Introduction
Intervertebral disc degeneration (IDD), a major
cause of lower back pain, is a serious problem with substantial
financial and health care implications worldwide (1,2).
Current clinical treatments for IDD include conservative approaches
and spinal surgery. Conservative treatments, including
non-steroidal anti-inflammatory drugs and physiotherapy, can often
alleviate the pain, however, the degenerative process of IDD cannot
be reversed (3). Spinal surgery to
remove the degenerated discs and fuse the adjacent spinal segments
may cause increased degeneration and instability of the neighboring
discs (4). Biological strategies
for IDD have been developed with promising prospects (5), and the injection of active substances
into the early degenerated disc has been suggested to be an ideal
approach for IDD regeneration (6).
The commonly applied active substances in IDD
include a variety of growth factors with potent effects on cell
proliferation and extracellular matrix (ECM) synthesis (7–9).
Currently, PRP, a growth factor cocktail, is suggested as the ideal
approach for early IDD regeneration (10). PRP is a fraction of whole blood
with a platelet concentration above baseline (11). When activated, multiple growth
factors, including transforming growth factor (TGF)-β1,
insulin-like growth factor-1, platelet-derived growth factor,
vascular endothelial growth factor and epidermal growth factore are
released from a-granules of platelets, with regenerative effects
(12). However, the short
half-life of growth factors may prohibit the continuous
regenerative process of IDD (11).
Cell transplantation is also an ideal approach for
IDD regeneration (13). Bone
marrow-derived mesenchymal stem cells (BMSCs), as a potential
substitute for native disc cells, represents another biological
strategy to restore the degenerated discs (14). BMSCs are capable of long-term
self-renewal and differentiation into other cell lineages,
exhibiting potent proliferation and differentiation potential
(15). BMSCs are reported to have
the ability to survive and proliferate within the microenvironment
of degenerated discs, which offers potential for the continuous
restoration of their normal structure and function (16). Considering these factors, the
present study hypothesized that the administration of
PRP-containing BMSCs into degenerated discs may have a synergistic
regenerative effect on the degenerated discs.
The overall purpose of the present study was to
investigate the regenerative effect of PRP-containing BMSCs on
early degenerated discs in vivo. To achieve this aim,
histological and magnetic resonance imaging (MRI) evaluations were
used to monitor changes in the treated discs.
Materials and methods
Laboratory animals and groups
All experimental procedures involving animals in the
present study conformed with the National Institutes of Health
Guidelines for the Care and Use of Laboratory Animals, and were
approved by the Administration Committee of Experimental Animals
(Jiangsu, China). A total of 40 male adult New Zealand white
rabbits (weight, 2.5–3.0 kg; age, ~3 months) were purchased from
the Jiangsu Academy of Agricultural Sciences (Jiangsu, China) for
use in the present study. The rabbits were maintained in cages at
the Animal Center of Southeast University (Nanjing, China) at 24°C
under a 12-h light/dark cycle with ad libitum access to food
and water. A total of 30 rabbits were used to establish IDD models,
and were subsequently randomly divided into three groups: 200
μl PRP or phosphate-buffered saline (PBS; Sigma-Aldrich, St.
Louis, MO, USA) were injected into the degenerated discs (PRP group
and PBS group, respectively) using a 26-gauge needle; 200 μl
PRP-containing BMSCs was also injected (PRP-BMSC group). Another 10
rabbits without intervention were used as the control group. The
present study was approved by the ethics committee of Southeast
University.
Isolation, culture and characterization
of BMSCs
The BMSCs were isolated from a New Zealand white
rabbit, as described previously (17). Briefly, 2 ml iliac bone marrow was
collected from the rabbits and immediately mixed with 2 ml PBS.
Following size-fractionation using a Ficoll-Paque Plus (1.077 g/ml;
GE Healthcare Life Sciences, Shanghai, China), BMSCs were isolated
by centrifugation at 400 × g for 30 min at room temperature. The
isolated BMSCs were seeded (105 cells/cm2)
into a 25 cm2 culture flask (Corning, Inc., Acton, MA,
USA), and cultured in Dulbecco's modified Eagle's medium with low
glucose (DMEM-LG; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), supplemented with 10% (v/v) fetal bovine serum (FBS;
Wisent., Inc., St-Jean-Baptiste, QC, Canada) at 37°C under a 5%
CO2 atmosphere. The culture medium was replaced every
2–3 days and BMSCs after three passages were used in the present
study. The expanded BMSCs were induced towards adipogenic,
osteogenic and chondrogenic lineages, as previously described
(18).
Adipogenic differentiation induction
BMSCs (2×103 cells/cm2) at
passage three were cultured in 6-well culture plates containing the
adipogenic induction medium, which consisted of 10% (v/v) FBS, 200
μM indomethacin, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) 1
μM dexamethasone, 10 μg/ml insulin (all: Cyagen
Biosciences, Inc., Santa Clara, CA, USA) and DMEM-LG. The culture
medium was changed every 3–4 days. After 4 weeks of induction, the
formation of oil droplets was tested using Oil Red O staining, in
which the induced BMSCs were fixed in 70% ethanol for 10 min and
stained with 0.3% fresh Oil Red O solution (Sigma-Aldrich) for 2 h
prior to observation under a microscope (Leica DMRXA2; Leica
Microsystems GmbH, Wetzlar, Germany).
Osteogenic dif ferentiation
induction
BMSCs (2×103 cells/cm2) at
passage three were cultured in 6-well culture plates containing the
osteogenic induction medium, which consisted of 10% (v/v) FBS, 100
nM dexamethasone, 10 mM β-glycerophosphate (Sigma-Aldrich), 0.05 mM
ascorbic acid (Sigma-Aldrich) and DMEM-LG. The culture medium was
changed every 3–4 days. After 4 weeks of induction, the formation
of calcium nudes were examined by Alizarin red staining, in which
the induced BMSCs were fixed with 70% ethanol for 10 min and
stained with 0.5% alizarin red solution (pH 4.1; Sigma-Aldrich) for
2 h, prior to observation under a microscope (Leica DMRXA2).
