Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide, with an age-standardized
world incidence rate of 44.7 for males and 19.6 for females per
100,000, and an age-standardized world mortality rate of 36.8 for
males and 14.3 for females per 100,000 in more developed areas
(1). In the past decade, great
advances have been made in the treatment of lung cancer using
surgery, chemotherapy and radiotherapy; however, the five-year
survival rate is still low (2).
The majority of patients with cancer develop drug resistance later
in later life resulting in metastatic cancer growth and,
ultimately, mortality. Therefore, drug resistance is a major
challenge in the treatment of lung cancer (3). It is imperative to elucidate the
underlying mechanisms and identify novel strategies to overcome
drug resistance.
Cisplatin, a platinum-containing anticancer drug,
has been widely used for the treatment of various types of human
cancer, including lung, bladder, ovarian, and head and neck cancer
(4). Cisplatin is a potent
chemotherapeutic agent, however, the development of cisplatin
resistance is a major obstacle for the successful treatment of lung
cancer. Cisplatin resistance in lung cancer may be a result of
multiple mechanisms, including excessive drug accumulation inside
cancer cells, drug inactivation, enhanced repair of DNA damage, as
well as abnormal activation of cell signaling pathways via growth
factors and cytokines (5).
However, the precise molecular mechanisms of cisplatin resistance
in lung cancer cells remains unclear.
Several studies have demonstrated the Wnt/β-catenin
signaling pathway to be important in cisplatin resistance of human
malignancies (6,7). β-catenin is highly expressed in
cisplatin-resistant A549 human lung adenocarcinoma cells
(A549/CDDP) (8). Furthermore,
other recent studies demonstrated that deregulation of
Wnt/β-catenin signaling was closely associated with cisplatin
resistance in lung cancer cells (9,10);
however, the underlying molecular mechanisms remain to be
determined. Ding et al (11) demonstrated that β-catenin
upregulates the expression of the anti-apoptotic protein B-cell
lymphoma-extra large (Bcl-xl) in CD4+CD25+
regulatory T cells. Evasion of apoptosis is a hallmark of cancer
cells and Bcl-xl has an important role in the prevention of cell
apoptosis. Furthermore, cisplatin induces DNA damage, which
activates the apoptotic cascade, killing cancer cells. This
suggests that the β-catenin signaling pathway may promote cisplatin
resistance in lung cancer cells by enhancing the expression of
Bcl-xl.
The present study examined the expression of
β-catenin and Bcl-xl in wild-type (A549/WT) and A549/CDDP lung
adenocarcinoma cells. In addition, the functional role of the
Wnt/β-catenin signaling pathway, and its association with Bcl-xl
expression, were investigated in cisplatin resistance of lung
adenocarcinoma cells.
Materials and methods
Reagents
The following reagents were used in the present
study: Fetal bovine serum (FBS), RPMI-1640 (Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA), lithium chloride (LiCl;
Sigma-Aldrich, St. Louis, MO, USA), CellTiter 96 AQueous One
Solution Cell Proliferation Assay (MTS assay; Promega Corporation,
Madison, WI, USA) and FITC Annexin V (BD Biosciences, San Jose, CA,
USA). Rabbit polyclonal anti-β-catenin (cat. no. sc-7199; 1:2,000
dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), mouse
monoclonal anti-β-actin (cat. no. sc-130065; Santa Cruz
Biotechnology, Inc.; 1:1,000 dilution), rabbit monoclonal
anti-phosphorylated (p)-β-catenin (cat. no. 4176; 1:1,000 dilution;
Cell Signaling Technology, Inc., Boston, MA, USA), rabbit
monoclonal anti-Bcl-xl (cat. no. 2764; Cell Signaling Technology,
Inc.; 1:1,000 dilution), horseradish peroxidase (HRP)-conjugated
polyclonal goat anti-mouse IgG (cat. no. SA0001-1; 1:5,000
dilution; ProteinTech Group, Inc., Chicago, IL, USA) and goat
anti-rabbit IgG (cat. no. SA0001-2; 1:5,000 dilution; ProteinTech
Group, Inc.) antibodies were used for western blotting. Small
interfering RNA (siRNA; Shanghai GenePharma Co., Shanghai, China)
and Lipofectamine 2000 transfection reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) were also used.
Cell culture and drug treatment
A549/WT and A549/CDDP cells were obtained from the
Chinese Academy of Medical Sciences (Beijing, China) and the Cancer
Hospital of Peking Union Medical College, Chinese Academy of
Medical Sciences (Beijing, China), respectively. Cells were
cultured in RPMI-1640 culture medium supplemented with 10% FBS, 100
U/ml penicillin and 100 µg/ml streptomycin (GE Healthcare
Life Sciences). A549/CDDP cells were grown in complete culture
medium containing 2 mg/l cisplatin. Cell cultures were maintained
in a 5% CO2-humidified incubator at 37°C. To activate
β-catenin signaling, cells were incubated with 10 mM LiCl for 24 or
48 h.
MTS assay
An MTS assay was used to determine the proliferative
potential of cells. In brief, cells were seeded onto 96-well plates
at a density of 5×103 cells/well. Following different
treatments, 20 µl of MTS solution was added to cells. After
incubation for 1 h at 37°C, the absorbance was measured at 490 nm
using an Infinite M200 microplate spectrophotometer (Tecan Group
Ltd., Männedorf, Switzerland). The growth inhibition rate was
calculated using the following equation: Growth inhibition rate (%)
= (1 − ODSample / ODControl) × 100. The half maximal inhibitory
concentration (IC50) value was calculated using the
least-squares method. The IC50 values and nonlinear
regression graph were calculated using the GraphPad
Prism® (version 5.0; GraphPad Software, Inc., La Jolla,
CA, USA) by plotting the log concentration of the cisplatin versus
the growth inhibition rate of cells.
Determination of apoptosis
Cellular apoptosis was evaluated using FITC Annexin
V/propidium iodide (PI; BD Biosciences) double staining followed by
flow cytometric analysis. Cells were treated with 0, 5 or 10 mg/l
cisplatin for 24 h. Subsequently, cells were collected and
resuspended in 100 µl binding buffer containing 5 µl
Annexin V-FITC and 5 µl PI. After 15 min incubation in
darkness at room temperature (20–25°C), a further 400 µl of
binding buffer was added to the cell suspension and the samples
were analyzed by a FACScan flow cytometer (BD Biosciences).
