Introduction
Patients with cancer often develop anemia as a
result of the complex interaction of various factors, which renders
treatment of the disease somewhat unpredictable (1,2).
Among the factors contributing to anemia include hemodilution,
bleeding, hypersplenism and hemophagocytosis, hemolysis,
nutritional deficiencies, bone marrow damage, chemotherapy,
radiotherapy and the anemia of the cancer itself (3–6). One
of the causes of anemia in these patients may be due to the
frequently lower than expected levels of circulating erythropoietin
(EPO) for the degree of anemia (7–9).
EPO is a heavily glycosylated glycoprotein produced
in the peritubular cells of the kidneys in response to hypoxia
(10) and is vital as a
hematopoietic hormone regulating erythrocyte production. The
hormone binds to the EPO receptor to cause proliferation,
differentiation and survival of erythoid progenitors. Each EPO
binds to two EPO receptors on the erythroid cell surface to cause
an effect (11–13).
Doxorubicin is the optimal known systemic
chemotherapy, which may be used alone or in combination with a
variety of agents, including, epirubicin, mitoxantrone, cisplatin
and etoposide, in the treatment of breast cancer (14,15).
However, doxorubicin frequently induces anemia in patients with
breast cancer (16,17) by causing systemic changes,
primarily through hemolysis or other conditions that reduce
hemoglobin concentration (18–20).
In patients with breast cancer, the management of anemia is
beneficial for improvement of the survival rates of these
patients.
Previously, EPO was found not to interfere with
tamoxifen or Taxol in the treatment of MCF-7 or MDA-MB231 cells
(21). Whether EPO can similarly
affect the efficacy of doxorubicin in the treatment of breast
cancer remains to be elucidated. Therefore, the present study
investigated the effect of EPO treatment in combination with
doxorubicin on MDA-MB231 and MCF-7 cells. The present study also
examined the mechanism underlying the cytotoxicity of this
combination in these cancer cell lines. The present study aims to
improve cancer therapeutics and provide potential insights to
possible application of the recombinant human erythropoietin and
doxorubicin combination in cancer therapy.
Materials and methods
Cell culture
The three cell lines used in the present study were
obtained from American Type Culture Collection (Rockville, MD,
USA). These cell lines comprised the estrogen receptor-positive
MCF-7 and estrogen receptor-negative MDA-MB-231 human breast cancer
lines, and the normal MCF-10A breast cell line, which were
characterized to be virus negative. These cells grow as an adherent
monolayer of tightly knit epithelial cells.
Cytotoxicity MTT assay
The MCF-7, MDA-MB 231 and MCF-10A cells were seeded
at 1×104 cells/well by adding 200 µl of a
5×104 cells/ml suspension to each well of a 96-well
tissue culture plate. The cells were cultured at 37°C for 24 h in
the presence of 5% CO2 until cell density of 50%
confluence was obtained. The cells were then treated with either 1
µg/ml doxorubicin (Sigma-Aldrich, St. Louis, MO, USA), 1 IU
EPO (Sigma-Aldrich) or a combination of 1 µg/ml doxorubicin
and 1 IU EPO. Following 72 h incubation at 37°C, 20 µl MTT
solution (Sigma-Aldrich; 5 mg/ml) was added to each well and the
plates were re-incubated for 4 h at 37°C. The microplates were
swiftly turned to discard the medium and the formazan precipitate
was dissolved in dimethyl sulfoxide (100%; Ajax Finechem PTY Ltd.,
Sydney, Australia). The microplates were then gently agitated in
the dark for 30 min and the absorbance was determined using a
microtiter plate reader at 570 and 630 nm for background (Model
550; Bio-Rad Laboratories, Inc., Hercules, CA, USA). All
experiments were performed in triplicate. The half maximal
inhibitory concentration (IC50) was determined from
dose-response curves constructed for each cell line.
Neutral red (NR) uptake
The cells were seeded at 1×104 cells/well
into 96-well plates until they reached 40–60% confluence, and were
subsequently incubated overnight at 37°C in the presence of 5%
CO2 for cell attachment. After 24 h, the medium was
removed and replaced with 200 µl fresh growth medium,
containing either 1 µg/ml doxorubicin, 1 IU EPO or a
combination of 1 µg/ml doxorubicin and 1 IU EPO. The plates
were incubated at 37°C, in 5% CO2 for 72 h, following
which the cells were washed three times with 200 µl
phosphate-buffered saline (PBS) followed by the addition of 200
µl NR solution (Sigma-Aldrich). The cells were then
incubated for 3 h at 25°C. The NR solution was removed and the
cells were exposed to fixing solution (Promega Corporation,
Madison, WI, USA; 1% CaCl2 and 0.5% formaldehyde in
milliQ water) for 1–2 min, followed by two washing steps. The
washing solution (Promega Corporation) consisted of 1% acetic acid
and 50% ethanol in milliQ water. Following the second wash, the
plates were incubated for 10 min, following which the plates were
read using a microplate reader at 540 nm. A control experiment was
performed on untreated cells under the same conditions. The
intensity of NR staining was directly proportional to the number of
viable cells.
Lactate dehydrogenase (LDH) assay
To determine the effect of doxorubicin on the
membrane permeability of MCF-7 and MDA MB231 cells, an LDH release
assay was used. The cells were seeded in 96-well culture plates at
a density of 2×104 cells/well in 100 µl and
allowed to grow for 18 h prior to treatment. Treatments were
performed, as described above for the MTT assay. Following
incubation for 72 h, 40 µl of the supernatants were removed
and placed in a fresh 96-well for the determination of LDH release.
The original plate was replenished with 40 µl 6% Triton
X-100 (Sigma-Aldrich) for determination of the total LDH
concentration. An aliquot of 100 µl 4.6 mM pyruvic acid
(Sigma-Aldrich) in 0.1 M potassium phosphate buffer (pH 7.5;
Sigma-Aldrich) was dispensed into each well of the plate containing
the supernatant only, and was mixed via repeated pipetting.
Subsequently, 100 µl of 0.4 mg/ml reduced β-NADH
(Sigma-Aldrich) in 0.1 M potassium phosphate buffer (pH 7.5) was
added to the wells, and the kinetic change, based on the loss of
NADH due to its oxidation to NAD+ as pyruvate is
converted into lactate, was determined. The change in absorbance at
340 nm was read for 1 min using an ELISA microplate reader (Model
550). This procedure was repeated using 40 µl of the total
cell lysate from the original plate containing cells to determine
the total LDH concentration. A change of 0.001 absorbance U/min was
equivalent to 1 U/l of LDH activity (22). The percentage of LDH release was
determined by dividing the LDH released in the supernatant by the
total LDH in the respective cell lysate. Untreated cells retained
LDH and exhibited minimal loss over the time.
