Introduction
Prostate cancer is a common cancer of the male
genitourinary system, with the 3rd highest morbidity
worldwide (1). According to
GLOBOCAN 2008, a project that offered the estimated cancer
incidence, mortality and prevalence worldwide in 2008 by the World
Health Organization, the morbidity of prostate cancer in 2008 was
14%, ranking 2nd among male cancer morbidities
worldwide, just behind lung cancer (2). A significant difference of prostate
cancer morbidity emerged in different parts of the world, with a
differential of 25:1 (2). The age
standardized morbidity of prostate cancer in developing countries
is 0.012%, the cumulative incidence of 0–74-year-old male is 1.4%,
which is 6th highest worldwide (2). By contrast, the age standardized
morbidity in developed countries is 0.062% and the cumulative
incidence of 0–74-year-old male is 7.8%, which is the highest
worldwide (2).
Cofilin-1 is a major member of cofilin/actin
depolymerizing factors, the monomer of which combines with actin
monomers and depolymerizes them from actin bottoms in two ways; One
of which is increasing the depolymerization speed of actin monomer
from actin bottoms, the other is cutting the actin microfilament
into fragments (3). Reconstruction
of actin skeleton serves a decisive role in tumor cell invasion and
metastasis (4). Actin regulatory
systems, including cofilin-1, are crucial in regulating the
formation of cancer cell pseudopods (5). Changes in the expression and activity
of cofilin-1 were revealed in vivo, in tissues of oral
squamous cell carcinoma, renal cell carcinoma and ovarian cancer,
as well as in the carcinoma cell lines cultured in vitro
(5,6). A previous study demonstrated that the
activation of cofilin-1 led to the lamellipodia formation of breast
cancer cells (7). When the
expression of cofilin-1 in the mammary gland MTLn3 cell line was
restrained, the invasive pseudopods of breast cancer cells failed
to completely mature, which explained the important role of
cofilin-1 in the formation of cancer cell pseudopods (7,8).
As one of the downstream regulatory proteins of
focal adhesion kinase (FAK), paxillin is able to control cell
metastasis and migration (9).
Paxillin is closely associated with the mitogen activated protein
kinase (MAPK)/FAK signaling pathway, since paxillin and FAK are
required for the MAPK/FAK signaling pathway to regulate the
adhesion process of fibroblasts. Proepithelin is involved in the
migration and invasion of cancer cells by activating
extracellular-regulated kinase 1/2 and forming the paxillin-FAK
complex (10). However, the
mechanism by which the paxillin-FAK complex effects the processes
that prostaglandin 2 influences the attachment, migration and
invasion of cancer cells remains to be elucidated (10,11).
As a type of docetaxel drugs, docetaxel is the first
cytotoxic drug with a definite effect on hormone refractory
prostate cancer (12). Experiments
in vitro revealed that the growth of the prostate cancer
cell line was effectively inhibited by docetaxel, a drug whose
anticancer activity is higher than taxol because of higher
intracellular drug level and longer retention time (13). Besides, docetaxel is free of
serious adverse reactions, particularly to the heart (14). With the effects of inhibiting
prostate specific antigen increments, relieving pain and reducing
adverse reactions from chemotherapy, docetaxel can delay tumor
progression, reduce the symptoms and significantly prolong patient
lives (15). The present study
hypothesized that the anticancer effect of docetaxel induces the
apoptosis of prostate cancer via the cofilin-1 and paxillin
signaling pathways.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were acquired from Hyclone (St. Louis, MO, USA)
and Thermo Fisher Scientific, Inc. (Waltham, MA, USA),
respectively. Streptomycin, penicillin,
3.3-(4,5-dimeth-ylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) and lactate dehydrogenase (LDH) assays were acquired from
Sigma-Aldrich (St. Louis, MO, USA). The EnzChek Caspase-3 assay kit
was acquired from Molecular Probes (Eugene, OR, USA).
Cell culture and assays of cell
growth
The human androgen-sensitive human LNCaP prostate
cancer cells were obtained from the central laboratory of The
Second Hospital of Shandong University (Shandong, China) and were
grown in DMEM, supplemented with 10% FBS and antibiotics (100 mg/ml
streptomycin and 100 IU/ml penicillin) in a 5%
CO2-humidified incubator at 37°C.
Assays of cell growth and
cytotoxicity
LNCaP cells (5×103 cells/well) were
seeded into 96-well plates and were incubated with docetaxel (1–50
nM) for 24 h. Following treatment, 20 µl MTT was added into
each well and incubated in a 5% CO2-humidified incubator
at 37°C. Subsequently, 150 µl dimethyl sulfoxide was added
to each well and the cells were agitated for 20 min. Cell growth
was measured using a Synergy H1 plate reader (Bio-Tek, Seattle, WA,
USA) at 540 nm. Meanwhile, LNCaP cells (5×103
cells/well) were seeded into 96-well plates and incubated with
docetaxel (1–50 nM) for 24 h. Following treatment, the cells were
lysed using 0.1% (w/v) Triton-X-100 in (0.9%) NaCl. LDH was
subsequently added to each well and the cytotoxicity was determined
spectrophotometrically at 490 nm on a Synergy H1 plate reader,
according to the manufacturer's protocol.
Assays of cell apoptosis
LNCaP cells (1×106 cells/well) were
seeded into 6-well plates and incubated with docetaxel (5, 7.5 or
10 nM) for 24 h. Following treatment, cell apoptosis was measured
on a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA)
using Annexin V-fluorescent isothiocyanate (FITC)/propidium iodide
(PI) staining (BestBio, Shanghai, China), according to the
manufacturer's protocol. LNCaP cells were washed twice with cold
phosphate-buffered saline (PBS) and resuspended using 500 µl
binding buffer (BestBio). Annexin V-FITC and PI (5 µl) were
added and incubated for 30 min at 4°C in the dark. The samples were
analyzed by flow cytometry and data was analyzed with FACSDiva™
software, version 7.0 (BD Biosciences).