Western blotting analysis
Total protein was extracted from cells using
radioimmunoprecipitation assay lysis buffer (Cell Signaling
Technology, Inc.) and the protein concentration was measured using
BCA Protein Assay kit, according to manufacturer's instructions
(Pierce Biotechnology, Inc., Rockford, IL, USA). Equal quantities
of protein extract were separated by 10% SDS-PAGE (Sigma-Aldrich).
The gel was run for 20 min at 80 V followed by 100 min at 120 V.
Proteins were transferred to a polyvinylidene fluoride or
nitrocellulose membrane (EMD Millipore, Billerica, MA, USA).
Membranes were blocked with 5% w/v non-fat dry milk (Sigma-Aldrich)
dissolved in Tris-buffered saline plus 0.1% Tween-20 (TBS-T; pH
8.3; Sigma-Aldrich) and probed with primary antibodies at 4°C
overnight. After washing with TBS-T, membranes were incubated with
HRP-labeled secondary antibodies for 1–2 h at room temperature.
Immunobands were detected using an enhanced chemiluminescence kit,
according to manufacturer's instructions (KPL, Inc., Gaithersburg,
MD, USA). The densitometric values of target proteins were analyzed
by Quantity One (version 4.62; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) or ImageJ (version 1.48; National Institutes of
Health, Bethesda, MD, USA) software.
DAPI staining and cell counting
Cells were fixed with 4% paraformaldehyde (PFA;
Sigma-Aldrich) for 15 min, permeabilized in 0.1% Triton-X100
(Sigma-Aldrich) for 15 min, and stained with DAPI (1 µg/ml;
Sigma-Aldrich) for 5 min in the dark. After washing with PBS, cell
samples were imaged using an IX51 fluorescent microscope (Olympus
Corporation, Tokyo, Japan) at ×100 magnification. Three fields were
randomly captured from each sample and the mean number of
DAPI-positive cells was calculated.
Immunocytochemical analysis
Cells were fixed with 4% PFA for 20 min. After
washing with PBS, cells were treated with PBS containing 0.5%
Triton-20 (Sigma-Aldrich) for 10 min. Cell samples were blocked
with PBS supplemented with 4% bovine serum albumin (Sigma-Aldrich)
for 1 h at room temperature and probed with primary antibodies at
4°C overnight or at 37°C for 3 h. After PBS washing, samples were
incubated with FITC-labeled secondary antibody for 1 h at 37°C. The
nuclei were counterstained with 5 µg/ml DAPI and the samples
were visualized using confocal laser scanning microscopy (Leica TCS
SP5II; Leica Microsystems GmbH, Wetzlar, Germany).
RNA interference
To knock down β-catenin expression, A549 cells were
seeded onto six-well plates at a density of 1×105
cells/well and maintained in 500 µl antibiotic-free culture
medium. After 24 h, Lipofectamine 2000 and 33 nM siRNA diluted in
250 µl serum- and antibiotic-free culture medium were added
to the cell culture. The culture medium was replaced with fresh
medium 4–6 h after transfection. The sequences of siRNAs targeting
β-catenin were as follows: Sense, 5′-GGACACAGCAGCAAUUUGU-3′ and
anti-sense, 5′-ACAAAUUGCUGCUGUCCTT-3′. Control cells were
transfected with negative control siRNA with the following
sequences: Sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and anti-sense,
5′-ACGUGACACGUUCGGAGAATT-3′.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using an RNeasy Mini kit,
according to the manufacturer's instructions (TianGen Biotech Co.,
Ltd.). Total RNA (2 µl) was reverse transcribed in a
20-µl reaction system using a Quant Reverse Transcriptase
kit (TianGen Biotech Co., Ltd.). qPCR was performed using a 7500
Real-Time PCR system (Thermo Fisher Scientific, Inc.) and a
SuperReal PreMix (SYBR Green) kit (TianGen Biotech Co., Ltd.).
Primers used for qPCR amplification were as follows: Bcl-xl,
forward, 5′-CCTGAATGACCACCTAGAGCCTT-3′ and reverse,
5′-TCATGCCCGTCAGGAACCAG-3′; 18S rRNA, forward,
5′-GTAACCCGTTGAACCCCATT-3′ and reverse, 5′-CCATCC
AATCGGTAGTAGCG-3′. The cycling conditions used were as follows:
Pre-denaturation at 94°C for 2 min; denaturation at 94°C for 15
sec; annealing at 55°C for 20 sec; extension at 68°C for 35 sec. A
total of 40 cycles were performed. The relative expression of
Bcl-xl was normalized to 18S rRNA and was calculated using the
2−ΔΔCq method (12).
Statistical analysis
Data were calculated from three independent
experiments and are presented as means ± standard deviation. Data
were analyzed using SPSS software (version 19.0; IBM SPSS, Armonk,
NY, USA). Statistical significance was assessed using Student's
t-test, Wilcoxon rank-sum test and one-way analysis of variance
(ANOVA). Bonferroni correction and Fisher's Least Significant
Difference test were performed following one-way ANOVA. P<0.05
indicated a statistically significant difference. Figures were
constructed using GraphPad Prism (version 5.0; GraphPad Software,
Inc., La Jolla, CA, USA).