Trypan blue exclusion
The MCF-7 and MDA MB231 cells were first seeded at
1×104 cells/well into 6-well plates until they reached
40–60% confluence. Following 24 h of incubation to allow for cell
attachment, the cells were exposed to 1 µg/ml doxorubicin or
a combination of 1 µg/ml doxorubicin and 1 IU EPO. The
plates were then incubated at 37°C in 5% CO2 for 24, 48
and 72 h. Following incubation, the media was removed and the cells
washed with cold PBS to remove dead cells. Subsequently, 1 ml 0.05%
(2 mg/ml) trypsin-EDTA (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was added to each well. The plates were
re-incubated at 37°C for 10–15 min, until the majority of the cells
had detached. The cells were harvested, and the suspension was
centrifuged at 2,000 × g for 10 min at 4°C and the supernatant
discarded. The cell suspension (20 µl) was mixed with 20
µl 0.4% trypan blue solution (Sigma-Aldrich). The cells were
re-suspended and dye-excluded viable cells were microscopically
counted using a Neubauer hemocytometer (Hirschmann Laborgeräte GmbH
and Co. KG, Eberstadt, Germany).
Microscopic examination of cell
morphology
The MCF-7 and MDA MB231 cells (1×104
cells/well) were seeded into 6-well plates. Following incubation
for 24 h with Dulbecco's modified Eagle's medium (Sigma-Aldrich),
the cells were treated either with 1 µg/ml doxorubicin (1
µg/ml), 1 IU/ml EPO or a combination of 1 µg/ml
doxorubicin and 1 IU/ml EPO for 72 h. The untreated cells were used
as a negative control. General morphological and membrane changes
were examined under an inverted microscope (CMM 214; Nikon
Corporation, Tokyo, Japan).
Caspase-3/7 and -9 assays
The extent of caspases-3/7 and -9 activation in the
MDA-MB231 and MCF-7 cells were treated using the same formulations
in the assays described above, and were assessed using a
commercially available colorimetric assay kit, according to the
manufacturer's instructions (CaspACE™ assay system; Promega
Corporation). The caspase activity in a sample is proportional to
the quantity of paranitroaniline (pNA) product detected
spectrophoto-metrically (Lambda 35; PerkinElmer, Inc., Waltham, MA,
USA). This assay uses the caspase-specific substrate L-asp
artic-L-glutamic-L-valyl-L-aspartic acid paranitroaniline
(DEVD-pNA) and L-leucine-L-glutamyl-L-histidyl-L-asp
artic-p-nitroaniline acid amide (LEHD-pNA), labeled with pNA for
caspase-3/7 and-9, respectively. Cleavage of the substrate by the
specific cellular caspase yields free pNA, which can be detected
spectrophotometrically at 405 nm. The cells were plated at a
density of 1×106 cells/culture dish. Following
treatment, the cells were harvested by centrifugation at 2,000 × g
for 10 min at 4°C. The pellets were washed with PBS and lysed in 50
µl chilled cell lysis buffer (Promega Corporation) and
maintained on ice for 10 min. The lysate was centrifuged at 10,000
× g for 1 min at 4°C, and the supernatant was used to determine the
caspase activities, which were measured colorimetrically at 405 nm
by the production of pNA from the cleavage of DEVD-pNA and
LEHD-pNA.
DNA fragmentation
DNA fragmentation was quantitatively determined
using diphenylamine reagent. The cells were treated with
doxorubicin alone or in combination with EPO at different
time-points, and then harvested 12 and 24 h following treatment.
Subsequently, 108 µl of 5 M perchloric acid (Sigma-Aldrich)
was added to the samples, which were heated at 70°C for 15 min,
followed by the addition of two volumes of a solution containing
diphenylamine reagent (Flinn Scientific, Batavia, IL, USA). The
samples were stored at 4°C for 48 h. The colorimetric reaction was
quantified at 575 nm using an ultraviolet-visible thermo smart
orbit spectrophotometer (PerkinElmer, Inc.). DNA from the pellet
and supernatant were quantified. The degree of DNA fragmentation
was determined according to the following equation: Degree of DNA
fragmentation = (DNAsupernatant /
DNA(pellet+supernatant)) × 100% (1).
Statistical analysis
All experiments were completed in triplicate. The
data were expressed as the mean ± standard deviation and analyzed
using Minitab statistical software (version 15; Minitab Inc., State
College, PA, USA). Treatment effects were determined using one-way
analysis of variance followed by Tukey's post hoc analysis. A value
of P<0.05 was considered to indicate a statistically significant
difference.