Assays of caspase-3 activity
LNCaP cells (1×106 cells/well) were
seeded into 6-well plates and incubated with docetaxel (5, 7.5 and
10 nM) for 24 h. Following treatment, caspase-3 activity was
measured at 460 nm spectrophotometrically using EnzChek Caspase-3
assay kit, according to the manufacturer's protocol on a Synergy H1
plate reader.
Western blotting
LNCaP cells (1×106 cells/well) were
seeded into 6-well plates and incubated with docetaxel (5, 7.5 and
10 nM) for 24 h. Following treatment, LNCaP cells were incubated
with lysis buffer (PBS containing 1% Triton-X-100 and protease
inhibitors) for 20 min at 4°C. The supernatants were harvested by
centrifugation at 13,800 × g for 10 min at 4°C. A bicinchoninic
acid Protein Assay kit (Thermo Fisher Scientific, Inc.) was used to
measure the protein concentrations. Equal quantities (500
µg/ml) were separated using 10–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and the proteins were
transferred onto a polyvinylidene difluoride (PVDF) membrane by
standard procedures (Bio-Rad Laboratories, Inc., Munich, Germany).
The PVDF membrane was blocked with 5% non-fat dry milk in PBS
containing 0.1% Tween 20 (pH 7.6; PBST) for 2 h. The membrane was
subsequently incubated with the appropriate antibodies: mouse
monoclonal anti-Phosphorylated (p-) cofilin-1 (cat. no. sc-53934)
or mouse monoclonal p-paxillin (1:2,000; Santa Cruz
Biotechnologies, Inc., Santa Cruz, CA, USA; cat. no. sc-365379),
diluted in PBST overnight at 4°C. The membranes were washed three
times with PBST and incubated with a goat anti-mouse secondary
antibody (1:1,000; Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd., Beijing, China) diluted in PBST for 2 h at room temperature.
The membranes were incubated with an enhanced chemiluminescence kit
(Amer-sham Biosciences, Freiburg, Germany) and the protein levels
