Introduction
Uveal melanoma (UM) is the most common type of
primary malignant intraocular tumor in adults. Hematogenous
dissemination is the predominant avenue for UM metastasis due to
the lack of lymphatic vessels in the eyes (1). UM is highly malignant and the primary
site of UM metastasis is the liver. Approximately 50% of patients
with UM develop metastasis within 15 years after diagnosis of the
primary tumor and the mortality rate is 40–50% (2,3).
Therefore, it is necessary to discover prognostic markers to
identify UM cells with high invasive and metastatic potential and
to develop therapies to prevent the generation of metastasis from
these cells.
Legumain is a cysteine endopeptidase of the
asparaginyl endopeptidase family (4), which has been identified in parasites
(5) and mammals (6). Overexpression of legumain has been
reported in several types of human solid tumor, including colon and
breast cancers (7–9). Legumain has a highly restricted
specificity requiring an asparagine at the P1 site of its
substrates (10). It has also been
demonstrated to activate pro-gelatinase A, which is required for
extracellular matrix degradation (7,11).
Cells overexpressing legumain possess an increased migratory
activity in vitro and exhibit invasive and metastatic
phenotypes in vivo. Therefore, legumain has been suggested
to be involved in tumor invasion and metastasis by degrading
extracellular matrix proteins (7).
Due to the enrichment of legumain in tumors and its unique
restricted specificity, it has been considered to be a potential
target for inhibiting tumor metastasis (12–14)
and for pro-drug therapy (7,15–17).
However, to the best of our knowledge, the
expression of legumain and its impact on the prognosis of patients
with UM have not yet been investigated. The purpose of the present
study was to determine the expression of legumain in UM cell lines
and primary UM specimens, and to determine the association between
legumain expression and clinical and pathological characteristics
to ultimately reveal its impact on the prognosis of patients with
UM.
Materials and methods
Patients and samples
The records of primary UM cases treated at Beijing
Tongren Hospital (Beijing, China) and Tianjin Eye Hospital
(Tianjin, China) between 1996 and 2005 were retrieved for analysis.
The inclusion criteria for the present study were as follows:
Histological confirmation of primary UM, no previous history of UM,
radiotherapy, thermotherapy or chemotherapy, and the availability
of data from a five-year follow-up. A total of 82 cases, comprising
45 males (age, 48–72 years) and 37 females (age, 46–70 years) were
enrolled in the present study and all patients had provided written
informed consent to their tumor tissues being used for scientific
studies. Formalin-fixed and paraffin-embedded surgical specimens of
these 82 patients were obtained. The study protocols were approved
by the Medical Ethics Committee of Tianjin Eye Hospital (Tianjin,
China) and Beijing Tongren Hospital (Beijing, China).
Cell lines and culture
The highly invasive UM cell line MUM-2B and the
poorly invasive UM cell line MUM-2C at passage 6 were established
by Seftor et al (18) and
kindly provided by Dr Rong Xiang (College of Medicine, University
of Nankai, Tianjin, China). Cells were incubated in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in a humidified atmosphere containing 5%
CO2.
Reverse-transcription quantitative
polymerase chin reaction (RT-qPCR)
Total RNA was isolated from UM cell lines using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
cDNA was synthesized using the Superscript III First Strand
Synthesis system (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. PCR was performed
using IU Taq DNA Polymerase (Sigma-Aldrich, St. Louis, MO, USA) and
MgCl2 at a final concentration of 1.5 mM in a total
volume of 25 µl. qPCR was performed using Fast SYBR Green
Master mix, according to the manufacturer's instructions (Applied
Biosystems Inc., Foster City, CA, USA) and GAPDH was used as the
control gene. The following primers were used: Legumain, forward:
5′-GATGAACCACCTGCCGGATAA-3′ and reverse:
5′-CATCATAGTAACAGGCGTAGGACGA-3′; GAPDH, forward:
5′-GAACGGGAAGCTCACTGG-3′ and reverse: 5′-TCCACCACCCTGTTGCTGTA-3′.
The PCR primers were synthesized by Shanghai Invitrogen
Biotechnology Co., Ltd. (Shanghai, China). PCR cycles consisted of
initial denaturation at 95°C for 5 min followed by 35 amplification
cycles of denaturation at 94°C for 45 sec, annealing at 50°C for 45
sec and extension at 72°C for 45 sec, and final extension at 72°C
for 10 min. Each reaction was performed in triplicate. The
2−ΔΔCq method was used to calculate the relative
expression levels of the target gene (19). The expression level of Legumain was
log2-transformed and expressed as the legumain/GAPDH expression
ratio (2−ΔΔCT).
Western blot analysis
Whole-cell protein samples were prepared by
homogenization in RIPA lysis buffer (Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China) supplemented with a protease and phosphatase
inhibitor cocktail. The lysate samples were mixed thoroughly in
Eppendorf tubes with equal volumes of electrophoresis sample buffer
containing β-mercaptoethanol (Sigma-Aldrich), and boiled in a
thermomixer at 99°C for 5 min at 12,000 × g. The protein
concentration of the lysate was determined using a BCA protein
assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Equal
quantities of protein (40 µg/lane) were separated by 12% SDS
PAGE (Huaxingbio Science and Technology Co., Ltd., Beijing, China).
