Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide. Non-small cell lung cancer
(NSCLC) accounts for 80% of lung cancer cases, and the reported
overall 5-year survival rate is <5% (1). The main factors associated with NSCLC
are tumor invasion and metastasis; therefore, a better
understanding regarding the cellular and molecular mechanisms
underlying metastatic dissemination of NSCLC cells is required.
Annexin A1 (ANXA1) is a member of the annexin
superfamily of calcium and phospholipid-binding proteins, which has
been detected in various organisms, including vertebrates,
invertebrates and plants (2).
ANXA1 is an endogenous mediator of the anti-inflammatory effects of
glucocorticoids, which acts via the inhibition of phospholipase A2
(3). Functionally, ANXA1 has been
reported to be involved in intracellular signaling, cell growth and
cell differentiation (4). Growing
evidence has suggested that ANXA1 contributes to the pathological
consequence and sequelae of a number of severe human diseases,
including cancer (5). As a
potential marker for malignant progression, ANXA1 expression levels
have been demonstrated to be upregulated in breast cancer (6), and knockdown of ANXA1 by specific
small interfering (si)RNA resulted in a significant reduction in
the invasiveness of breast cancer cells (7). The expression of ANXA1 protein is
also upregulated in human hepatocellular carcinoma (5). Furthermore, a previous study
demonstrated that ANXA1 was overexpressed in melanoma, and may
promote metastasis via formyl peptide receptor stimulation and
matrix metalloproteinase 2 expression (8,9).
ANXA1 expression is also dysregulated in esophageal squamous cell
carcinoma (10). As a novel
mechanism of post-transcriptional regulation, microRNA (miR)-196a
targets ANXA1 expression, and miR-196a has been identified as a
marker of esophageal cancer (11).
Upregulation of ANXA1 expression is correlated with increased
recurrence rate and decreased overall survival in esophageal cancer
(12). Furthermore, the expression
of ANXA1 has been reported to be dysregulated in prostate
carcinogenesis, resulting in enhanced tumor aggressiveness via the
upregulation of interleukin-6 expression and activity (13). These previous studies have reported
diverse roles of ANXA1 in various types of human cancer; however,
the relevant biological function of ANXA1 in NSCLC remains to be
elucidated.
The present study aimed to investigate the effects
of ANXA1 on NSCLC, and determine the effects of ANXA1 knockdown on
cell proliferation and metastatic ability. The aim of this study
was to evaluate the relationship between ANXA1 expression and the
biological function of NSCLC.
Materials and methods
Ethics statement
The present study was approved by the institutional
review board (CWO) of Guangzhou Medical University (Guangzhou,
China). All patients provided written informed consent.
Tissue collection
Lung tumor tissue samples were harvested from 10
patients (aged, 64.9±6.8 years; male, n=8; female, n=2) at the
Cancer Center of Guangzhou Medical University on July 15, 2014
during surgery. Matched healthy paracarcinoma tissue samples were
also harvested from normal lung tissue.
Cell culture
The human NSCLC cell lines (BEAS-2B, A549, H460,
H1299, 95D, H520 and PAa) and 293T cells were purchased from
American Type Culture Collection (Manassas, VA, USA). All cells
were cultured as monolayers in RPMI-1640 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) in a
humidified atmosphere containing 5% CO2 at 37°C.
Preparation of ANXA1 siRNA lentiviral
vectors
The siRNA duplexes targeting ANXA1 (accession
number, NM_000700) were designed online (http://rnaidesigner.invitrogen.com/rnaiexpress/rnaiexpress.jsp).
