progression. An increasing amount
of attention has focused on understanding the molecular and genetic
factors that confer an intrinsic metastatic advantage to certain
tumor cells. In addition, changes occurring within distant tissues,
creating a 'soil' conducive for tumor invasion, have been largely
neglected. Bone marrow-derived stem cells emerged as key players in
initiating these early changes, creating a receptive
microenvironment at designated sites for distant tumor growth and
establishing the 'pre-metastatic niche' (32). This insight into the earliest
stages in the metastatic cascade revises our concept of the
metastatic 'microenvironment' to include physiological cells
recruited from the bone marrow. Understanding the cellular and
molecular cross-talk between 'seed' and 'soil' may improve our
understanding of the factors that govern site-specific patterning
in metastasis and the phenomenon of tumor dormancy. This may lead
to therapeutic strategies to detect and prevent metastasis at its
earliest inception. Human gastric MSCs and ovarian metastatic MSCs
may be one of the most important components of the cancer 'niche.'
On one hand, human GC MSCs may support the growth of tumor cells by
cell-cell interaction. Alternatively, MSCs within human ovarian
metastases of GC may contribute to the vasculature and
extracellular matrix for the development of these tumor cells
(33).
To the best of our knowledge, the present study is
the first to demonstrate a distinct population of benign ovarian
metastases of GC cells with stem cell-like properties. Further
studies on the role of these cells in the initiation, development
and/or progression of ovarian metastases from GC are warranted.
Acknowledgments
The present study was supported by the Hunan
Province Health Department of China (grant no. B2013101, to
Professor Chaohui Zuo) and Hunan Province Natural Science
Foundation of China (grant no. 2015JJ6063), and the National
Natural Science Foundation of China (grant no. 81500150, to Dr Man
Xia).
Abbreviations:
|
MSC
|
mesenchymal stem cells
|
|
GC
|
gastric cancer
|
|
HBM
|
human bone marrow
|
References
|
1
|
Bou Kheir T, Futoma-Kazmierczak E,
Jacobsen A, Krogh A, Bardram L, Hother C, Grønbæk K, Federspiel B,
Lund AH and Friis-Hansen L: miR-449 inhibits cell proliferation and
is down-regulated in gastric cancer. Mol Cancer. 10:292011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fock KM and Ang TL: Epidemiology of
Helicobacter pylori infection and gastric cancer in Asia. J
Gastroenterol Hepatol. 25:479–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh
KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, et al: Screening
for gastric cancer in Asia: Current evidence and practice. Lancet
Oncol. 9:279–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cunningham D and Chua YJ: East meets west
in the treatment of gastric cancer. N Engl J Med. 357:1863–1865.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yilmaz M and Christofori G: Mechanisms of
motility in metastasizing cells. Mol Cancer Res. 8:629–642. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vignot S and Soria JC: Discrepancies
between primary tumor and metastasis: Impact on personalized
medicine. Bull Cancer. 100:561–568. 2013.In French. PubMed/NCBI
|
|
8
|
Wang J, Shi YK, Wu LY, Wang JW, Yang S,
Yang JL, Zhang HZ and Liu SM: Prognostic factors for ovarian
metastases from primary gastric cancer. Int J Gynecol Cancer.
18:825–832. 2008. View Article : Google Scholar
|
|
9
|
Bianco P, Robey PG and Simmons PJ:
Mesenchymal stem cells: Revisiting history, concepts and assays.
Cell Stem Cell. 2:313–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato N, Hayasaka T, Takeda J, Osakabe M
and Kurachi H: Ovarian tumors with functioning stroma: A
clinicopathologic study with special reference to serum estrogen
level, stromal morphology and aromatase expression. Int J Gynecol
Pathol. 32:556–561. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calloni R, Cordero EA, Henriques JA and
Bonatto D: Reviewing and updating the major molecular markers for
stem cells. Stem Cells Dev. 22:1455–1476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Droujinine IA, Eckert MA and Zhao W: To
grab the stroma by the horns: From biology to cancer therapy with
mesenchymal stem cells. Oncotarget. 4:651–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cuiffo BG and Karnoub AE: Mesenchymal stem
cells in tumor development: Emerging roles and concepts. Cell Adh
Migr. 6:220–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang X, Hou J, Han Z, Wang Y, Hao C, Wei L
and Shi Y: One cell, multiple roles: Contribution of mesenchymal
stem cells to tumor development in tumor microenvironment. Cell
Biosci. 3:52013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu MH, Gao X, Luo D, Zhou XD, Xiong W and
Liu GX: EMT and acquisition of stem cell-like properties are
involved in spontaneous formation of tumorigenic hybrids between
lung cancer and bone marrow-derived mesenchymal stem cells. PLoS
One. 9:e878932014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ono M, Kosaka N, Tominaga N, Yoshioka Y,
Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K and Ochiya
T: Exosomes from bone marrow mesenchymal stem cells contain a
microRNA that promotes dormancy in metastatic breast cancer cells.