Denatured protein lysate was centrifuged at 12,000 × g and resolved
using gradient (4–20%) Tris-HCl gels (Bio-Rad Laboratories,
Hercules, CA, USA) in electrophoresis buffer (Takara Bio, Inc.,
Dalian, China) at 200 V and 350 mA for 1 h. The separated proteins
were immobilized onto polyvinylidene fluoride (PVDF) membranes at
100 V and 350 mA for 1 h according to the manufacturer's
instructions. The PVDF membranes were washed five times with
phosphate-buffered saline (PBS) containing Tween-20 (Sigma-Aldrich)
for 5 min, blocked with blocking buffer (5% non-fat dry milk) and
incubated for 2 h at room temperature with agitation. Subsequently,
the membranes were incubated with rabbit polyclonal anti-legumain
antibody (1:1,000 dilution; cat. no. HPA001426; Sigma-Aldrich) for
12 h at 4°C. Membranes were then incubated with
peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:5,000
dilution; cat. no. A0545; Sigma-Aldrich) in 3% non-fat dry milk for
2 h at room temperature with agitation. Between all incubation
steps, the membranes were washed five times with PBS containing
Tween-20 for 5 min with agitation. The immunoreactive bands were
visualized using ECL Western Blotting Reagents (RPN2106GE; GE
Healthcare, Little Chalfont, UK) and visu-alized on Medical X-ray
films (Kodak, Rochester, NY, USA). X-ray films were scanned in a
calibrated densitometer (GS-800; Bio-Rad Laboratories Inc.,
Hercules, CA, USA) and quantified with Image J software (version
1.49; National Institutes of Health, Bethesda, MD, USA). β-actin
(mouse monoclonal antibody; 1:1,000 dilution; cat. no. AA128;
Beyotime Institute of Biotechnology, Shanghai, China) was used as a
loading control, and its molecular weight ranged from 42 to 43
kDa.
Immunofluorescence staining
Melanoma cell lines (30,000 cells/chamber) were
grown on cover slips and fixed in 4% paraformaldehyde for 10 min at
room temperature, rinsed twice with PBS, permeabilized with 0.01%
Triton X-100 (Sigma-Aldrich) in PBS for 10 min and blocked in 3%
bovine serum albumin (Sigma-Aldrich)/PBS for 1 h. The cells were
incubated with 1:1,000 primary antibody (rabbit polyclonal
anti-legumain antibody; Sigma-Aldrich) overnight at 4°C, and
subsequent to washing, incubated with secondary antibody (Alexa
Fluor® 488 Goat Anti-Rabbit SFX kit; cat. no. A31627;
1:1,000 dilution; Invitrogen; Thermo Fisher Scientific, Inc.) at
room temperature for 2 h. Finally, the cells were mounted in
mounting medium containing 4′,6-diamidino-2-phenylindole
dihydrochloride (DAPI; Vectashield® Mounting Medium with
DAPI; Vector Laboratories, Inc., Burlingame, CA, USA). Fluorescence
images were captured and analyzed using a Nikon Eclipse 90i
microscope (Nikon Corp., Tokyo, Japan).
Immunohistochemistry
Immunohistochemical staining was performed on
4-µm formalin-fixed, paraffin-embedded sections. The
sections were incubated at 60°C for 12 h and de-paraffinized 2–3
times in xylene for 10 min each, followed by incubation in 100%
ethanol for 5 min, hydration in 95, 70, 50 and 30% ethanol for 5
min each, and de-pigmentation in kalium hypermanganicum for 30 min.
The sections were rinsed in ethanedioic acid and then in PBS three
times prior to transfer into citrate buffer. For antigen retrieval,
the sections were placed in a high-pressure cooker for 8 min and
then incubated at room temperature for 30 min. The sections were
then rinsed in PBS (pH 7.4) and incubated with 3%
H2O2 in methanol for 20 min to block
endogenous peroxidase activity. Non-specific binding of antibody
was prevented by pre-incubating the sections with goat serum for 60
min. After removal of the blocking solution, the sections were
incubated with rabbit polyclonal anti-legumain antibody (1:500
dilution; Sigma-Aldrich) in 4% goat serum at room temperature for 1
h. Subsequently, the sections were incubated with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin
(1:1,000 dilution; Sigma-Aldrich) for 60 min. The sections were
washed with PBS between each incubation step. Antibodies were
visualized using staining with a 3,3′-diaminobenzidine
tetrahydrochloride substrate kit (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) for 3 min. Next, the
sections were rinsed with water, followed by counterstaining with
hematoxylin and eosin (Beyotime Institute of Biotechnology).
Subsequently, the sections were de-hydrated in 80, 95 and 100%
ethanol for 5 min each, and three times in xylene for 3 min each
prior to being mounted.
Histological evaluation of legumain
staining in UM specimens
The expression levels of legumain in UM specimens
were quantified based on the extent and intensity of the staining.
The extent of legumain staining was quantified using the following
scale: 0, no staining; 1, staining observed in ≤40% of the viable
cells; 2, staining observed in >40% of the viable cells. The
intensity of legumain staining was scored as follows: 0, no
staining; 1, low-intensity staining; 2, high-intensity staining.
The specimens were analyzed by two pathologists blinded to the
study and the consistency of their scoring was 91%. Whenever
differences in the scoring were present, the average of the two
score was used as the result.
The total score was defined as the sum of the extent
and intensity of legumain staining, and the specimens were divided
into two groups based on their total scores: Negative or low
legumain expression was defined as a total score of 0–2, while high
legumain expression was defined as a total score of 3–4.
Evaluation of clinical and pathological
factors
Pathological analysis of the surgical specimens was
performed by two pathologists blinded to the study. The size of the
primary tumor was determined by measuring the largest basal
diameter (LBD) and the maximum tumor height (MTH). Tumors with
LBD<10.0 mm and MTH<2.5 mm were classified as small tumors,
while tumors with an LBD≥10.0 mm or MTH≥2.5 mm were classified as
large tumors. Specimens were examined for local tumor local
invasion. Negative local invasion was defined as the absence of
infiltration into the sclera or optic nerve; otherwise, positive
local invasion was considered to be present. Metastasis was defined
as positive whenever it occurred during a five-year post-operative
follow-up period.