were quantitatively analyzed using ImageJ software (imagej.nih.gov/ij/).
Small interfering (si)RNA complex
formation
Cofilin-1 siRNA and control siRNA were purchased
from Sangon Biotech (Shanghai, China). LNCaP cells
(1×106 cells/well) were seeded into 6-well plates and
were allowed to grow to 70–80% confluence prior to transfection.
The medium was replaced with fresh medium and the LNCaP cells were
transfected with a mixture of 100 nmol/l siRNA and Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol.
Statistical analysis
All statistical analyses were performed using SPSS
18.0 software (IBS SPSS, Chicago, IL, USA) and are expressed as the
mean ± standard deviation from at least three experiments.
Comparisons between two groups were performed using Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Anticancer effect of docetaxel on cell
growth in LNCaP cells
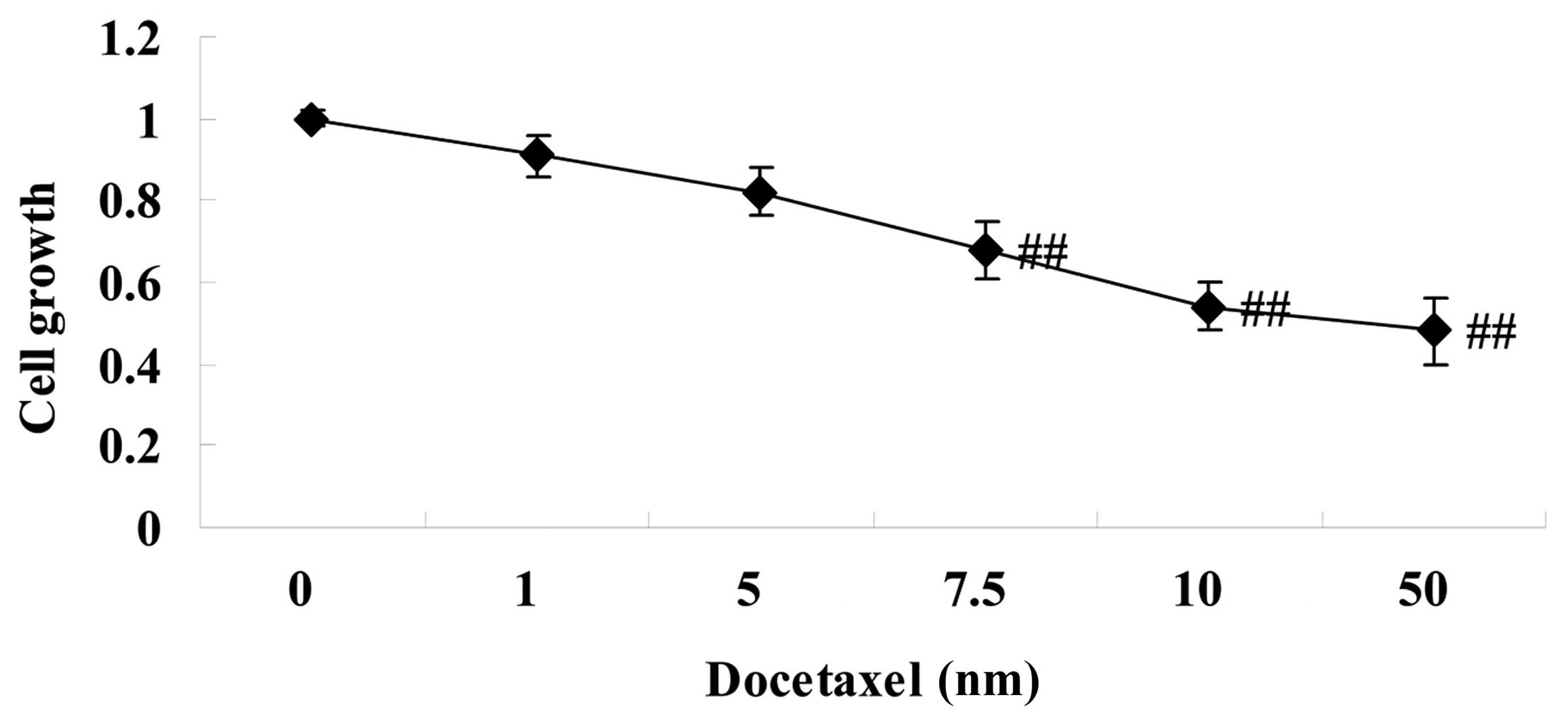
To address the hypothesis that docetaxel exerts
anticancer effects on human prostate cancer, the present study
initially investigated the effect of pretreatment with docetaxel
(1–50 nM) on LNCaP cells for 24 h. The effects of increasing drug
concentrations were first measured using MTT assays. At 24 h
post-exposure, cell growth of LNCaP cells was suppressed in a
dose-dependent manner, as measured by MTT assays (Fig. 1). Notably, 7.5–50 nm docetaxel
significantly suppressed cell growth of LNCaP cells, compared with
the 0 nm docetaxel group (Fig.
1).
Anticancer effect of docetaxel on
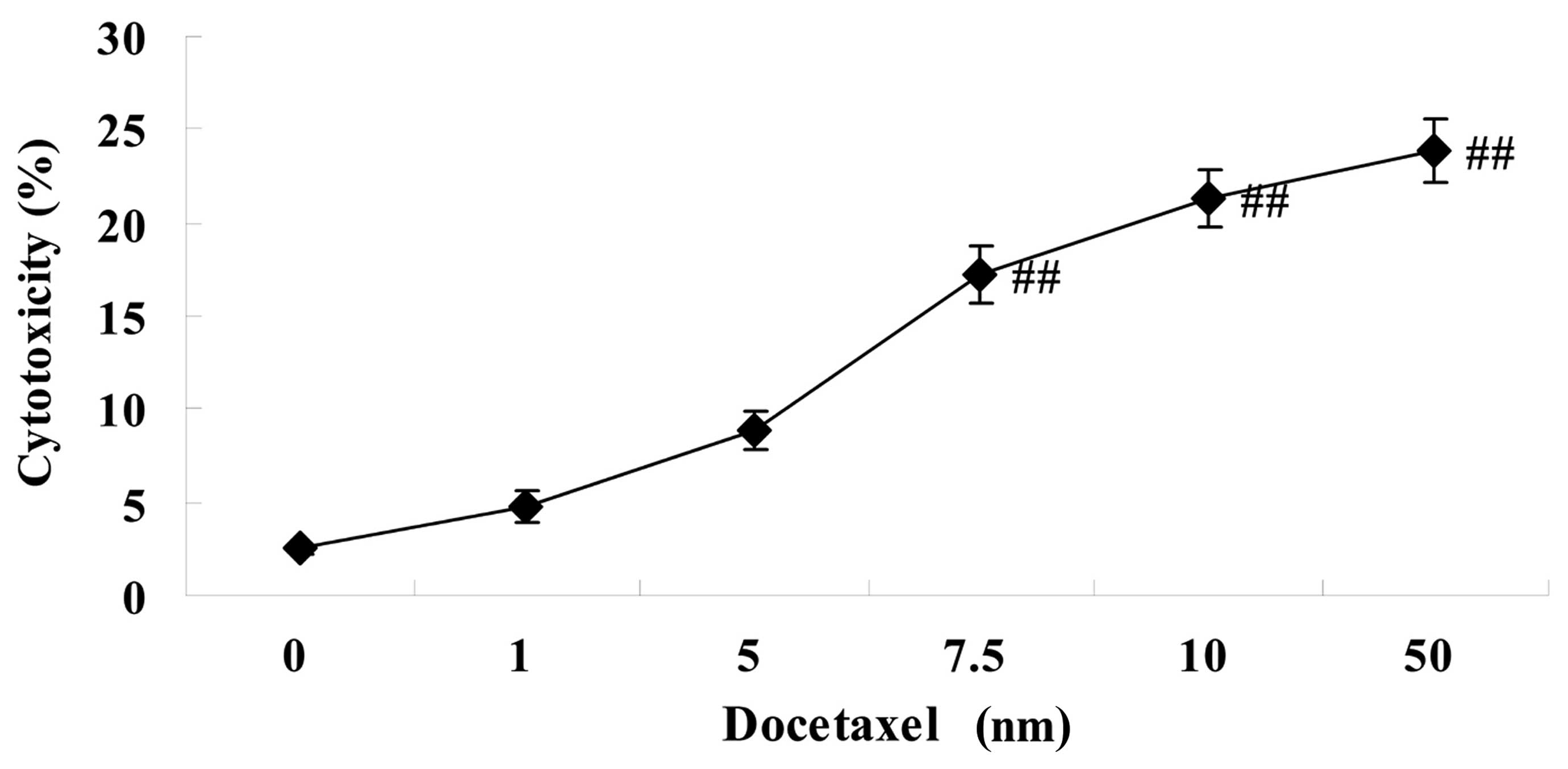
cytotoxicity in LNCaP cells
As shown in Fig. 2,
these docetaxel-mediated anticancer effects on cytotoxicity were
enhanced, as demonstrated using an LDH assay. Notably, 7.5–50 nm
docetaxel significantly increased the cytotoxicity of LNCaP cells,
compared with the 0 nm docetaxel group (Fig. 2).
