1. Introduction
MicroRNAs (miRNAs) are small, single-stranded, 21–23
nucleotide-long, independent functional units of noncoding RNA
(1–3). Often referred to as the
'micromanagers of gene expression', miRNAs are evolutionarily
well-conserved. Mature miRNAs regulate the expression of ~30% of
all human genes involved in fundamental biological processes at a
post-transcriptional level by sequence-specific binding to
3′-untranslated regions (3′-UTRs) of multiple target messenger RNAs
(mRNAs), leading to their degradation or translational suppression
(4,5). Increasing evidence has suggested that
miRNAs are important in a broad range of biological processes,
including embryonic development, cellular proliferation,
differentiation, apoptosis and other physiological processes
(6,7).
miRNAs are synthesized in a precisely coordinated
manner. Briefly, the miRNA gene is transcribed by RNA polymerase
II, resulting in a hairpin-shaped primary miRNA (pri-miRNA), which
is ~500–3,000 base pairs in length. This pri-miRNA is further
processed by Drosha/Pasha (DGCR8) to form a 60–70 nucleotide-long
precursor miRNA (pre-miRNA), which is transported from the nucleus
to the cytoplasm through nuclear pore complexes, with the
assistance of Exportin-5 (XPO5) (6,8). The
pre-miRNA is further cleaved in the cytoplasm by the RNase III
endonuclease, Dicer, to release two complementary short RNA
molecules (9). The argonaut
protein complex selectively binds to the guide strand and
facilitates the formation of a miRNA-mRNA-induced silencing complex
(RISC) assembly, which consists of HIWI, GEMIN3 and GEMIN4. Upon
miRNA binding, the RISC complex is activated and, by a mechanism
that remains to be fully elucidated, locates its binding site in
the 3′-UTR of the target mRNA and contributes to regulation of the
expression of the gene (8–10).
Advancements in investigations of miRNA have
indicated the involvement of miRNAs in the genesis, progression
(proliferation, migration and invasion) and prognosis of multiple
types of human malignancy (11).
Of note, ~50% of all annotated human miRNA genes are located in
fragile sites or areas of the genome, which are frequently deleted,
amplified and mis-expressed in human cancer (12). The conditional deletion or
overexpression of a single miRNA is sufficient to drive
tumorigenesis in mice (13). It
has been suggested that the single nucleotide polymorphisms (SNPs)
in miRNAs, which encode their biogenesis pathway and target binding
sites, may affect the regulatory capacity of miRNAs by affecting
miRNA processing and/or miRNA-mRNA interactions (14). Polymorphisms in miRNA regulatory
pathways may result in the loss or gain of an miRNA function, which
can act as an oncogene or tumor suppressor. Previously, several
studies have demonstrated a marked association between
miRNA-polymorphisms and the risk, treatment response and outcome in
patients with cancer (15,16). Polymorphisms in miRNA regulatory
networks are a novel class of functional polymorphisms in the human
genome (11). These enable
investigation of the biology of cancer and have the potential for
use as biomarkers in cancer diagnosis and prognosis. The present
review provides a brief outlook on the biogenesis and biology of
miRNAs, and the functional effects of miRNA-associated SNPs.
2. Genetic polymorphisms in the miRNA
biogenesis pathway
Several proteins and protein complexes are involved
in various stages of miRNA biogenesis, including miRNA
transcription, processing, export and targeting (7). These proteins include the RNA
polymerase II complex, Drosha/Pasha, Exportin-5, nuclear pore
complexes, Dicer and the Argonaut protein/RISC complex, as shown in
Fig. 1. As the underexpression or
overexpression of miRNA may have serious consequences in a cell,
polymorphisms in core components of miRNA biogenesis may impair or
enhance miRNA processing efficiency or function, resulting in
altered levels of mature miRNAs and deleterious effects (4). Several lines of evidence have
supported that SNPs in the biogenesis pathway of miRNAs are
associated with development and progression in a several types of
tumor (Table I).
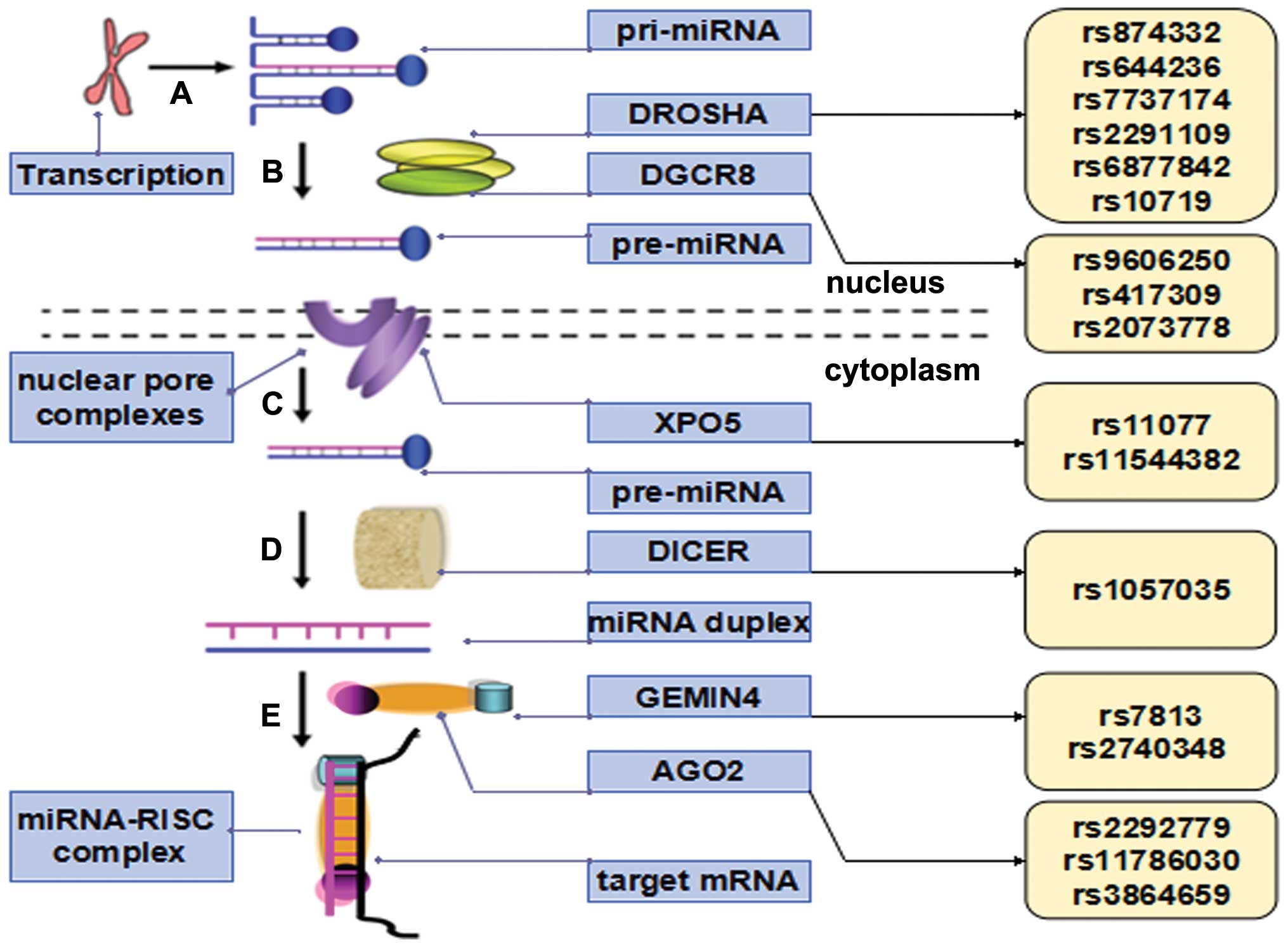 | Figure 1Illustrative overview of
polymorphisms in miRNA biogenesis pathways. (A) miRNA gene is
transcribed by RNA polymerase II; (B) pri-miRNA is further
processed by Drosha/Pasha; (C) pre-miRNA is transported to the
cytoplasm by Exportin-5; (D) pre-miRNA is cleaved by Dicer,
releasing two complementary short RNA molecules; (E) miRNA-RISC
complex binds to the 3′-untranslated reguin of the target mRNA and
contributes to translational inhibition or mRNA degradation.