Results
β-catenin expression is increased in
A549/CDDP cells
The current study initially determined the
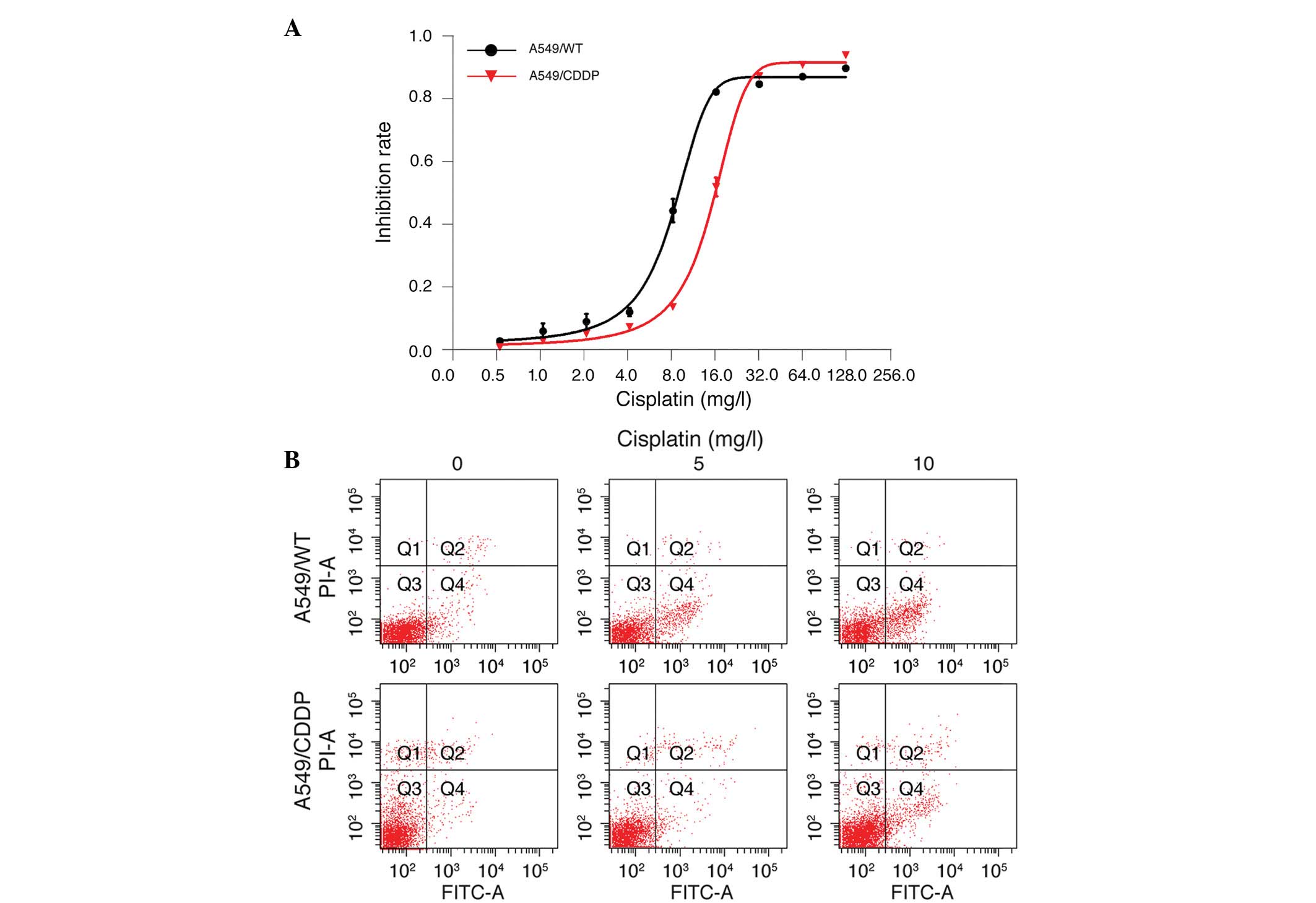
sensitivity of human lung adenocarcinoma A549/WT and A549/CDDP
cells to different concentrations of cisplatin. An MTS assay
demonstrated that cisplatin dose-dependently inhibited the
proliferation of A549/WT and A549/CDDP cells; however A549/WT cells
were more sensitive to cisplatin exposure. The IC50
value of A549/CDDP cells was two-fold higher than that of A549/WT
cells (14.67±0.66 vs. 7.87±0.57 mg/l; n=3; Fig. 1A). In addition, 5 and 10 mg/l
cisplatin treatment induced apparent apoptosis in A549/WT and
A549/CDDP cells, with an increased number of apoptotic cells among
A549/WT cells as compared with A549/CDDP cells (Fig. 1B). To investigate the potential
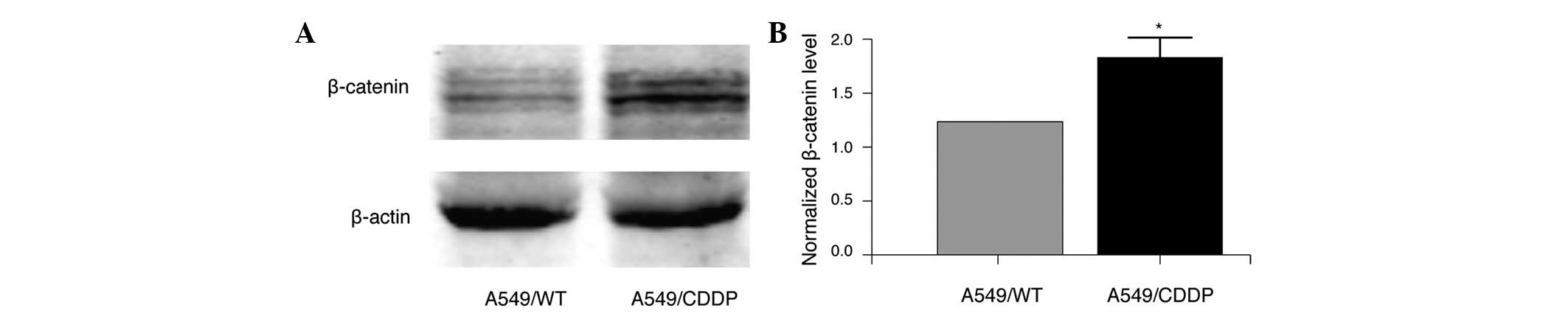
role of Wnt/β-catenin signaling in cisplatin resistance, the
protein expression of β-catenin in A549/WT and A549/CDDP cells was
determined by immunoblotting. As demonstrated in Fig. 2, a significant upregulation of
β-catenin was detected in A549/CDDP cells compared with A549/WT
cells (P<0.05), suggesting that β-catenin may participate in the
cisplatin resistance of lung adenocarcinoma cells.