Anticancer effect of docetaxel on cell
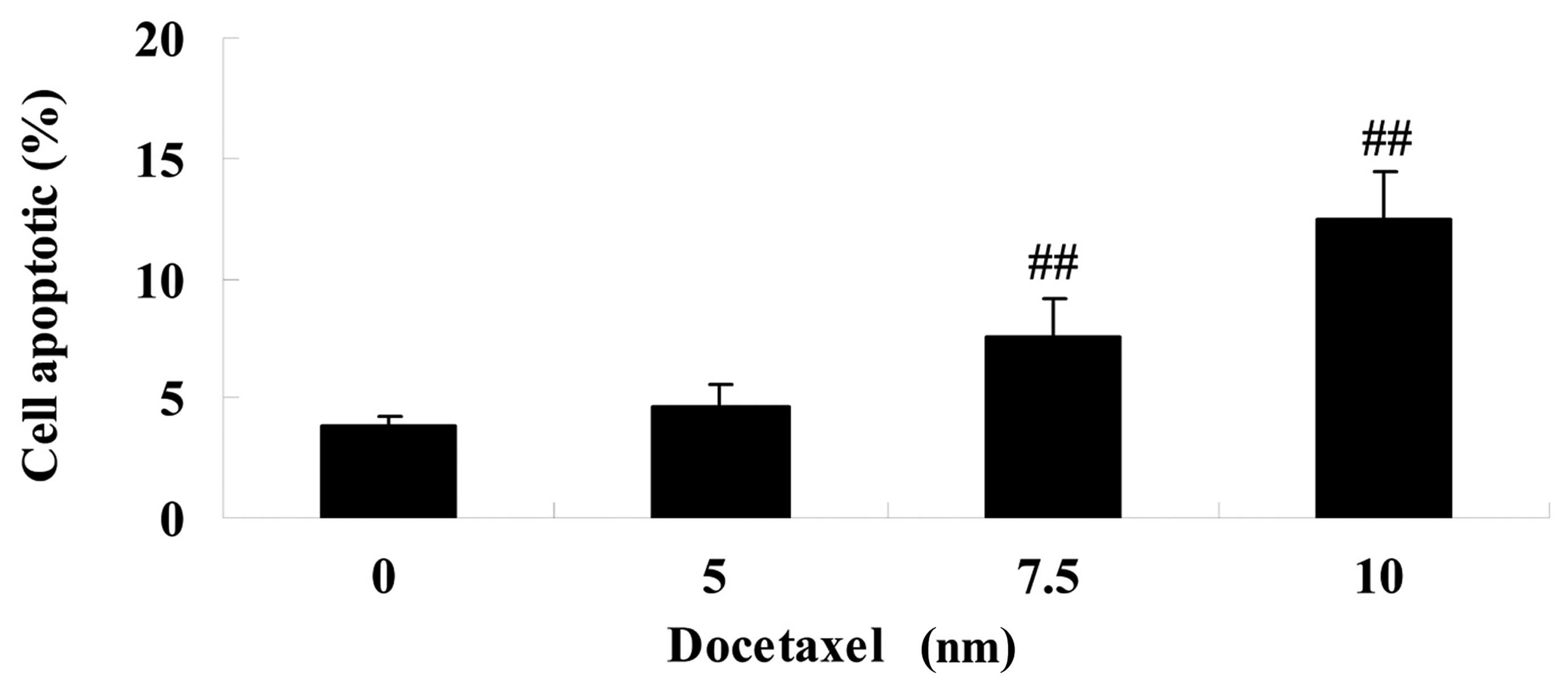
apoptosis in LNCaP cells
To determine whether docetaxel has anticancer
effects on human prostate cancer, the effect of treatment with
docetaxel (5, 7.5 and 10 nM) was assessed in LNCaP cells for 24 h.
Compared with the 0 nm docetaxel group, treatment with docetaxel
(7.5 and 10 nM) significantly promoted cell apoptosis in LNCaP
cells (Fig. 3).