Chondrogenic differentiation
induction
A pellet culture system was applied to confirm the
chondrogenesis of the isolated BMSCs. Briefly, 2.5×105
cells were centrifuged at 450 × g for 10 min at room temperature to
form a small pellet and cultured in 15 ml polypropylene tubes
(Sigma-Aldrich). The chondrogenic induction medium, including 10 nM
dexamethasone, 10 ng/ml TGF-β3 (Cyagen Biosciences, Inc.), 50 mg/ml
ascorbic acid and 50 mg/ml ITS+Premix (Cyagen Biosciences, Inc.) in
DMEM with high glucose was used, and replaced every 3 days.
Following culturing in the chondrogenic induction medium at 37°C
under a 5% CO2 atmosphere for 4 weeks, the pellets were
fixed in formalin (Sigma-Aldrich) and sectioned for staining with
1% toluidine blue solution (Seebio Biotech, Inc., Shanghai, China)
for 2 h. Following washing with PBS, the sections were mounted
using neutral gum (China Sinopharm International, Co., Ltd.,
Shanghai, China) for observation under a microscope (Leica
DMRXA2).
Preparation of autologous PRP and the
PRP-BMSC mixture
The PRP was prepared, according to a method
described by Landesberg et al (19) with modifications. Briefly, under
general anesthesia with pentobarbitone sodium (30 mg/kg;
Sigma-Aldrich), 6 ml fresh blood was obtained from the central
artery of the rabbits ear using a syringe containing 0.6 ml acid
citrate dextrose-A solution (Kermel Chemical Reagent Co., Ltd.,
Tianjin, China) as a anticoagulant. Subsequently, 0.2 ml whole
blood was obtained to perform a platelet count. The obtained whole
blood was primarily centrifuged by a centrifuge (SC-04; Zhongke
Meiling Cryogenics Co., Ltd., Hefei, China) at 200 g for 10 min.
Subsequently, the plasma fraction was collected and further
centrifuged at 1,000 g for another 10 min. The upper three-quarters
of supernatant plasma was carefully removed, and the remaining
plasma and platelets were gently agitated and designated as PRP
(~0.6 ml). Subsequently, 0.2 ml of the prepared PRP was drawn for
platelet counting, and the remaining PRP (~0.4 ml) was retained for
further use. To prepare the PRP-BMSC mixture, the BMSCs were
trypsinized (Sigma-Aldrich) and diluted in PBS to 1×106
cells/ml, and 0.2 ml volumes of cell suspension were carefully
mixed with the previously prepared PRP (~0.4 ml).
Establishment and evaluation of IDD
models in rabbits
The rabbit lumbar IDD models of the PRP, PBS and
PRP-BMSC groups were established by needle puncture into the
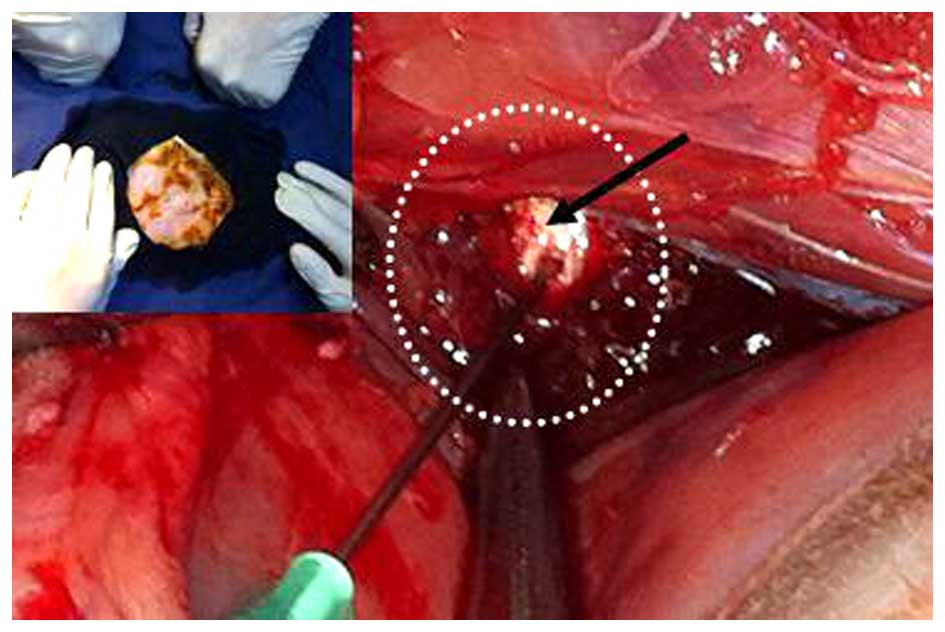
annulus fibrosis (Fig. 1).
Briefly, under general anesthesia, the L4–5 and L5–6 lumbar discs
were exposed using a contralateral side approach under a sterile
environment. Following puncture of the discs with a 21-gauge needle
to the depth of 5 mm, the surgical wound was immediately sutured.
To confirm early degeneration of the punctured discs, the spines of
the rabbits were evaluated using a 3.0-T MRI scan 2 weeks following
post-needle puncture), the targeted discs were exposed again for
the respective treatments. At weeks 1, 2, and 8 following the
different treatments, the targeted discs of the groups were scanned
using 3.0-T MRI. At week 8 following intervention, the rabbits in
each group were sacrificed by intravenous injection with an
overdose of pentobarbitone sodium (120 mg/kg). The vertebral
body-disc-vertebral units of the targeted segments were carefully
cut off and fixed in 10% (v/v) formalin in neutral buffer for
paraffin (Sigma-Aldrich) embedding. The paraffin blocks were
sectioned at a thickness of 5 μm. Type II collagen
immunohistochemistry and hematoxylin and eosin (H&E;
Sigma-Aldrich) staining were performed to evaluate the histological
changes in the treated discs. At 200× magnification, five visual
fields were randomly selected from each sample for evaluation under
a microscope (Leica DMRXA2). The Image-Pro Plus 6.0 image analysis
system (Media Cybernetics, Inc., Rockville, MD, USA) was utilized
to analyze the integrated absorbance (IA) value of the
yellow-stained type II collagen.
