Introduction
Mesenchymal stem cells (MSCs) have been reported to
have therapeutic applications in tissue injury (1,2). In
lung tissue, the ability of the lung epithelium to restore itself
is of clinical importance, which is correlated with alveolar fluid
clearance (AFC). Impaired AFC in patients with acute lung injury
and acute respiratory distress syndrome has been demonstrated to be
associated with high morbidity and mortality (3,4).
AT-II cells, as one of the key types of pulmonary epithelium, are
responsible for the secretion of surfactant in addition to active
sodium transport from the alveolar surface to the pulmonary
interstitium via sodium channels and the
Na+-K+-adenosine triphosphatase (ATPase)
transporter. AT-II cells have been reported to be important in
numerous lung diseases and exert vital functions in the prevention
of pulmonary inflammatory formation (5,6).
Despite research into MSC administration for lung injury, the
effect of MSCs on AT-II cells in an inflammatory microenviroment
remains unclear.
Despite initial interest in the multipotency
capabilities of MSCs, the differentiation of MSCs into pulmonary
epithelium does not appear to serve a key role in lung injury
repair. Previous studies have indicated that MSCs may be engrafted
to injured pulmonary epithelial cells and express the specific
biomarker of the pulmonary epithelium (7–9).
However, the engraftment rate of MSCs to alveoli was observed to be
too low to replace the damaged cells (10–12),
suggesting that direct engraftment and differentiation into
pulmonary epithelial cells was unlikely to be the key therapeutic
mechanism. At present, it has been suggested that MSCs function in
tissue repair in the lungs, and this is predominantly mediated
through paracrine factors (13).
In the current study, AT-II cells were exposed to
major inflammatory cytokines which led to impairments including
damaged cell morphology, and reduced cell proliferation and
expression of surfactant protein A (SP-A) and the α1 subunit. In
order to study the potential benefits of MSCs on injured AT-II
cells and the possible mechanisms underlying this, AT-II cells and
MSCs were co-cultured using a Transwell system under inflammatory
stimulation. Due to the fact that keratinocyte growth factor (KGF)
is a specific epithelial growth factor secreted by MSCs (13,14),
KGF was knocked down by small interfering RNA (siRNA) to further
investigate whether the therapeutic effects on AT-II cell repair
were due to the MSCs (13).
The current study suggested that MSCs ameliorated
AT-II cell impairments by increasing cell proliferation and the
expression levels of SP-A and the α1 subunit of the
Na+-K+-ATPase transporter. In addition, the
phosphoinositide 3-kinase (PI3K) signaling pathway was involved in
this process. Using KGF siRNA knockdown, it was identified that
MSCs increased the expression levels of SP-A and the α1 subunit in
injured AT-II cells in part via a KGF-dependent PI3K/protein kinase
B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway.
Materials and methods
Primary culture of AT-II cells
All animal procedures were approved in advance by
the Animal Care Committee of Chinese People's Liberation Army
General Hospital (Beijing, China). A total of 50 male 8-week old
Sprague-Dawley rats (average weight, 175 g; Beijing HFK Bioscience
Co., Ltd., Beijing, China) were housed in conditions of 40%
humidity and 23°C with a 12 h light/dark cycle and ad
libitum access to food and water. Rats were anesthetized by 2%
pentobarbital (50 mg/kg; Cascade Biologics; Thermo Fisher
Scientific, Inc., Portland, OR, USA), anticoagulated with heparin
sodium (ToYongBio, Shanghai, China), disinfected with 75% alcohol
and plated on a Superclean bench (Shanghai Boxun Industry &
Commerce Co., Ltd., Shanghai, China). The thorax of the rats was
opened and the pulmonary microcirculation was flushed through the
right ventricle to remove remaining blood subsequent to sacrifice
of the rats by exsanguination. The lungs were removed and lavaged
with phosphate-buffered saline (PBS). The distal airspaces were
then lavaged 10 times and intubated with 20 ml trypsase (0.25%;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China). The lobes were ground in the presence of fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
then digested with DNase (500 µg/ml; Beijing Solarbio
Science & Technology Co., Ltd.) at 37°C for 60 min. The
cell-rich fraction was filtered through a 200 meshstrainer (Beijing
Solarbio Science & Technology Co., Ltd.). The filtrate was
centrifuged at 400 × g for 20 min at 4°C, and the supernatant was
removed. The deposit was resuspended with PBS and red blood cell
lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.)
was added into suspension for 5 min subsequent to mixing. The
suspension was centrifuged at 400 × g for 5 min at 4°C subsequent
to completely dissolving the red blood cells and removing the
supernatant. Cells were resuspended, counted and added into culture
dishes coated with rat polyclonal IgG antibody (1:500; SP5-10;
Beijing Solarbio Science & Technology Co., Ltd.) in an
incubator (37°C and 5% CO2) for one hour. The unattached
remaining cells were transferred to a centrifuge tube and
centrifuged at 400 × g for 10 min at 4°C. The deposit was
resuspended and cultured in a dish with (Dulbecco's modified
Eagle's medium (DMEM)/F12 containing 2% FBS, 100 U/ml penicillin
and 100 U/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.)
for the experiments. AT-II cells were identified using rabbit
polyclonal alveolar SP-A (1:100; sc-13977; Santa Cruz
Biotechnology, Inc., Heidelberg, Germany) and monoclonal
fluorescien isothiocyanate labeled goat anti-rabbit secondary
antibody (1:500; A0562; Beyotime Institute of Biotechnology), which
exhibited green fluorescence under confocal fluorescence microscopy
(Leica TCS SP5; Leica Microsystems, Wetzlar, Germany).
MSC culture and identification
Tibiaes and femurs were excised from rats following
anaesthesia. MSCs were flushed with DMEM/F12 and isolated from the
tibiae and femur marrow of 8-week old male SD rats (15). bone marrow-derived MSCs were
cultured with DMEM/F12 containing 1% glutamine, 2% FBS, 100 U/ml
penicillin and 100 U/ml streptomycin in incubator (37°C and 5%
CO2). As cells reached 80–90% confluence, MSCs were
passaged every 3–4 days by trypsinization (Beijing Solarbio Science
& Technology Co., Ltd.) and cells from the 3rd to 8th passage
were used for experiments. Cells (5×105) in a plate were
cultured with adipogenic or osteogenic induction media (Cyagen
Biosciences, Guangzhou, China) every 3 days. After 2 weeks, cells
reached 90% confluence and were stained with oil red O or alizarin
red (Cyagen Biosciences) in a culture plate. MSCs exhibited
osteogenic and adipogenic differentiation. Biological cell surface
markers of MSCs, including CD29, CD44 (both
allophycocyanin-labeled), CD90, CD45 and CD34 (all
phycoerythrin-labeled), were detected by flow cytometry (BD
FACSCalibur; BD Biosciences, San Jose, CA, USA).