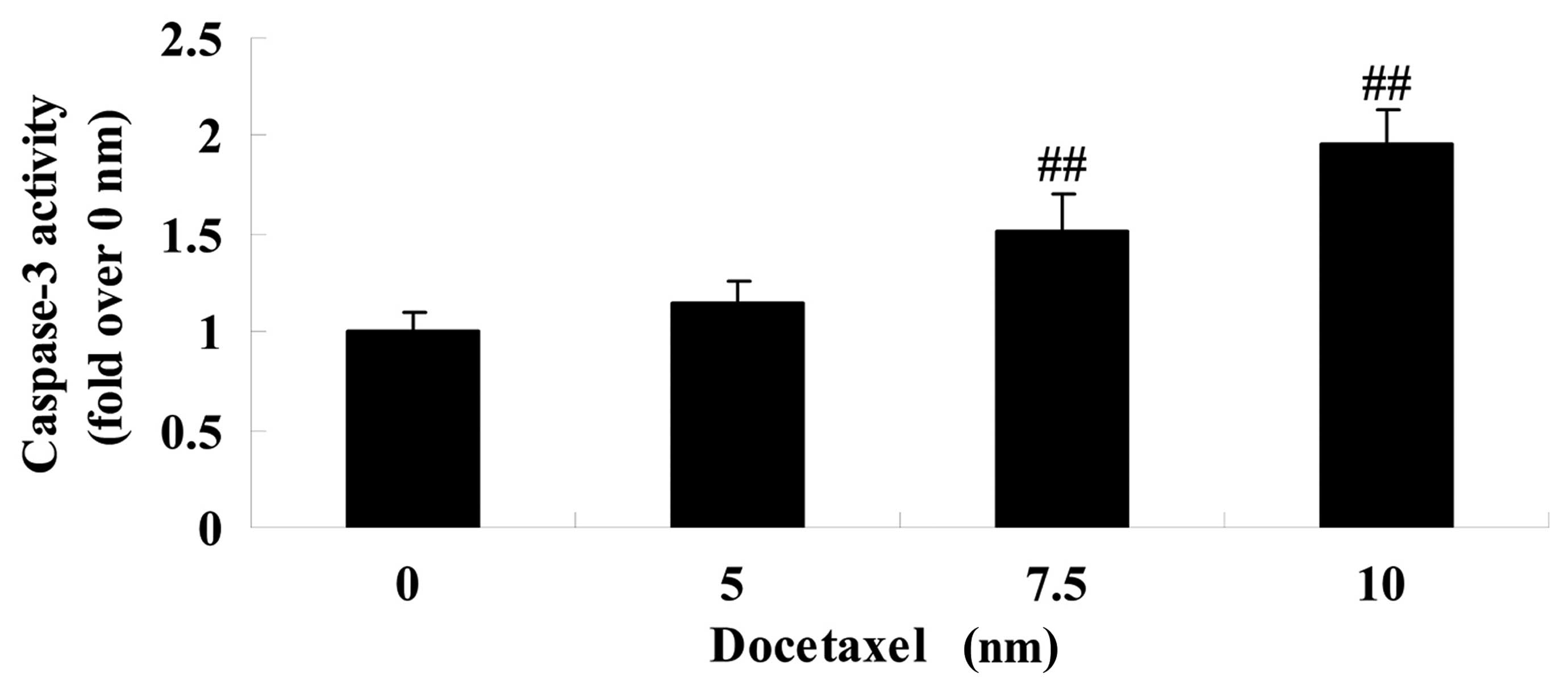
Anticancer effect of docetaxel on the
activity of caspase-3 in LNCaP cells
To investigate the molecular mechanism of docetaxel
on human prostate cancer, the effect of docetaxel on the activity
of caspase-3 was assessed in LNCaP cells following 24 h exposure.
As shown in Fig. 4, treatment with
docetaxel (7.5 and 10 nM) significantly increased the activity of
caspase-3 in LNCaP cells compared with the 0 nm docetaxel group
(Fig. 4).
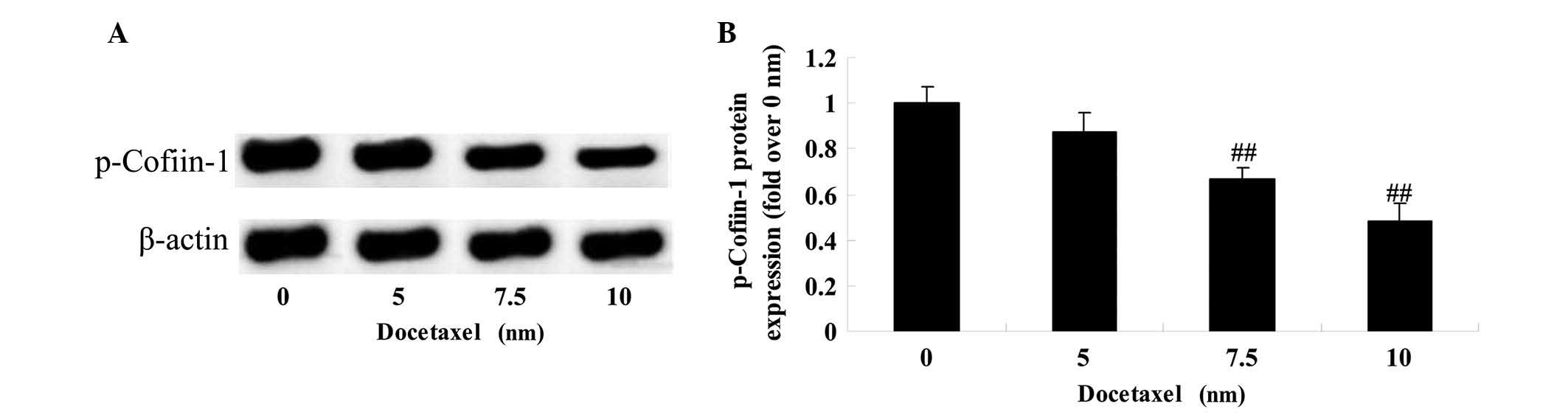
Anticancer effect of docetaxel on
cofilin-1 in LNCaP cells
To analyze the molecular mechanism of docetaxel on
human prostate cancer, the effect of docetaxel on the protein
expression of p-cofilin-1 was assessed in LNCaP cells at 24 h
post-exposure. As shown in Fig. 5,
treatment with docetaxel (7.5 and 10 nM) significantly suppressed
the protein expression of p-cofilin-1 in LNCaP cells.