MRI evaluation
MRI was performed using a 3.0T imager unit (Verio
3.0T MR; Siemens Medical Solutions, Erlangen, Germany). Following
general anesthesia with pentobarbitone sodium (30 mg/kg), the
rabbits were placed in the supine position on a quadrature surface
coil for MRI scanning. The parameters of the T2-weighted images
were as follows: Time-to-repeat=1,800 ms; time-to-echo=71 ms; field
of view=140×140 mm; slice thickness=2 mm; averages=9; image
matrix=288×384. The signal intensity of the discs was evaluated
using T2-weighted images. IDD grade was evaluated using the
modified Thompson classification (20) of grades I–IV, as follows. Grade I,
normal signal intensity; grade II, minimal decreased signal
intensity without obvious narrowing of high signal area; grade III,
moderate decrease of signal intensity; grade IV, severe decrease of
signal intensity. Two senior radiologists evaluated the images to
reach a consensus.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software (IBM SPSS, Armonk, NY, USA). Data of all treatment groups
are presented as the mean ± standard error of the mean and were
analyzed using one way analysis of variance. Pairwise comparisons
were analyzed using a Student-Newman-Kuells test. The level data of
the MRI images were analyzed using a Mann-Whitney U-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
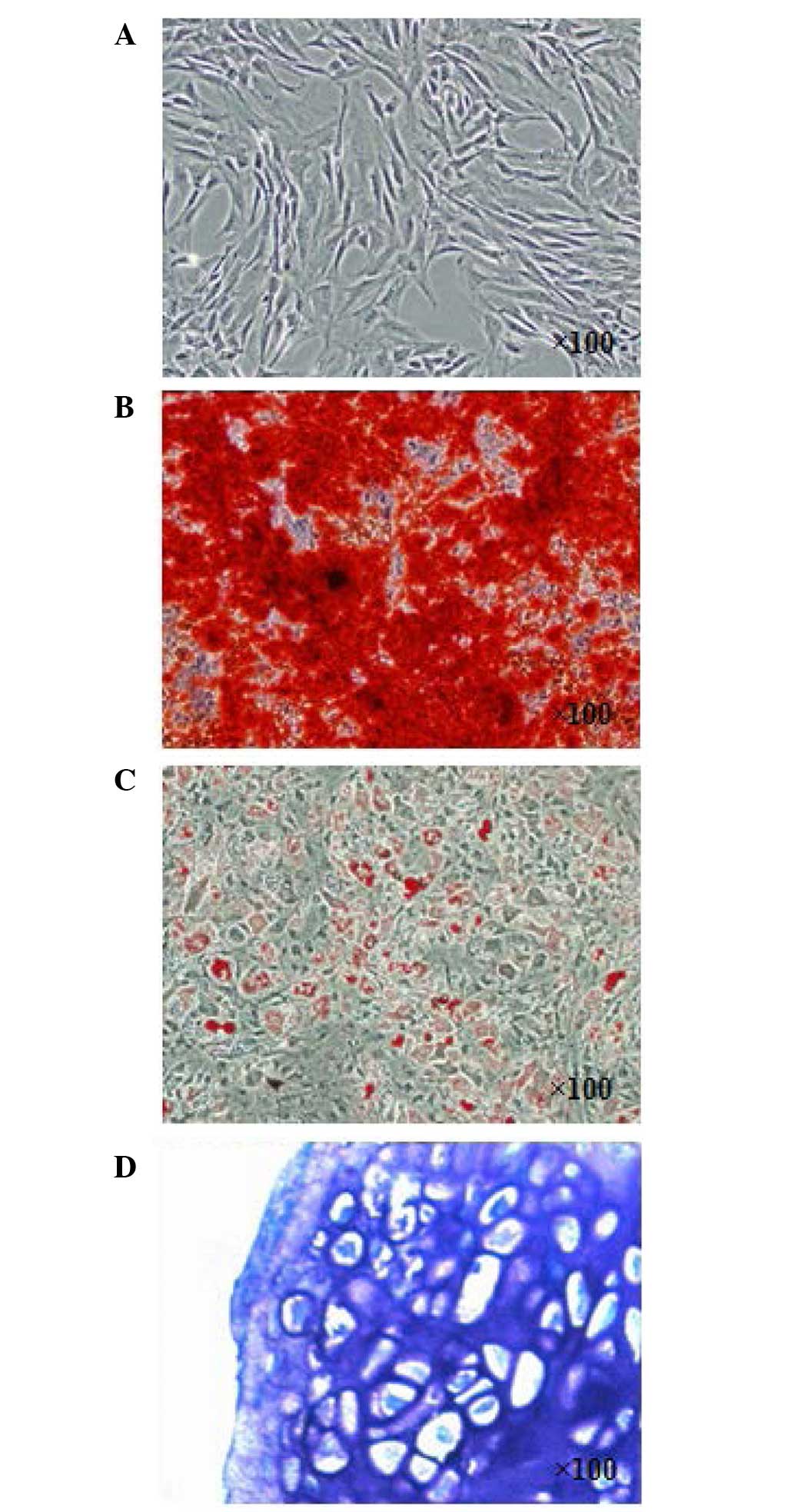
Characterization of isolated BMSCs
At 3 weeks post-induction, the isolated BMSCs
(Fig. 2A) were successfully
induced toward adipogenic, osteogenic and chondrogenic lineages.
The osteogenic differentiation potential of the isolated BMSCs was
confirmed using Alizarin-red staining (Fig. 2B). Lipid droplets were formed and
confirmed using Oil Red O staining (Fig. 2C). As for chondrogenic induction of
the BMSCs, the cell pellets sectioned for toluidine blue staining
(Fig. 2D) were positive,
indicating the chondrogenic potential of the isolated BMSCs.
Platelet concentration of the PRP
The platelet concentration of the prepared PRP was
776±48×109/l, whereas the concentration in the whole
blood was only 159±21×109/l. The PRP prepared in the
present study contained almost five times the number of platelets
in the whole blood.