Impairment assay of AT-II cells
subsequent to inflammatory exposure
To injure the cells, primary cultures of AT-II cells
were exposed to inflammatory cytokines containing 1.7 ng/ml tumor
necrosis factor (TNF)-α, 87.6 ng/ml IL-6 and 4.4 ng/ml IL-1β
(PeproTech, Inc., Rocky Hill, NJ, USA), which were determined
according to a previous study (16). Cell morphology was observed and
cell proliferation were analyzed with the Cell Counting Kit-8
(CCK-8) kit (Beyotime Institute of Biotechnology, Jiangsu, China)
in a 96-well plate at 72 h. The medium was replaced by 90 µl
fresh DMEM/F12 mixed with 10 µl CCK-8 solution at a final
volume of 0.1 ml. Subsequently, cells were incubated (37°C, 5%
CO2) for 2 h. The optical density in each well was
measured with a microplate reader (Spectra MR; Dynex Technologies,
Inc., Chantilly, VA, USA). Protein expression levels of SP-A and
the α1 subunit of Na+-K+-ATPase were
evaluated by western blotting.
Co-culture system development and KGF
detection
To detect KGF secretion by MSCs, a co-culture system
was developed using a 6-well Transwell plate (0.4 µm pore
size insert; Corning Incorporated, Corning, NY, USA). MSCs
(5×105) were exposed to the inflammatory cytokines or
co-cultured with AT-II cells (5×105) in Transwell for 48
h (n=3 per group). The concentration of KGF was detected under
following conditions: i) MSCs alone; ii) cytokine-exposed MSCs;
iii) cytokine-exposed MSCs + AT-II cells in Transwell; and iv)
cytokine-exposed AT-II cells. In advance, MSCs were starved for 24
h using serum-free medium (Gibco; Thermo Fisher Scientific, Inc.)
prior to inflammatory exposure. The supernatant of the culture
medium was obtained at 48 h and the KGF concentration was measured
with the rat specific Enzyme-Linked Immunosorbent Assay (ELISA) kit
(R&D Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol.
MSC transfection and efficiency
assay
Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to transfect MSCs (5×105)
with siRNA to knockdown KGF secretion in a dish (60 mm × 15 mm).
Lipofectamine 2000 (5 µl) and siRNA (5 µl) were
supplemented into 125 µl Opti-MEM (Gibco; Thermo Fisher
Scientific, Inc.), then after five min, the two liquids were mixed
and placed at room temperature for 20 min. Subsequent to washing of
MSCs with PBS, the 260 µl mixture and Opti-MEM were
respectively added to the MSC culture plate at final volume of 2
ml. Cells were then cultured in an incubator (37°C and 5%) for 6 h
and the transfection liquid was replaced with fresh DMEM/F12
containing 2% FBS, 1% glutamine, 100 U/ml penicillin and 100 U/ml
streptomycin. The siRNA against rat KGF
[3′-dTdTCGCUGUGUGCUCUUCAAUA-5′ (siG1312692455)] was provided by
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). MSCs were
transfected with the negative siRNA (Leica TCS SP8; Leica
Microsystems) or transfected only with Lipofectamine 2000
(MSCs-Lipof). To test the transfection efficiency, MSCs were
transfected with cy5-labeled siRNA (Guangzhou RiboBio Co., Ltd.),
which was imaged by confocal microscopy. The knocking down
efficiency in each group was evaluated by RT-PCR and ELISA assays
at 48 h subsequent to transfection.
Proliferation assay of AT-II cells by
CCK-8
Cell proliferation was measured using a CCK-8 assay
kit in a 6-well transwell plate. AT-II cells were grown on the
upper compartment (5×105), while MSCs were plated in the
bottom compartment (5×105). To injure cells, AT-II cells
were exposed to the inflammatory cytokines. The cell proliferation
assay was conducted under the following conditions at 0, 12, 24,
36, 48 and 72 h: i) Cytokine-exposed AT-II cells + PBS; ii)
cytokine-exposed AT-II cells + MSCs; iii) cytokine-exposed AT-II
cells + MSCs-KGF siRNA; and iv) cytokine-exposed AT-II cells +
MSCs-Lipof.
Total protein isolation and western blot
analysis
To further investigate the effects of MSCs on AT-II
cells in vitro, the protein levels of the α1 subunit and
SP-A were evaluated under the same experimental conditions. In
advance, MSC transfection was conducted in the lower Transwell
chamber. Subsequent to exposure of AT-II cells to the inflammatory
cytokines for 4 h, AT-II cells were co-cultured with MSCs according
to the following experimental conditions: i) Cytokine-exposed AT-II
cells + PBS (control group); ii) cytokine-exposed AT-II cells +
MSCs; iii) cytokine-exposed AT-II cells + MSCs-KGF siRNA; and iv)
cytokine-exposed AT-II cells + MSCs-Lipof. Subsequent to culture
for 72 h at 37°C, total protein was extracted from the AT-II cells
using 0.2 ml radioimmunoprecipitation assay lysate (Applygen
Technologies, Inc., Beijing, China) per well. Samples mixed with
10% sodium dodecyl sulfate-polyacrylimide gel electrophoresis
(SDS-PAGE) were denatured at 95°C for 10 min. Protein levels were
measured with a bicinchoninic acid protein assay kit (Applygen
Technologies, Inc.). Western blotting was conducted according to
the following protocol. The proteins were transferred onto a
polyvinylidene difluoride membrane (PVDF; Shanghai Jiang Lai
Biotechnology Co., Ltd., Shanghai, China) and blocked with 5% dried
skimmed milk in PBS with Tween-20 at a density of 0.1% for 1.5 h.
The PVDF membrane was then exposed to the primary antibody
overnight at 4°C. The following three primary antibodies were used:
Monoclonal mouse anti-Na+/K+-ATPase α1
(1:200; sc-21712; Santa Cruz Biotechnology, Inc.), monoclonal mouse
anti-β-actin antibody (1:800; TA-09; OriGene Technologies, Inc.,
Beijing, China) and polyclonal rabbit anti-SP-A (1:200; sc-13977;
Santa Cruz Biotechnology, Inc.). The PVDF membrane was incubated
for 2 h with monoclonal goat anti-mouse (1:5,000; ZB2305) or goat
anti-rabbit secondary antibodies (1:5,000; ZB2301) (both purchased
from ZSGB-BIO, Beijing, China). The protein blots on the PVDF
membranes were visualized by enhanced chemiluminescence reagent
detection reagents (Applygen Technologies, Inc.) and
semi-quantified with Image J2x software (version 2.1.4.7; Rawak
Software, Inc., Germany). Expression of SP-A and the α1 subunit was
normalized to β-actin expression. Protein expression levels were
detected using monoclonal rabbit anti-mouse AKT (1:1,000; 4685),
monoclonal rabbit anti-mouse phosphorylated AKT (p-AKT) (1:1,000;
4058), polyclonal rabbit anti-mouse mTOR (1:1,000; 2972) and
polyclonal rabbit anti-mouse p-mTOR (1:1,000; 2974) (all purchased
from Cell Signaling Technologies, Inc., Danvers, MA USA) in the
PI3K/AKT/mTOR signalling pathway were also analyzed.