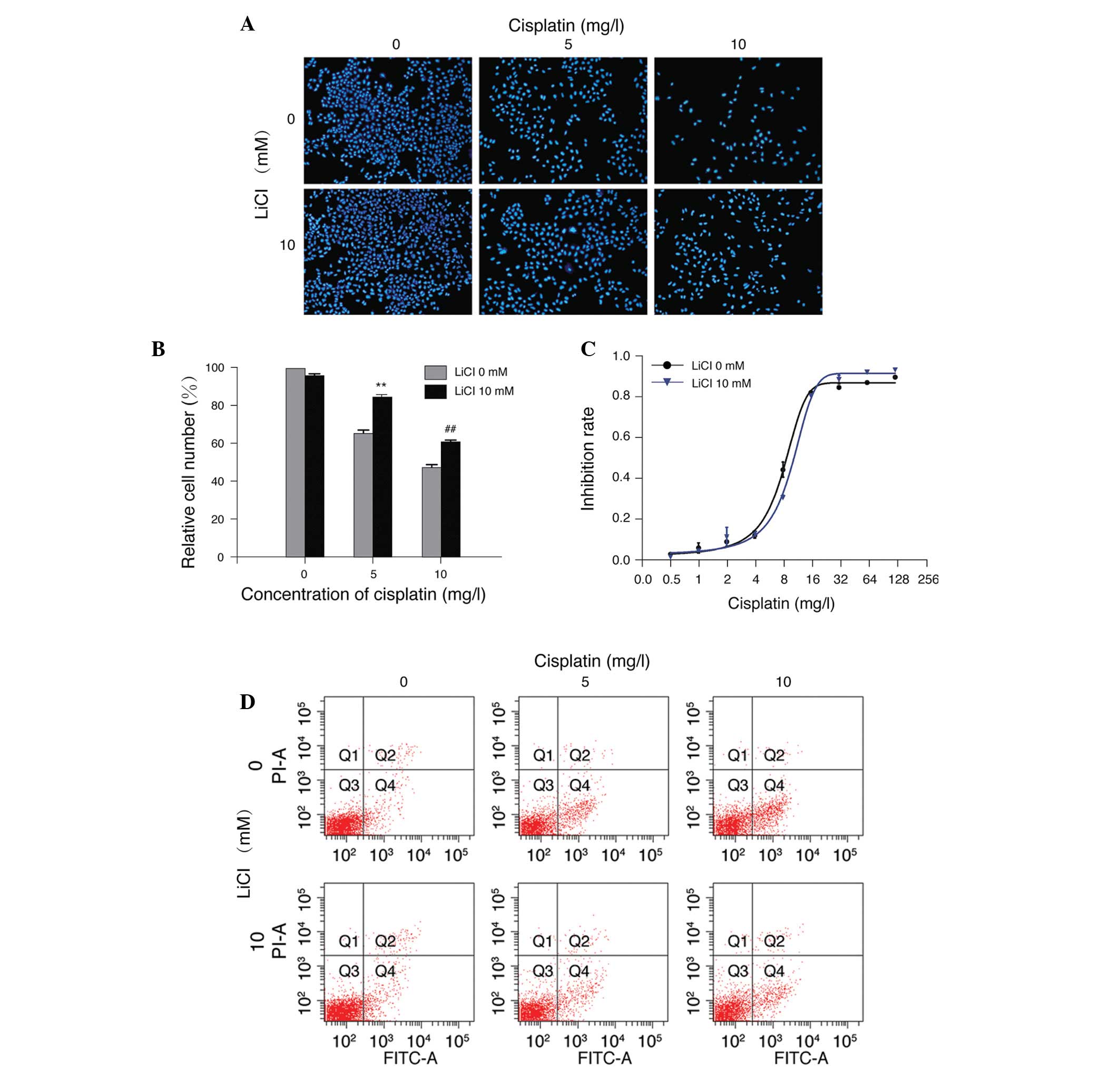
LiCl reduces the sensitivity of A549/WT
cells to cisplatin
LiCl has previously been demonstrated to confer
chemotherapy resistance in several tumor cell types, such as
hepatoblastoma (13), ovarian
carcinoma (14) and A549 lung
cancer cells (8). In the current
study, treatment of A549/WT cells with 5 or 10 mg/l cisplatin
resulted in a marked loss of cell viability, which was abolished by
treatment with 10 mM LiCl (P<0.01 compared with cisplatin
treatment alone; Fig. 3A and B).
Furthermore, 10 mM LiCl reduced the sensitivity of A549/WT cells to
different concentrations of cisplatin. The IC50 value of
the LiCl plus cisplatin treatment group was elevated compared with
that of cisplatin treatment alone (9.79±0.76 vs. 7.87±0.57 mg/l;
n=3; Fig. 3C). In addition, LiCl
markedly reduced cellular apoptosis induced by cisplatin (Fig. 3D). These results indicate that LiCl
reduced the sensitivity of A549/WT cells to cisplatin, suggesting
that LiCl-conferred drug resistance may be a general phenomenon in
cancer cells.
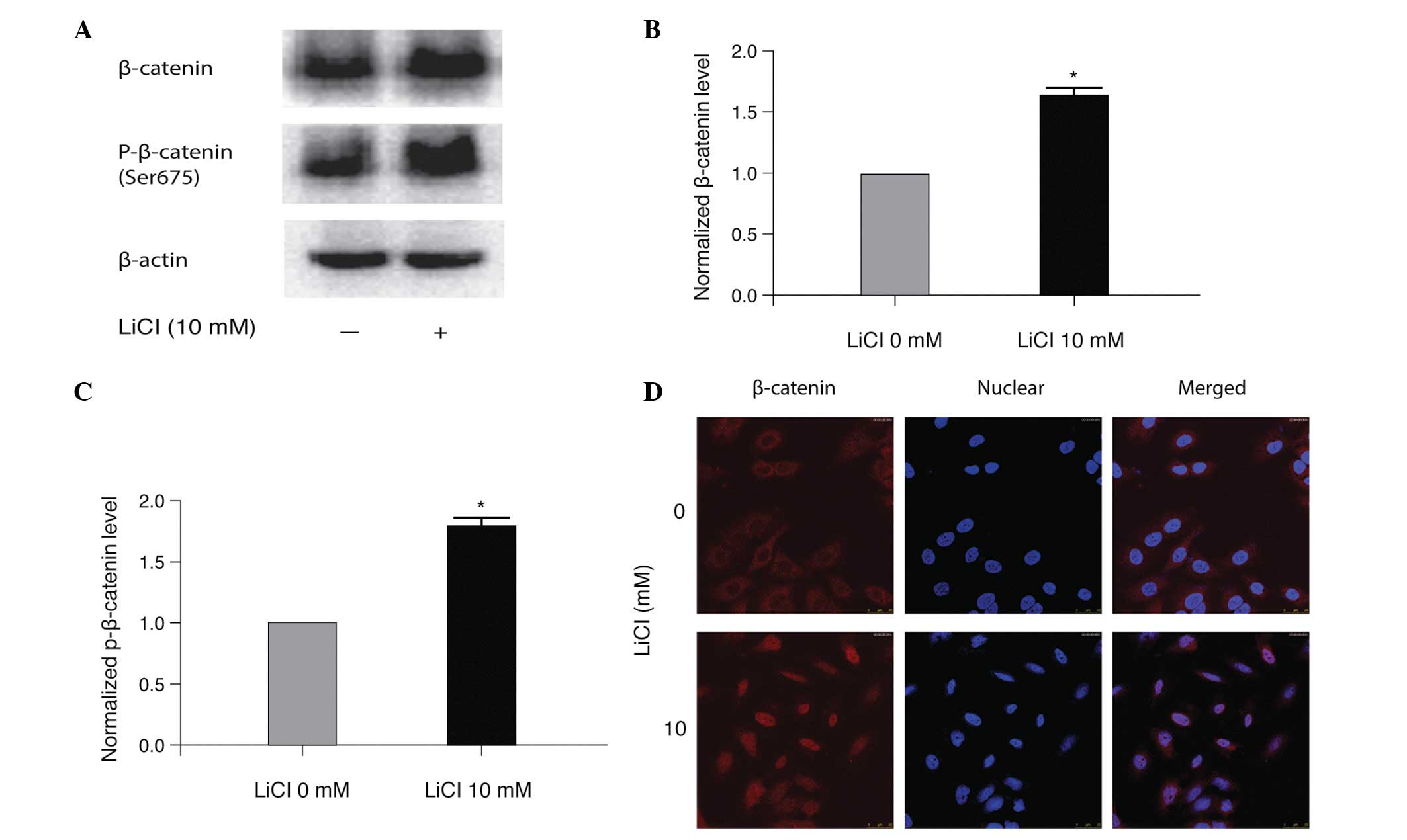
LiCl treatment activates Wnt/β-catenin
signaling in A549/WT cells
To assess the molecular mechanism by which LiCl
promoted cisplatin resistance in A549/WT cells, the protein
expression level of total and p-β-catenin in LiCl-treated A549/WT
cells was determined. As demonstrated in Fig. 4A–C, LiCl significantly increased
β-catenin and p-β-catenin compared with the non-treated controls