Results and Discussion
Cell sensitivity to combination of
doxorubicin and EPO
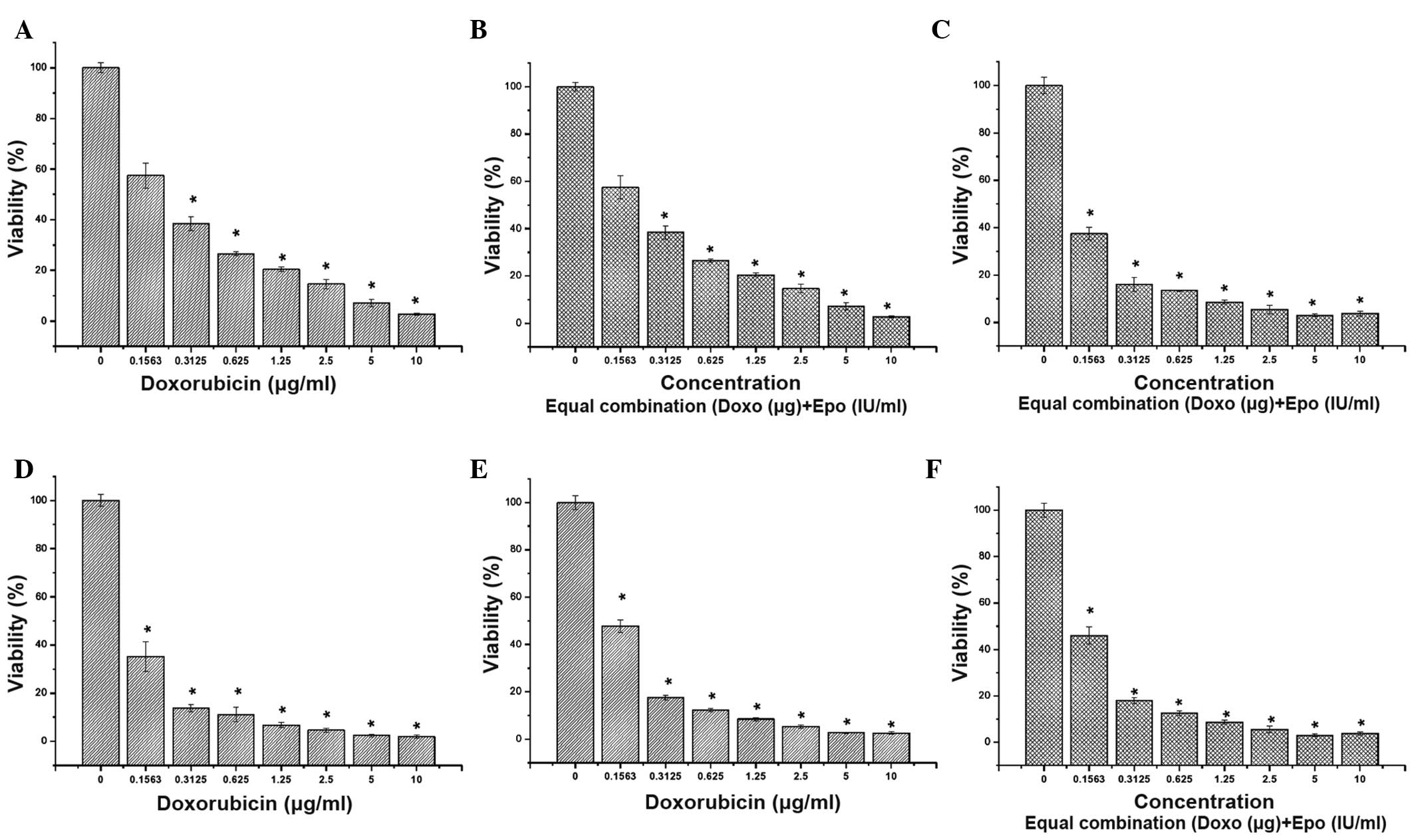
The present study determined the sensitivity of the
MDA-MB-231, MCF-7 and MCF-10A cells to doxorubicin by evaluating
their survival following exposure for 72 h. Doxorubicin reduced the
survival of all three cells in a dose-dependent manner (Fig. 1). Notably, the effect of
doxorubicin was not selective towards breast cancer cells and also
targeted the rapidly dividing normal cells. To investigate the
effect of the combination treatment of doxorubicin and EPO, an
equal combination of the two drugs were used. The combination
treatment affected the cytotoxicity of doxorubicin. The estimated
IC50 values for doxorubicin and the combination of
doxorubicin with EPO were between 0.140 and 0.260 µg/ml
following 72 h treatment (Tables I
and II).
 | Table IIC50 values of DOX-EPO
treatment of breast cancer cells lines for 72 h. |
Table I
IC50 values of DOX-EPO
treatment of breast cancer cells lines for 72 h.
| Treatment | IC50
(µg/ml)
|
|---|
| MCF-7 | MDA-MB231 | MCF-10a |
|---|
| DOX (1
µg/ml) | 0.217 | 0.121 | 0.149 |
| DOX-EPO (1
µg/ml–1 IU/ml) | 0.258 | 0.125 | 0.145 |
 | Table IIIC50 values of DOX-EPO
treatment of breast cancer cells lines for 72 h, determined by
neutral red exclusion assay. |
Table II
IC50 values of DOX-EPO
treatment of breast cancer cells lines for 72 h, determined by
neutral red exclusion assay.
| Treatment | IC50
(µg/ml)
|
|---|
| MCF-7 | MDA-MB231 |
|---|
| DOX (1
µg/ml) | 0.127 | 0.118 |
| DOX-EPO (1
µg/ml-1 IU/ml) | 0.143 | 0.120 |
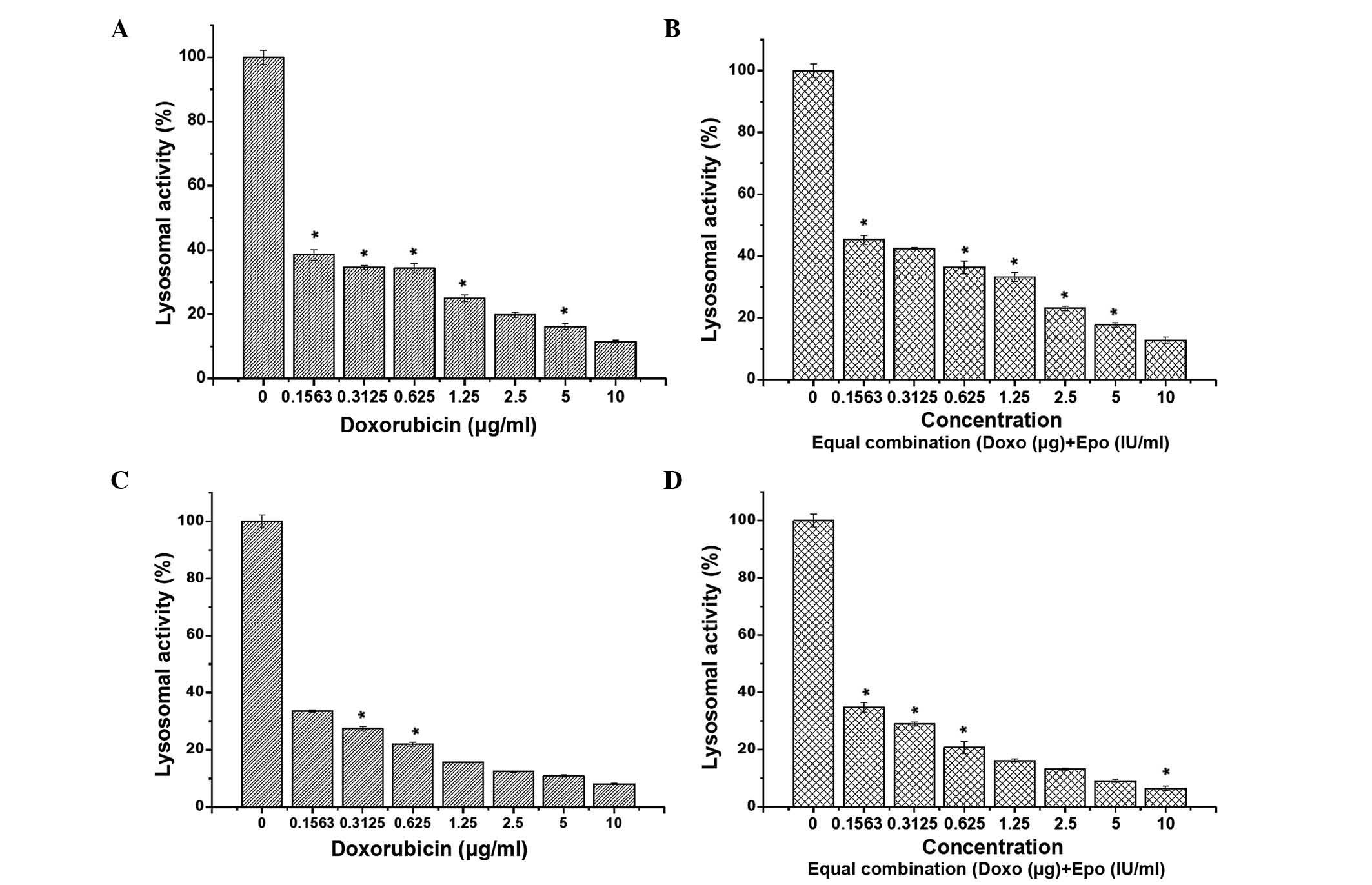
Lysosomal membrane activity
Lysosomes function as digestive system cells
(23), where degrading enzymes are
located (24). In the NR cell
uptake assay, the uptake of dye is considered to occur by passive
diffusion across the viable cell membrane through proton pumps
(25,26). The present study demonstrated that,
in the MCF-7 cells, the lysosomal activity was proportionally
lower, compared with the levels observed in the human breast cancer
cells following treatment with doxorubicin or a combination of
doxorubicin and EPO. This suggested that certain cells that
appeared viable may have lost the ability to accumulate the NR dye,
possibly through the loss of lysosomal membrane stability (Fig. 2). However, in the MDA-MB-231 cells,
this effect was not evident, suggesting that the effect of
doxorubicin and its combination with EPO differed between these
human breast cancer cell lines.