Statistical analysis
Values are expressed as the mean ± the standard
deviation. The association of the legumain expression with clinical
and pathological features was tested using the χ2 and
Fisher's exact test. The association of legumain expression with
five-year survival was evaluated using the log-rank test and
displayed in Kaplan-Meier plots. A two-tailed P<0.05 was
considered to indicate a statistically significant differences. All
statistical analyses were performed using SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
Legumain expression is upregulated in
invasive UM cells
Overexpression of legumain has been observed in
several solid tumors and has been indicated to be correlated with
tumor invasion and metastasis (7).
To investigate whether legumain expression was differentially
expressed in invasive or non-invasive UM cells, the MUM-2B and
MUM-2C cell lines were subjected to immunofluorescence, western
blot and RT-qPCR analyses.
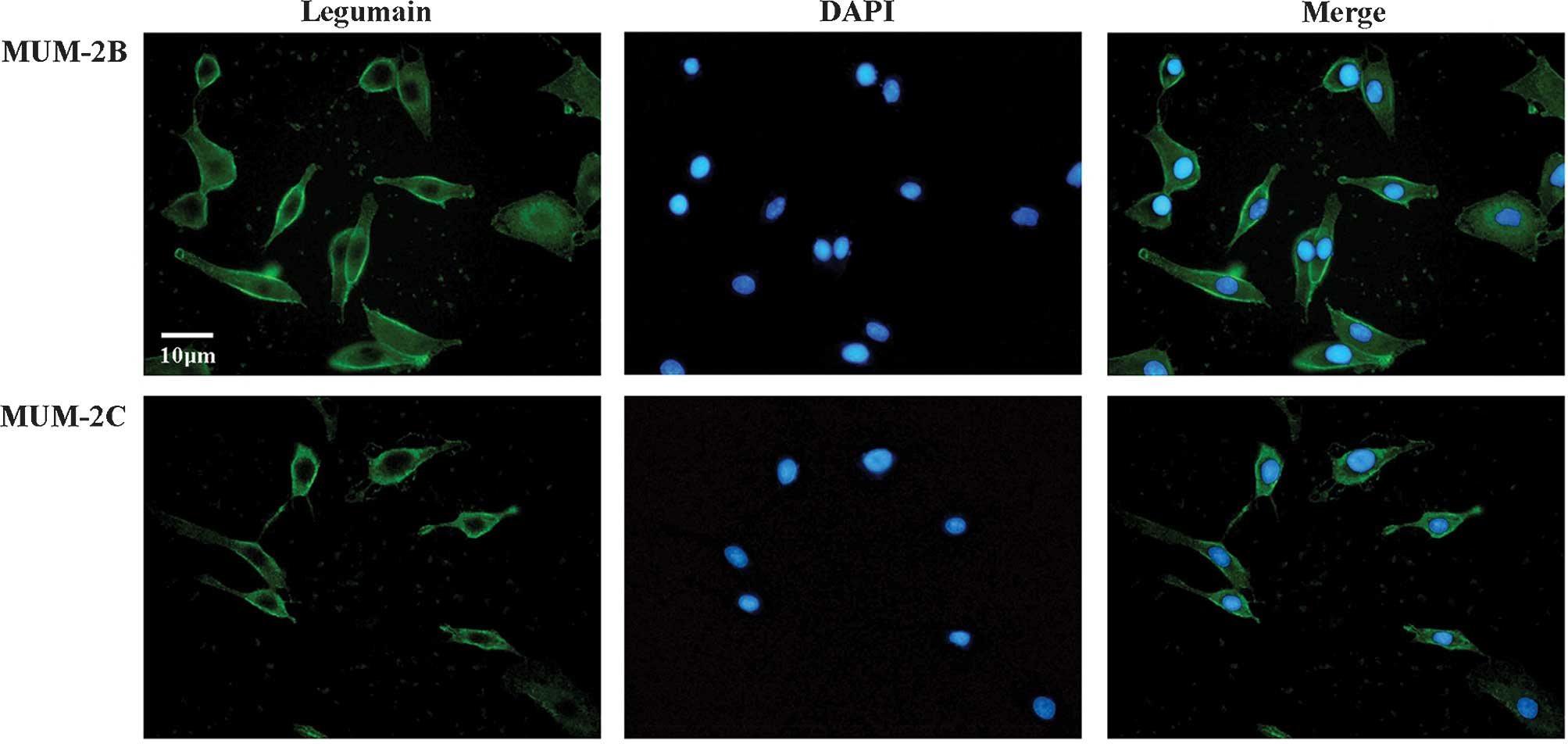
Immunofluorescence staining revealed that legumain
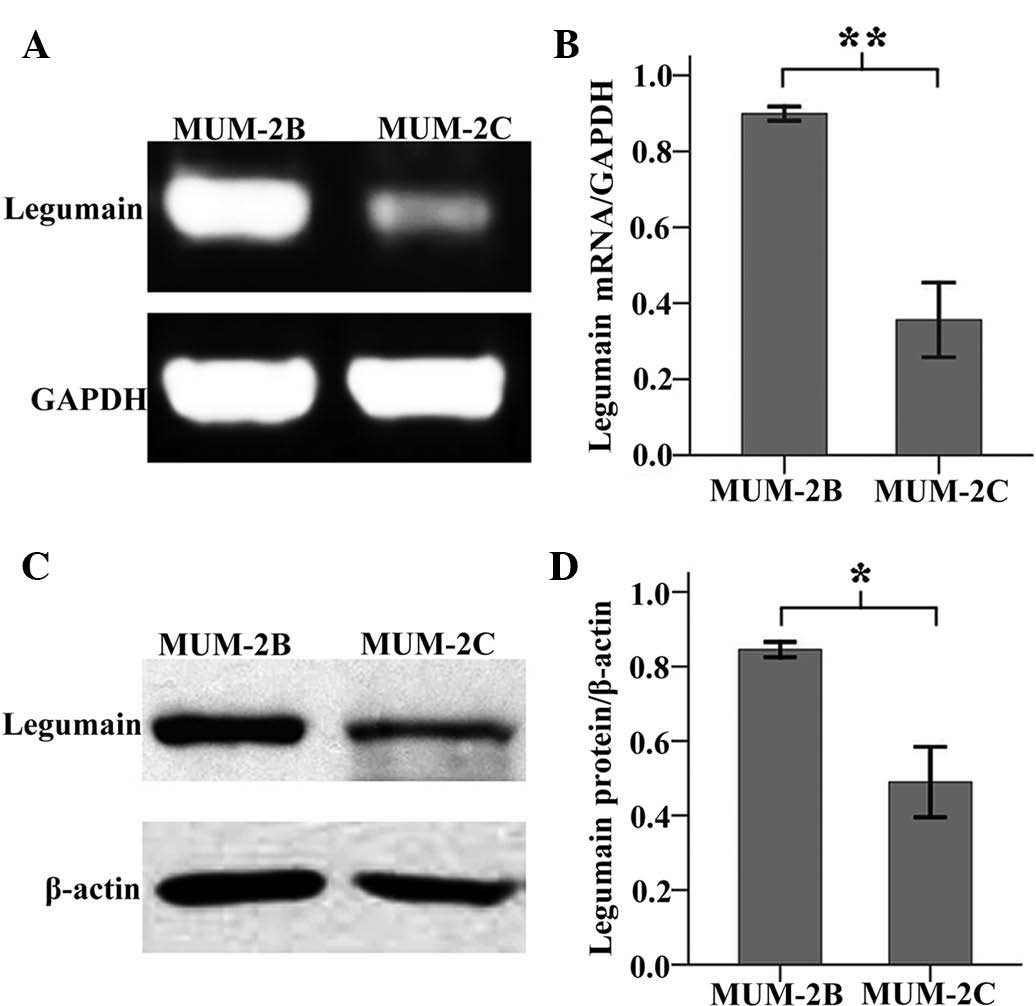
was expressed in MUM-2B and MUM-2C cells (Fig. 1). Furthermore, semi-quantitative
analysis showed that the expression of legumain mRNA and protein in
MUM-2B cells was elevated compared with that in MUM-2C cells
(Fig. 2). In Fig 2B, the mRNA levels in MUM-2B and
MUM-2C cells were 0.89±0.03 and 0.37±0.11, respectively. In
Fig. 2D, the expression of
legumain protein in MUM-2B and MUM-2C cells was 0.82±0.02 and
0.48±0.09, respectively. Since MUM-2B cells are highly invasive,
while MUM-2C display low invasiveness (18), the results suggested that legumain
may be a biological marker of invasive UM-cell behavior, and may
thus be a potential prognostic factor of UM.
Legumain is differentially expressed
among UM-cell types
To investigate the prognostic value of legumain in