(P<0.05). In addition, LiCl treatment led to nuclear
translocation of β-catenin in A549/WT cells, indicating the
activation of β-catenin signaling (Fig. 4D). These results suggest that
activation of Wnt/β-catenin signaling may be one of the mechanisms
by which LiCl promotes cisplatin resistance in A549/WT cells.
LiCl upregulates Bcl-xl expression in
A549/WT cells
Bcl-xl is a member of the Bcl-2 protein family and
functions as a pro-survival factor, inhibiting cellular apoptosis
(15,16). As evasion of apoptosis is the key
to drug resistance in cancer treatment, the current study examined
the potential involvement of Bcl-xl in cisplatin resistance of lung
adenocarcinoma cells in the presence and absence of LiCl. The
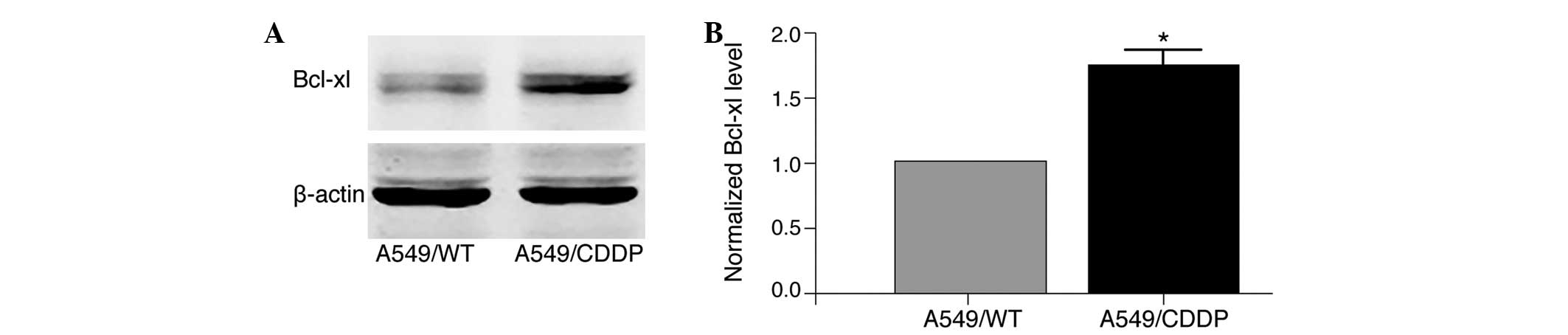
results demonstrated that the protein expression level of Bcl-xl
was significantly elevated in A549/CDDP cells compared with A549/WT
cells (P<0.05; Fig. 5).
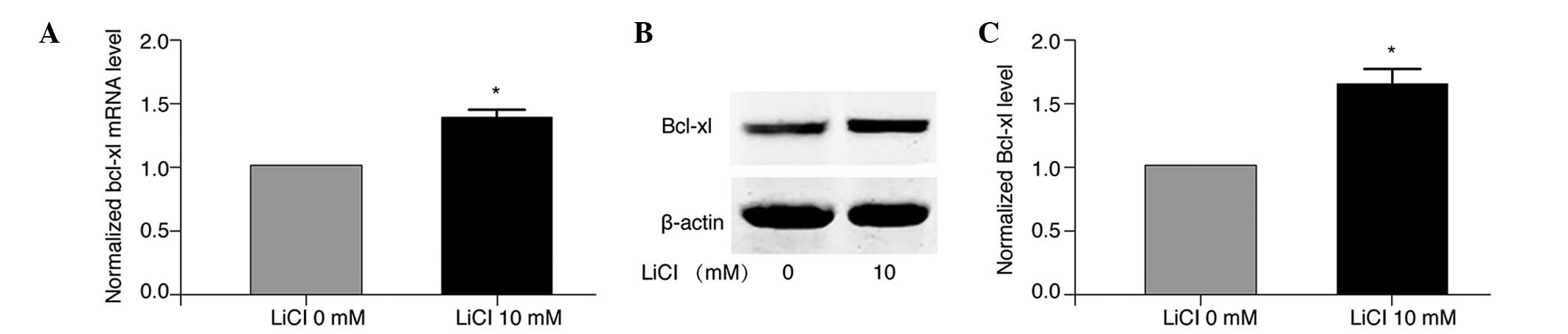
Furthermore, RT-qPCR analysis revealed that LiCl treatment
significantly increased the mRNA expression levels of Bcl-xl in
A549/WT cells compared with the non-treated control cells
(P<0.05; Fig. 6A). Consistent
with the upregulation of Bcl-xl mRNA, western blot analysis
demonstrated that treatment with 10 mM LiCl significantly elevated
the protein expression level of Bcl-xl in A549/WT cells (P<0.05;
Fig. 6B and C). Thus, LiCl
treatment of A549/WT cells resulted in increased expression of
Bcl-xl at the mRNA and protein level.