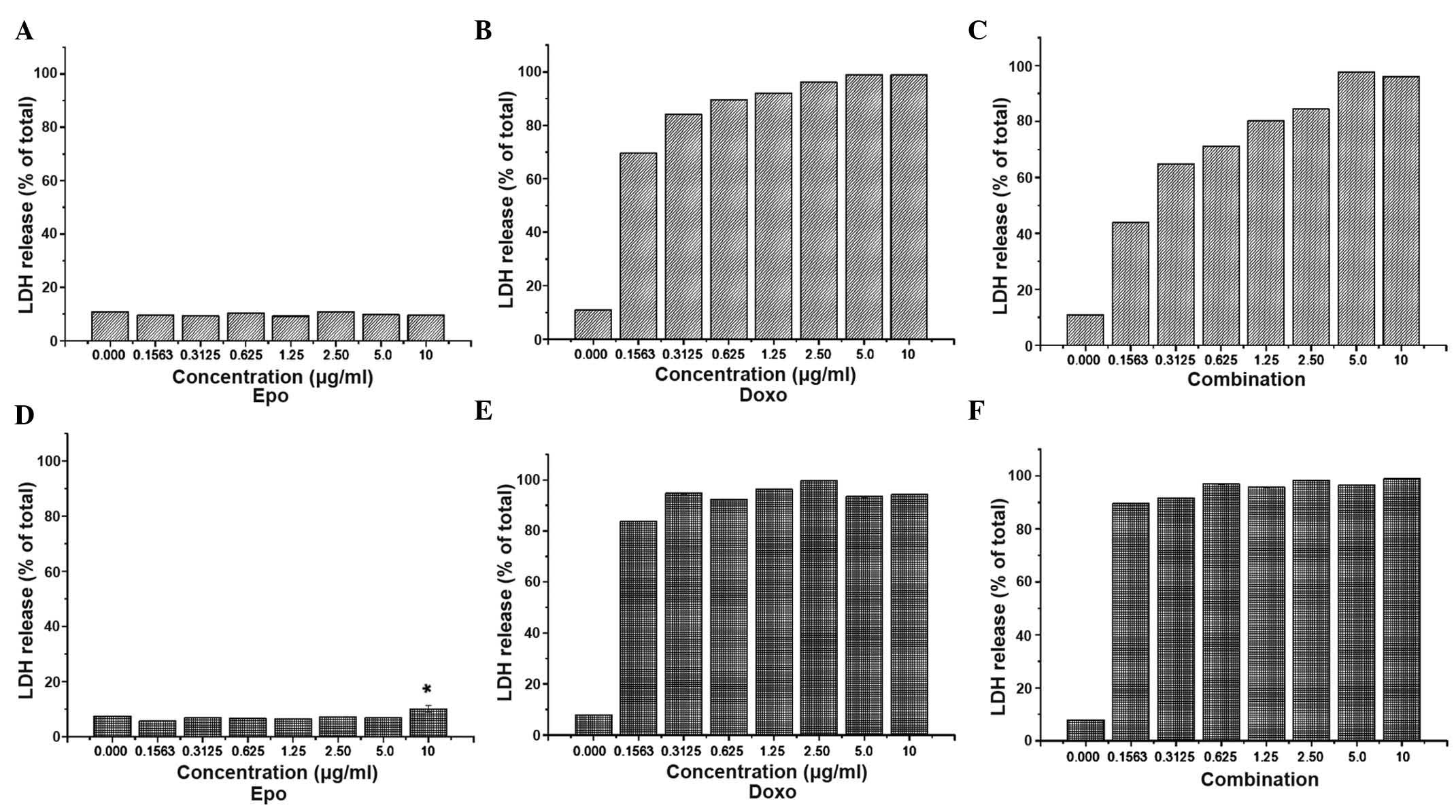
LDH release
LDH is a leakage enzyme, which is released by dead
cells. Thus, the measurement of LDH activity is another indicator
of cell viability. As shown in Fig.
3, LDH release into the culture medium was examined following
72 h exposure to doxorubicin or the combination of doxorubicin with
EPO. The exposure of MCF-7 to 0.31 µg/ml doxorubicin
increased LDH leakage by up to 84%, compared with the increase of
68% observed following treatment with 0.15 µg/ml
doxorubicin. However, increasing the doxorubicin concentration
between 1.25 and 10 µg/ml caused only a marginal increase in
LDH leakage, between 92 and 98%. Following treatment with 0.15
µg/ml doxorubicin and 0.15 IU/ml EPO, MCF-7 cell death
decreased by 43%, which was lower than that observed in the Triton
X-treated cells treated with 0.15 µg/ml doxorubicin (68%).