UM, the expression of legumain was examined in UM specimens by
immunohistochemical analysis. Legumain was expressed in most of the
UM specimens; based on the total score of legumain staining, 35 of
the 82 specimens exhibited high expression, while the other 47
exhibited low expression. The results showed that the expression
levels of legumain were independent of age and gender in the high-
and low-expression groups, while it was dependent on the type of UM
cells.
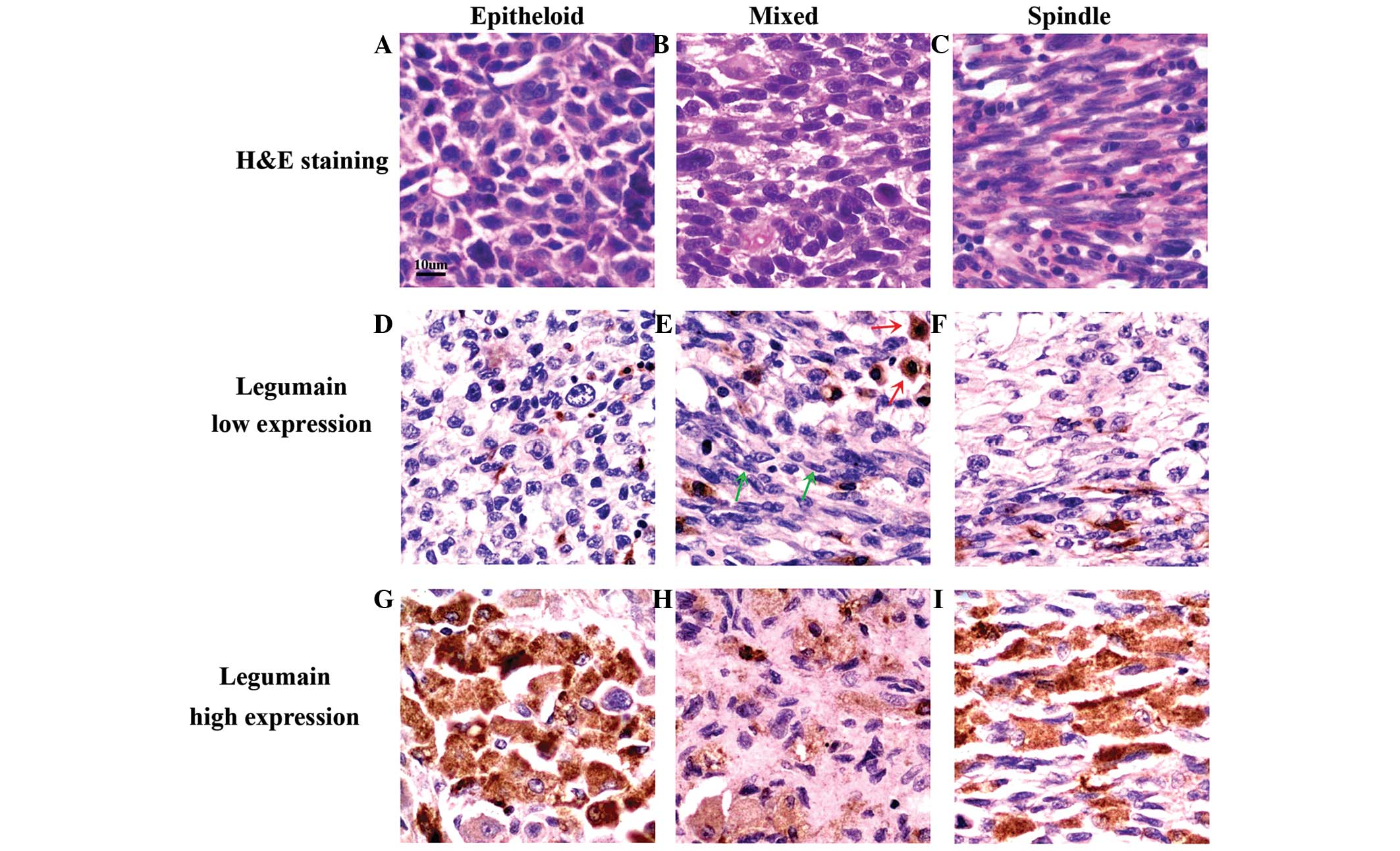
Within each of the three types of UM cells
(epithelioid, mixed or spindle-like) in the tissue specimens, the
expression of legumain was found to be either high or low (Fig. 3). However, high expression of
legumain was observed to be more common in epithelioid UM cells
than in spindle-like UM cells (Table
I). In mixed-type UM cells, legumain was preferentially
expressed in epithelioid cells (Fig.
3E; red arrows) rather than in spindle-like cells (Fig. 3E; green arrows). The observed
preferential expression of legumain in epithelioid cells suggested
that legumain may be associated with the enhanced malignant
behavior of epitheloid-cell UM (1).
 | Table IExpression of legumain in the three
uveal melanoma cell types. |
Table I
Expression of legumain in the three
uveal melanoma cell types.
| Cell type | Cases, n (%) | Legumain expression
| P-value |
|---|
| Low/negative, n
(%) | High, n (%) |
|---|
| Spindle | 30 (36) | 24 (29) | 6 (7) | 0.004 |
| Mixed | 25 (31) | 13 (16) | 12 (15) | |
| Epithelioid | 27 (33) | 10 (12) | 17 (21) | |
Association of legumain expression with
clinical and pathological characteristics
In analogy with other tumor types, the prognosis of
UM is affected by more than one factor. Negative
clinicopathological prognostic factors in UM include
epithelioid-cell morphology, large tumor size as well as the