MRI evaluation
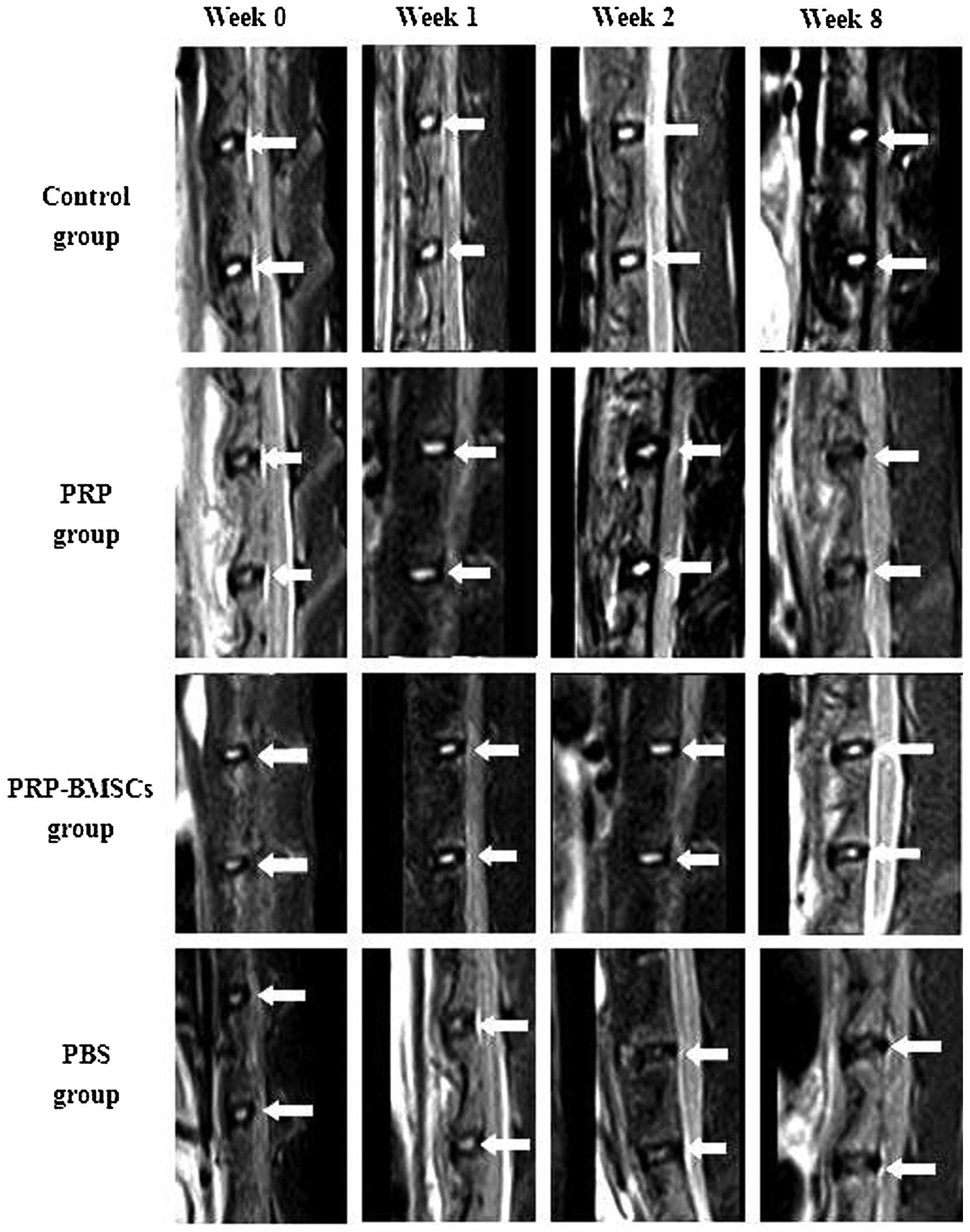
Representative sagittal MRI images showed the
variation of the targeted discs with high T2-weighted signal
intensity in all groups (Fig. 3).
T2 signal intensity was stable at each time point in the control
group. At 2 weeks following needle puncture (week 0), the injected
discs experienced mild signal loss. The treated discs of the PBS
group experienced continuous T2 signal loss during the 8-week
period, whereas the discs in the PRP-BMSC group showed an increase
in signal intensity over time. PRP exhibited a regenerative effect
on the degenerated discs at 1 and 2 weeks following treatment by
increasing the signal intensity. However, at week 8 post-treatment,
the discs of the PRP group exhibited decreased signal intensity. To
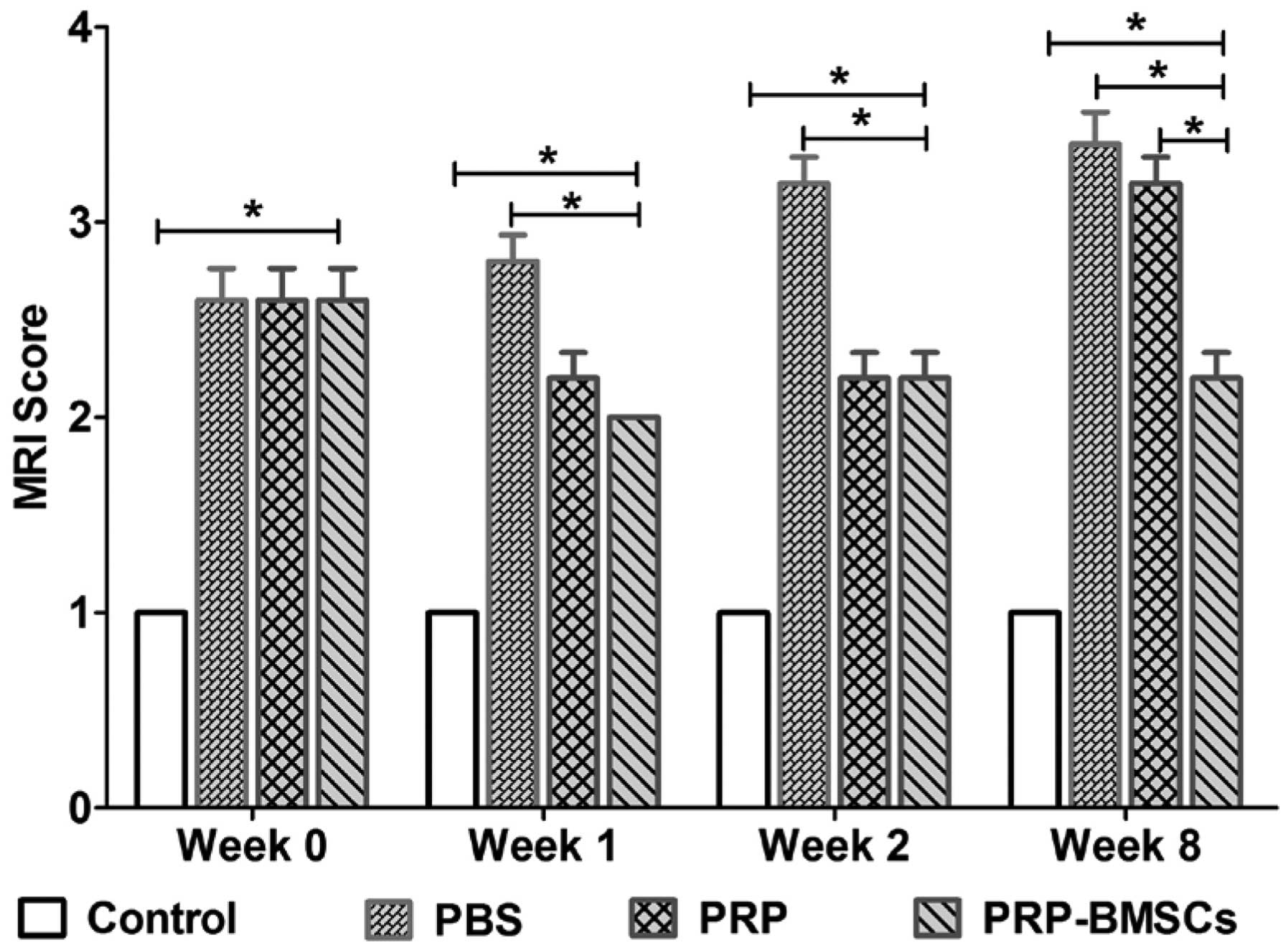