Therefore, the MDA-MB-231 cells were more sensitive to doxorubicin,
compared with the MCF-7 cells. In the MDA-MB-231 cells, LDH leakage
was 84% following treatment with ~0.15 µg/ml doxorubicin for
72 h, suggesting that this cell line was more sensitive to the
cytotoxic effect of doxorubicin, compared with the MCF-7 cells.
None of the concentrations of the doxorubicin-EPO combination
treatment suppressed the cytotoxicity of doxorubicin alone in the
MDA-MB-231 cell line.
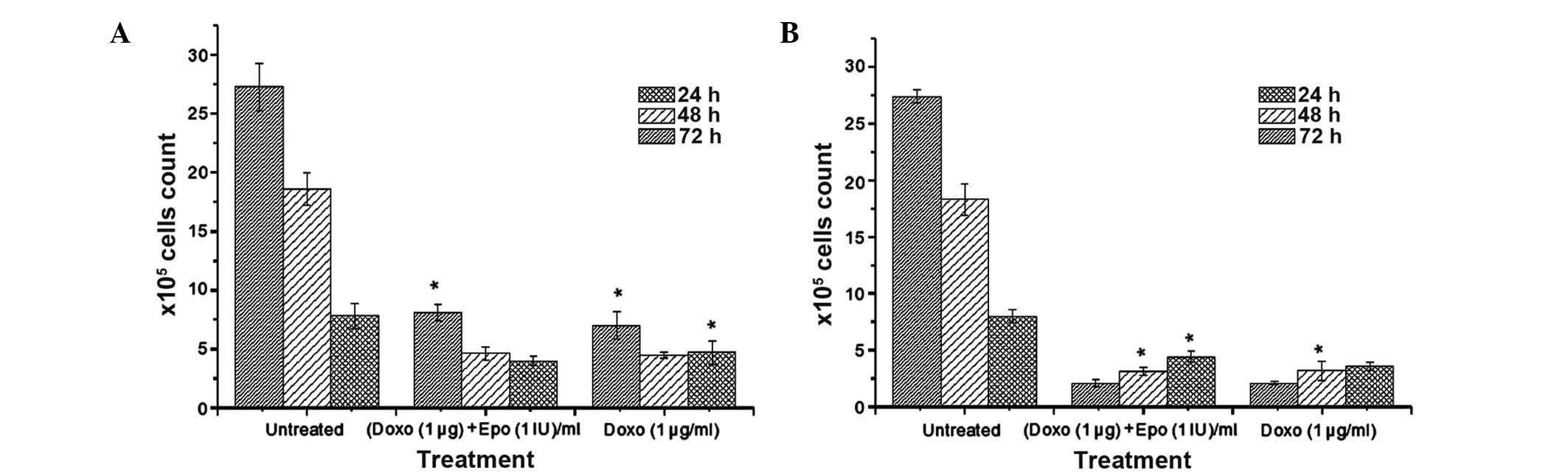
Antiproliferation assay
The results of the antiproliferative effect of
doxorubicin either alone or in a combination with EPO on the MCF-7
and MDA-MB-231 cells following 24, 48 and 72 h of incubation are
shown in Fig. 4. Consistent with
the findings from the MTT assay, the combination of doxorubicin and
EPO was marginally less cytotoxic to the MCF-7 cells, compared with
doxorubicin treatment alone. The results also demonstrated that the
MDA-MB-231 cells were more sensitive to doxorubicin, compared with
the MCF-7 cells. The viable cell counts for the untreated
MDA-MB-231 following 24, 48 and 72 h incubation were 8.0, 18.3 and
27.4×105/ml, respectively. As expected, the percentage
survival of the doxorubicin-treated MDA-MB-231 cells decreased
markedly with increase in exposure duration, which was in contrast
to the untreated cells. The ratio of the viable cell to untreated
cell counts at 24 and 72 h in the doxorubicin-EPO combination
treatment group did not differ significantly to that in the
doxorubicin alone treatment group.
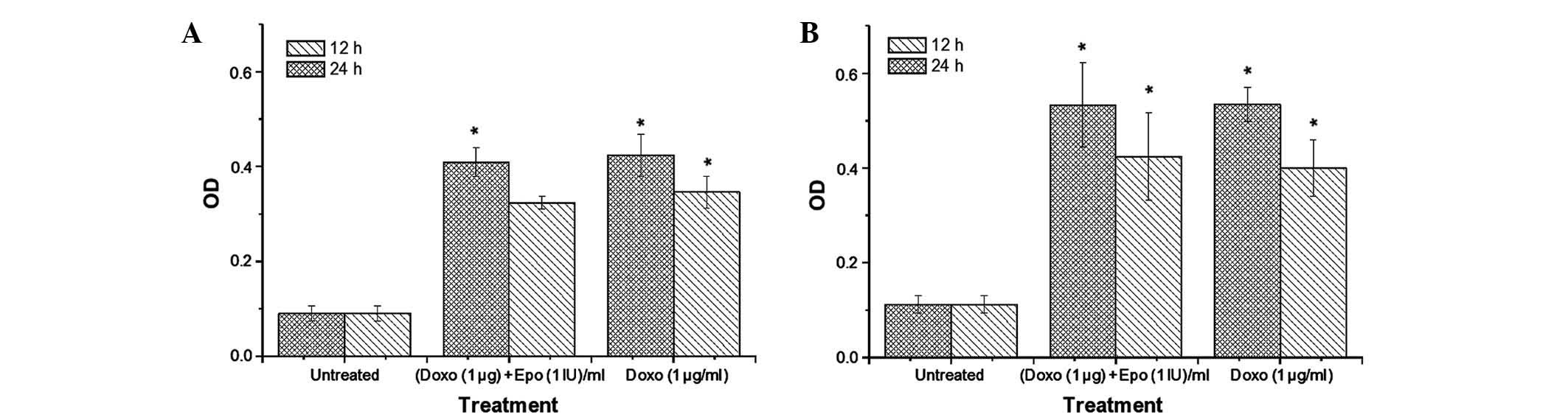
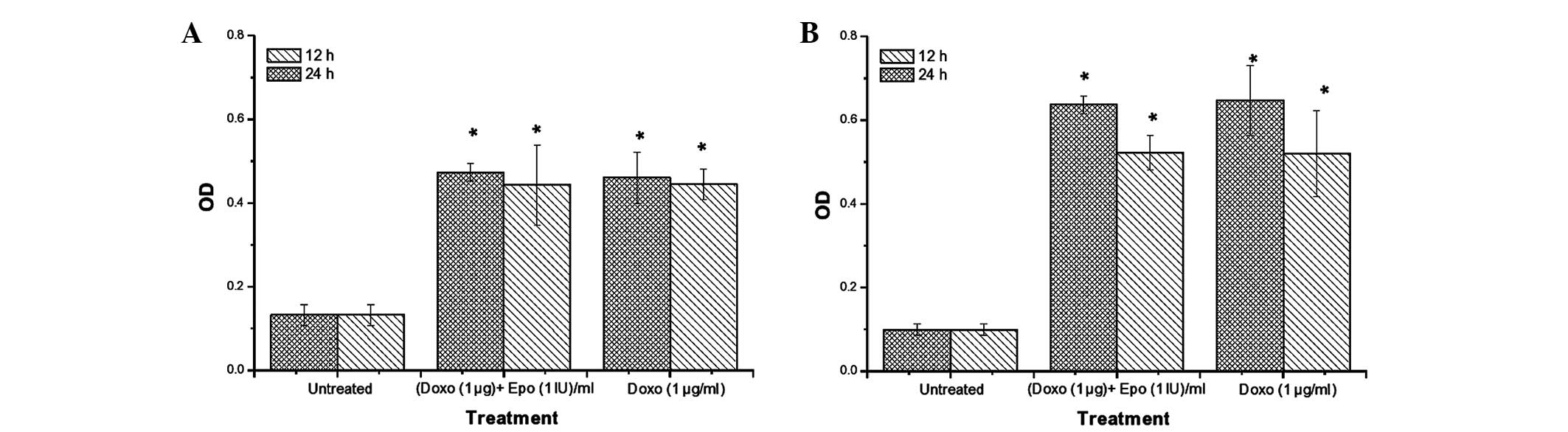
Caspase-3/7 and -9 activities
Caspase-9 is an initiator of the
mitochondria-mediated (intrinsic) apoptotic pathway (27), whereas caspase-3 is a major
enzymatic marker of apoptosis (28). In the present study, the activities
of caspase-3/7 (Fig. 5) and -9
(Fig. 6) increased significantly
in the MCF-7 and MDA-MB-231 cells, compared with the untreated