presence of local invasion and metastasis (1). In order to elucidate the role of
legumain in the malignant behavior of UM and assess its prognostic
value, it is required to determine the association between the
expression of legumain and these clinicopathological factors.
Preliminary analysis showed legumain expression was
associated with large tumor size, local invasion and metastasis
(Table II). However, tumor size,
local invasion and metastasis were all associated with the UM-cell
type (data not shown). Therefore, legumain expression and
prognostic factors may be dependent on the UM-cell type.
 | Table IIAssociation of legumain expression
with histological and clinical factors. |
Table II
Association of legumain expression
with histological and clinical factors.
| Prognostic
factor | Cases, n (%) | Legumain expression
| P-value |
|---|
| Low/negative, n
(%) | High, n (%) |
|---|
| Tumor size | | | | 0.010 |
| Small | 44 (54) | 31 (38) | 13 (16) | |
| Large | 38 (46) | 16 (19) | 22 (27) | |
| Local invasion | | | | <0.001 |
| Negative | 28 (34) | 27 (33) | 1 (1) | |
| Positive | 54 (66) | 20 (25) | 34 (41) | |
| Metastasis | | | | 0.004 |
| Negative | 66 (80) | 43 (52) | 23 (28) | |
| Positive | 16 (20) | 4 (5) | 12 (15) | |
To further specify the association of legumain
expression with UM tumor behavior, further analysis was performed
on each UM-cell type. In contrast to the preliminary results,
legumain expression was found to be associated with local invasion
(Table III), but not with tumor
size or metastasis in every UM-cell type (Table IV). These results suggested that
legumain is primarily associated with local invasion of UM.
 | Table IIILegumain expression is associated
with local invasion in each type of uveal melanoma. |
Table III
Legumain expression is associated
with local invasion in each type of uveal melanoma.