Total RNA extraction and semiquantitative
reverse transcription-polymerase chain reaction (RT-PCR)
analysis
RT-PCR analysis of SP-A and α1 subunit mRNA levels
was conducted. Total RNA of the α1 subunit and SP-A in AT-II cells
was extracted from AT-II cells under the following conditions: i)
Cytokine-exposed AT-II cells + PBS; ii) cytokine-exposed AT-II
cells + MSCs; iii) cytokine-exposed AT-II cells + MSCs-KGF siRNA;
and iv) cytokine-exposed AT-II cells + MSCs-Lipof. Cells were
extracted using TRIzol reagent (Life Technologies; Thermo Fisher
Scienctific, Inc.) according to manufacturer's instruction and RNA
quality was assessed by the 260/280 ratio. RT-PCR was conducted
following the two step manufacturer's protocol of the PrimeScript™
RT-PCR kit (Takara Bio, Inc., Otsu, Japan) using the C1000 Thermal
cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). First, the
cDNA was prepared using the C1000 Thermal cycler, and the PCR
reaction system was prepared in tubes with 1 µl dNTP mixture
(25 mM), 1 µl Oligo (dt) primers (2.5 µM), 1
µg template RNA (or positive control RNA) and DEPC-treated
water to a final volume of 10 µl. Tubes were placed into the
C1000 Thermal Cycler at 65°C for 5 min for denaturation and
annealing. Subsequently, reverse transcription was conducted by
preparing the following reagent mixture including 10 µl
reaction mixture from denaturation and annealing, 4 µl 5X
PrimeScript buffer, 0.5 µl RNase inhititor, 0.5 µl
PrimeScript RTase and 5 µl RNase DEPC-treated water to a
final volume of 20 µl reaction mixture. Tubes were placed
into the Thermal Cycler at 42°C for 30 min and at 4°C for 10 min.
Subsequently, a 50 µl reaction consisting of 5 µl 10X
PCR buffer II, 2 µl dNTP Mixture (10 mM), 0.5 µl
upstream primer (0.2 µM), 0.5 µl downstream primer
(0.2 µM), Takara Ex TaqHS (5 u/µl), 5 µl
reverse transcriptant and DEPC-treated water to a final volume of
50 µl was conducted. Subsequent to mixing, all tubes were
placed in the thermal cycler at 94°C for 30 sec, 60°C for 30 sec
and 72°C for 1 min. This program was run for 30 cycles. Primer