Polymorphisms, which affect the expression of proteins involved in
miRNA action and biogenesis, including Drosha, Pasha, Dicer,
Exportin 5 and proteins in the RISC complex, may affect
miRNA-mediated regulation of mRNA expression and protein
translation in the cell, consequently, contributing to the
susceptibility and prognosis of various types of cancer. miRNA,
microRNA; pri-miRNA, primary miRNA; pre-miRNA, precursor miRNA;
RISC. RNA-induced silencing complex. |
 | Table IPolymorphisms in microRNA biogenesis
pathways and functional variations. |
Table I
Polymorphisms in microRNA biogenesis
pathways and functional variations.
| Biogenesis
gene | SNP site | Tumor type
(population, n) | Description | Risk (95% CI) | Ref |
|---|
| DROSHA | rs874332
(C>T) | Breast cancer
(Korean, 488) | OS | HR=2.24
(1.21–4.17) | 20 |
| rs644236
(C>T) | Breast cancer
(Korean, 559/567) | Susceptibility | OR=1.27
(0.94–1.73) | 18 |
| rs7737174
(A>G) | Breast cancer
(Korean, 559/567) | Susceptibility | OR=1.63
(1.01–2.64) | 18 |
| rs2291109
(A>T) | Breast cancer
(Chinese, 878/900) | Susceptibility | OR=0.81
(0.66–0.99) | 19 |
| rs6877842
(C>G) | Renal cell
carcinoma (Caucasian, 316) | Recurrence | HR=0.36
(0.13–0.98) | 22 |
| rs10719
(C>T) | Malignant
peripheral nerve sheath tumor (Chinese, 156/200) | Susceptibility | OR=1.64
(1.23–2.20) | 23 |
| DGCR8 | rs9606250
(A>T) | Breast cancer
(Korean, 488) | DFS | HR=0.21
(0.05–0.84) | 20 |
| rs417309
(A>G) | Breast cancer
(Chinese, 878/900) | Susceptibility | OR=1.50
(1.16–1.93) | 19 |
| rs2073778
(C>T) | Bladder cancer
(non-Hispanic, 421) | Progression | HR=4.00
(1.53–10.46) | 24 |
| XPO5 | rs11077
(A>C) | NSCLC (Chinese,
112) | OS | RR=0.457
(0.251–0.831) | 27 |
| | SCLC (Chinese,
42) | OS | RR=2.469
(1.088–5.603) | 28 |
| | Hepatocellular
carcinoma (Chinese, 108) | OS | RR=0.395
(0.167–0.933) | 29 |
| | Renal cell
carcinoma (Caucasian, 316) | Recurrence | HR=0.36
(0.16–0.85) | 22 |
| rs11544382
(A>G) | Breast cancer
(Caucasian, 441/479) | Susceptibility | OR=1.59
(1.06–2.39) | 30 |
| DICER | rs1057035
(C>T) | Head and neck
cancer (Chinese, 397/900) | Susceptibility | OR=0.65
(0.46–0.92) | 31 |
| | Cervical carcinoma
(Chinese, 1,486/1,549) | Susceptibility | OR=0.962
(0.805–1.149) | 32 |
| | Hepatocellular
carcinoma (Chinese, 1300/1344) | Susceptibility | OR=0.79
(0.64–0.96) | 33 |
| | Breast cancer
(Korean, 488) | DFS | HR=1.72
(0.99–2.99) | 20 |
| | | OS | HR=2.08
(1.01–4.28) | |
| GEMIN4 | rs7813
(C>T) | Renal cell
carcinoma (Caucasian, 316) | OS | HR=1.74
(1.15–2.62) | 22 |
| | Prostate cancer
(Chinese, 300/244) | Susceptibility | OR=2.53
(1.07–6.28) | 34 |
| | Malignant
peripheral nerve sheath tumor (Chinese, 156/200) | Susceptibility | OR=0.50
(0.34–0.72) | 23 |
| | Ovarian cancer
(Caucasian, 339/349) | Susceptibility | OR=0.71
(0.57–0.87) | 35 |
| rs2740348
(C>G) | Renal cell
carcinoma (Caucasian, 279/278) | Susceptibility | OR=0.67
(0.47–0.96) | 36 |
| | Prostate cancer
(Chinese, 300/244) | Susceptibility | OR=0.68
(0.47–0.98) | 34 |
| AGO2 | rs2292779
(C>G) | Breast cancer
(Korean, 488) | DFS | HR=1.42
(1.06–1.92) | 20 |
| | | OS | HR=2.94
(1.52–5.69) | |
| rs11786030
(A>G) | Breast cancer
(Korean, 488) | DFS | HR=2.62
(1.41–4.88) | 20 |
| | | OS | HR=2.41
(1.05–5.50) | |
| rs3864659
(A>C) | Breast cancer
(Korean, 559/567) | Susceptibility | OR=0.67
(0.46–0.96) | 18 |
DROSHA
Drosha is an RNAse III enzyme, which mediates the
processing of pri-miRNAs into pre-miRNAs with DGCR8 (8). In a previous in vitro
functional investigation, a reduction in miRNA processing efficacy,
which was induced by the knockdown of DROSHA, was found to
reduce the levels of mature forms of tumor-suppressive miRNAs and
facilitate the invasion of breast cancer cells (17). Several studies have indicated the
role of Drosha in breast cancer. A case-control study demonstrated
that two SNPs in DROSHA, rs644236 and rs7737174, may
contribute to the risk of breast cancer in postmenopausal women
(18). Jiang et al also
suggested rs2291109 as a predictor for breast cancer risk,
however, the association was not confirmed (19). In addition, patients with breast
cancer carrying the DROSHA rs874332 C allele are at
increased risk of mortality (20).
As rs874332 is located in the 3′-UTR of DROSHA mRNA
and a predicted miRNA binding site, it is possible that
rs874332 may be correlated with the translational repression
and mRNA destabilization of DROSHA through an miRNA-mRNA
interaction. However, data from Sung et al (21) involving east Asian women, including
5,066 cases and 4,337 controls, failed to identify an association
between the SNPs in DROSHA and breast cancer risk. In
addition, DROSHA rs6877842 has been reported to reduce the
risk of recurrence in patients with renal cell carcinoma by 36%,
and haplotypes of DROSHA (rs6877842/rs10719) have
been associated with survival rates (22). rs10719 may also affect the
risk of malignant peripheral nerve sheath tumors through increasing
the expression level of DROSHA (23).