cells. Notably, the increase in caspase-3/7 activity appeared to be
time-dependent. The highest caspase activities were observed in the
MDA-MB-231 cells treated with doxorubicin. Doxorubicin-EPO
combination treatment caused no significant difference in caspase
activities, compared with the Dox alone group. After 24 h, the
activity of caspase-3/7 in the MDA-MB-231 were 571 and 476% higher,
compared with the untreated cells following doxorubicin and
doxorubicin-EPO combination treatments respectively. By contrast,
the same treatments produced 471 and 571% increases in caspase-3/7
activities, respectively, in the MCF-7 cells. Doxorubicin and its
combination with EPO appeared to induce a more marked increase in
caspase-9 activity in the MDA-MB-231 cells, compared with the MCF-7
cells. Following treatment for 24 h, the caspase activity in the
MDA-MB-231 cells following doxorubicin and doxorubicin-EPO
combination treatments were 645 and 635% higher than in the
untreated cells, whereas the activities of this enzyme were 345 and
356% for the respective treatments in the MCF-7 cells. These
results suggested that EPO did not alter the stimulatory effect of
doxorubicin on the activities of caspases-3/7 and -9 in the breast
cancer cell lines.
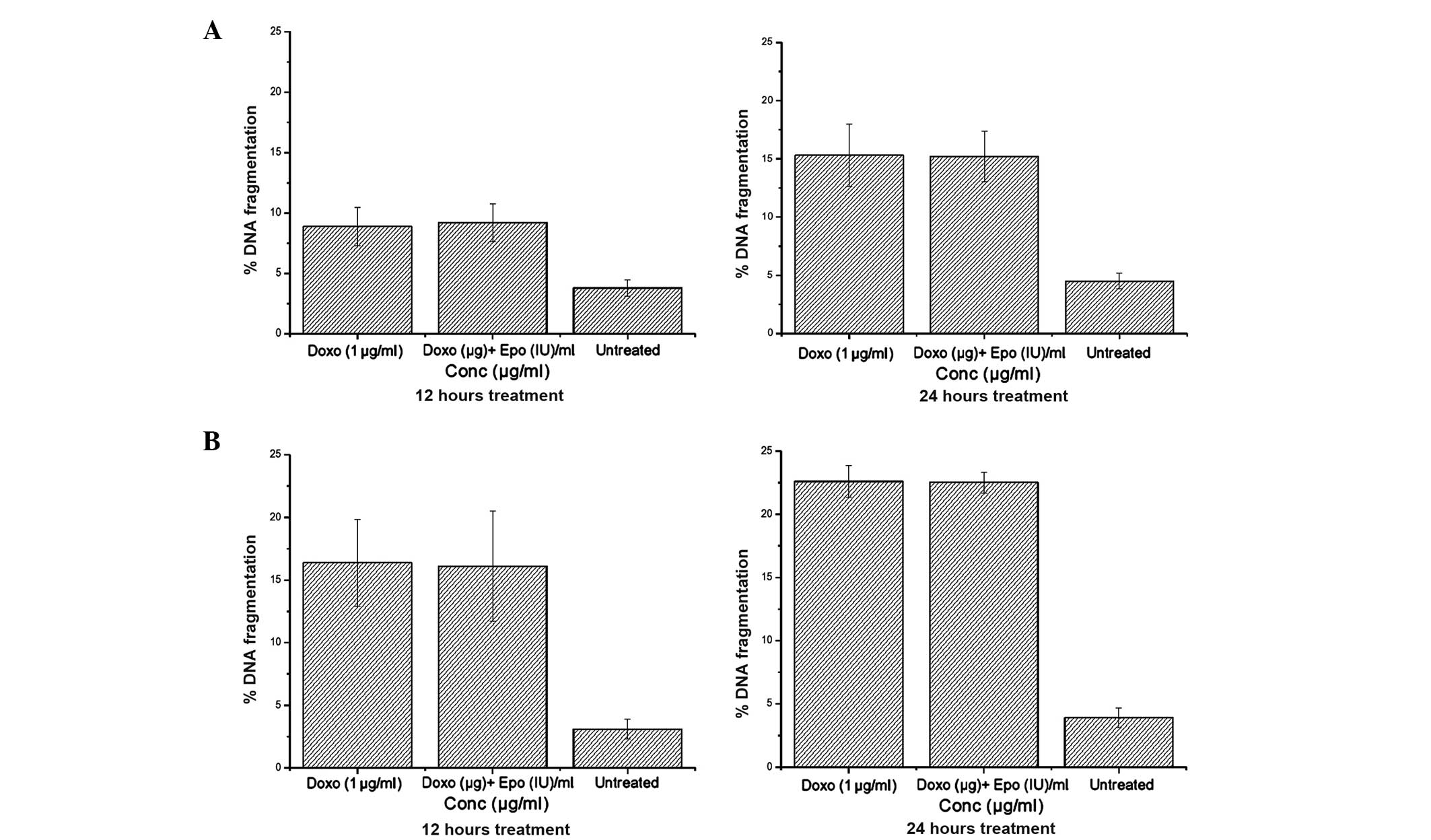
DNA fragmentation
The relative quantity of small DNA fragments in the
cells treated with 1 µg/ml doxorubicin is shown in Fig. 7. In the two breast cancer cell
lines, doxorubicin treatment increased DNA fragmentation in a
time-dependent manner. No difference in DNA fragmentation was
observed between the cells treated with doxorubicin alone or with
doxorubicin in combination with EPO, however, these treatments
caused 338 and 573% increases in DNA fragmentation in the MCF-7 and
MDA-MB-231, respectively, compared with the untreated cells. These
results suggested that doxorubicin was more sensitive to the
estrogen-negative MDA-MB-231 cells than the estrogen-positive MCF-7
cells, and EPO did not modify the effect of doxorubicin.