sequences for the α1 subunit, SP-A and β-actin were provided by
Sangon Biotech Co., Ltd. (Shanghai, China) (Table I).
 | Table IPrimer sequences used in
experiment. |
Table I
Primer sequences used in
experiment.
| Gene | Sequence |
|---|
| ATP-α1 F |
5′-CTCCTTCTGCCTGACGAACA-3′ |
| ATP-α1 R |
5′-ATCAAGCTCAACCGAGTGCT-3′ |
| SP-A F |
5′-ATCAAGCTCAACCGAGTGCT-3′ |
| SP-A R |
5′-TGGACAGGTAGGACGTTTGG-3′ |
| KGF F |
5′-AGCGATCAACTCAAGGTCCA-3′ |
| KGF R |
5′-TATGGTGCCCACAAGACAGA′-3′ |
| Actin F |
5′-CTAAGGCCAACCGTGAAAAGA-3′ |
| Actin R |
5′-CCAGAGGCATACAGGGACAAC-3′ |
Statistical analysis
All experiments were conducted three times for each
group. Results are expressed as the mean ± standard deviation.
Comparisons between two groups were made using the unpaired
two-tailed t-test. Multiple comparisons between more than two
groups were made using one-way analysis of variance using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
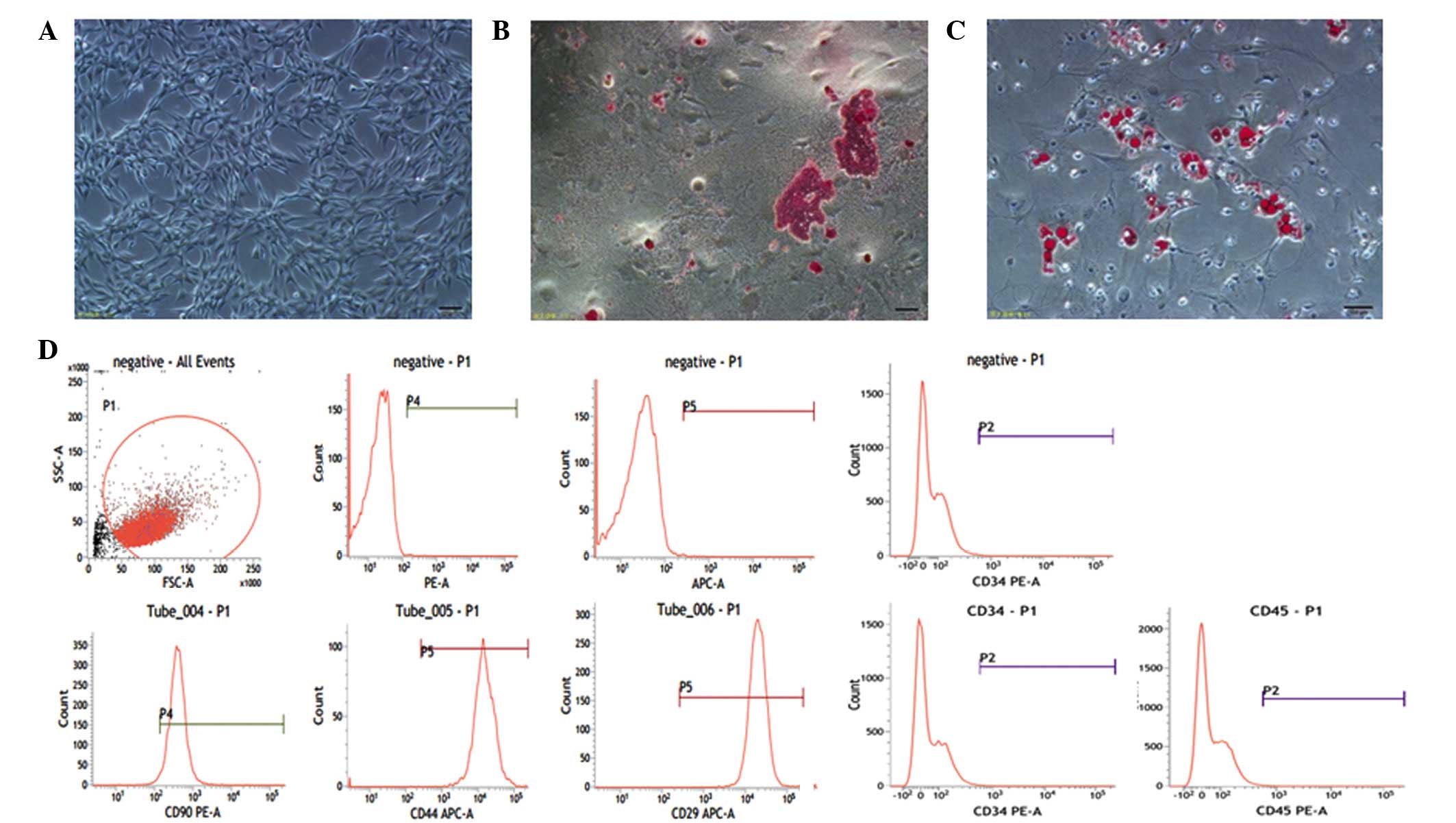
Characterization of MSCs and AT-II
cells
Rat MSCs are adherent and spindle-like cells, which
were observed to differentiate into the predominant mesenchymal
lineages, adipocytes and osteocytes. Rat MSCs were identified to
positively express the cell surface markers CD29, CD44, CD90 and
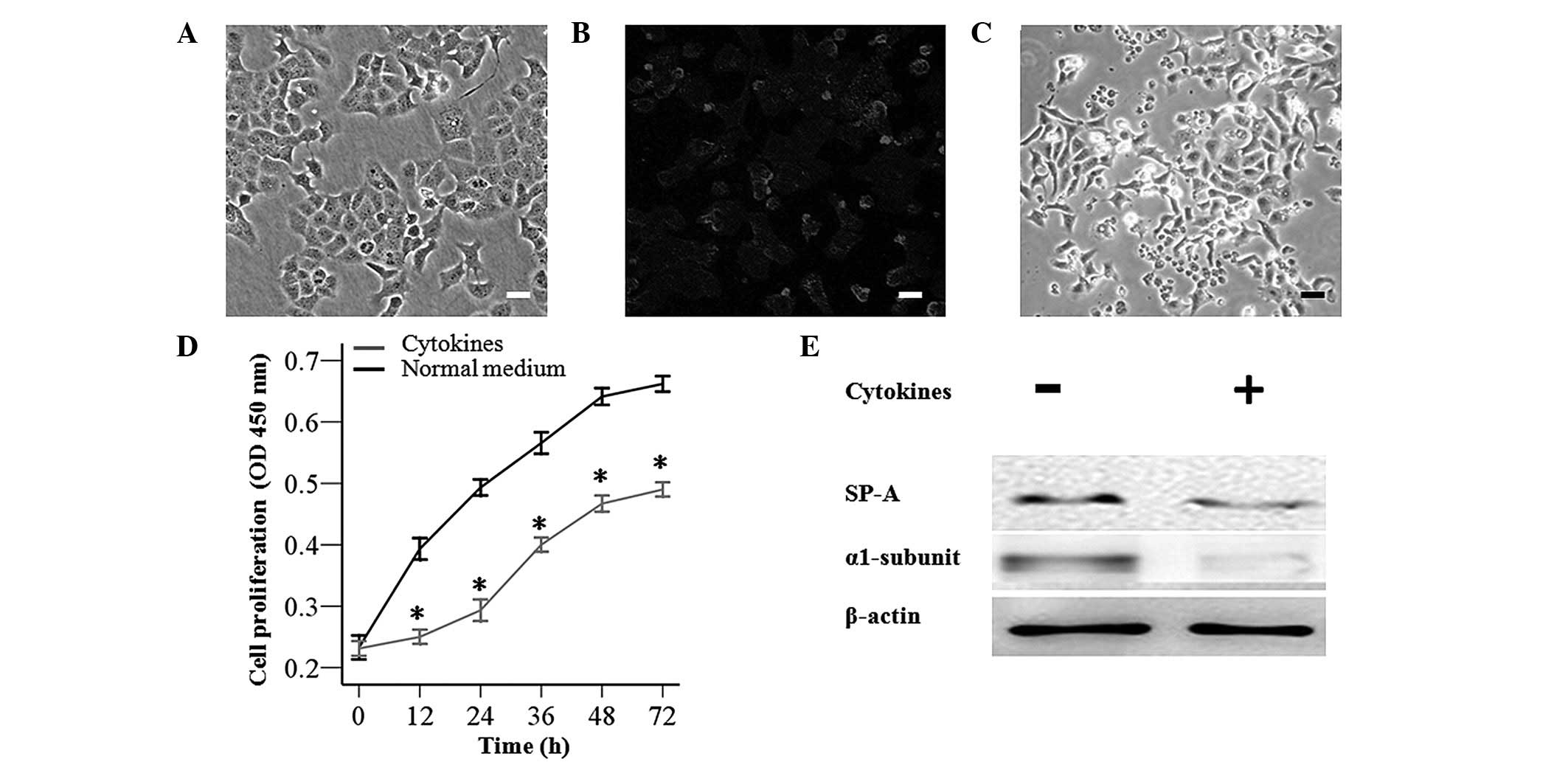
negatively express CD34 and CD45 by flow cytometry (Fig. 1). The primary culture of AT-II
cells was observed to exhibit adherent and round cells in the
culture plate, which were identified by the fact that SP-A was
bound to the fluorescein isothiocyanate-labeled secondary antibody.
Green fluorescence from AT-II cells was detected in the cell
membrane and cytoplasm by confocal fluorescence microscopy
(Fig. 2A and B).
Impairments of AT-II cells caused by
inflammatory cytokines
To injure AT-II cells, AT-II cells were exposed to
inflammatory cytokines (TNF-α, IL-6 and IL-1β) for 72 h, which
resulted in impaired cell morphology, delayed cell proliferation
after 12 h (Fig. 2C and D) and a
downregulation in protein expression of SP-A and the α1 subunit
compared with AT-II cells cultured in normal medium (Fig. 2E).