DGCR8 (Pasha)
DGCR8, as a component of the multiprotein complex
with the RNAse III enzyme, Drosha, is a double stranded RNA-binding
protein, which is involved in the processing of pri-miRNAs into
pre-miRNAs (9). Impaired miRNA
processing through the knockdown of DGCR8 also facilitates
the invasion of breast cancer cells (17). The rs9605062 in DGCR8
may upregulate the level or timing of gene expression (20), and it has been reported that
rs9606250 is significantly associated with poor disease-free
survival (DFS) rates in breast cancer (20). In addition, the interruption of
miRNA binding of rs417309, located at the binding sites of
miR-106b and miR-579 in the 3′-UTR of DGCR8, has been found
to increased the risk of breast in the Chinese population (19). Another two linked SNPs,
rs2073778 and rs720012, in DGCR8 have also
been shown to be significantly associated with tumor progression in
bladder cancer (24).
XPO5
XPO5 is located in the nuclear membrane, and
mediates the transport of pre-miRNAs to regulate miRNA expression
(10). The XPO5-mediated nuclear
export of pre-miRNAs may be a rate-limiting step in miRNA
biogenesis. The over-expression of XPO5 has been shown to
result in enhanced miRNA activity (25), whereas the loss of XPO5
leads to reduced expression and function of pre-miRNAs (26). Among the SNPs in XPO5,
rs11077 has received the most attention. Located in the
3′-UTR of XPO5, rs11077 may affect mRNA stability,
alter the expression of XPO5 and, consequently, affect the
expression of miRNAs, including those specific for drug metabolism,
altering the response to chemotherapy and affecting survival rates
of patients with advanced non-small-cell lung cancer (NSCLC) and
small-cell lung cancer (27,28).
In addition, rs11077 has been associated with poor
progression in hepatocellular carcinoma and renal cell carcinoma
(22,29). A mutation in rs11544382 in a
functionally conserved region of XPO5 may also alter the
protein structure of XPO5, resulting in altered nucleocytoplasmic
transport activity (30), and this
SNP has been associated with an increased risk of breast cancer
(30).
DICER
Dicer is an enzyme responsible for the cleavage of
miRNA precursors, and has been implicated in the oncogenic process
of several types of cancer. Increasing evidence has supported the
role of DICER rs1057035 in cancer susceptibility. This SNP
has been associated with a decreased risk of oral cancer (31), cervical carcinoma (32) and hepatocellular carcinoma
(33). This polymorphism is
located in the 3′-UTR of DICER and a predicted binding site
of miR-574-3p, which may affect the binding of miR-574-3p, and
result in decreased mRNA expression levels of DICER
(31). Of note, this SNP has been
shown to be associated with a 1.72- and 2.08-fold increased risk of
progression and cancer-associated mortality, respectively, among
patients with breast cancer (20).
GEMIN4
The GEMIN4 protein is referred to as an important
molecule in the RISC complex, which is involved in the maturation
process of miRNAs, and the recognition and repression of target
mRNAs (7). The protein expression
level of GEMIN4 is closely associated with the biogenesis of
associated miRNAs (7).
Rs7813 in the exons of GEMIN4 has been frequently
identified as a predictive biomarker in several types of cancer,
including renal cell carcinoma (22), prostate cancer (34), malignant peripheral nerve sheath
tumor (23) and ovarian cancer
(35). Another non-synonymous SNP,
rs2740348, which is located in the functional region of the
GEMIN4 gene has been demonstrated to decrease the risks of
prostate cancer and renal cell carcinoma by 36 and 33%,
respectively (34,36). Notably, Wan et al found that
rs2740348 and rs7813 were significantly associated
with cell growth and DNA repair in a heptacellular carcinoma cell
line (37), suggesting that the
amino acid changes caused by these SNPs may have a physiological
significance on the development of cancer.
AGO2
AGO2 is important in miRNA-mediated gene silencing,
as a component of the RISC complex that directly binds miRNAs and
mediates the cleavage of target mRNAs (7). Emerging evidence from in vitro
analysis and clinical samples has indicated that the abnormal
expression or enzymatic function of AGO2 is associated with cancer
development and progression. In breast cancer cell lines, the
overexpression of AGO2 induces the transformed phenotype (38). Sung et al indicated that
AGO2 rs3864659 may have a protective effect on breast cancer
risk (18). In addition, two
further SNPs in AGO2 rs11786030 and rs2292779 have
been significantly associated with poor DFS and poor overall
survival (OS) rates in breast cancer (20). Variations in the genomic structure
of AGO2, including changes in copy number or frameshift
mutations, have also been reported to be associated with several
types of cancer, including multiple myeloma, gastric cancer and
colorectal cancer (39,40).
3. Genetic polymorphisms in miRNA genes
SNPs in miRNA genes are considered to exert their
effects by one of three mechanisms: Through transcription of the
primary transcript; through pri-miRNA and pre-miRNA processing; and
through effects on miRNA-mRNA interactions (11). In general, sequence variations in
miRNA genes, including pri-miRNAs, pre-miRNAs and mature miRNAs,
have the potential of affect the processing efficiency and/or
target selection of miRNAs, leading to aberrant expression of
hundreds of genes in different biological pathways (11). As miRNAs are highly conserved, SNPs
in miRNA genes are relatively rare. The majority of studies have
followed a biologically-based candidate gene approach to identify
SNPs in miRNAs, which may affect cancer susceptibility, relying on
a knowledge of the functional link between a particular miRNA and
gene target (Table II).
 | Table IIPolymorphisms in miRNA genes and
their clinical significance. |
Table II
Polymorphisms in miRNA genes and
their clinical significance.
| miRNA | SNP site | Tumor type
(population, n) | Description | Risk (95% CI) | Ref |
|---|
| pre-miR-27a | rs895819
(A>G) | Breast cancer
(Chinese, 264/255) | Susceptibility | OR=0.535
(0.321–0.891) | 42 |
| | Renal cell
carcinoma (Chinese, 594/600) | Susceptibility | OR=0.71
(0.56–0.90) | 44 |
| | NSCLC (Chinese,
576) | OS | HR=1.71
(1.12–2.26) | 45 |
| | |
Chemotherapyresponse | OR=0.54
(0.32–0.91) | |
| miR-196a2 | rs11614913
(C>T) | Breast cancer
(meta, 2588/3260) | Susceptibility | OR=0.906
(0.825–0.995) | 53 |
| | Lung cancer (meta,
2219/2232) | Susceptibility | OR=1.13
(0.98–1.29) | 55 |
| | Hepatocellular
carcinoma (meta, 3437/3437) | Susceptibility | OR=0.90
(0.83–0.98) | 56 |
| | Digestive system
cancers (meta, 4999/7606) | Susceptibility | OR=1.29
(1.10–1.50) | 58 |
| | NSCLC (Korean,
388) | RFS | HR=0.60
(0.38–0.94) | 59 |
| miR-146a | rs2910164
(C>G) | Acute-on-chronic
hepatitis B liver failure (Chinese, 717/251) | Susceptibility | OR=0.496
(0.309–0.797) | 65 |
| | NSCLC (Korean,
388) | RFS | HR=0.48
(0.28–0.80) | 59 |
| | Cervical squamous
cell carcinoma (Chinese, 226/309) | Susceptibility | OR = 2.10
(1.22–3.59) | 66 |
| miR-499 | rs3746444
(C>T) | Cervical squamous
cellcarcinoma (Chinese, 226/309) | Susceptibility | OR= 1.78
(1.24–2.56) | 66 |
| | Head and neck
cancer (non-Hispanic white, 1109/1130) | Susceptibility | OR=0.83
(0.69–0.99) | 68 |
| pri-miR-218 | rs11134527
(A>G) | Cervical carcinoma
(Chinese, 1584/1394) | Susceptibility | OR=0.77
(0.63–0.95) | 71 |
| | Hepatocellular
carcinoma (Chinese, 302/513) | Susceptibility | OR=2.96
(1.16–7.56) | 72 |
Pre-miR-27a
The pre-miR-27a, rs895819, has been
frequently investigated in the development of cancer, however, the
results remain contradictory rather than conclusive (41–46).