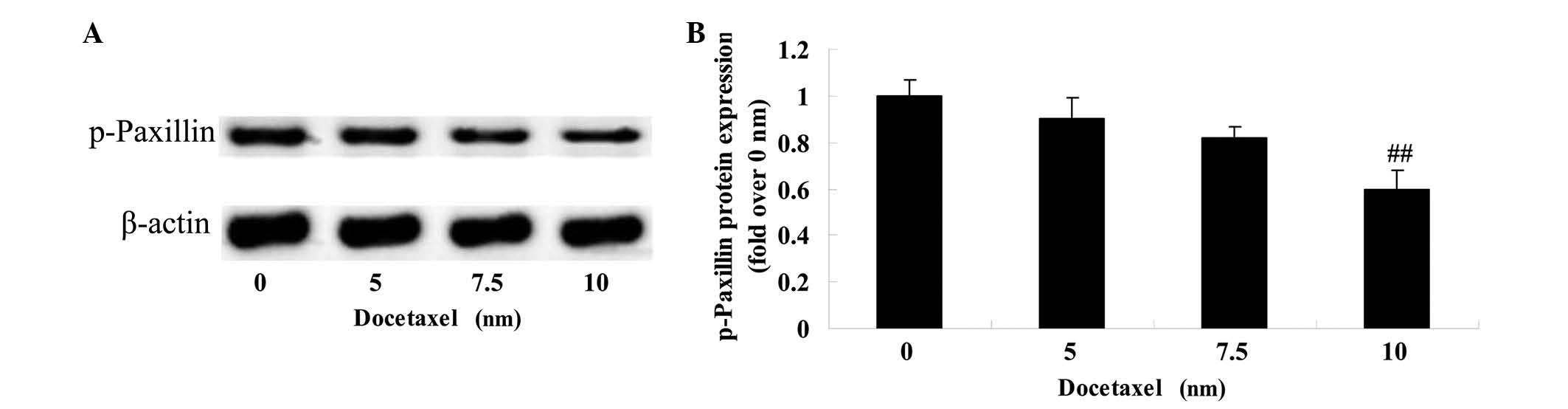
Anticancer effect of docetaxel on
paxillin in LNCaP cells
To research the molecular mechanism of docetaxel on
human prostate cancer, the effect of docetaxel on the protein
expression of p-paxillin was assessed in LNCaP cells after 24 h
docetaxel treatment. A significant inhibition of the protein
expression of p-paxillin was observed in the LNCaP cells following
treatment with 10 nM docetaxel compared with the 0 nm docetaxel
group (Fig. 6).
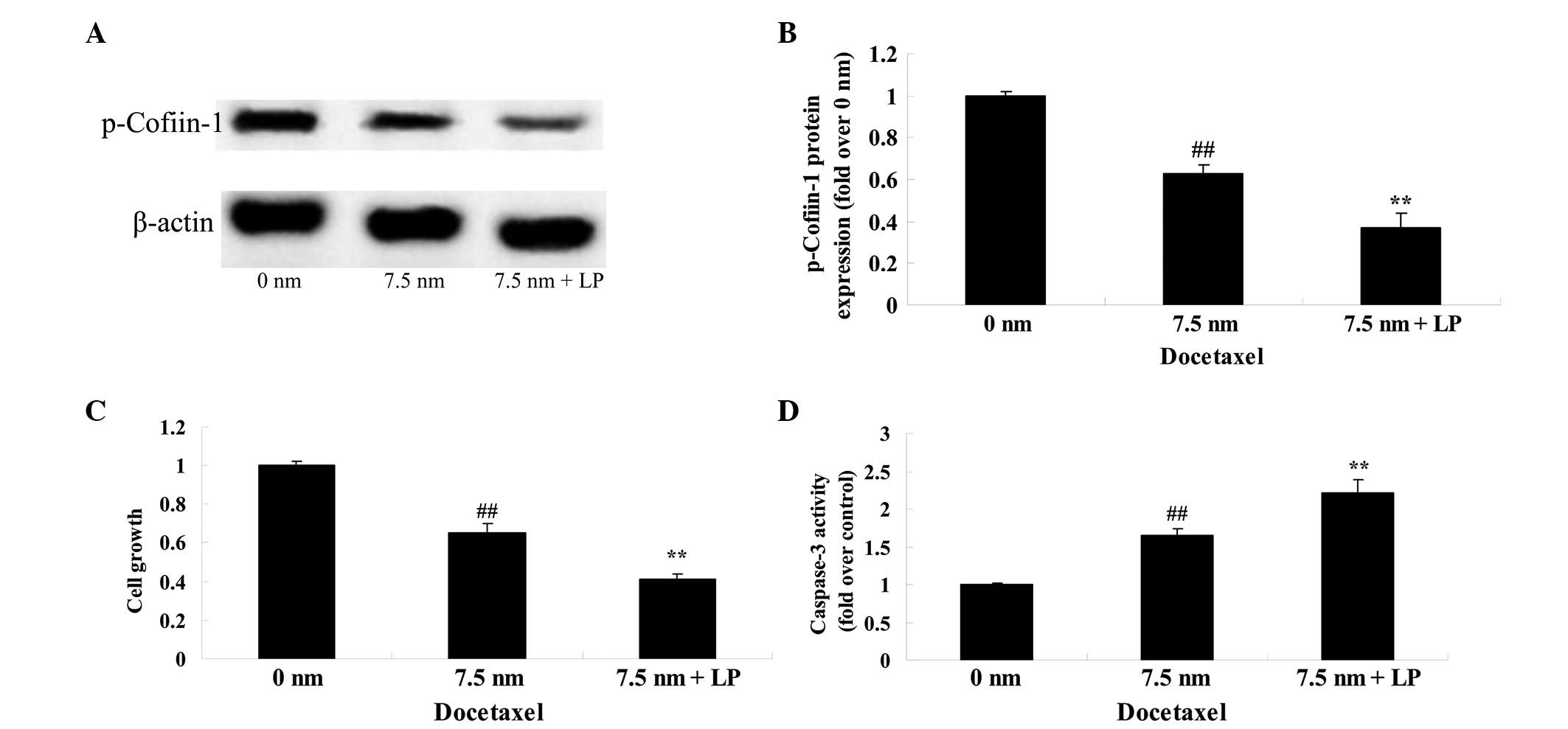
Knockdown of cofilin-1 enhances cell
death and causes apoptosis
To further investigate the molecular mechanism of
docetaxel on human prostate cancer, the effect of knockdown of
cofilin-1 on the anticancer effect of docetaxel was assessed in
LNCaP cells. Following treatment with docetaxel (7.5 nM) and also
cofilin-1 siRNA, a significant decrease in the protein expression
of p-cofilin-1 was observed in the LNCaP cells (Fig. 7). However, the effect of docetaxel
on cell growth and cell apoptosis were significantly reduced,
however, were increased by the combined exposure, compared with the
0 nm docetaxel group (Fig. 7).