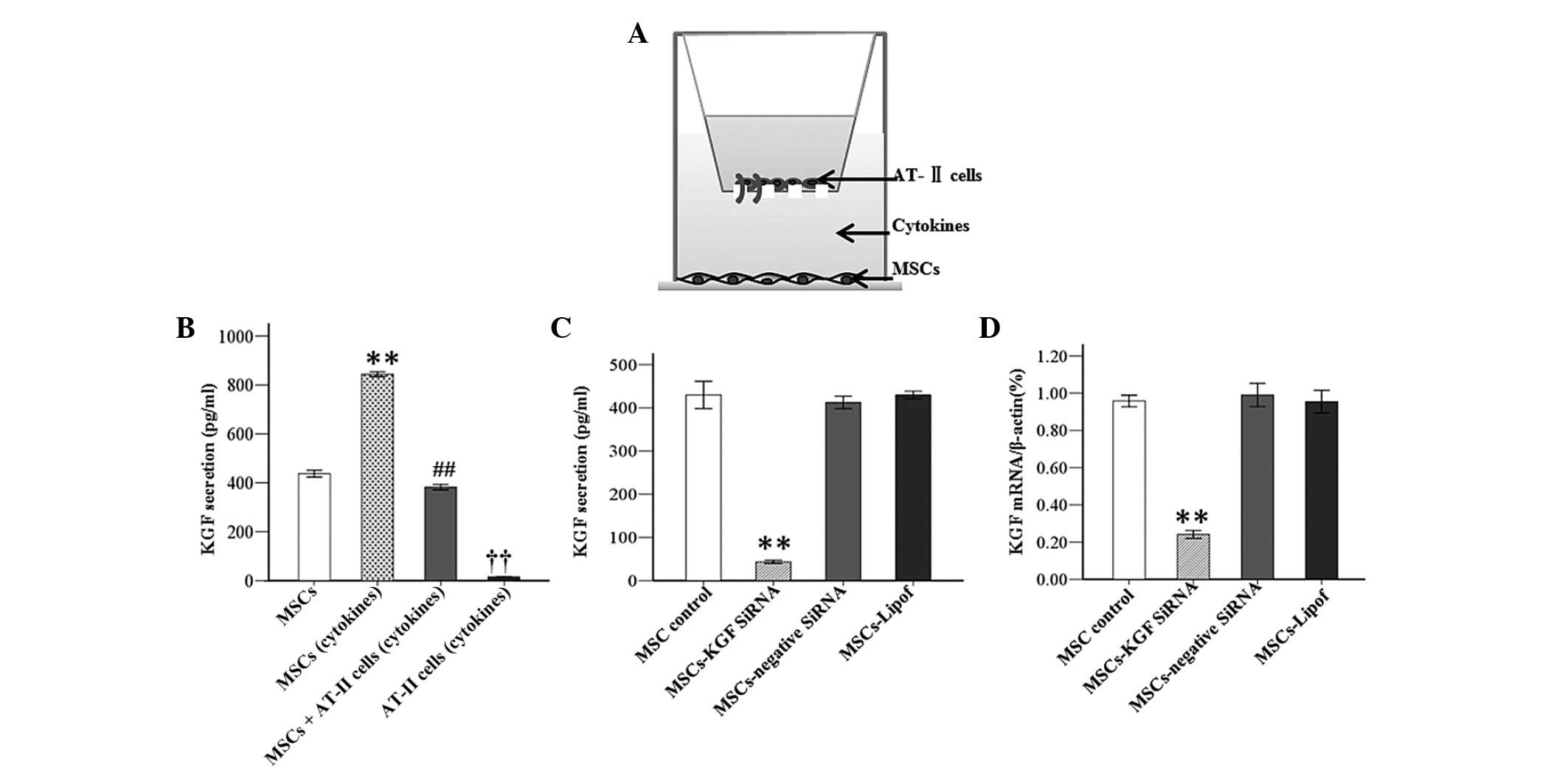
KGF secretion by MSCs
KGF secretion reached 884.17 pg/ml in MSCs that were
cultured for 48 h subsequent to exposure to inflammatory cytokines
in comparison with 437.65 pg/ml in MSCs cultured in normal medium
(P<0.01). However, the KGF concentration was detected to be
382.37 pg/ml in the co-culture system of AT-II cells and MSCs under
the condition of inflammation. The results indicated that AT-II
cells produced significantly less KGF under inflammatory condition
vs. MSCs under the same conditions (Fig. 3A and B).
Knockdown efficiency of KGF siRNA
The knockdown efficiency was confirmed by RT-PCR and
ELISA at 48 h after transfection of MSCs with KGF siRNA. According
to the results, the concentration of KGF in MSC culture medium was
significantly reduced 48 h subsequent to transfection with KGF
siRNA in comparison to MSCs without transfection. In addition, it
was identified that KGF expression at the mRNA level was
significantly downregulated by KGF siRNA. Protein or mRNA
expression levels of mock-transfected MSCs were unchanged compared
with those transfected with negative-siRNA (Fig. 3C and D).
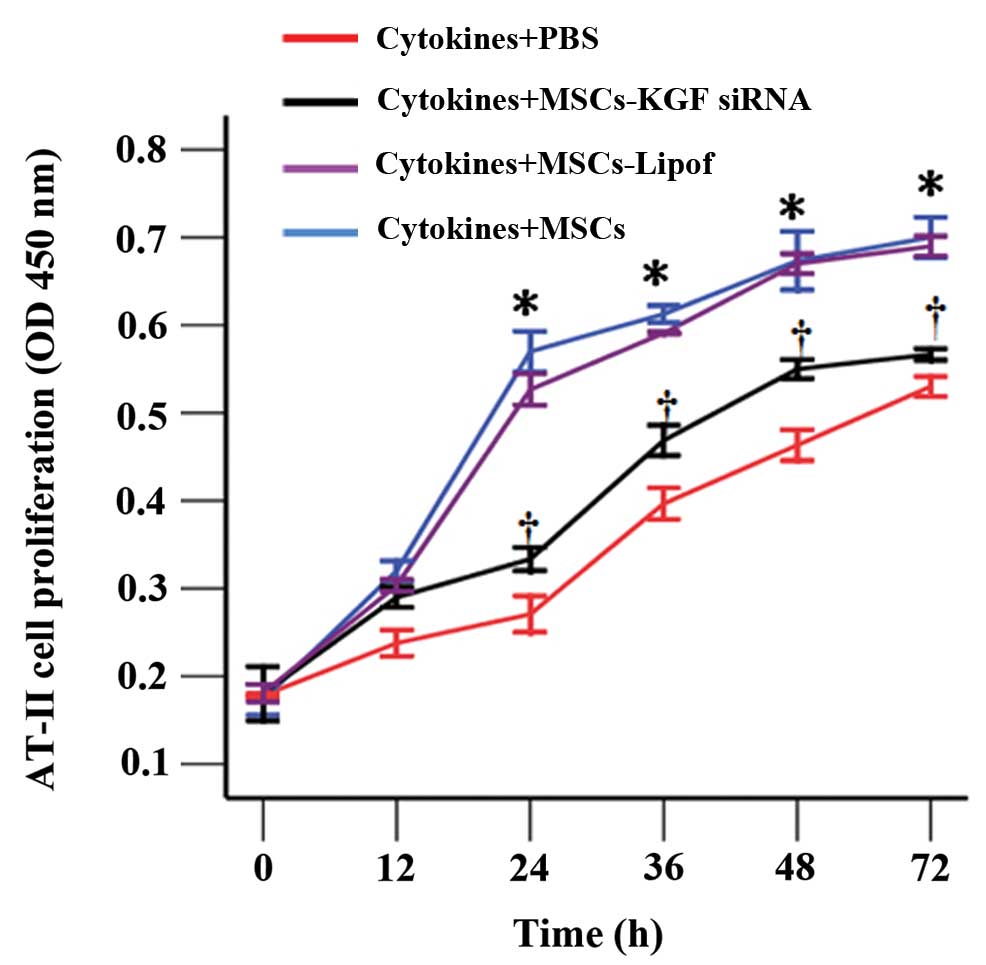
MSCs ameliorated AT-II cell
impairment
The CCK-8 assay demonstrated that AT-II cell
proliferation was delayed subsequent to the exposure of cells to
inflammatory cytokines. The reduced AT-II cell proliferation was
increased with co-culture with MSCs, in particular between 24 h and
72 h. In addition, AT-II cell proliferation was reduced in AT-II
cells cultured with MSCs-KGF siRNA when compared with AT-II cells
cultured with MSCs (Fig. 4).