determine the degenerative changes of the targeted discs, the mean
MRI score of each group was presented (Fig. 4). At weeks 1 and 2, no significant
differences were found in the MRI grading between the PRP and
PRP-BMSC groups (P<0.05). At week 8, the PRP-BMSC group showed a
significantly higher MRI grading score, compared with the PRP group
(P<0.05).
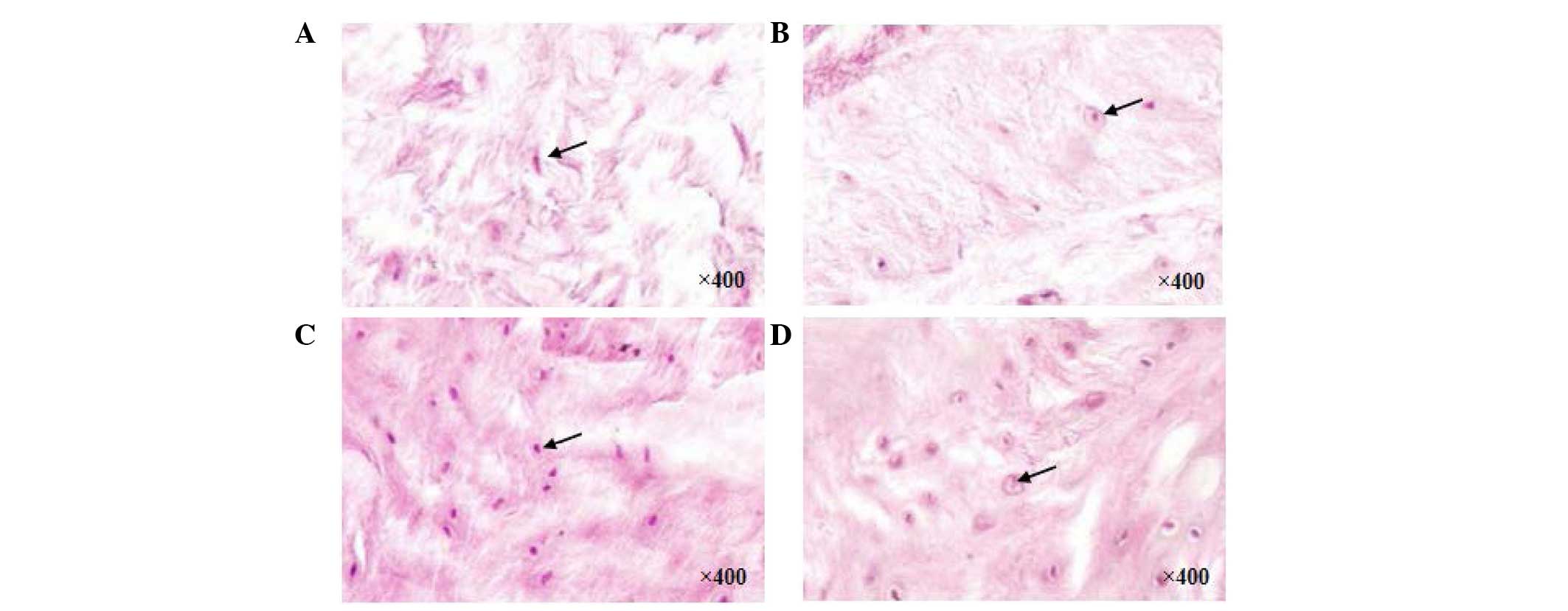
H&E staining assessment
In the control group, discs without any
interventions exhibited a high density of ECM. At 8 weeks following
the different treatments, the discs in the PRP (Fig. 5A) and PBS (Fig. 5B) groups exhibited decreased ECM.
In the PRP-BMSC group (Fig. 5C),
ECM and cell density were well preserved, however, fewer NP cells
were observed in the PRP and PBS groups, compared with the control
group (Fig. 5D).