| Cell type | Cases, n (%) | Legumain expression
| P-value |
|---|
| Low/negative, n
(%) | High, n (%) |
|---|
| Spindle | | | | 0.017 |
| Negative | 15 (50) | 15 (50) | 0 (0) | |
| Positive | 15 (50) | 9 (30) | 6 (20) | |
| Mixed | | | | 0.030 |
| Negative | 8 (32) | 7 (28) | 1 (4) | |
| Positive | 17 (68) | 6 (24) | 11 (44) | |
| Epithelioid | | | | 0.003 |
| Negative | 5 (18) | 5 (18) | 0 (0) | |
| Positive | 22 (81) | 5 (18) | 17 (81) | |
 | Table IVLegumain expression is not associated
with tumor size or metastasis in each type of uveal melanoma. |
Table IV
Legumain expression is not associated
with tumor size or metastasis in each type of uveal melanoma.
| Site/cell type | Size/presence | Cases, n (%) | Legumain expression
| P-value |
|---|
| Low/negative, n
(%) | High, n (%) |
|---|
| Tumor | | | | | |
| Spindle | Small | 23 (77) | 18 (60) | 5 (17) | 1.000 |
| Large | 7 (23) | 6 (20) | 1 (3) | |
| Mixed | Small | 15 (60) | 9 (36) | 6 (24) | 0.428 |
| Large | 10 (40) | 4 (16) | 6 (24) | |
| Epithelioid | Small | 6 (22) | 4 (15) | 2 (7) | 0.153 |
| Large | 21 (78) | 6 (22) | 15 (56) | |
| Metastasis | | | | | |
| Spindle | Negative | 28 (94) | 23 (77) | 5 (17) | 0.366 |
| Positive | 2 (6) | 1 (3) | 1 (3) | |
| Mixed | Negative | 19 (76) | 12 (48) | 7 (28) | 0.073 |
| Positive | 6 (24) | 1 (4) | 5 (20) | |
| Epithelioid | Negative | 19 (70) | 8 (30) | 11 (40) | 0.666 |
| Positive | 8 (30) | 2 (7) | 6 (22) | |
To confirm that legumain was primarily associated
with local invasion, but not with tumor size or metastasis, its
expression was compared between tumors with or without local
invasion. The results showed that legumain expression was
independent of the tumor size or the presence of metastasis, while
it was highly asssociated with local invasion (Table V). The association of legumain with
tumor size and metastasis shown in Table II is most likely due to its
association with local invasion.
 | Table VLegumain expression is not associated
with tumor size or metastasis. |
Table V
Legumain expression is not associated
with tumor size or metastasis.
| Site | Local invasion | Size/presence | Cases, n (%) | Legumain expression
| P-value |
|---|
| Low/negative, n
(%) | High, n (%) |
|---|
| Tumor | Negative | Small | 24 (86) | 24 (86) | 0 (0) | 0.143 |
| | Large | 4 (14) | 3 (11) | 1 (3) | |
| Positive | Small | 20 (37) | 7 (13) | 13 (24) | 0.812 |
| | Large | 34 (63) | 13 (24) | 21 (39) | |
| Metastasis | Negative | Negative | 27 (97) | 26 (94) | 1 (3) | 1.000 |
| | Positive | 1 (3) | 1 (3) | 0 (0) | |
| Positive | Negative | 39 (72) | 17 (31) | 22 (41) | 0.108 |
| | Positive | 15 (28) | 3 (6) | 12 (22) | |
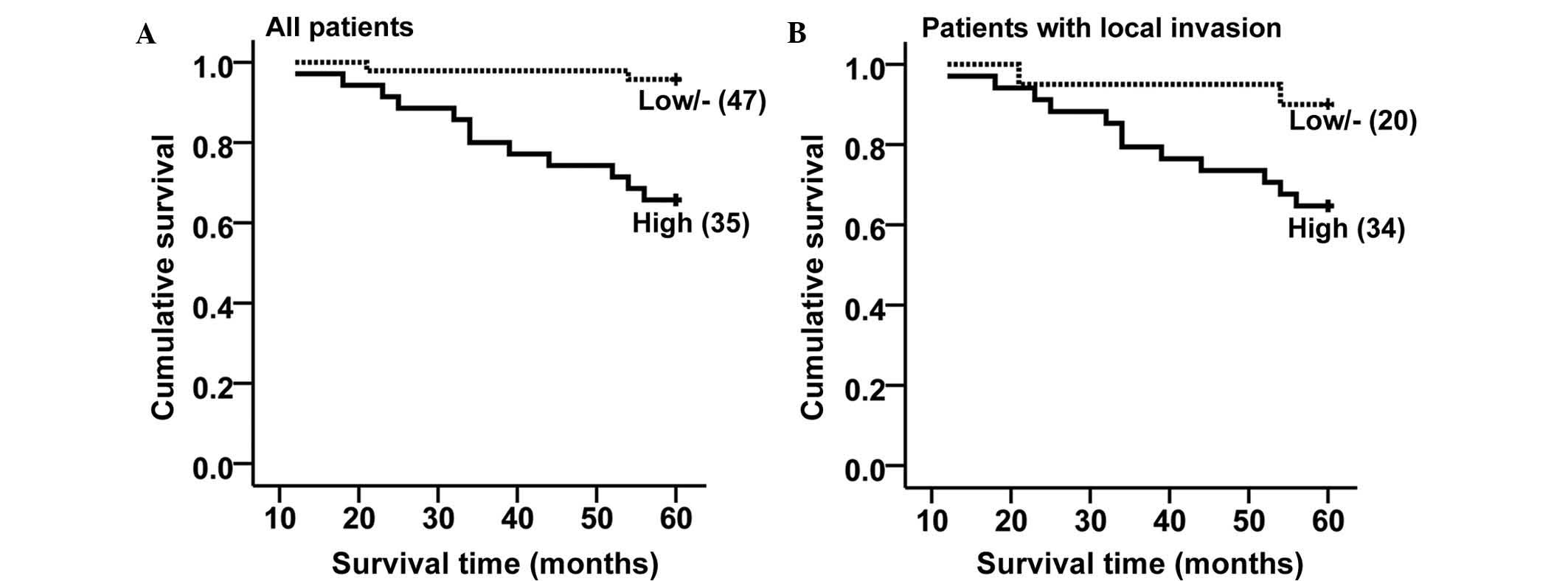
High expression of legumain is associated
with poor outcome of UM
To determine the prognostic value of legumain in UM,
the five-year survival rates were compared between patients with
high and those with low/negative legumain expression. The overall
survival analysis revealed that the patients with high legumain
expression exhibited poorer survival as compared to that of
patients with low/negative legumain expression (Fig. 4A; log-rank test, P<0.001). Since
legumain expression was shown to be associated with local invasion,
the negative impact of legumain expression on patient survival is
likely to be due to legumain-induced local invasion of UM.