However, proliferation in the MSCs-KGF siRNA group was still
enhanced compared with the proliferation of cytokine-exposed AT-II
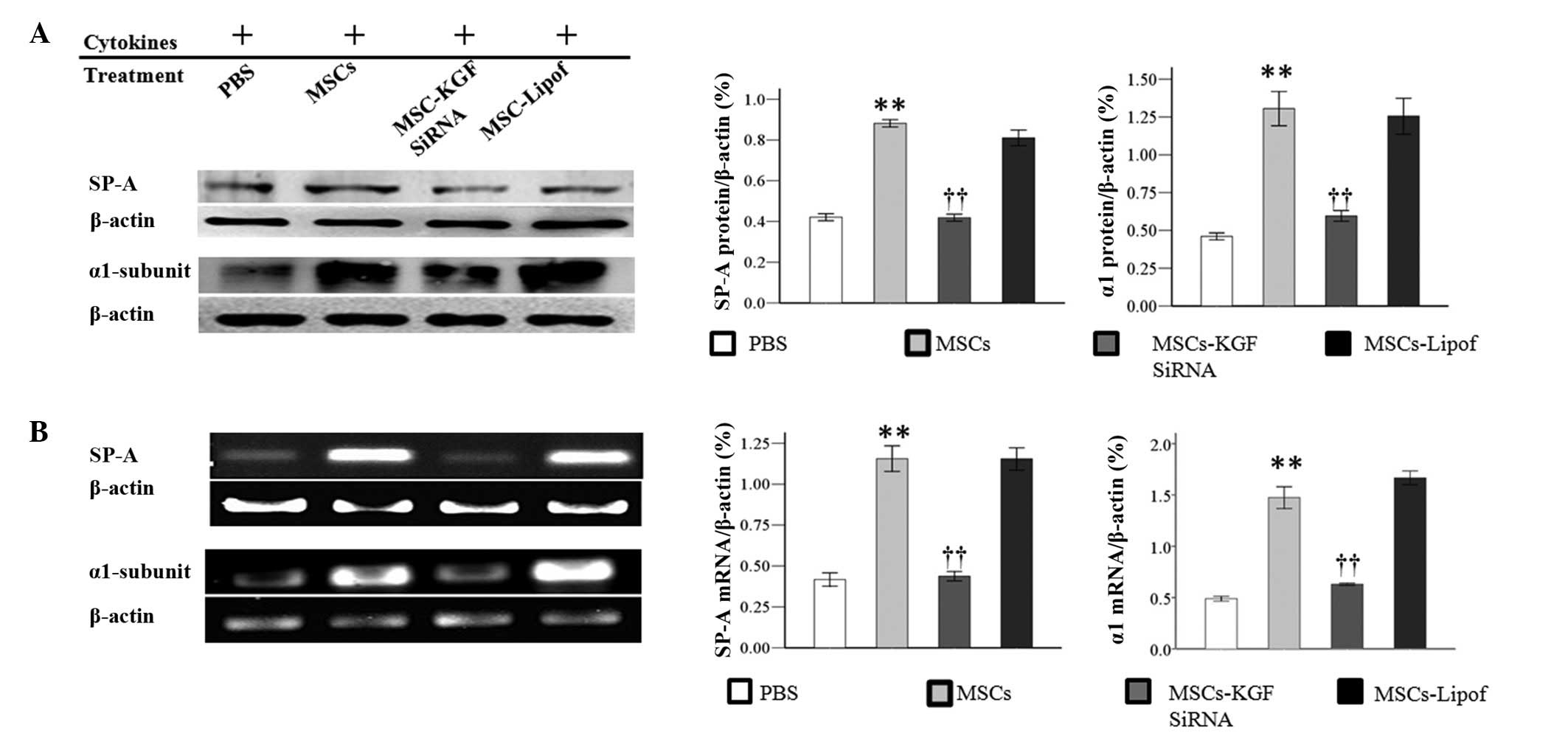
cells. Results of SP-A and α1 subunit expression by western blot
analysis indicated that co-culture with MSCs increased the protein
expression levels, which were reduced by MSCs-KGF siRNA. However,
the protein levels of the α1 subunit and SP-A were observed to be
unchanged between the AT-II cells treated with MSCs-Lipof and those
treated with MSCs alone (Fig. 5A).
Therapeutic benefits were further investigated by RT-PCR.
Co-culture with MSCs was observed to increase the mRNA expression
levels of the α1 subunit and SP-A in AT-II cells. However,
co-culture with MSCs-KGF siRNA attenuated the increased expression
of α1 subunit and SP-A. It was observed that there was no
significant difference in the mRNA levels of SP-A and the α1
subunit between AT-II cells treated with MSCs-Lipof and MSCs alone
(Fig. 5B).
MSCs activated PI3K signaling pathway by
KGF secretion
To further investigate the underlying mechanisms,
the proteins levels of AKT, p-AKT, mTOR and p-mTOR in the
PI3K/AKT/mTOR pathway were measured by western blotting. Treatment
with MSCs significantly increased the protein expression levels of
p-AKT and p-mTOR at 72 h. MSCs-KGF siRNA significantly attenuated
the protein increase of p-AKT and p-mTOR. Protein levels of AKT and
mTOR were not markedly altered apparently under the same
experimental conditions (Fig.