Silencing of β-catenin downregulates
Bcl-xl and sensitizes A549/WT cells to cisplatin
In order to explore the potential association
between Bcl-xl and β-catenin signaling pathways in cisplatin
resistance of lung adenocarcinoma cells, β-catenin expression was
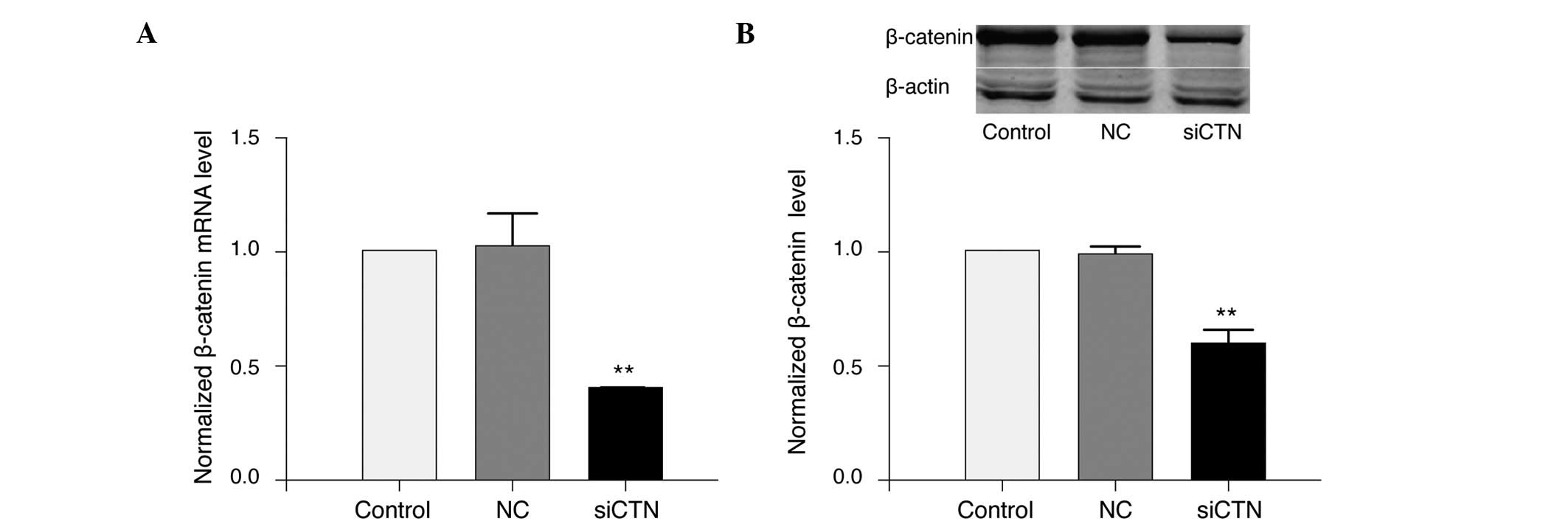
silenced using siRNA in A549/WT cells. Transfection of siRNA
targeting β-catenin efficiently down-regulated the mRNA (Fig. 7A) and protein (Fig. 7B) levels of β-catenin in A549/WT
cells compared with negative control siRNA (P<0.01).
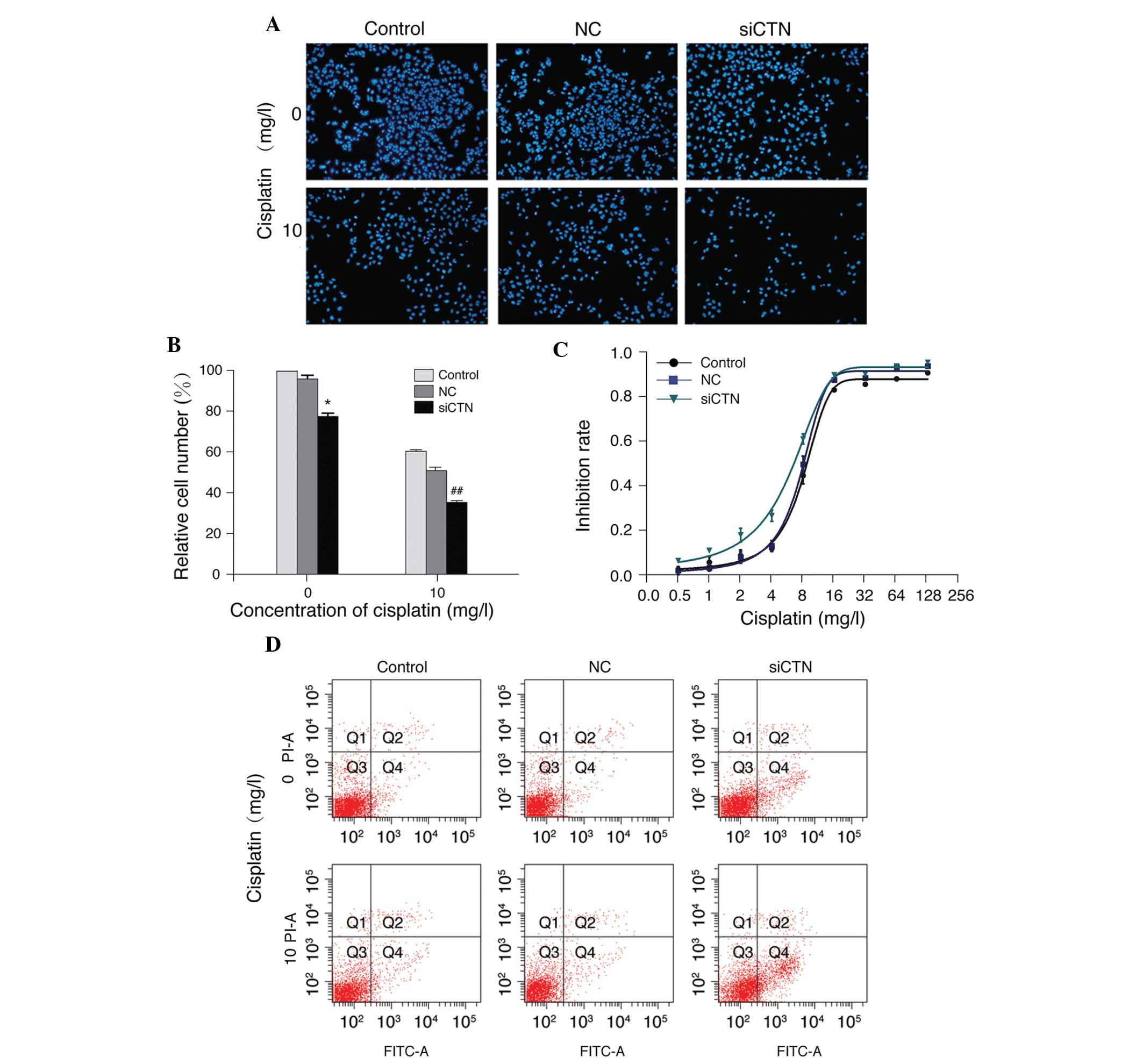
Additionally, β-catenin siRNA significantly inhibited the growth of
A549/WT cells (P<0.05) and caused a further reduction of
cisplatin-induced growth inhibition (P<0.01; Fig. 8A and B). Furthermore, silencing of
β-catenin with siRNA decreased the inhibition rate of cisplatin, as
demonstrated by MTS assay. The IC50 value of cisplatin
was reduced in cells transfected with β-catenin siRNA (β-catenin
siRNA, 4.98±1.37 mg/l; control, 7.87±0.57 mg/l; negative control,
7.45±0.49 mg/l; Fig. 8C).