The sequences are shown in Table
I. Hairpin DNA oligomers were synthesized and annealed.
According to the manufacturers protocol (Takara LA Taq; Takara Bio,
Inc., Otsu, Japan), each PCR reaction mixture (10 µl)
contained the following reagents: 5 µl 10X LA PCR Buffer II,
1.6 µl dNTP mixture, 0.2 µM of each primer (forward:
5′-AGC GTC AAC AGA TCA AAG CAG CAT-3′ and reverse: 5′-AGA CCC TGT
TAA TGT CTC TGA TTT-3′), 1.0 µl LA Taq and 171 ng genomic
DNA. The PCR cycling conditions were as follows: Initial
denaturation at 94°C for 5 min followed by 30 cycles with
denaturation at 98°C for 10 sec, annealing at 55°C for 30 sec,
extension at 72°C for 60 sec; then a final extension at 72°C for 10
min. All PCR products were purified and sequenced by Sangon Biotech
Co., Ltd. (Shanghai, China) using the ABI 3730XL DNA Sequencer
(Applied Biosystems, Foster City, CA, USA). The annealed
double-stranded siRNA oligonucleotides were then cloned into a
GV115 (Shanghai Genechem Co., Ltd., Shanghai, China) lentiviral
vector that was driven by the U6 promoter and carried the transgene
for green fluorescent protein. A control siRNA unrelated to human
gene sequences was used as a negative control (5′-TTC TCC GAA CGT
GTC ACGT-3′) (NC). The accuracy of the inserted vector sequences
was verified by sequencing. The most efficient recombinant vector
was selected following transfection of A549 cells with
LV-ANXA1-RNAi-A, LV-ANXA1-RNAi-B and LV-ANXA1-RNAi-C. After around
24 h, single cells were transfected using an established protocol
(Lipofectamine® 2000 Reagent kit; Invitrogen; Thermo
Fisher Scientific, Inc.), Briefly, cells were exposed to 30 nM
concentration of either scrambled siRNAs (Shanghai Genechem Co.,
Ltd.) and diluted in Opti-MEM (Life Technologies; Thermo Fisher
Scientific, Inc.) along with DharmaFECT (GE Dharmacon, Lafayette,
CO, USA) in transfection medium (complete RPMI medium without
antibiotics and BSA). Cells were incubated with these transfection
complexes for 24 h and then the medium was changed back to complete
RPMI. After 4 days, the protein was harvested and extracted using
lysis buffer and inhibition levels were detected using western
blotting. The selected vector was subsequently denoted as ANXA1
siRNA. The 293T cells were infected with ANXA1 siRNA and NC
lentiviral vectors using virion-packaging elements (pHelper 1.0 and
pHelper 2.0; Shanghai Genechem Co., Ltd.) (14). ANXA1 siRNA and the NC vector were
separately cotransfected into 293T cells with packing plasmids by
calcium phosphate precipitation. Following a 24 h culture, the
viral supernatant of each clone was collected and the viral titer
was measured according to standard protocols.
 | Table ISequences of ANXA1 siRNA. |
Table I
Sequences of ANXA1 siRNA.
| siRNA | Accession no. | Target sequence | CDS | GC % |
|---|
|
LV-ANXA1-RNAi-A | NM_000700 |
ATTCTATCAGAAGATGTAT | 75..1115 | 26.32 |
|
LV-ANXA1-RNAi-B | NM_000700 |
CTTGTATGAAGCAGGAGAA | 75..1115 | 42.11 |
|
LV-ANXA1-RNAi-C | NM_000700 |
AGCGCAATTTGATGCTGAT | 75..1115 | 42.11 |
|
LV-ANXA1-RNAi-NC | |
TTCTCCGAACGTGTCACGT | | |
Selection of cells in which lentiviral
vectors exhibit stable expression
The 293T cells were washed and resuspended in
complete medium following transfection. Stable cell lines
containing ANXA1 siRNA and NC lentiviral vectors were selected and
25 µg/ml puromycin was added to the medium. Following 6
weeks of culturing in the presence of puromycin, the remaining
cells were isolated and transferred into 24-well dishes. An aliquot
of the selected clones was removed for subsequent experimentation,
and the remaining clones were frozen for future use.