6).
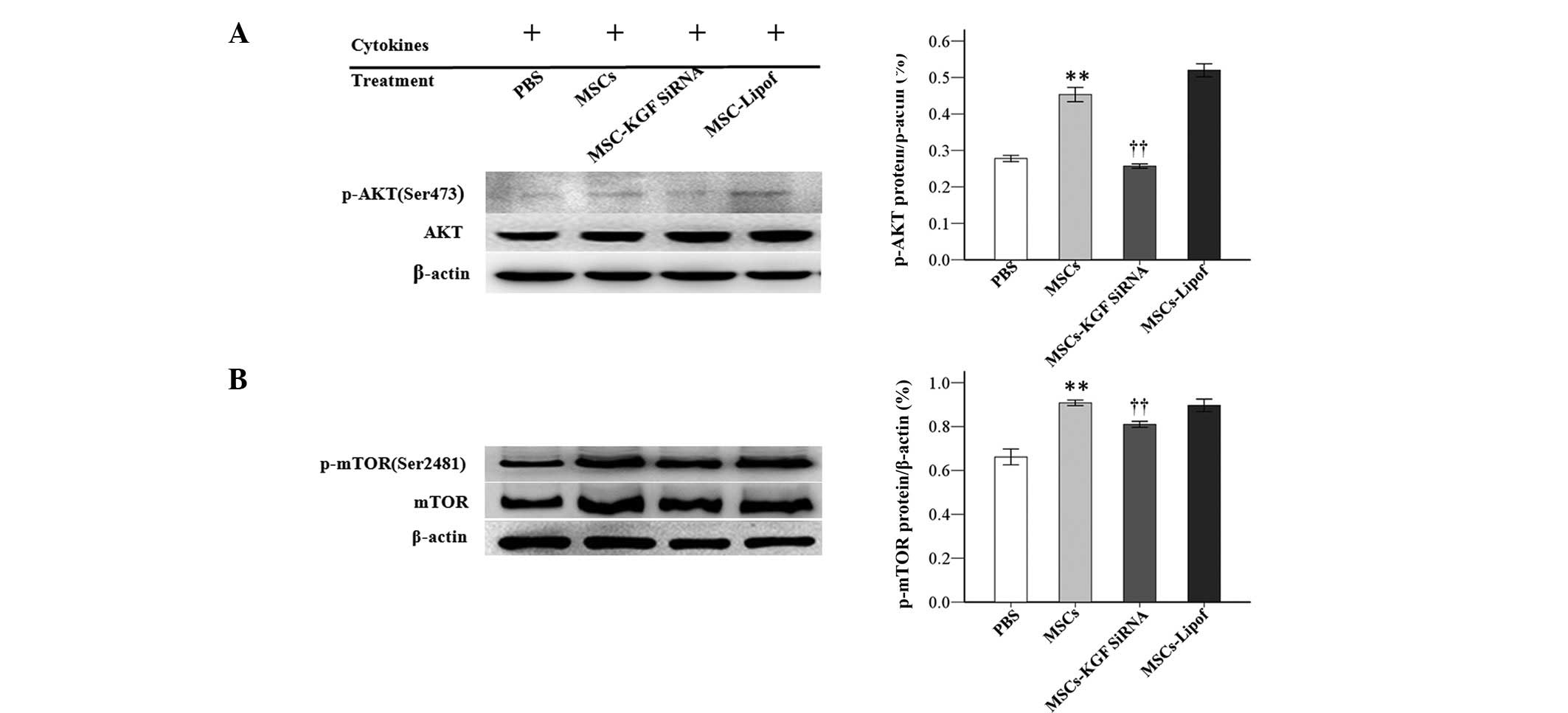 | Figure 6MSCs increased protein expression
levels of p-AKT and p-mTOR in AT-II cells. Protein expression of
(A) p-AKT and AKT, and (B) p-mTOR and mTOR were analyzed by western
blotting subsequent to treatment of AT-II cells with
phosphate-buffered saline, MSCs, MSC-KGF siRNA or MSC-Lipof.
Experiments were run three times and protein levels were normalized
to β-actin expression. Data are presented as the mean ± standard
deviation. **P<0.01 vs. the PBS group;
††P<0.01 vs. the MSC group. MSCs, mesenchymal stem
cells; p-, phosphorylated; AKT, protein kinase B; mTOR, mechanistic
target of rapamycin; AT-II cell, alveolar type II epithelial cell;
KGF, keratinocyte growth factor; siRNA, small interfering RNA;
Lipof, lipofectamine. |
Discussion
The key observations of the current study are
summarized as following: i) To the best of our current knowledge,
this is the first study investigating AT-II cell impairments
characterized by impaired cell morphology accompanied by reductions
in cell proliferation and the expression of SP-A and the α1 subunit
following an inflammatory insult in vitro. ii) Co-culture
with MSCs was observed to increase AT-II cell proliferation and
expression levels of SP-A and the α1 subunit of
Na+-K+-ATPase significantly via activation of
the KGF-dependent PI3K/AKT/mTOR pathway.
As has been previously demonstrated, AT-II cell
impairment is involved in multiple inflammation-associated lung
diseases (6). To imitate
inflammation, the AT-II cells were exposed to the key inflammatory
cytokines including TNF-α, IL-6 and IL-1β at concentrations of 1.7,
87.6 and 4.4 ng/ml, respectively (16). The results of the current study
demonstrated that exposure to these inflammatory cytokines led to
impairment of cell morphology, and reductions in cell proliferation
and the expression levels of SP-A and the α1 subunit, suggesting
that the impairments of AT-II cells by inflammation had been
reproduced in vitro.
As hypothesized, co-culture with MSCs in Transwell
enhanced AT-II cell proliferation subsequent to exposure to
inflammation, which suggested that certain paracrine factors may
serve a role in improving AT-II cell growth. Among the previously
reported paracrine factors produced by MSCs, KGF has been regarded
as the key epithelial promoter (17,18).
The results of the current study indicated that the KGF
concentration in MSC culture medium was higher under inflammatory
conditions than that of normal medium, thus suggesting that MSCs
may produce increased quantities of KGF in response to an
inflammatory stimulus. However, KGF concentration was observed to
be reduced in the MSC and AT-II cell co-culture medium, which
indicated that MSC-secreted KGF may be consumed in the crosstalk
between MSCs and AT-II cells.
Notably, AT-II cells alone produced significantly
less KGF in comparison with MSCs, which suggested that MSCs are
likely to be the primary source of KGF in the co-culture system.