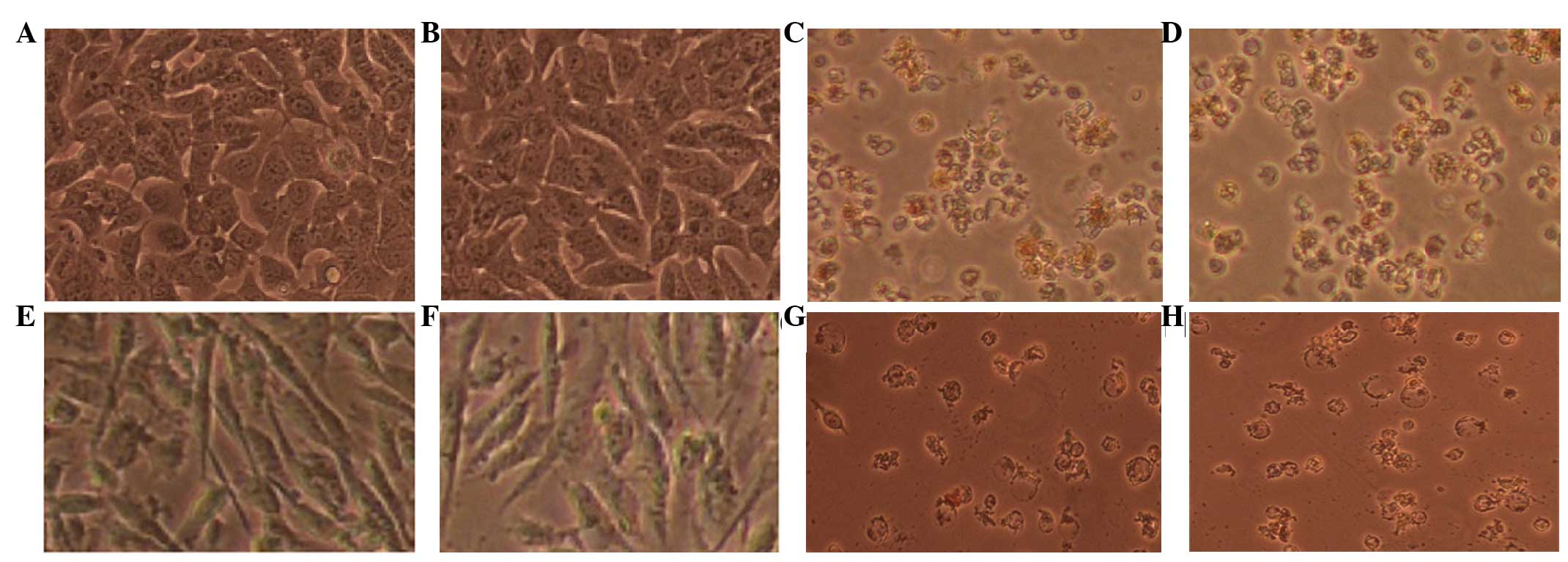
Morphological alterations
The detachment of dead cells following exposure to
doxorubicin was monitored using microscopic technique. Healthy
cells remain elongated, whereas dying or dead cells are rounded and
lose their adhesion to the culture plate. In the present study,
inverse and fluorescence microscopy was performed following 72 h
treatment of the MCF-7 and MDA MB231 cells with doxorubicin, which
revealed marked morphological changes (Fig. 8). Cellular extensions were
detected, cells were rounded and partially detached from the
culture flask, and cellular membranes exhibited extensive
blebbings.
Following 72 h incubation with 1.0 µg/ml
doxorubicin and its combination with EPO, the majority of the
adhered MCF-7 cells were spherical and exhibited a markedly
different morphology. The morphological changes caused by
doxorubicin in the MCF-7 cells included detachment and floating of
the cells, the presence of shrunken and dispersed cells, and a
reduction in the formation of a monolayer. After 72 h, 1
µg/ml doxorubicin caused substantial morphological changes
when added to the MDA MB231 cells, with cells being detached,
shrunken and dispersed, and membrane blebbing and cytoplasmic
shrinkage observed. EPO (1 IU/ml) did not exhibit any changes in
either of these cell lines. The above-mentioned changes were more
apparent in the MDA MB231 cells, compared with the MCF-7 cells when
subjected to the doxorubicin treatment for 72 h.
The effect of doxorubicin or its combination with
EPO were not selective for breast cancer cells as it also affected
rapidly dividing normal breast cancer cells. However, the results
of the cell viability investigations demonstrated that the MDA
MB231 cells were more sensitive to the effect of doxorubicin or
doxorubicin-EPO combination, compared with the MCF-7 cells. The
results of the present study suggested that treatment with
doxorubicin, either alone or in combination with EPO, induced
apoptosis of the MCF-7 and MDA-MB-231 cells in a time-dependent
manner, more through the caspase-9 than the caspase-3 pathway. This
finding correlates with the observed increase in DNA fragmentation
in the breast cancer cell lines following treatment. Of note, EPO
did not modify the cytotoxicity of doxorubicin in the breast cancer
cell lines, suggesting that these drugs can be safely used in
combination in patients with breast cancer exhibiting symptoms of
anemia.
Acknowledgments
The authors would like to thank the Institute of
Bioscience and the University Putra Malaysia (Serdang, Malaysia)
for supporting the present study.