Transfection of A549 and H1299 cells with
ANXA1 siRNA
A549 and H1299 cells were subcultured into 6-well
plates, at a density of 8×104/cm2 in a volume
of 1 ml. Subsequently, 3.2×109 TU/ml lentiviral vector
and NC-infected cells, together with 10 µl polybrene. The
cells were allowed to adhere overnight in serum-containing
antimicrobial-free RPMI-1640 medium in an atmosphere containing 5%
CO2 at 37°C. After 24 h, the transduction medium was
replaced with serum-containing medium.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Approximately 1 µg
total RNA was used to generate cDNA using the Primescript RT-PCR
kit (Takara Bio, Inc.). According to the manufacturers protocol,
each RT reaction mixture (10 µl) contained the following
reagents: 2 µl 5X primescript buffer, 0.5 µl
primescript RT enzyme mix I, 0.5 µl oligo-dT primer (50
µM), 0.5 µl random 6 mers (100 µM) and 500 ng
genomic DNA. The conditions were as follows: 37°C for 15 min, 85°C
for 5 sec and 4°C for 10 min. All PCR products were synthesized by
Biometra (Göttingen, Germany). The following primers (Takara Bio,
Inc.) were used in the present study: ANXA1, upstream 5′-AGC GTC
AAC AGA TCA AAG CAG CAT-3′, downstream 5′-AGA CCC TGT TAA TGT CTC
TGA TTT-3′; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH),
upstream 5′-CCA TCA CCA TCT TCC AGGAG-3′ and downstream 5′-CCT GCT
TCA CCA CGT TCTTG-3′. According to the manufacturers protocol
(SYBR® Premix Ex Taq™ II; Tli RNaseH Plus; Takara
Bio Inc., Japan), each PCR reaction mixture (25 µl)
contained the following reagents: 12.5 µl 2X SYBR Premix Ex
Taq II, 1.0 µl PCR forward primer (10 µM), 1.0
µl PCR reverse primer (10 µM), 0.5 µl 50X
Reference Dye II and 2 µl genomic DNA. PCR was performed
using the Applied Biosystems 7500 Fast Real-Time PCR System
(Applied Biosystems, Foster City, CA, USA). PCR was performed using
Platinum Taq polymerase (Invitrogen; Thermo Fisher
Scientific, Inc.) under the following conditions: 37°C for 15 min,
85°C for 5 sec followed by 40 cycles at 95°C for 30 sec, 95°C for 5
sec, 60°C for 34 sec, 95°C for 15 sec, 60°C for 1 min and 95°C for
15 sec. GAPDH was amplified as an internal control. Data were
analyzed using the comparative quantification cycle method
(2−ΔΔCq) (15). Three
separate experiments were performed.
Western blot analysis
Cells from each group were harvested and proteins
were extracted using lysis buffer [1 ml 1 mol/l Tris-HCl, 4 ml 10%
sodium dodecyl sulphate (SDS), 40 µl 0.5 mol/l EDTA, 10
µl protease inhibitor and 14.96 ml ddH2O]. The
protein content was quantified using a Pierce BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Briefly, a working reagent was prepared by mixing 50
parts of BCA Reagent A and 1 part BCA Reagent B. The PRP pellet was
resus-pended into 25 µl of mammalian protein extraction
reagent, and 200 µl of working reagent was added to the
solution. After 30 min of incubation, the absorbance was measured
at 562 nm on a BioTek Synergy 2 96-well plate reader (BioTek,
Winooski, VT, USA) and converted to a concentration using a
calibration curve. Cell extracts were boiled for 5 min in loading
buffer, and an equal amount of protein (40 µg) was separated
by 10% SDS-polyacrylamide gel electrophoresis (PAGE). Separated
protein bands were transferred onto nitrocellulose membranes
(8-µm pores; Millipore, Billerica, MA, USA) and the
membranes were blocked in 5% skimmed milk powder. Standard western
blotting was performed using a rabbit polyclonal anti-ANXA1
antibody (cat. no. ab137745; 1:1,000 dilution; 4°C for 16 h; Abcam,
Cambridge, UK) and a horseradish peroxidase-conjugated rabbit
anti-rat IgG H&L polyclonal antibody (cat. no. ab6734; 1:5,000
dilution; at room temperature for 1 h; Abcam). Equal protein sample
loading was monitored by probing the same membrane filter with
mouse monoclonal anti-β-actin antibody (cat. no. ab6276; 1:5,000
dilution; at 4°C for 16 h; Abcam), rabbit polyclonal anti-GAPDH
antibody (cat. no. ab70699; 1:2,000 dilution; 4°C for 16 h; Abcam)
and rabbit polyclonal anti-tubulin antibody (cat. no. ab150729;
1:1,000 dilution; 4°C for 16 h; Abcam), which was used as an
internal control. Blots were visualized using enhanced
chemiluminescence (Millipore) and were exposed to chemiluminescent
film (Pierce; Thermo Fisher Scientific, Inc.). Data were measured
using ImageJ 1.48u software (National Institutes of Health,
Bethesda, MD, USA).