To integrate all individual studies and comprehensively analyze the
role of rs895819 in tumorigenesis, several meta-analysis
have been performed. Previous overall meta-analysis suggested no
association between the pre-miR-27a rs895819 polymorphism
and cancer susceptibility (47–49).
In a stratified analysis, according to the type of cancer,
individuals with the variant G allele were consistently found to be
at a reduced risk of breast, renal cell and nasopharyngeal cancer,
but at an increased risk of digestive tract cancer (47). In addition, subgroup analysis
according to ethnicity revealed that the rs895819 AG
genotype was associated with a decreased risk of cancer in
Caucasian individuals (48). As
this SNP is located at the terminal loop of pre-miR-27a, it may
have an effect on the secondary structure of pre-miR-27a (42). The substitution of G for A in
rs895819 may reduce the size of the loop and alter the
minimum free energy, consequently inhibiting cleavage and resulting
in low expression levels of mature miR-27a (42).
miR-196a2
rs11614913 in the mature sequence of
miR-196a2 has been increasingly identified a predictor for various
types of cancer (50–52). The results from several
meta-analyses, each containing thousands of subjects, have
demonstrated that rs11614913 may contribute to the risk of
developing breast cancer (53),
lung cancer (54,55), hepatocellular carcinoma (56) and cancer of the digestive system
(57,58). In addition, the SNP was positively
correlated with improved recurrence-free survival (RFS) in patients
with stage II and stage III NSCLC (59). The polymorphism may negatively
affect endogenous processing of either miR-196a2 precursor to its
mature form, and the levels of mature miR-196a2 are lower in CC
carriers, compared with TT carriers (60). Furthermore, binding assays have
revealed that this SNP can affect the binding of mature miR-196a2
to its target mRNA (60).
miR-146a
miR-146a, first identified in the mouse, has been
shown to be important in tumorigenesis, by promoting cell
proliferation and colony formation in NIH/3T3 cells (61). However, it also exhibits an
antitumor property, by suppressing metastatic ability, in breast
cancer and prostate cancer (62,63).
The G-C substitution (rs2910164), located in the middle of
the stem hairpin on the passenger strand of the precursor miR-146a,
has a lower transcriptional activity due to decreased nuclear
processing efficiency, leading to low level of mature miR-146a in
cells (64). Although three
meta-analyses consistently found that rs2910164 was not
involved in overall cancer risk, stratified analysis by ethnicity
has shown a close association between rs2910164 and overall
cancer risk in the Caucasian population (50–52).
Jiang et al reported that the rs2910164 GG homozygote
was a protective genotype, in terms of susceptibility to
acute-on-chronic hepatitis B liver failure (65). However, results from another
meta-analysis showed the C variant to be associated with decreased
hepatocellular carcinoma risk (56). As for patients with NSCLC, variants
of rs2910164 were found to be positively correlated with RFS
(59). However, in the development
of cervical squamous cell carcinoma, the G allele of
rs2910164 was associated with a significantly increased
risk, as well as reduced tumor differentiation and a decline in
lymph node status (66).
miR-499
It is known that the secondary structure of miRNA is
critical to mRNA-miRNA interactions and gene regulation (67). The rs3746444 polymorphism
may affect miR-499 maturation and regulate the expression of its
target genes through directly altering its secondary structure.
Zhou et al provided evidence that rs3746444 may
contribute to the susceptibility to cervical squamous cell
carcinoma (66). Of note, Liu
et al demonstrated that rs3746444 has a protective
effect in the development of head and neck cancer (68). In addition, the T allele of
rs3746444 was associated with a decreased risk of breast
cancer among Asian individuals, however, a follow-up meta-analysis
suggested risk was increased in Caucasians individuals, suggesting
ethnic differences in the consequences of SNPs (69). A further meta-analysis failed to
identify any significant correlation between the miR-499
polymorphism and risk of hepatocellular carcinoma (56).
miR-218
The expression level of miR-218 is associated with
infection with high-risk human papilloma virus (HPV), and is
involved in the pathogenesis of cervical cancer (70). The rs11134527 in miR-218 has
been shown to upregulate the expression of miR-218, and inhibit the
expression of its target gene, LAMB3, by interfering with
the mRNA-miRNA interaction. The overexpression of LAMB3
induces carcinogenesis by increasing carcinoma cell migration and
disturbing tumor microenvironment, therefore, this polymorphism has
been implicated in the infective process of high-risk HPV, thus
contributing to cervical carcinogenesis (66,71).
Another study evaluated the role of rs11134527 in
hepatocellular carcinoma, which noted that the AG genotype of
rs11134527 was associated with family history and elevated
levels of serum α-fetoprotein, suggesting that the AG
genotype may be associated with genetic predisposition in patients
with hepatocellular carcinoma (72).
4. Genetic polymorphisms in miRNA target
sites
The disruption of miRNA-dependent regulation by SNPs
in the miRNA binding site of target mRNAs has been confirmed as a
mechanism for altered gene expression in cancer. In contrast to the
miRNA-polymorphisms in the miRNA biogenesis pathway, the
polymorphisms located at the 3′-UTR of an miRNA target gene are
more abundant in the human genome, and affect only the expression
of the target gene and its downstream effectors, resulting in a
more defined and limited range of effects (73). The majority of the miRNA binding
sites in the 3′-UTRs of a target mRNA lack a complex secondary
structure, thereby facilitating access for an miRNA. Polymorphisms
at or close to these binding sites, through creating or eradicating
secondary structure, may affect the accessibility of an miRNA-RISC
complex, and the coordination of miRNAs with other regulatory
elements in the 3′-UTR of the target transcript (11). Among the 120,000 known SNPs that
occur in 3′-UTRs, ~17% destroy putative conserved or non-conserved
miRNA-binding sites, and 8.6% create novel predicted target sites,
according to the Patrocles database (74). Several examples of SNPs located in
the 3′-UTR of target mRNAs, and their clinical significance, are
presented in Table III.
 | Table IIIPolymorphisms in miRNA target sites
and the effects of the variability. |
Table III
Polymorphisms in miRNA target sites
and the effects of the variability.