References
|
1
|
Barrett-Lee P, Bokemeyer C, Gascón P,
Nortier JW, Schneider M, Schrijvers D and Van Belle S; ECAS
Advisory Board and Participating Centers: Management of
cancer-related anemia in patients with breast or gynecologic
cancer: New insights based on results from the European Cancer
Anemia Survey. Oncologist. 10:743–757. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cella D: The functional assessment of
cancer therapy-Anemia (FACT-An) scale: A new tool for the
assessment of outcomes in cancer anemia and fatigue. Semin Hematol.
34(3 Suppl 2): 13–19. 1997.PubMed/NCBI
|
|
3
|
Mughal TI: Current and future use of
hematopoietic growth factors in cancer medicine. Hematol Oncol.
22:121–134. 2004. View
Article : Google Scholar
|
|
4
|
Beguin Y: Prediction of response to
optimize outcome of treatment with erythropoietin. Semin Oncol.
25(Suppl 7): 27–34. 1998.PubMed/NCBI
|
|
5
|
Beguin Y: Erythropoiesis and
erythropoietin in multiple myeloma. Leuk Lymphoma. 18:413–421.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beguin Y and Vanstraelen G: Prediction of
response to recombinant human erythropoietin in the anemia of
cancer. Recombinant Human Erythropoietin (rhEPO) in Clinical
Oncology. Nowrousian MR: 2nd edition. Springer; New York, NY: pp.
541–582. 2008, View Article : Google Scholar
|
|
7
|
Leonard RC, Untch M and Von Koch F:
Management of anaemia in patients with breast cancer: Role of
epoetin. Ann Oncol. 16:817–824. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aapro M, Leonard RC, Barnadas A, Marangolo
M, Untch M, Malamos N, Mayordomo J, Reichert D, Pedrini JL, Ukarma
L, et al: Effect of once-weekly epoetin beta on survival in
patients with metastatic breast cancer receiving
anthracycline-and/or taxane-based chemotherapy: Results of the
breast cancer-anemia and the value of erythropoietin (BRAVE) study.
J Clin Oncol. 26:592–598. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seal S, Thompson D, Renwick A, Elliott A,
Kelly P, Barfoot R, Chagtai T, Jayatilake H, Ahmed M, Spanova K, et
al: Truncating mutations in the Fanconi anemia J gene BRIP1 are
low-penetrance breast cancer susceptibility alleles. Nat Genet.
38:1239–1241. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hale SA, Wong C and Lounsbury KM:
Erythropoietin disrupts hypoxia-inducible factor signaling in
ovarian cancer cells. Gynecol Oncol. 100:14–19. 2006. View Article : Google Scholar
|
|
11
|
Fisher JW: Erythropoietin: Physiology and
pharmacology update. Exp Biol Med (Maywood). 228:1–14. 2003.
|
|
12
|
Fu P and Arcasoy MO: Erythropoietin
protects cardiac myocytes against anthracycline-induced apoptosis.
Biochem Biophys Res Commun. 354:372–378. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lappin TR, Maxwell AP and Johnston PG:
EPO's alter ego: Erythropoietin has multiple actions. Stem Cells.
20:485–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Henderson IC, Berry DA, Demetri GD,
Cirrincione CT, Goldstein LJ, Martino S, Ingle JN, Cooper MR, Hayes
DF, Tkaczuk KH, et al: Improved outcomes from adding sequential
paclitaxel but not from escalating Doxorubicin dose in an adjuvant
chemotherapy regimen for patients with node-positive primary breast
cancer. J Clin Oncol. 21:976–983. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ayers M, Symmans WF, Stec J, Damokosh AI,
Clark E, Hess K, Lecocke M, Metivier J, Booser D, Ibrahim N, et al:
Gene expression profiles predict complete pathologic response to
neoadjuvant paclitaxel and fluorouracil, doxorubicin and
cyclophosphamide chemotherapy in breast cancer. J Clin Oncol.
22:2284–2293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kirshner J, Hatch M, Hennessy DD, Fridman
M and Tannous RE: Anemia in stage II and III breast cancer patients
treated with adjuvant doxorubicin and cyclophosphamide
chemotherapy. Oncologist. 9:25–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Melichar B, Solichova D, Melicharova K,
Cermanova M, Urminska H and Ryska A: Systemic immune activation,
anemia and thrombocytosis in breast cancer patients treated by
doxorubicin and paclitaxel. Pteridines. 17:107–114. 2006.
View Article : Google Scholar
|
|
18
|
Shuai X, Ai H, Nasongkla N, Kim S and Gao
J: Micellar carriers based on block copolymers of poly
(epsilon-caprolactone) and poly (ethylene glycol) for doxorubicin
delivery. J Control Release. 98:415–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lewin SN, Mutch DG, Whitcomb BP, Liapis H
and Herzog TJ: Three cases of hemolytic uremic syndrome in ovarian
cancer patients treated with combination gemcitabine and pegylated
liposomal doxorubicin. Gynecol Oncol. 97:228–233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang KW, Chun MK, Kim O, Subedi RK, Ahn
SG, Yoon JH and Choi HK: Doxorubicin-loaded solid lipid
nanoparticles to overcome multidrug resistance in cancer therapy.
Nanomedicine. 6:210–213. 2010.PubMed/NCBI
|
|
21
|
Gewirtz DA, Di X, Walker TD and Sawyer ST:
Erythropoietin fails to interfere with the antiproliferative and
cytotoxic effects of antitumor drugs. Clin Cancer Res.
12:2232–2238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jemmerson R, Laplante B and Treeful A:
Release of intact, monomeric cytochrome c from apoptotic and
necrotic cells. Cell Death Differ. 9:538–548. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Novikoff AB: Lysosomes in the physiology
and pathology of cells: contributions of staining methods. Ciba
Foundation Symposium-Lysosomes. de Reuck AVS and Cameron MP: pp.
36–77. 2008
|
|
24
|
Bonifacino JS and Traub LM: Signals for
sorting of transmembrane proteins to endosomes and lysosomes. Annu
Rev Biochem. 72:395–447. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lloyd JB: Lysosome membrane permeability:
Implications for drug delivery. Adv Drug Deliv Rev. 41:189–200.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Macintyre AC and Cutler DJ: The potential
role of lysosomes in tissue distribution of weak bases. Biopharm
Drug Dispos. 9:513–526. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dörrie J, Gerauer H, Wachter Y and Zunino
SJ: Resveratrol induces extensive apoptosis by depolarizing
mitochondrial membranes and activating caspase-9 in acute
lymphoblastic leukemia cells. Cancer Res. 61:4731–4739.
2001.PubMed/NCBI
|
|
28
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|