Sci Signal. 7:ra632014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sung SY, Liao CH, Wu HP, Hsiao WC, Wu IH,
Jinpu Yu, Lin SH and Hsieh CL: Loss of let-7 microRNA upregulates
IL-6 in bone marrow-derived mesenchymal stem cells triggering a
reactive stromal response to prostate cancer. PLoS One.
8:e716372013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Konno Y, Dong P, Xiong Y, Suzuki F, Lu J,
Cai M, Watari H, Mitamura T, Hosaka M, Hanley SJ, et al:
MicroRNA-101 targets EZH2, MCL-1 and FOS to suppress proliferation,
invasion and stem cell-like phenotype of aggressive endometrial
cancer cells. Oncotarget. 5:6049–6062. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Z, Jiang Z, Huang J, Huang S, Li Y,
Sheng F, Yu S, Yu S and Liu X: Mesenchymal stem cells show little
tropism for the resting and differentiated cancer stem cell-like
glioma cells. Int J Oncol. 44:1223–1232. 2014.PubMed/NCBI
|
|
20
|
Ochs K, Sahm F, Opitz CA, Lanz TV, Oezen
I, Couraud PO, von Deimling A, Wick W and Platten M: Immature
mesenchymal stem cell-like pericytes as mediators of
immunosuppression in human malignant glioma. J Neuroimmunol.
265:106–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bian ZY, Li G, Gan YK, Hao YQ, Xu WT and
Tang TT: Increased number of mesenchymal stem cell-like cells in
peripheral blood of patients with bone sarcomas. Arch Med Res.
40:163–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kirkland SC: Type I collagen inhibits
differentiation and promotes a stem cell-like phenotype in human
colorectal carcinoma cells. Br J Cancer. 101:320–326. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marrelli M, Paduano F and Tatullo M: Cells
isolated from human periapical cysts express mesenchymal stem
cell-like properties. Int J Biol Sci. 9:1070–1078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao H, Xu W, Qian H, Zhu W, Yan Y, Zhou H,
Zhang X and Xu X, Li J, Chen Z and Xu X: Mesenchymal stem cell-like
cells derived from human gastric cancer tissues. Cancer Lett.
274:61–71. 2009. View Article : Google Scholar
|
|
25
|
Mamidi MK, Pal R, Govindasamy V, Zakaria Z
and Bhonde R: Treat the graft to improve the regenerative ability
of the host. Med Hypotheses. 76:599–601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu J, Wang W, Kapila Y, Lotz J and Kapila
S: Multiple differentiation capacity of
STRO-1+/CD146+ PDL mesenchymal progenitor
cells. Stem Cells Dev. 18:487–496. 2009. View Article : Google Scholar :
|
|
27
|
Ribitsch I, Burk J, Delling U, Geißler C,
Gittel C, Jülke H and Brehm W: Basic science and clinical
application of stem cells in veterinary medicine. Adv Biochem Eng
Biotechnol. 123:219–263. 2010.PubMed/NCBI
|
|
28
|
Dezawa M, Ishikawa H, Itokazu Y, Yoshihara
T, Hoshino M, Takeda S, Ide C and Nabeshima Y: Bone marrow stromal
cells generate muscle cells and repair muscle degeneration.
Science. 309:314–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barberini DJ, Freitas NP, Magnoni MS, Maia
L, Listoni AJ, Heckler MC, Sudano MJ, Golim MA, da Cruz
Landim-Alvarenga F and Amorim RM: Equine mesenchymal stem cells
from bone marrow, adipose tissue and umbilical cord:
Immunophenotypic characterization and differentiation potential.
Stem Cell Res Ther. 5:252014. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cuiffo BG and Karnoub AE: Mesenchymal stem
cells in tumor development: Emerging roles and concepts. Cell Adh
Migr. 6:220–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thirant C, Bessette B, Varlet P, Puget S,
Cadusseau J, Tavares Sdos R, Studler JM, Silvestre DC, Susini A,
Villa C, et al: Clinical relevance of tumor cells with stem-like
properties in pediatric brain tumors. PLoS One. 6:e163752011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaplan RN, Riba RD, Zacharoulis S, Bramley
AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et
al: VEGFR1-positive haematopoietic bone marrow progenitors initiate
the pre-metastatic niche. Nature. 438:820–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lescarbeau RM, Seib FP, Prewitz M, Werner
C and Kaplan DL: In vitro model of metastasis to bone marrow
mediates prostate cancer castration resistant growth through
paracrine and extracellular matrix factors. PLoS One. 7:e403722012.
View Article : Google Scholar : PubMed/NCBI
|