| Target gene | SNP site | miRNA | Tumor type
(population, n) | Description | Risk (95% CI) | Ref |
|---|
| FAS | rs2234978
(C>T) | miR-561 | NSCLC (Caucasian,
535) | OS | HR=0.59
(0.44–0.77) | 74 |
| FZD4 | rs713065
(A>G) | miR-494 | NSCLC (Caucasian,
535) | OS | HR=0.46
(0.32–0.65) | 74 |
| SP1 | rs17695156
(C>T) | miR-545 | NSCLC (Caucasian,
535) | Recurrence | HR=3.36
(1.62–6.69) | 74 |
| MDM4 | rs4245739
(A>C) | miR-191 | Esophageal squamous
cell carcinoma (Chinese, 1128/1150) | Susceptibility | OR=0.54
(0.35–0.82) | 76 |
| | miR-191 | Ovarian carcinoma
(Caucasian, 113) | Mortality | HR=5.5
(1.5–20.5) | 77 |
| | | | Recurrence | HR=4.1
(1.2–13.5) | |
| SGSM3 | rs56228771
(insertion/deletion) | miR-151-5p | Hepatocellular
carcinoma (Chinese, 502/513) | Susceptibility | OR=0.55
(0.42–0.73) | 78 |
| COL1A2 | rs3917
(insertion/deletion) | miR-382 let-7g | Hepatocellular
carcinoma (Chinese, 207/245) | Susceptibility | OR=1.76
(1.03–3.01) | 79 |
FAS is a cell surface receptor of the tumor necrosis
family, which is important in the regulation of apoptosis (75). The rs2234978 SNP in the
3′-UTR of FAS has been reported to create a novel
miRNA-binding site for miR-561, and ultimately result in decreased
expression of FAS. Patients with NSCLC, who carry the variant
allele, appear to have a better overall survival (OS), independent
of treatment regimen (75). This
may be explained by higher expression levels of FAS due to
the SNP, which may increase tumor cell death. Similarly,
rs713065 in the 3′-UTR of FZD4 may downregulate the
expression of FZD4 by creating an miR-494 binding site, leading to
enhanced survival through decreased WNT signaling (76). By contrast, rs17695156 in
the 3′-UTR of SP1 is predicted to disrupt a conserved
miR-545 binding site and alter the expression of SP1 by affecting
mRNA stability or post-transcriptional regulation, and patients
with NSCLC patients carrying at least one variant allele of
rs17695156 have a shorter median RFS, compared with patients
with a common homozygous genotype (75). The rs4245739 SNP in the
3′-UTR of MDM4 has been noted to create an miR-191 target
site and results in decreased expression of MDM4 (76). As MDM4 is key in the P53 tumor
suppressor pathway, by negatively regulating P53 function, this
polymorphism may contribute to reduced susceptibility to esophageal
squamous cell carcinoma (77). In
addition, AA genotype carriers, who do not express the estrogen
receptor, have a 4.2-fold increased risk of recurrence and a
5.5-fold increased risk of tumor-associated mortality in ovarian
cancer (78).
In addition to SNPs, the insertion/deletion
polymorphisms in a target gene can also create or destroy a binding
site. SGSM3 is involved in the small G protein-coupled receptor
signal transduction pathway. It has been reported that a 4-bp
insertion/deletion polymorphism (rs56228771) in the 3′-UTR
of SGSM3 can affect the susceptibility of hepatocellular
carcinoma, reducing decreased risk of ins/del+ins/ins genotypes by
~45% (79). In addition, a 7-base
pair deletion polymorphism (rs3917) in the 3′-UTR of
COL1A2 has been associated with a 1.73-fold increased risk
of hepatocellular carcinoma (80).
The rs3917 lies within a predicted binding site for miR-382
and let-7g, and the deletion allele may alter the affinity of
miRNA-mRNA binding, by disrupting the local structure of
COL1A2 mRNA, possibly upregulating the expression of COL1A2
(80,81).
5. Scope and challenges
Further investigations
To date, the majority of the studies in this field
are case-control studies, based on a candidate gene approach
(15). Although several positive
results have been reported, inconsistent findings and
non-replication of previous results have frequently occurred
(15). This may be attributed to
several reasons, including the heterogeneity of patient groups,
different experimental designs, insufficient sample size or unclear
disease biology (15).
Heterogeneity in clinical confounding factors and
endpoint phenotypes between initial and replication studies can
undermine the opportunity to compare among them. It is essential to
account for all confounding factors, which may predispose to a
given phenotype, in order to estimate the residual phenotype that
is likely due to genetics. As a small sample size can provide
imprecise or incorrect estimates of the magnitude of an observed
effect, sufficient sample size is necessary to accurately
distinguish a suggested effect from a lack of effect (82). As several initial studies have been
reported in populations of European descent, the challenge remains
to extend investigations to include other ethnic populations
(82).
Well-planned investigations are required, providing
sufficient statistical power and stringency to detect and quantify
a modest impact of the investigated SNPs. Follow-up epidemiological
association investigations are important to validate previous
findings in multiple independent large and homogenous samples. The
National Cancer Institute-National Human Genome Research Institute
working group on replication in association studies has published a
comprehensive set of guidelines, providing a number of essential
criteria for establishing positive replication studies (83).
Investigation of biological
mechanisms
In addition, further functional investigations are
required to clarify the underlying mechanism. Several miRNAs are
found in CpG islands, and miRNA expression can also be affected by
DNA methylation and histone deacetylase inhibitors, providing
another example of the bivalent roles of how miRNAs in malignancy
(84). For example, two
well-defined tumor suppressors, miR-124 and miR-34, are subject to
epigenetic silencing by aberrant DNA hypermethylation, affecting
cell cycle pathways in tumors, whereas the downregulation of miR-34
affects the Notch pathway, which is involved in cell invasion and
apoptosis (85–87). Furthermore, DNA methylation
profiles in miRNA promoter regions can be useful as a diagnostic
and prognostic marker. For example, miR-23b, an miRNA with tumor
suppressor activity in prostate cancer, is downregulated through
DNA hypermethylation of its promoter region, and its expression
level is correlated with OS and RFS (88). In addition to SNPs, structural
variations, including insertions, deletions, inversions and copy
number variants, with important implications on tumor biology
(79,80). Evaluating the link among genetic
variants, epigenetic modifications and disease predispositions is
currently an active area of investigation (79,80).
Of note, there are several ways in which the
processes of miRNA production, stability and maturation can be
orchestrated (89–91). A semi-miRNA of12 nucleotides in
length, which correspond to the 5′ region of the miRNA, let-7, is
generated along the miRNA pathway, and may be involved in the
control of gene expression by regulating the activity of mature
miRNAs in vivo (92). Novel
mechanisms for miRNA biogenesis have been described, and may be
important as cancer drivers (93).
Winter et al provided the first evidence that a small number
of miRNAs are generated from single-stranded loop regions of human
pre-miRNA hairpins, termed loop-miRs (94). In addition, an alternative miRNA
processing pathway has been found in Drosophila melanogaster
and Caenorhabditis elegans, which bypasses DROSHA and
uses a splicing technique to generate miRNA precursors from short
intronic sequences (95,96). The genetic polymorphisms and the
functional implications of these novel pathways require further
investigation.
6. Conclusion
The present review focused on the predictive role
of genetic variations in miRNA regulatory networks on inherited
cancer risk and progression. Although the biological mechanisms
underlying their effects on miRNA maturation and cancer development
remain to be fully elucidated, our knowledge of the myriad of
pathways in malignancy has improved, and further investigations of
miRNA polymorphisms hold promise in advancing knowledge in the
field of pharmacogenomics, molecular epidemiology and personalized
medicine.
Acknowledgments
This review was supported by the National Natural
Science Foundation of China (grant nos. 81273595, 81522048 and
81573511) and the National High Technology Research and Development
Program (grant nos. 2012AA02A518 and 2012AA02A517).
References
|
1
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee RC and Ambros V: An extensive class of
small RNAs in Caenorhabditis elegans. Science. 294:862–864. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Selbach M, Schwanhäusser B, Thierfelder N,
Fang Z, Khanin R and Rajewsky N: Widespread changes in protein
synthesis induced by microRNAs. Nature. 455:58–63. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baek D, Villén J, Shin C, Camargo FD, Gygi
SP and Bartel DP: The impact of microRNAs on protein output.
Nature. 455:64–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambros V: MicroRNA pathways in flies and
worms: Growth, death, fat, stress and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J,
Lee J, Provost P, Rådmark O, Kim S and Kim VN: The nuclear RNase
III Drosha initiates microRNA processing. Nature. 425:415–419.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cullen BR: Transcription and processing of
human microRNA precursors. Mol Cell. 16:861–865. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lund E, Güttinger S, Calado A, Dahlberg JE
and Kutay U: Nuclear export of microRNA precursors. Science.