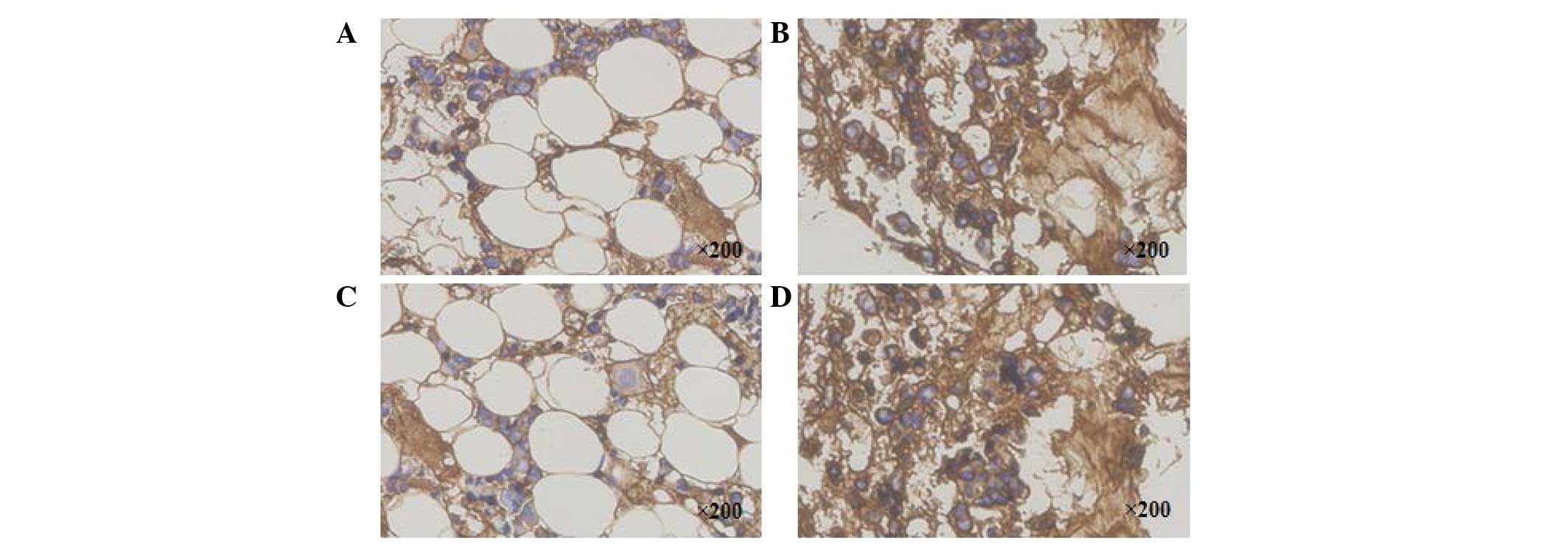
Immunohistochemical staining of type II
collagen
Representative immunohistochemistry of type II
collagen staining exhibited brown staining. The type II collagen
staining of the targeted discs in the PRP group (Fig. 6A) became weakly positive, whereas
the staining in the PRP-BMSC group (Fig. 6B) was strongly positive at week 8.
As with the PRP group, the staining in the PBS group (Fig. 6C) became weakly positive at week 8,
whereas staining in the control group was strongly positive
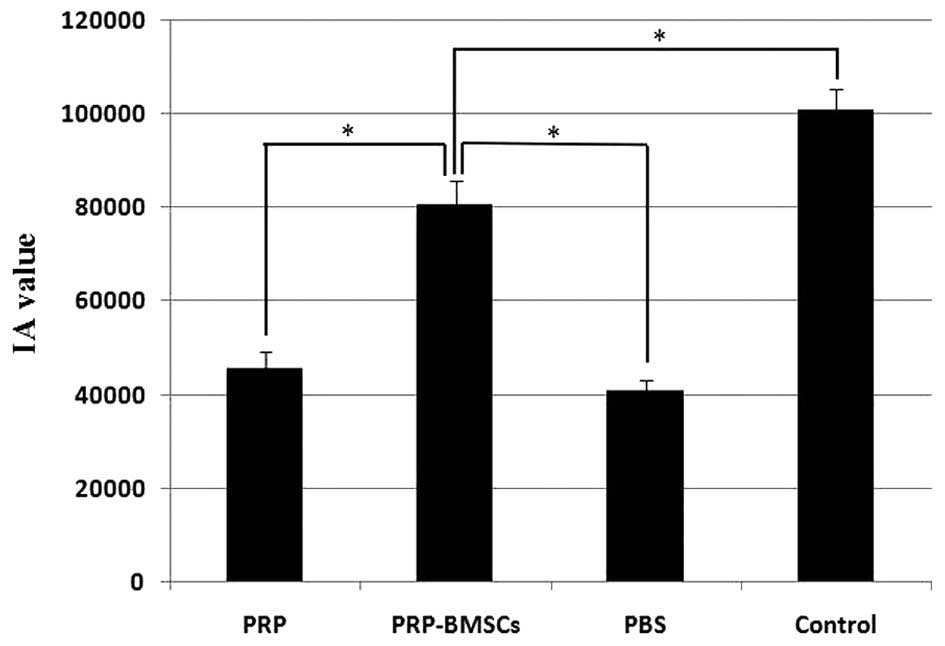
(Fig. 6D). The IA values of the
control group (100910.06±4319.57) and PRP-BMSC group
(80620.30±5030.62) were significantly higher, compared with those
of the PRP group (45682.05±3501.89) and PBS group
(43768.22±2275.67), as shown in Fig.
7 (P<0.05).
Discussion
The present study investigated the efficacy of
PRP-containing BMSCs on the regeneration of early degenerated discs
induced by needle puncture. The results of the present study
demonstrated that the injection of PRP-containing BMSCs was
effective in restoring the early degenerated discs on histological
evaluation. MRI examination at different time points revealed the
reparative effects of the PRP-BMSCs on the early degenerated
discs.