Furthermore, β-catenin siRNA increased cisplatin-induced apoptosis
in A549/WT cells (Fig. 8D). In
accordance with a previous observation that β-catenin promoted
transcription of Bcl-xl (17), the
present study observed that silencing of β-catenin in A549/WT cells
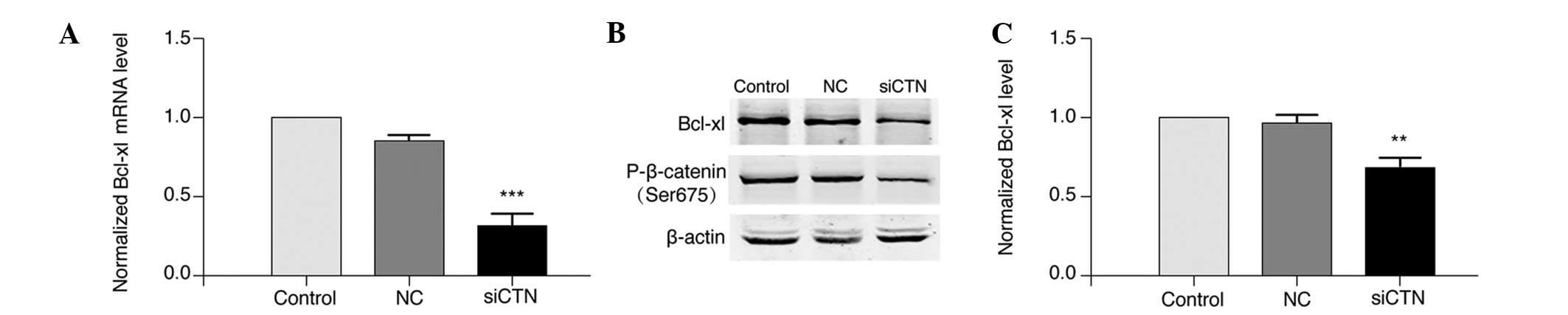
significantly decreased the mRNA (P<0.001; Fig. 9A) and protein (P<0.01; Fig. 9B and C) expression of Bcl-xl
compared with negative control cells. In addition, knockdown of
β-catenin reduced β-catenin phosphorylation in A549/WT cells
(Fig. 9B). These results indicate
that silencing of β-catenin sensitized A549/WT cells to cisplatin
and this effect may be mediated by downregulation of Bcl-xl
expression.
Discussion
Cisplatin resistance is a major challenge during
lung cancer treatment. The molecular mechanism of cisplatin
resistance in lung cancer cells is largely unknown, therefore,
there are few efficient strategies to overcome such resistance. The
current study revealed the Wnt/β-catenin signaling pathway and
anti-apoptotic protein Bcl-xl to be involved in cisplatin
resistance of human A549 cells. These findings indicate that
molecular targeting of Wnt/β-catenin signaling may sensitize lung
cancer cells to cisplatin.
Platinum derivatives, such as cisplatin, are widely
used chemotherapeutic agents during lung cancer treatment, however,
their efficiency can be affected by a number of factors (5). Various genes are abnormally expressed
in drug resistant cancer cells, such as increased levels of
anti-apoptotic genes (such as Bcl-xl, Bcl-2 and Survivin) and
reduced levels of pro-apoptotic genes (such as Bax and
Bcl-2-associated death promoter) (18). In addition, multiple signaling
pathways are activated during cisplatin resistance, including the
Wnt/β-catenin pathway (8–10). However, the cross-talk that occurs
among these signaling pathways and mediators is still unclear.
The Wnt/β-catenin signaling pathway has been shown
to be an essential signal transduction pathway in tumorigenesis and
progression of various types of cancer (19,20).
In the present study, a significant upregulation of β-catenin was
observed in A549/CDDP cells compared with A549/WT cells, which is
in accordance with a previous observation (8). Furthermore, interference of β-catenin
expression by siRNA suppressed cell growth and increased cisplatin
sensitivity in A549/WT cells. Consistent with the findings of the
current study, a previous investigation observed that
downregulation of β-catenin expression reversed resistance to
cisplatin in A2780 ovarian cancer cells (21). The same in vivo study
demonstrated that silencing of β-catenin inhibited the progression
of ovarian cancer in mice (21).
Other studies have proposed the Wnt/β-catenin pathway as a
potential therapeutic target in hepatocellular carcinoma (HCC)
(22,23). These lines of evidence suggest that
targeting Wnt/β-catenin signaling is a promising strategy for
combating cisplatin resistance in lung cancer.
It is widely accepted that defects in the apoptotic
pathway contribute to tumor cell survival and resistance to
anticancer therapy (18).
Therefore, targeting endogenous apoptotic inhibitor proteins, such
as the Bcl-2 family, is considered to be a promising strategy for
the treatment of cancer and overcoming chemotherapeutic resistance
(16,24). Bcl-xl, a member of the Bcl-2
family, is a pro-survival protein that prevents apoptosis by
inhibiting the release of cytochrome c from mitochondria
(15,16). In the present study, increased
expression of Bcl-xl mRNA was detected in A549/CDDP cells and LiCl
significantly elevated the mRNA and protein levels of Bcl-xl in
A549/WT cells. Notably, silencing of β-catenin increased the
sensitivity of A549/WT cells to cisplatin and downregulated Bcl-xl
expression. These results indicate that Bcl-xl may be a critical
target gene of Wnt/β-catenin signaling in cisplatin-resistant lung
cancer.