Discussion
As a common male malignancy in Occident, prostate
cancer has the 2nd highest mortality among malignant
tumor types (1). The latest data
shows that in America, 217,730 newly diagnosed cases of prostate
cancer occurred in 2010, accounting for 28% of all tumor types. Of
this, 32,050 patients succumbed to prostate cancer in 2010,
accounting for 11% of cancer mortality (16). The prostate cancer morbidity in
China is markedly less compared with in Western countries, however,
its trend has escalated perpendicularly in the past 10 years due to
the westernized lifestyle, aging population and the popularization
of prostate specific antigen screening (17). As a result of the significant rises
in morbidity and mortality at the end of 20th century,
prostate cancer has become a major disease influencing Chinese
males, and requires increased attention (18). The present in vitro
investigation clearly revealed that pretreatment with docetaxel
significantly suppressed cell growth, increased the cytotoxicity,
increased the apoptosis, and induced the activity of caspase-3 in
the LNCaP cells.
Cofilin-1 is a type of eukaryon low molecular weight
protein, combining with actin. Cofilin-1 is important in cell
migration since it produces lamellipodia by highly localized
activities and determines the direction of cell movement (5). It was shown previously that cofilin-1
is a type of regulatory factor for cancer cell metastasis and
invasion, whose overexpression in protein raises the tumor
migration rate (5). Therefore,
inhibiting its expression can significantly reduce cancer cell
invasion (3). Cofilin-1 was found
by researches to be overexpressed in several types of tumor
(8). The present findings
supported an earlier study showing that treatment with docetaxel
significantly reduced the protein expression of p-cofilin-1 in
LNCaP cells. Following knockdown of cofilin-1 expression, docetaxel
significantly inhibited cell growth and promoted the apoptosis of
LNCaP cells. Pérez-Martínez et al (19) suggested that docetaxel enhances
cytotoxicity through knockdown of cofilin-1 in human prostate
cancer cells (19). Additionally,
it was also shown that docetaxel induced apoptosis via the
targeting of cofilin-1 pathways in prostate cancer cells.
Paxillin is expressed in human muscular tissue and
other tissues, with the exception of nervous tissue and blood
platelets. Cytoskeletal proteins combined with paxillin, including
actin, tubulin, vinculin and actopaxin, are essential for embryonic
development, damage repair and tumor associated cell migration
(20). A previous study has shown
that paxillin has regulatory functions for adhesion plaque, cell
migration and cell dissemination (21). There exists a certain association
between paxillin, and the invasion and metastasis of tumor cells
since the invasion and metastasis of tumor cells are directly
associated with changes of adhesive force and locomotive ability.
The biological function of paxillin and the specific binding
proteins requires further investigation, however paxillin is likely
to become a novel target of tumor treatments (22). Therefore, the present findings
suggested that docetaxel significantly inhibited the protein
expression of p-paxillin in LNCaP cells. Lu et al (23) indicated that docetaxel inhibits
vascular endothelial growth factor through suppression of the
phosphorylation of paxillin in human endo-thelial cell migration
(23).
In conclusion, the present studies revealed that the
anticancer effect of docetaxel suppressed cell growth, increased
cytotoxicity, induced apoptosis and activated caspase-3 activity in
human LNCaP prostate cancer cells, therefore leading to protein
expression levels of cofilin-1 and paxillin. The present study
suggested that therapies using novel specific signaling molecules
may prove useful for the anticancer effect of docetaxel on prostate
cancer or other cancer types.
Acknowledgments
The present study was financially supported by the
Seed Fund of The Second Hospital of Shandong University (Shandong,
China; grant no. S2014010010).
References
|
1
|
Yang F, Song L, Wang H, Wang J, Xu Z and
Xing N: Quercetin in prostate cancer: Chemotherapeutic and
chemopreventive effects, mechanisms and clinical application
potential (Review). Oncol Rep. 33:2659–2668. 2015.PubMed/NCBI
|
|
2
|
Crocker-Buque T and Pollock AM: Appraising
the quality of sub-Saharan African cancer registration systems that
contributed to GLOBOCAN 2008: A review of the literature and
critical appraisal. J R Soc Med. 108:57–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang L, Kuwahara I and Matsumoto K: EWS
represses cofilin 1 expression by inducing nuclear retention of
cofilin 1 mRNA. Oncogene. 33:2995–3003. 2014. View Article : Google Scholar
|
|
4
|
Sundram V, Chauhan SC, Ebeling M and Jaggi
M: Curcumin attenuates β-catenin signaling in prostate cancer cells
through activation of protein kinase D1. PLoS One. 7:e353682012.