Therefore, KGF was knocked down in MSCs using siRNA, in order to
confirm whether the therapeutic effect was due to MSCs and
dependent on its secretion of KGF. The results demonstrated that
the increase in SP-A and α1 subunit expression was reduced by the
addition of MSCs pretreated with KGF siRNA. Thus, this suggested
that MSCs-secreted KGF may contribute to this therapeutic effect in
addition to cell proliferation.
In order to investigate the underlying mechanisms,
the critical proteins of the PI3K pathway that are associated with
cell prolifieration, p-AKT and p-mTOR, were detected (19–21).
It was identified that co-culture with MSCs increased the protein
expression levels of p-AKT and p-mTOR under inflammatory
conditions, while these effects were reduced by KGF siRNA
pretreatment. This also implied that the PI3K/AKT/mTOR signaling
pathway may be activated by MSC-secreted KGF. Notably, it was
identified that the increases in expression of the α1 subunit and
SP-A were in line with alterations of p-AKT and p-mTOR under the
same experimental conditions. This indicated that MSCs may enhance
the expression of the α1 subunit and SP-A via a KGF-dependent
PI3K/AKT/mTOR signaling pathway.
In conclusion, MSCs were identified to be able to
ameliorate AT-II cell impairment by increasing cell proliferation
and the expression levels of SP-A and the α1 subunit. Furthermore,
KGF secretion may account in part for the protective benefits of
MSCs by activating the PI3K/AKT/mTOR signaling pathway. The current
study explored the mechanism of MSCs in reducing the impairment of
AT-II cells caused by inflammatory cytokines. These observations
provide a novel insight into MSC-based cell therapy for treating
acute lung injury and acute respiratory distress syndrome.
Acknowledgments
The present study was supported partly by the
National Nature Science Foundation of China (grant nos. 81121004,
81230041 and 81372066) and the National Basic Science and
Development Program (973 Program; grant no. 2012CB518105).
References
|
1
|
Matthay MA, Goolaerts A, Howard JP and Lee
JW: Mesenchymal stem cells for acute lung injury: Preclinical
evidence. Crit Care Med. 38(Suppl 10): S569–S573. 2010. View Article : Google Scholar
|
|
2
|
Lee JW, Gupta N, Serikov V and Matthay MA:
Potential application of mesenchymal stem cells in acute lung
injury. Expert Opin Biol Ther. 9:1259–1270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JW, Fang X, Dolganov G, Fremont RD,
Bastarache JA, Ware LB and Matthay MA: Acute lung injury edema
fluid decreases net fluid transport across human alveolar
epithelial type II cells. J Biol Chem. 282:24109–24119. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berthiaume Y and Matthay MA: Alveolar
edema fluid clearance and acute lung injury. Respir Physiol
Neurobiol. 159:350–359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mutlu GM and Sznajder JI: Mechanisms of
pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol.
289:L685–L695. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fehrenbach H: Alveolar epithelial type II
cell: Defender of the alveolus revisited. Respir Res. 2:33–46.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kotton DN, Ma BY, Cardoso WV, Sanderson
EA, Summer RS, Williams MC and Fine A: Bone marrow-derived cells as
progenitors of lung alveolar epithelium. Development.
128:5181–5188. 2001.PubMed/NCBI
|
|
8
|
Krause DS, Theise ND, Collector MI,
Henegariu O, Hwang S, Gardner R, Neutzel S and Sharkis SJ:
Multi-organ, multi-lineage engraftment by a single bone
marrow-derived stem cell. Cell. 105:369–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang G, Bunnell BA, Painter RG, Quiniones
BC, Tom S, Lanson NA Jr, Spees JL, Bertucci D, Peister A, Weiss DJ,
et al: Adult stem cells from bone marrow stroma differentiate into
airway epithelial cells: Potential therapy for cystic fibrosis.
Proc Natl Acad Sci USA. 102:186–191. 2005. View Article : Google Scholar :
|
|
10
|
Mei SH, McCarter SD, Deng Y, Parker CH,
Liles WC and Stewart DJ: Prevention of LPS-induced acute lung
injury in mice by mesenchymal stem cells overexpressing
angiopoietin-1. PLoS Med. 4:e2692007. View Article : Google Scholar
|
|
11
|
Ortiz LA, Dutreil M, Fattman C, Pandey AC,
Torres G, Go K and Phinney DG: Interleukin 1 receptor antagonist
mediates the antiinflammatory and antifibrotic effect of
mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA.
104:11002–11007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kotton DN, Fabian AJ and Mulligan RC:
Failure of bone marrow to reconstitute lung epithelium. Am J Respir
Cell Mol Biol. 33:328–334. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JW, Fang X, Krasnodembskaya A, Howard
JP and Matthay MA: Concise review: Mesenchymal stm cells for acute
lung injury: Role of paracrine soluble factors. Stem Cells.
29:913–919. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayes M, Curley G and Laffey JG:
Mesenchymal stem cells - a promising therapy for acute respiratory
distress syndrome. F1000. Med Rep. 4:22012.
|
|
15
|
Yang Y, Cheng Y, Lian QQ, Yang L, Qi W, Wu
DR, Zheng X, Liu YJ, Li WJ, Jin SW and Smith FG: Contribution of
CFTR to alveolar fluid clearance by lipoxin A4 via PI3K/Akt pathway
in LPS-induced acute lung injury. Mediators Inflamm.
2013:8626282013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semaeva E, Tenstad O, Bletsa A, Gjerde EA
and Wiig H: Isolation of rat trachea interstitial fluid and
demonstration of local cytokine production in
lipopolysaccharide-induced systemic inflammation. J Appl Physiol
(1985). 104:809–820. 2008. View Article : Google Scholar
|
|
17
|
Crosby LM and Waters CM: Epithelial repair
mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol.
298:L715–L731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Panos RJ, Bak PM, Simonet WS, Rubin JS and
Smith LJ: Intratracheal instillation of keratinocyte growth factor
decreases hyperoxia-induced mortality in rats. J Clin Invest.
96:2026–2033. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hubbard PA, Moody CL and Murali R:
Allosteric modulation of Ras and the PI3K/AKT/mTOR pathway:
Emerging therapeutic opportunities. Front Physiol. 5:4782014.
View Article : Google Scholar
|
|
20
|
LoPiccolo J, Blumenthal GM, Bernstein WB
and Dennis PA: Targeting the PI3K/Akt/Mtor pathway: Effective
combinations and clinical considerations. Drug Resist Updat.
11:32–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang L, Graham PH, Hao J, Ni J, Bucci J,
Cozzi PJ, Kearsley JH and Li Y: Acquisition of
epithelial-mesenchymal transition and cancer stem cell phenotypes
is associated with activation of the PI3K/Akt/mTOR pathway in
prostate cancer radioresistance. Cell Death Dis. 4:e8752013.
View Article : Google Scholar : PubMed/NCBI
|