To test whether legumain expression alone had any
impact on UM prognosis, further survival analysis was performed in
patients with or without local invasion. Among the 28 patients
without local invasion, there was only one case of high legumain
expression, and all patients had survived at five years following
surgery; therefore, it was not possible to perform any survival
analysis. Among the 54 patients with local invasion, patients with
high legumain expression exhibited poorer survival than patients
with low/negative legumain expression (Fig. 4B; log-rank test, P<0.046). These
results indicated that high legumain expression is a negative
prognostic factor for UM.
Discussion
The prognosis of UM is largely dependent on tumor
behavior, which is essentially determined by the tumor-cell type.
The UM cell line MUM-2B is highly invasive, while MUM-2C exhibits
low invasiveness (18);
furthermore, patients diagnosed with epithelioid-cell UM exhibit
poorer survival than patients with spindle-cell UM due to the
increased invasiveness of the former (1,3). In
the present study, legumain was shown to be expressed in MUM-2B as
well as MUM-2C, while it was significantly higher in the more
invasive MUM-2B cell line compared to that in MUM-2C. Furthermore,
legumain expression was observed to be higher in epitheloid-cell UM
specimens compared to that in spindle-like UM cells. These results
supported the hypothesis that upregulation of legumain is
associated with malignant behavior of UM.
While a preliminary experiment indicated that high
expression of legumain was associated with large tumor size and the
presence of metastasis, it was subsequently revealed that this
observation was due to the sole association of legumain with local
invasion, while it was independent of tumor size and metastasis.
Considering the general mechanism of tumor progression, legumain
expression may have a causal association with metastasis by
promoting UM metastasis via enhancing the local invasion of UM
cells. While no causal association was present between large tumor
size and high legumain expression, the likelihood of local invasion
may have been increased in large-size tumors, which was promoted by
legumain in parallel.
The results of the present study, combined with the
results of a previous study (20),
indicated that the negative impact of legumain on the prognosis of
UM may be attributed to its effect of promoting local invasion.
However, survival analysis of the 54 patients with local invasion
showed that, by contrast to patients with low/negative legumain
expression, high legumain expression was associated with poor
survival of UM patients. Since in patients with local invasion,
legumain expression was not associated with tumor size or
metastasis, it is indicated that legumain may exert its negative
effects on UM prognosis through other pathways.
Little is known regarding other biological functions
of legumain. A previous study showed that legumain is involved in
nucleoplasmic calcium signaling pathways that regulate cell
proliferation, and it was suggested that increased expression of
legumain might be involved in carcinogenesis (21). Legumain was also shown to be highly
expressed in tumor-associated macrophages (TAMs) in the tumor
microenvironment (12). TAMs have
an important role in tumor angiogenesis and metastasis (22); however, the possible implication of
legumain in this process remains elusive. A recent study reported
that legumain induced chemotaxis of primary human monocytes and
human umbilical vein endothelial cells (23), suggesting that legumain may also
have a role in recruiting macrophages and angiogenesis.
Legumain has been shown to be present not only
intracellularly in endosome/lysosome systems, but also on the
surface of tumor cells and endothelial cells, as well as in the
extracellular matrix (7,16). The enrichment of legumain in tumor
microenvironments provides opportunities for several potential
therapeutic approaces. A novel legumain-activated, cell-impermeable
doxorubicin pro-drug, LEG-3, was shown to completely arrest the
growth of a variety of neoplasms, including multidrug-resistant
tumors, in vivo, and significantly prolonged survival
without evidence of myelosuppression or cardiac toxicity (16). Several other novel
legumain-activated pro-drugs have also shown promising potency
against tumors (15). The
enrichment of legumain in the tumor microenvironment also makes it
a target for tumor immunotherapy. Studies have shown that a DNA
vaccine targeting legumain-expressing cells suppressed tumor growth
and dissemination by inducing a specific CD8+ T-cell response
against legumain-expressing cells in vivo (9,12,13).
Due to the importance of legumain in the promotion of tumor
invasion, the suppression of legumain expression or inhibition of
its function may also be feasible approaches for anti-tumor
therapies.
The present study revealed that legumain has a
negative impact on the prognosis of patients with UM. Patients with
UM tissues exhibiting high and extensive staining of legumain
exhibited shorter survival than those with weak and confined
legumain staining. However, only the combination of high intensity
and extent of legumain expression had a significant negative impact
on patient survival, suggesting that both factors should be
considered when posing a prognosis for UM patients.
The present study indicated that legumain was
overexpressed in epithelioid UM cells with high potential of
invasion. Legumain was revealed to be a prognostic biomarker for UM
with a significant association with local invasion, which requires
further clinical evaluation. Furthermore, legumain-activated
pro-drugs and anti-legumain immunotherapy may represent novel
approaches for UM therapy.