View Article : Google Scholar
|
|
5
|
Zhou J, Wang Y, Fei J and Zhang W:
Expression of cofilin 1 is positively correlated with the
differentiation of human epithelial ovarian cancer. Oncol Lett.
4:1187–1190. 2012.PubMed/NCBI
|
|
6
|
Li M, Yin J, Mao N and Pan L: Upregulation
of phosphorylated cofilin 1 correlates with taxol resistance in
human ovarian cancer in vitro and in vivo. Oncol Rep. 29:58–66.
2013.
|
|
7
|
Tahtamouni LH, Shaw AE, Hasan MH, Yasin SR
and Bamburg JR: Non-overlapping activities of ADF and cofilin-1
during the migration of metastatic breast tumor cells. BMC Cell
Biol. 14:452013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quintela-Fandino M, Arpaia E, Brenner D,
Goh T, Yeung FA, Blaser H, Alexandrova R, Lind EF, Tusche MW,
Wakeham A, et al: HUNK suppresses metastasis of basal type breast
cancers by disrupting the interaction between PP2A and cofilin-1.
Proc Natl Acad Sci USA. 107:2622–2627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Le Devedec SE, Geverts B, de Bont H, Yan
K, Verbeek FJ, Hout-smuller AB and van de Water B: The residence
time of focal adhesion kinase (FAK) and paxillin at focal adhesions
in renal epithelial cells is determined by adhesion size, strength
and life cycle status. J Cell Sci. 125:4498–4506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishibe S, Joly D, Liu ZX and Cantley LG:
Paxillin serves as an ERK-regulated scaffold for coordinating FAK
and Rac activation in epithelial morphogenesis. Mol Cell.
16:257–267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomas PE, Peters-Golden M, White ES,
Thannickal VJ and Moore BB: PGE(2) inhibition of TGF-beta1-induced
myofi-broblast differentiation is Smad-independent but involves
cell shape and adhesion-dependent signaling. Am J Physiol Lung Cell
Mol Physiol. 293:L417–L428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beer TM, Myrthue A and Eilers KM:
Rationale for the development and current status of calcitriol in
androgen-independent prostate cancer. World J Urol. 23:28–32. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Che CL, Zhang YM, Zhang HH, Sang YL, Lu B,
Dong FS, Zhang LJ and Lv FZ: DNA microarray reveals different
pathways responding to paclitaxel and docetaxel in non-small cell
lung cancer cell line. Int J Clin Exp Pathol. 6:1538–1548.
2013.PubMed/NCBI
|
|
14
|
Guillen KP, Restuccia A, Kurkjian C and
Harrison RG: Annexin V-Directed enzyme prodrug therapy plus
docetaxel for the targeted treatment of pancreatic cancer.
Pancreas. 44:945–952. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tamatani T, Ferdous T, Takamaru N, Hara K,
Kinouchi M, Kuribayashi N, Ohe G, Uchida D, Nagai H, Fujisawa K and
Miyamoto Y: Antitumor efficacy of sequential treatment with
docetaxel and 5-fluorouracil against human oral cancer cells. Int J
Oncol. 41:1148–1156. 2012.PubMed/NCBI
|
|
16
|
Quann P, Jarrard DF and Huang W: Current
prostate biopsy protocols cannot reliably identify patients for
focal therapy: Correlation of low-risk prostate cancer on biopsy
with radical prostatectomy findings. Int J Clin Exp Pathol.
3:401–407. 2010.PubMed/NCBI
|
|
17
|
Niu WB, Gui SL, Lin YL, Fu XL, Ma JG and
Li WP: Promoter methylation of protocadherin8 is an independent
prognostic factor for biochemical recurrence of early-stage
prostate cancer. Med Sci Monit. 20:2584–2589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng WS, Tao H, Hu EP, Liu S, Cai HR, Tao
XL, Zhang L, Mao JJ and Yan DL: Both genes and lncRNAs can be used
as biomarkers of prostate cancer by using high throughput
sequencing data. Eur Rev Med Pharmacol Sci. 18:3504–3510.
2014.PubMed/NCBI
|
|
19
|
Pérez-Martínez FC, Carrión B, Lucío MI,
Rubio N, Herrero MA, Vázquez E and Ceña V: Enhanced
docetaxel-mediated cyto-toxicity in human prostate cancer cells
through knockdown of cofilin-1 by carbon nanohorn delivered siRNA.
Biomaterials. 33:8152–8159. 2012. View Article : Google Scholar
|
|
20
|
Robertson LK and Ostergaard HL: Paxillin
associates with the microtubule cytoskeleton and the immunological
synapse of CTL through its leucine-aspartic acid domains and
contributes to microtubule organizing center reorientation. J
Immunol. 187:5824–5833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu YL and Chien S: Dynamic motion of
paxillin on actin filaments in living endothelial cells. Biochem
Biophys Res Commun. 357:871–876. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamauchi J, Miyamoto Y, Murabe M, Fujiwara
Y, Sanbe A, Fujita Y, Murase S and Tanoue A: Gadd45a, the gene
induced by the mood stabilizer valproic acid, regulates neurite
outgrowth through JNK and the substrate paxillin in N1E-115
neuroblastoma cells. Exp Cell Res. 313:1886–1896. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu H, Murtagh J and Schwartz EL: The
microtubule binding drug laulimalide inhibits vascular endothelial
growth factor-induced human endothelial cell migration and is
synergistic when combined with docetaxel (taxotere). Mol Pharmacol.
69:1207–1215. 2006. View Article : Google Scholar : PubMed/NCBI
|