β-catenin is a key component of Wnt/β-catenin
signaling. Activated β-catenin translocates into the nucleus and
binds to T-cell factors to regulate transcription. Accumulating
evidence has suggested that tumor cells depend on β-catenin for
survival and downregulation of β-catenin results in tumor cell
apoptosis, which is associated with reduced levels of Bcl-xl
(25). β-catenin was identified to
induce Bcl-xl expression in CD8+ T cells, whereas
upregulated β-catenin reduced Bcl-xl in cultured HepG2 HCC cells
(26). This contradiction may be
attributed to the varied responses and signaling mechanisms in
different types of cancer cells. In addition, other members of the
Bcl-2 protein family, such as Bcl-2, Bax and Bcl-2 homologous
antagonist/killer, may also be involved in anticancer drug
resistance regulated by Wnt/β-catenin signaling.
In summary, the present study demonstrated that
A549/CDDP cells expressed high levels of β-catenin and Bcl-xl, and
interference of β-catenin by siRNA enhanced cisplatin sensitivity
in A549/WT cells by downregulation of Bcl-xl. The findings suggest
that targeting Wnt/β-catenin signaling may be a valuable strategy
for overcoming cisplatin resistance in lung cancer. Future studies
should investigate the effects of β-catenin silencing in
vivo using animal models of lung cancer.
Acknowledgments
This study was supported by the Specialized Research
Fund for the Doctoral Program of Higher Education (grant no.
20111107110003).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prokop M: Lung cancer screening: The
radiologist's perspective. Semin Respir Crit Care Med. 35:91–98.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
MacDonagh L, Gray SG, Finn SP, Cuffe S,
O'Byrne KJ and Barr MP: The emerging role of microRNAs in
resistance to lung cancer treatments. Cancer Treat Rev. 41:160–169.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang G, Reed E and Li QQ: Molecular basis
of cellular response to cisplatin chemotherapy in non-small cell
lung cancer (Review). Oncol Rep. 12:955–965. 2004.PubMed/NCBI
|
|
6
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar
|
|
7
|
Xia Y, He Z, Liu B, Wang P and Chen Y:
Downregulation of Meg3 enhances cisplatin resistance of lung cancer
cells through activation of the WNT/β-catenin signaling pathway.
Mol Med Rep. 12:4530–4537. 2015.PubMed/NCBI
|
|
8
|
Teng Y, Wang X, Wang Y and Ma D:
Wnt/beta-catenin signaling regulates cancer stem cells in lung
cancer A549 cells. Biochem Biophys Res Commun. 392:373–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie C, Pan Y, Hao F, Gao Y, Liu Z, Zhang
X, Xie L, Jiang G, Li Q and Wang E: C-Myc participates in
β-catenin-mediated drug resistance in A549/DDP lung adenocarcinoma
cells. APMIS. 122:1251–1258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao Y, Liu Z, Zhang X, He J, Pan Y, Hao F,
Xie L, Li Q, Qiu X and Wang E: Inhibition of cytoplasmic GSK-3β
increases cisplatin resistance through activation of Wnt/β-catenin
signaling in A549/DDP cells. Cancer Lett. 336:231–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding Y, Shen S, Lino AC, Curotto de
Lafaille MA and Lafaille JJ: Beta-catenin stabilization extends
regulatory T cell survival and induces anergy in nonregulatory T
cells. Nat Med. 14:162–169. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
13
|
Beurel E, Kornprobst M, Blivet-Van
Eggelpoel MJ, Ruiz-Ruiz C, Cadoret A, Capeau J and Desbois-Mouthon
C: GSK-3beta inhibition by lithium confers resistance to
chemotherapy-induced apoptosis through the repression of CD95
(Fas/APO-1) expression. Exp Cell Res. 300:354–364. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai G, Wang J, Xin X, Ke Z and Luo J:
Phosphorylation of glycogen synthase kinase-3 beta at serine 9
confers cisplatin resistance in ovarian cancer cells. Int J Oncol.
31:657–662. 2007.PubMed/NCBI
|
|
15
|
Volkmann N, Marassi FM, Newmeyer DD and
Hanein D: The rheostat in the membrane: BCL-2 family proteins and
apoptosis. Cell Death Differ. 21:206–215. 2014. View Article : Google Scholar :
|
|
16
|
Xiong S, Mu T, Wang G and Jiang X:
Mitochondria-mediated apoptosis in mammals. Protein Cell.
5:737–749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie H, Huang Z, Sadim MS and Sun Z:
Stabilized beta-catenin extends thymocyte survival by up-regulating
Bcl-xL. J Immunol. 175:7981–7988. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
Targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arend RC, Londono-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holland JD, Klaus A, Garratt AN and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Curr
Opin Cell Biol. 25:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao H, Wei W, Sun Y, Gao J, Wang Q and
Zheng J: Interference with the expression of β-catenin reverses
cisplatin resistance in A2780/DDP cells and inhibits the
progression of ovarian cancer in mouse model. DNA Cell Biol.
34:55–62. 2015. View Article : Google Scholar
|
|
22
|
Dahmani R, Just PA and Perret C: The
Wnt/β-catenin pathway as a therapeutic target in human
hepatocellular carcinoma. Clin Res Hepatol Gastroenterol.
35:709–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yeh CT, Rao YK, Ye M, Wu WS, Chang TC,
Wang LS, Wu CH, Wu AT and Tzeng YM: Preclinical evaluation of
destruxin B as a novel Wnt signaling target suppressing
proliferation and metastasis of colorectal cancer using
non-invasive bioluminescence imaging. Toxicol Appl Pharmacol.
261:31–41. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kamal A, Faazil S and Malik MS:
Apoptosis-inducing agents: A patent review 2010–2013. Expert Opin
Ther Pat. 24:339–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi PS, Li Y and Felsher DW: Addiction to
multiple oncogenes can be exploited to prevent the emergence of
therapeutic resistance. Proc Natl Acad Sci USA. 111:E3316–3324.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li W, Tong H, Huang X, Wang W, Wu H and
Lin S: High levels of β-catenin promote IFNγ-induced apoptosis in
hepatocellular carcinoma cells. Oncol Lett. 4:1092–1096.
2012.PubMed/NCBI
|