303:95–98. 2004. View Article : Google Scholar
|
|
11
|
Mishra PJ and Bertino JR: MicroRNA
polymorphisms: The future of pharmacogenomics, molecular
epidemiology and individualized medicine. Pharmacogenomics.
10:399–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Medina PP, Nolde M and Slack FJ: OncomiR
addiction in an in vivo model of microRNA-21-induced pre-B-cell
lymphoma. Nature. 467:86–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mishra PJ, Mishra PJ, Banerjee D and
Bertino JR: MiRSNPs or MiR-polymorphisms, new players in microRNA
mediated regulation of the cell: Introducing microRNA
pharmacogenomics. Cell Cycle. 7:853–858. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ryan BM, Robles AI and Harris CC: Genetic
variation in microRNA networks: The implications for cancer
research. Nat Rev Cancer. 10:389–402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salzman DW and Weidhaas JB: SNPing cancer
in the bud: MicroRNA and microRNA-target site polymorphisms as
diagnostic and prognostic biomarkers in cancer. Pharmacol Ther.
137:55–63. 2013. View Article : Google Scholar :
|
|
17
|
Noh H, Hong S, Dong Z, Pan ZK, Jing Q and
Huang S: Impaired MicroRNA processing facilitates breast cancer
cell invasion by upregulating Urokinase-Type plasminogen activator
expression. Genes Cancer. 2:140–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sung H, Lee KM, Choi JY, Han S, Lee JY, Li
L, Park SK, Yoo KY, Noh DY, Ahn SH and Kang D: Common genetic
polymorphisms of microRNA biogenesis pathway genes and risk of
breast cancer: A case-control study in Korea. Breast Cancer Res
Treat. 130:939–951. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Y, Chen J, Wu J, Hu Z, Qin Z, Liu X,
Guan X, Wang Y, Han J, Jiang T, et al: Evaluation of genetic
variants in microRNA biosynthesis genes and risk of breast cancer
in Chinese women. Int J Cancer. 133:2216–2224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sung H, Jeon S, Lee KM, Han S, Song M,
Choi JY, Park SK, Yoo KY, Noh DY, Ahn SH and Kang D: Common genetic
polymorphisms of microRNA biogenesis pathway genes and breast
cancer survival. Bmc Cancer. 12:1952012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sung H, Zhang B, Choi JY, Long J, Park SK,
Yoo KY, Noh DY, Ahn SH, Zheng W and Kang D: Common genetic variants
in the microRNA biogenesis pathway are not associated with breast
cancer risk in Asian women. Cancer Epidemiol Biomarkers Prev.
21:1385–1387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin J, Horikawa Y, Tamboli P, Clague J,
Wood CG and Wu X: Genetic variations in microRNA-related genes are
associated with survival and recurrence in patients with renal cell
carcinoma. Carcinogenesis. 31:1805–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weng Y, Chen Y, Chen J, Liu Y and Bao T:
Common genetic variants in the microRNA biogenesis pathway are
associated with malignant peripheral nerve sheath tumor risk in a
Chinese population. Cancer Epidemiol. 37:913–916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ke HL, Chen M, Ye Y, Hildebrandt MA, Wu
WJ, Wei H, Huang M, Chang DW, Dinney CP and Wu X: Genetic
variations in micro-RNA biogenesis genes and clinical outcomes in
non-muscle-invasive bladder cancer. Carcinogenesis. 34:1006–1011.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yi R, Doehle BP, Qin Y, Macara IG and
Cullen BR: Overexpression of exportin 5 enhances RNA interference
mediated by short hairpin RNAs and microRNAs. Rna. 11:220–226.
2005. View Article : Google Scholar
|
|
26
|
Zeng Y and Cullen BR: Structural
requirements for pre-microRNA binding and nuclear export by
Exportin 5. Nucleic Acids Res. 32:4776–4785. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding C, Li C, Wang H, Li B and Guo Z: A
miR-SNP of the XPO5 gene is associated with advanced non-small-cell
lung cancer. Onco Targets Ther. 6:877–881. 2013.PubMed/NCBI
|
|
28
|
Guo Z, Wang H, Li Y, Li B, Li C and Ding
C: A microRNA-related single nucleotide polymorphism of the XPO5
gene is associated with survival of small cell lung cancer
patients. Biomed Rep. 1:545–548. 2013.
|
|
29
|
Liu S, An J, Lin J, Liu Y, Bao L, Zhang W
and Zhao JJ: Single nucleotide polymorphisms of microRNA processing
machinery genes and outcome of hepatocellular carcinoma. PLoS One.
9:e927912014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leaderer D, Hoffman AE, Zheng T, Fu A,
Weidhaas J, Paranjape T and Zhu Y: Genetic and epigenetic
association studies suggest a role of microRNA biogenesis gene
exportin-5 (XPO5) in breast tumorigenesis. Int J Mol Epidemiol
Genet. 2:9–18. 2011.PubMed/NCBI
|
|
31
|
Ma H, Yuan H, Yuan Z, Yu C, Wang R, Jiang
Y, Hu Z, Shen H and Chen N: Genetic variations in key microRNA
processing genes and risk of head and neck cancer: A case-control
study in Chinese population. PLoS One. 7:e475442012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen J, Qin Z, Pan S, Jiang J, Liu L, Liu
J, Chen X, Hu Z and Shen H: Genetic variants in RAN, DICER and HIWI
of microRNA biogenesis genes and risk of cervical carcinoma in a
Chinese population. Chin J Cancer Res. 25:565–571. 2013.PubMed/NCBI
|
|
33
|
Liu L, An J, Liu J, Wen J, Zhai X, Liu Y,
Pan S, Jiang J, Wen Y, Liu Z, et al: Potentially functional genetic
variants in microRNA processing genes and risk of HBV-related
hepatocellular carcinoma. Mol Carcinog. 52(Suppl 1): E148–E154.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Liu J, Wei M, He Y, Liao B, Liao G,
Li H and Huang J: Genetic variants in the microRNA machinery gene
GEMIN4 are associated with risk of prostate cancer: A case-control
study of the Chinese Han population. Dna Cell Biol. 31:1296–1302.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang D, Meyer L, Chang DW, Lin J, Pu X,
Ye Y, Gu J, Wu X and Lu K: Genetic variants in MicroRNA
biosynthesis pathways and binding sites modify ovarian cancer risk,
survival and treatment response. Cancer Res. 70:9765–9776. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Horikawa Y, Wood CG, Yang H, Zhao H, Ye Y,
Gu J, Lin J, Habuchi T and Wu X: Single nucleotide polymorphisms of
microRNA machinery genes modify the risk of renal cell carcinoma.
Clin Cancer Res. 14:7956–7962. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wan D, He M, Wang J, Qiu X, Zhou W, Luo Z,
Chen J and Gu J: Two variants of the human hepatocellular
carcinoma-associated HCAP1 gene and their effect on the growth of
the human liver cancer cell line Hep3B. Genes Chromosomes Cancer.
39:48–58. 2004. View Article : Google Scholar
|
|
38
|
Adams BD, Claffey KP and White BA:
Argonaute-2 expression is regulated by epidermal growth factor
receptor and mitogen-activated protein kinase signaling and
correlates with a transformed phenotype in breast cancer cells.