Currently, there remains no comprehensively accepted
classification standard of IDD. Thompson et al (21) suggested a grading system, according
to the gross morphology of the human intervertebral discs (IVDs).
Subsequently, Pfirrmann et al (22) established a new grading system of
IDD, based on MRI (modified Thompson classification). In these two
grading systems, normal IVD is defined as grade I. As the severity
of the degeneration increases, the degeneration increases between
grades II and V. Zhang et al (23) suggested IDD grades II and III be
defined as early stage IDD. In the present study, the efficacy of
21-gauge needle puncture in inducing the early degeneration of IVD
was confirmed using the modified Thompson classification. The
results revealed that the 21-gauge needle induced more severe
degeneration of the punctured discs, compared with that of a
previous study reported by Masuda et al (20). However, the rabbits used in the
present study weighed less, suggesting the discs were of smaller
size, which may be the cause of the inconsistent result.
PRP is a small fraction of the plasma with a high
platelet concentration. The regenerative effect of PRP is based on
its growth factor cocktail effect (24,25).
BMSCs, as a promising therapeutic strategy, have also been widely
investigated in IDD repair and regeneration (26). The results of the present study
indicated that the combined use of PRP and BMSCs exerted a superior
effect, compared with the use of PRP alone. Over the 8 week period,
MRI T2 signal intensity was well maintained in the PRP-BMSC group,
whereas this signal intensity was lower in the PRP group at week 8.
However, at week 2, the regenerative effect of PRP was similar to
that of the PRP-BMSCs, as indicated by MRI. These variations in IDD
regeneration may be a result of the following pathways.
Firstly, subsequent to the intradiscal injection of
the PRP-BMSCs, the activation of PRP contributed to the release of
multiple bio-active growth factors, which assisted in promoting the
restoration of the degenerated discs for the initial 2 weeks.
During this period, the proliferation and migration of the BMSCs
embedded in the PRP did not exhibit such a significant regenerative
effect. However, with degradation of the growth factors, PRP did
not maintain this regenerative effect, whereas BMSCs proliferated
to continuously repair the degenerated discs.
Secondly, the injected PRP may have been activated
by the surrounded tissue, which formed a three-dimensional scaffold
for the BMSCs. Growth factors released from the PRP may effectively
stimulate BMSCs to produce type II collagen and aggrecan, as
indicated by a previous study of Xie et al (27). The increased collagen may provide
tensile strength and anchor the tissue to the bone, and aggrecan
may contribute to high osmotic pressure for the absorption of
water. Thus, the increased ECM may effectively assist in restoring
the biological and mechanical functions of the degenerated
discs.
Thirdly, the residual NP cells within the discs may
interact with the injected BMSCs to repair the degenerated discs.
In a previous in vitro study, Strassburg et al
(28) suggested that cellular
interactions between MSCs and degenerate NP cells stimulate the
endogenous degenerated NP cell population to regain a
non-degenerate phenotype and consequently enhance matrix synthesis
for self-repair. In addition, the BMSCs may also exhibit the
NP-like phenotype with the stimulation of NP cells.
In rabbit IDD models, Obata et al (29) injected PRP-releasate into the
degenerated discs, which contributed to the structural restoration
of IVD over an 8-week period. These results were different from
those of the present study, as PRP only exhibited a regenerative
effect in the initial 2 weeks. However, similar to the present
study, Nagae et al (30)
suggested that PRP alone was not able to effectively suppress the
degenerative trend of IDD. To slow the release of growth factors
from PRP, PRP was injected within gelatin hydrogel microspheres
(PRP-GHMs), therefore, the immobilized PRP was released in a
gradual manner, accompanied by degradation of the microspheres
(31). The PRP-GHMs exhibited a
superior regenerative effect, compared with PRP alone. However, the
different processing methods of the PRP and IDD models in the
present study may account for the conflicting results.
A limitation of the present study is that the rabbit
IDD model used does not reflect the natural course of human IDD. In
addition, the PRP-containing BMSCs were effective in the early
degenerated discs in the present study, however, whether this
therapy can also be effective for severely degenerated discs
remains to be fully elucidated. In the present study, the PRP-BMSCs
were confirmed to exert a regenerative effect, however, the treated
discs were not restored to the level of the normal discs in control
group, following histological and radiographical evaluations within
the 8 weeks. The regenerative trend induced by the PRP-BMSCs in the
long term remains to be elucidated. Currently, there is no
comprehensively accepted PRP preparation or evaluation method, and
the quality of the PRP prepared using commonly applied laboratory
centrifugation procedures is biased. Therefore, the present study
provided preliminary support for PRP-containing BMSCs as a more
effective alternative to PRP in the restoration of early
degenerated discs.
In conclusion, the present study showed that the
administration of autologous PRP-containing BMSCs was more
effective than PRP alone in restoring early degenerated discs
induced by annular needle puncture in a rabbit model. The PRP
injected into the discs was activated by the surrounding tissue,
and formed a three-dimensional scaffold for the proliferation and
differentiation of BMSCs, maintaining a continuous regenerative
effect. The results of the present study suggested that
PRP-containing BMSCs offer an effective and promising therapeutic
strategy for IVD regeneration, providing valuable information for
the clinical use of PRP, based on BMSCs.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81572188) and the
Jiangsu Provincial Special Program of Medical Science (grant no. BL
20122004).
References
|
1
|
Dagenais S, Caro J and Haldeman S: A
systematic review of low back pain cost of illness studies in the
United States and internationally. Spine J. 8:8–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McBeth J and Jones K: Epidemiology of
chronic musculoskeletal pain. Best Pract Res Clin Rheumatol.
21:403–425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mirza SK and Deyo RA: Systematic review of
randomized trials comparing lumbar fusion surgery to nonoperative
care for treatment of chronic back pain. Spine. 32:816–823. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karppinen J, Shen FH, Luk KD, Andersson
GB, Cheung KM and Samartzis D: Management of degenerative disk
disease and chronic low back pain. Orthop Clin North Am.
42:513–528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Chee A, Thonar EJ and An HS:
Intervertebral disk repair by protein, gene, or cell injection: a
framework for rehabilitation-focused biologics in the spine. PM R.