Acknowledgments
The authors would like to thank Dr Rong Xiang
(College of Medicine, University of Nankai, Tianjin, China) for
kindly providing the UM cell lines, and Tianjin Eye Hospital
(Tianjin, China) and Beijing Tongren Hospital (Beijing, China) for
providing the patient samples and data. This study was supported by
grants from the National Natural Science Foundation of China (grant
no. NSFC 81441027).
References
|
1
|
Mudhar HS, Parsons MA, Sisley K, Rundle P,
Singh A and Rennie IG: A critical appraisal of the prognostic and
predictive factors for uveal malignant melanoma. Histopathology.
45:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh AD and Borden EC: Metastatic uveal
melanoma. Ophthalmol Clin North Am. 18:143–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Desjardins L, Levy-Gabriel C,
Lumbroso-Lerouic L, Sastre X, Dendale R, Couturier J,
Piperno-Neumann S, Dorval T, Mariani P, Salmon R, et al: Prognostic
factors for malignant uveal melanoma. Retrospective study on 2,241
patients and recent contribution of monosomy-3 research. J Fr
Ophtalmol. 29:741–749. 2006.In French. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kembhavi AA, Buttle DJ, Knight CG and
Barrett AJ: The two cysteine endopeptidases of legume seeds:
Purification and characterization by use of specific fluorometric
assays. Arch Biochem Biophys. 303:208–213. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dalton JP, Hola-Jamriska L and Brindley
PJ: Asparaginyl endopeptidase activity in adult Schistosoma
mansoni. Parasitology. 111:575–580. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen JM, Dando PM, Rawlings ND, Brown MA,
Young NE, Stevens RA, Hewitt E, Watts C and Barrett AJ: Cloning,
isolation and characterization of mammalian legumain, an
asparaginyl endopeptidase. J Biol Chem. 272:8090–8098. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu C, Sun C, Huang H, Janda K and
Edgington T: Overexpression of legumain in tumors is significant
for invasion/metastasis and a candidate enzymatic target for
prodrug therapy. Cancer Res. 63:2957–2964. 2003.PubMed/NCBI
|
|
8
|
Murthy RV, Arbman G, Gao J, Roodman GD and
Sun XF: Legumain expression in relation to clinicopathologic and
biological variables in colorectal cancer. Clin Cancer Res.
11:2293–2299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gawenda J, Traub F, Lück HJ, Kreipe H and
von Wasielewski R: Legumain expression as a prognostic factor in
breast cancer patients. Breast Cancer Res Treat. 102:1–6. 2007.
View Article : Google Scholar
|
|
10
|
Barrett AJ and Rawlings ND: Evolutionary
lines of cysteine peptidases. Biol Chem. 382:727–733. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen JM, Fortunato M, Stevens RA and
Barrett AJ: Activation of progelatinase A by mammalian legumain, a
recently discovered cysteine proteinase. Biol Chem. 382:777–783.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo Y, Zhou H, Krueger J, Kaplan C, Lee
SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA and Xiang R:
Targeting tumor-associated macrophages as a novel strategy against
breast cancer. J Clin Invest. 116:2132–2141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lewēn S, Zhou H, Hu HD, Cheng T, Markowitz
D, Reisfeld RA, Xiang R and Luo Y: A legumain-based minigene
vaccine targets the tumor stroma and suppresses breast cancer
growth and angiogenesis. Cancer Immunol Immunother. 57:507–515.
2008. View Article : Google Scholar
|
|
14
|
Xiang R, Luo Y, Niethammer AG and Reisfeld
RA: Oral DNA vaccines target the tumor vasculature and
microenvironment and suppress tumor growth and metastasis. Immunol
Rev. 222:117–128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bajjuri KM, Liu Y, Liu C and Sinha SC: The
legumain protease-activated auristatin prodrugs suppress tumor
growth and metastasis without toxicity. Chem Med Chem. 6:54–59.
2011. View Article : Google Scholar
|
|
16
|
Wu W, Luo Y, Sun C, et al: Targeting
cell-impermeable prodrug activation to tumor microenvironment
eradicates multiple drug-resistant neoplasms. Cancer Res.
66:970–980. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stern L, Perry R, Ofek P, Many A, Shabat D
and Satchi-Fainaro R: A novel antitumor prodrug platform designed
to be cleaved by the endoprotease legumain. Bioconjug Chem.
20:500–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seftor EA, Meltzer PS, Kirschmann DA,
Pe'er J, Maniotis AJ, Trent JM, Folberg R and Hendrix MJ: Molecular
determinants of human uveal melanoma invasion and metastasis. Clin
Exp Metastasis. 19:233–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Briggs JJ, Haugen MH, Johansen HT, Riker
AI, Abrahamson M, Fodstad Ø, Maelandsmo GM and Solberg R: Cystatin
E/M suppresses legumain activity and invasion of human melanoma.
BMC Cancer. 10:172010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Andrade V, Guerra M, Jardim C, Melo F,
Silva W, Ortega JM, Robert M, Nathanson MH and Leite F:
Nucleoplasmic calcium regulates cell proliferation through
legumain. J Hepatol. 55:626–635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bingle L, Brown NJ and Lewis CE: The role
of tumour-associated macrophages in tumour progression:
Implications for new anticancer therapies. J Pathol. 196:254–265.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clerin V, Shih HH, Deng N, Hebert G,
Resmini C, Shields KM, Feldman JL, Winkler A, Albert L, Maganti V,
et al: Expression of the cysteine protease legumain in vascular
lesions and functional implications in atherogenesis.
Atherosclerosis. 201:53–66. 2008. View Article : Google Scholar : PubMed/NCBI
|