Endocrinology. 150:14–23. 2009. View Article : Google Scholar :
|
|
39
|
Kim MS, Oh JE, Kim YR, Park SW, Kang MR,
Kim SS, Ahn CH, Yoo NJ and Lee SH: Somatic mutations and losses of
expression of microRNA regulation-related genes AGO2 and TNRC6A in
gastric and colorectal cancers. J Pathol. 221:139–146. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou Y, Chen L, Barlogie B, Stephens O, Wu
X, Williams DR, Cartron MA, van Rhee F, Nair B, Waheed S, et al:
High-risk myeloma is associated with global elevation of miRNAs and
overexpression of EIF2C2/AGO2. Proc Natl Acad Sci USA.
107:7904–7909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Q, Jie Z, Ye S, Li Z, Han Z, Wu J,
Yang C and Jiang Y: Genetic variations in miR-27a gene decrease
mature miR-27a level and reduce gastric cancer susceptibility.
Oncogene. 33:193–202. 2014. View Article : Google Scholar
|
|
42
|
Zhang N, Huo Q, Wang X, Chen X, Long L,
Jiang L, Ma T and Yang Q: A genetic variant in pre-miR-27a is
associated with a reduced breast cancer risk in younger Chinese
population. Gene. 529:125–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang R, Schlehe B, Hemminki K, Sutter C,
Bugert P, Wappenschmidt B, Volkmann J, Varon R, Weber BH,
Niederacher D, et al: A genetic variant in the pre-miR-27a oncogene
is associated with a reduced familial breast cancer risk. Breast
Cancer Res Treat. 121:693–702. 2010. View Article : Google Scholar
|
|
44
|
Shi D, Li P, Ma L, Zhong D, Chu H, Yan F,
Lv Q, Qin C, Wang W, Wang M, et al: A genetic variant in
pre-miR-27a is associated with a reduced renal cell cancer risk in
a Chinese population. PLoS One. 7:e465662012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu J, Yin Z, Shen H, Gao W, Qian Y, Pei D,
Liu L and Shu Y: A genetic polymorphism in pre-miR-27a confers
clinical outcome of non-small cell lung cancer in a Chinese
population. PLoS One. 8:e791352013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hezova R, Kovarikova A, Bienertova-Vasku
J, Sachlova M, Redova M, Vasku A, Svoboda M, Radova L, Kiss I,
Vyzula R and Slaby O: Evaluation of SNPs in miR-196-a2, miR-27a and
miR-146a as risk factors of colorectal cancer. World J
Gastroenterol. 18:2827–2831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu Q, He CY, Liu JW and Yuan Y:
Pre-miR-27a rs895819A/G polymorphisms in cancer: A meta-analysis.
PLoS One. 8:e652082013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Z, Lai J, Wang Y, Nie W and Guan X:
The Hsa-miR-27a rs895819 (A>G) polymorphism and cancer
susceptibility. Gene. 521:87–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhong S, Chen Z, Xu J, Li W and Zhao J:
Pre-mir-27a rs895819 polymorphism and cancer risk: A meta-analysis.
Mol Biol Rep. 40:3181–3186. 2013. View Article : Google Scholar
|
|
50
|
Xu W, Xu J, Liu S, Chen B, Wang X, Li Y,
Qian Y, Zhao W and Wu J: Effects of common polymorphisms rs11614913
in miR-196a2 and rs2910164 in miR-146a on cancer susceptibility: A
meta-analysis. PLoS One. 6:e204712011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang J, Wang Q, Liu H, Shao N, Tan B,
Zhang G, Wang K, Jia Y, Ma W, Wang N and Cheng Y: The association
of miR-146a rs2910164 and miR-196a2 rs11614913 polymorphisms with
cancer risk: A meta-analysis of 32 studies. Mutagenesis.
27:779–788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Srivastava K and Srivastava A:
Comprehensive review of genetic association studies and
meta-analyses on miRNA polymorphisms and cancer risk. PLoS One.
7:e509662012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang PY, Gao ZH, Jiang ZH, Li XX, Jiang BF
and Xie SY: The associations of single nucleotide polymorphisms in
miR-146a, miR-196a and miR-499 with breast cancer susceptibility.
PLoS One. 8:e706562013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen Z, Xu L, Ye X, Shen S, Li Z, Niu X
and Lu S: Polymorphisms of microRNA sequences or binding sites and
lung cancer: A meta-analysis and systematic review. PLoS One.
8:e610082013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yuan Z, Zeng X, Yang D, Wang W and Liu Z:
Effects of common polymorphism rs11614913 in Hsa-miR-196a2 on lung
cancer risk. PLoS One. 8:e610472013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xu Y, Li L, Xiang X, Wang H, Cai W, Xie J,
Han Y, Bao S and Xie Q: Three common functional polymorphisms in
microRNA encoding genes in the susceptibility to hepatocellular
carcinoma: A systematic review and meta-analysis. Gene.
527:584–593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wan D, Gu W, Xu G, Shen C, Ding D, Shen S,
Wang S, Gong X, He S and Zhi Q: Effects of common polymorphisms
rs2910164 in miR-146a and rs11614913 in miR-196a2 on susceptibility
to colorectal cancer: A systematic review meta-analysis. Clin
Transl Oncol. 16:792–800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guo J, Jin M, Zhang M and Chen K: A
genetic variant in miR-196a2 increase digestive system cancer
risks: A meta-analysis of 15 case-control studies. PLoS One.
7:e305852012. View Article : Google Scholar
|
|
59
|
Yoon KA, Yoon H, Park S, Jang HJ, Zo JI,
Lee HS and Lee JS: The prognostic impact of microRNA sequence
polymorphisms on the recurrence of patients with completely
resected non-small cell lung cancer. J Thorac Cardiovasc Surg.
144:794–807. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hu Z, Chen J, Tian T, Zhou X, Gu H, Xu L,
Zeng Y, Miao R, Jin G, Ma H, et al: Genetic variants of miRNA
sequences and non-small cell lung cancer survival. J Clin Invest.
118:2600–2608. 2008.PubMed/NCBI
|
|
61
|
Li L, Chen XP and Li YJ: MicroRNA-146a and
human disease. Scand J Immunol. 71:227–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bhaumik D, Scott GK, Schokrpur S, Patil
CK, Campisi J and Benz CC: Expression of microRNA-146 suppresses
NF-kappaB activity with reduction of metastatic potential in breast
cancer cells. Oncogene. 27:5643–5647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lin SL, Chiang A, Chang D and Ying SY:
Loss of mir-146a function in hormone-refractory prostate cancer.
RNA. 14:417–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jazdzewski K, Murray EL, Franssila K,
Jarzab B, Schoenberg DR and de la Chapelle A: Common SNP in
pre-miR-146a decreases mature miR expression and predisposes to
papillary thyroid carcinoma. Proc Natl Acad Sci USA. 105:7269–7274.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jiang H, He X, Li J, Xie Q, Lin J and
Chang Y: Association of a single-nucleotide polymorphism within the
miR-146a gene with susceptibility for acute-on-chronic hepatitis B
liver failure. Immunogenetics. 65:257–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhou X, Chen X, Hu L, Han S, Qiang F, Wu
Y, Pan L, Shen H, Li Y and Hu Z: Polymorphisms involved in the
miR-218-LAMB3 pathway and susceptibility of cervical cancer, a
case-control study in Chinese women. Gynecol Oncol. 117:287–290.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kertesz M, Iovino N, Unnerstall U, Gaul U
and Segal E: The role of site accessibility in microRNA target
recognition. Nat Genet. 39:1278–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu Z, Li G, Wei S, Niu J, El-Naggar AK,
Sturgis EM and Wei Q: Genetic variants in selected pre-microRNA
genes and the risk of squamous cell carcinoma of the head and neck.