3(6 Suppl 1): S88–S94. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masuda K: Biological repair of the
degenerated intervertebral disc by the injection of growth factors.
Eur Spine J. 17(Suppl 4): S441–S451. 2008. View Article : Google Scholar
|
|
7
|
Xie X, Zhang C and Tuan RS: Biology of
platelet-rich plasma and its clinical application in cartilage
repair. Arthritis Res Ther. 16:2042014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Finnson KW, McLean S, Di Guglielmo GM and
Philip A: Dynamics of transforming growth factor beta signaling in
wound healing and scarring. Adv Wound Care (New Rochelle).
2:195–214. 2013. View Article : Google Scholar
|
|
9
|
Spencer EM, Tokunaga A and Hunt TK:
Insulin-like growth factor binding protein-3 is present in the
alpha-granules of platelets. Endocrinology. 132:996–1001.
1993.PubMed/NCBI
|
|
10
|
Longo UG, Petrillo S, Franceschetti E,
Maffulli N and Denaro V: Growth factors and anticatabolic
substances for prevention and management of intervertebral disc
degeneration. Stem Cells Int. 2012:8971832012.PubMed/NCBI
|
|
11
|
Wang SZ, Rui YF, Tan Q and Wang C:
Enhancing intervertebral disc repair and regeneration through
biology: Platelet-rich plasma as an alternative strategy. Arthritis
Res Ther. 15:2202013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marx RE: Platelet-rich plasma (PRP): What
is PRP and what is not PRP? Implant Dent. 10:225–228. 2001.
View Article : Google Scholar
|
|
13
|
Huang YC, Leung VY, Lu WW and Luk KD: The
effects of microenvironment in mesenchymal stem cell-based
regeneration of intervertebral disc. Spine J. 13:352–362. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benneker LM, Andersson G, Iatridis JC,
Sakai D, Härtl R, Ito K and Grad S: Cell therapy for intervertebral
disc repair: Advancing cell therapy from bench to clinics. Eur Cell
Mater. 27:5–11. 2014.PubMed/NCBI
|
|
15
|
Ikehara S and Li M: Stem cell
transplantation improves aging-related diseases. Front Cell Dev
Biol. 2:162014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakai D: Stem cell regeneration of the
intervertebral disk. Orthop Clin North Am. 42:555–562. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai F, Wu XT, Xie XH, Wang F, Hong X,
Zhuang SY, Zhu L, Rui YF and Shi R: Evaluation of intervertebral
disc regeneration with implantation of bone marrow mesenchymal stem
cells (BMSCs) using quantitative T2 mapping: A study in rabbits.
Int Orthop. 39:149–159. 2015. View Article : Google Scholar
|
|
18
|
Schilling T, Nöth U, Klein-Hitpass L,
Jakob F and Schütze N: Plasticity in adipogenesis and osteogenesis
of human mesenchymal stem cells. Mol Cell Endocrinol. 271:1–17.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Landesberg R, Roy M and Glickman RS:
Quantification of growth factor levels using a simplified method of
platelet-rich plasma gel preparation. J Oral Maxillofac Surg.
58:297–300. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Masuda K, Aota Y, Muehleman C, Imai Y,
Okuma M, Thonar EJ, Andersson GB and An HS: A novel rabbit model of
mild, reproducible disc degeneration by an anulus needle puncture:
Correlation between the degree of disc injury and radiological and
histological appearances of disc degeneration. Spine (Phila Pa
1976). 30:5–41. 2005. View Article : Google Scholar
|
|
21
|
Thompson JP, Pearce RH, Schechter MT,
Adams ME, Tsang IK and Bishop PB: Preliminary evaluation of a
scheme for grading the gross morphology of the human intervertebral
disc. Spine. 15:411–415. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine. 26:1873–1878. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Chee A, Thonar EJ and An HS:
Intervertebral disk repair by protein, gene, or cell injection: A
framework for rehabilitation-focused biologics in the spine. PM R.
3(6 Suppl 1): S88–S94. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fréchette JP, Martineau I and Gagnon G:
Platelet-rich plasmas: Growth factor content and roles in wound
healing. J Dent Res. 84:434–439. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abrams GD, Frank RM, Fortier LA and Cole
BJ: Platelet-rich plasma for articular cartilage repair. Sports Med
Arthrosc. 21:213–219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang SZ, Rui YF, Lu J and Wang C: Cell and
molecular biology of intervertebral disc degeneration: Current
understanding and implications for potential therapeutic
strategies. Cell Prolif. 47:381–390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie X, Wang Y, Zhao C, Guo S, Liu S, Jia
W, Tuan RS and Zhang C: Comparative evaluation of MSCs from bone
marrow and adipose tissue seeded in PRP-derived scaffold for
cartilage regeneration. Biomaterials. 33:7008–7018. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Strassburg S, Richardson SM, Freemont AJ
and Hoyland JA: Co-culture induces mesenchymal stem cell
differentiation and modulation of the degenerate human nucleus
pulposus cell phenotype. Regen Med. 5:701–711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Obata S, Akeda K, Imanishi T, Masuda K,
Bae W, Morimoto R, Asanuma Y, Kasai Y, Uchida A and Sudo A: Effect
of autologous platelet-rich plasma-releasate on intervertebral disc
degeneration in the rabbit anular puncture model: A preclinical
study. Arthritis Res Ther. 14:R2412012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagae M, Ikeda T, Mikami Y, Hase H, Ozawa
H, Matsuda K, Sakamoto H, Tabata Y, Kawata M and Kubo T:
Intervertebral disc regeneration using platelet-rich plasma and
biodegradable gelatin hydrogel microspheres. Tissue Eng.
13:147–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sawamura K, Ikeda T, Nagae M, Okamoto S,
Mikami Y, Hase H, Ikoma K, Yamada T, Sakamoto H, Matsuda K, et al:
Characterization of in vivo effects of platelet-rich plasma and
biodegradable gelatin hydrogel microspheres on degenerated
intervertebral discs. Tissue Eng Part A. 15:3719–3727. 2009.
View Article : Google Scholar : PubMed/NCBI
|