Cancer. 116:4753–4760. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen QH, Wang QB and Zhang B: Ethnicity
modifies the association between functional microRNA polymorphisms
and breast cancer risk: A HuGE meta-analysis. Tumour Biol.
35:529–543. 2014. View Article : Google Scholar
|
|
70
|
Li Y, Liu J, Yuan C, Cui B, Zou X and Qiao
Y: High-risk human papillomavirus reduces the expression of
microRNA-218 in women with cervical intraepithelial neoplasia. J
Int Med Res. 38:1730–1736. 2010. View Article : Google Scholar
|
|
71
|
Shi TY, Chen XJ, Zhu ML, Wang MY, He J, Yu
KD, Shao ZM, Sun MH, Zhou XY, Cheng X, et al: A pri-miR-218 variant
and risk of cervical carcinoma in Chinese women. Bmc Cancer.
13:192013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang LS, Liang WB, Gao LB, Li HY, Li LJ,
Chen PY, Liu Y, Chen TY, Han JG, Wei YG, et al: Association between
pri-miR-218 polymorphism and risk of hepatocellular carcinoma in a
Han Chinese population. Dna Cell Biol. 31:761–765. 2012. View Article : Google Scholar
|
|
73
|
Cipollini M, Landi S and Gemignani F:
MicroRNA binding site polymorphisms as biomarkers in cancer
management and research. Pharmgenomics Pers Med. 7:173–191.
2014.PubMed/NCBI
|
|
74
|
Georges M, Clop A, Marcq F, Takeda H,
Pirottin D, Hiard S, Tordoir X, Caiment F, Meish F, Bibé B, et al:
Polymorphic microRNA-target interactions: A novel source of
phenotypic variation. Cold Spring Harb Symp Quant Biol. 71:343–350.
2006. View Article : Google Scholar
|
|
75
|
Pu X, Roth JA, Hildebrandt MA, Ye Y, Wei
H, Minna JD, Lippman SM and Wu X: MicroRNA-related genetic variants
associated with clinical outcomes in early-stage non-small cell
lung cancer patients. Cancer Res. 73:1867–1875. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
McEvoy J, Ulyanov A, Brennan R, Wu G,
Pounds S, Zhang J and Dyer MA: Analysis of MDM2 and MDM4 single
nucleotide polymorphisms, mRNA splicing and protein expression in
retino-blastoma. PLoS One. 7:e427392012. View Article : Google Scholar
|
|
77
|
Zhou L, Zhang X, Li Z, Zhou C, Li M, Tang
X, Lu C, Li H, Yuan Q and Yang M: Association of a genetic
variation in a miR-191 binding site in MDM4 with risk of esophageal
squamous cell carcinoma. PLoS One. 8:e643312013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wynendaele J, Böhnke A, Leucci E, Nielsen
SJ, Lambertz I, Hammer S, Sbrzesny N, Kubitza D, Wolf A, Gradhand
E, et al: An illegitimate microRNA target site within the 3′ UTR of
MDM4 affects ovarian cancer progression and chemosensitivity.
Cancer Res. 70:9641–9649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang C, Zhao H, Zhao X, Wan J, Wang D, Bi
W, Jiang X and Gao Y: Association between an insertion/deletion
polymorphism within 3′UTR of SGSM3 and risk of hepatocellular
carcinoma. Tumour Biol. 35:295–301. 2014. View Article : Google Scholar
|
|
80
|
Zhu Z, Jiang Y, Chen S, Jia S, Gao X, Dong
D and Gao Y: An insertion/deletion polymorphism in the 3′
untranslated region of type I collagen a2 (COL1A2) is associated
with susceptibility for hepatocellular carcinoma in a Chinese
population. Cancer Genet. 204:265–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jin Y, Xu G, Huang J, Zhou D, Huang X and
Shen L: Analysis of the association between an insertion/deletion
polymorphism within the 3′ untranslated region of COL1A2 and
chronic venous insufficiency. Ann Vasc Surg. 27:959–963. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ioannidis JP, Ntzani EE, Trikalinos TA and
Contopoulos-Ioannidis DG: Replication validity of genetic
association studies. Nat Genet. 29:306–309. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chanock SJ, Manolio T, Boehnke M,
Boerwinkle E, Hunter DJ, Thomas G, Hirschhorn JN, Abecasis G,
Altshuler D; NCI-NHGRI Working Group on Replication in Association
Studies; et al: Replicating genotype-phenotype associations.
Nature. 447:655–660. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Fabbri M, Ivan M, Cimmino A, Negrini M and
Calin GA: Regulatory mechanisms of microRNAs involvement in cancer.
Expert Opin Biol Ther. 7:1009–1019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Silber J, Lim DA, Petritsch C, Persson AI,
Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello
JF, et al: MiR-124 and miR-137 inhibit proliferation of
glioblastoma multiforme cells and induce differentiation of brain
tumor stem cells. BMC Med. 6:142008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Agirre X, Vilas-Zornoza A, Jiménez-Velasco
A, Martin-Subero JI, Cordeu L, Gárate L, San José-Eneriz E,
Abizanda G, Rodríguez-Otero P, Fortes P, et al: Epigenetic
silencing of the tumor suppressor microRNA Hsa-miR-124a regulates
CDK6 expression and confers a poor prognosis in acute lymphoblastic
leukemia. Cancer Res. 69:4443–4453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Pang RT, Leung CO, Ye TM, Liu W, Chiu PC,
Lam KK, Lee KF and Yeung WS: MicroRNA-34a suppresses invasion
through downregulation of Notch1 and Jagged1 in cervical carcinoma
and choriocarcinoma cells. Carcinogenesis. 31:1037–1044. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Majid S, Dar AA, Saini S, Arora S,
Shahryari V, Zaman MS, Chang I, Yamamura S, Tanaka Y, Deng G and
Dahiya R: MiR-23b represses proto-oncogene Src kinase and functions
as methylation-silenced tumor suppressor with diagnostic and
prognostic significance in prostate cancer. Cancer Res.
72:6435–6446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zisoulis DG, Kai ZS, Chang RK and
Pasquinelli AE: Autoregulation of microRNA biogenesis by let-7 and
Argonaute. Nature. 486:541–544. 2012.PubMed/NCBI
|
|
90
|
Juvvuna PK, Khandelia P, Lee LM and
Makeyev EV: Argonaute identity defines the length of mature
mammalian microRNAs. Nucleic Acids Res. 40:6808–6820. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yang JS, Maurin T and Lai EC: Functional
parameters of Dicer-independent microRNA biogenesis. RNA.
18:945–957. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Plante I, Plé H, Landry P, Gunaratne PH
and Provost P: Modulation of microRNA activity by Semi-microRNAs.
Front Genet. 3:992012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Faller M and Guo F: MicroRNA biogenesis:
There's more than one way to skin a cat. Biochim Biophys Acta.
1779:663–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Winter J, Link S, Witzigmann D,
Hildenbrand C, Previti C and Diederichs S: Loop-miRs: Active
microRNAs generated from single-stranded loop regions. Nucleic
Acids Res. 41:5503–5512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Berezikov E, Liu N, Flynt AS, Hodges E,
Rooks M, Hannon GJ and Lai EC: Evolutionary flux of canonical
microRNAs and mirtrons in Drosophila. Nat Genet. 42:6–9. 2010.
View Article : Google Scholar
|
|
96
|
Ruby JG, Jan CH and Bartel DP: Intronic
microRNA precursors that bypass Drosha processing. Nature.
448:83–86. 2007. View Article : Google Scholar : PubMed/NCBI
|