Wound healing assay
Transduced cells were incubated until they had
reached 90–100% confluence. The cells were scratched using a P-10
pipette tip, and were then incubated for various durations. Phase
contrast images were captured at 0 and 24 h using a Nikon
microscopy system (Nikon Eclipse Ti-s; Nikon Corporation, Tokyo,
Japan). The wound healing distance was measured using ImageJ
software (1.48u; National Institutes of Health). All assays were
conducted in triplicate, and the mean values were calculated.
Migration and invasion assays
The migratory ability of human A549 and H1299 NSCLC
cells transduced with ANXA1 siRNA and NC siRNA vectors was
determined using Corning Transwell insert chambers (Corning, Inc.,
Corning, NY, USA). Briefly, during the logarithmic growth phase,
cells were trypsinized with 1X trypsin, and were resuspended in 200
µl (2×105 cell/ml) serum-free RPMI-1640 medium.
The cells were placed in the upper chamber of the insert without
Matrigel. Medium containing 5% FBS was added to the lower chamber
as a chemoattractant. Following a 24 h incubation, the cells on the
upper membrane were carefully removed, and cells that had migrated
through the membrane were manually counted at 200x magnification
from 10 fields per filter using a Nikon microscope (Nikon Eclipse
Ti-s; Nikon Corporation). All experiments were independently
repeated at least three times.
The invasive ability of human A549 and H1299 NSCLC
cells transduced with ANXA1 siRNA and NC siRNA vectors was
determined using Matrigel-coated cell culture chambers (8 µm
pore size; EMD Millipore, Billerica, MA, USA). Briefly, the cells
were transduced and cultured to ~90% confluence in 24-well dishes.
Subsequently, the cells were resuspended in 200 µl
(1×106 cell/ml) serum-free RPMI-1640 medium and were
placed in the upper chamber of the insert with Matrigel. Medium
containing 5% FBS was added to the lower chamber as a
chemoattractant. Following a 24 h incubation, the cells that
remained on the upper membrane were carefully removed. Cells that
had invaded through the membrane were manually counted at 200×
magnification from 10 fields per filter using a Nikon microscope
(Nikon Eclipse Ti-s; Nikon Corporation). All experiments were
independently repeated at least three times.
Cell proliferation assay
Cells were seeded into 96-well plates at a density
of 2×103 cells/well. Cell viability was assessed using
the Cell Counting kit (CCK)-8 assay (Beyotime Institute of
Biotechnology, Shanghai, China). Briefly, cells were seeded into
96-well plates (2.0×103 cells per well) and incubated in
α-MEM supplemented with 10% FBS for 4 days. CCK-8 reagent (10
µl, 1 mg/ml) was added and incubated for 3 h at 37°C. The
absorbance of each well was measured using a spectrophotometer
(51119200; Thermo Fisher Scientific, Inc.) at 450 nm. Three
independent experiments were performed.
Clone formation assay
For the clone formation assay, 125 cells/4 ml were
plated onto 6-well plates and were incubated at 37°C. Once the
cells grew to visible colonies, the colonies were washed once with
phosphate-buffered saline and were fixed with 4% paraformaldehyde
for 20 min. Subsequently, the cells were stained with crystal
violet (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China), and the number of clones per well was counted. All
assays were conducted in triplicate, and the mean values were
calculated.
Statistical analysis
All assays were conducted in triplicate, and the
mean values were calculated. Data are presented as the mean ±
standard deviation. All statistical analyses were performed using
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Unpaired sets of data
were compared using unpaired Student's t-test (two-tailed).
P<0.05 was considered to indicate a statistically significant
difference.
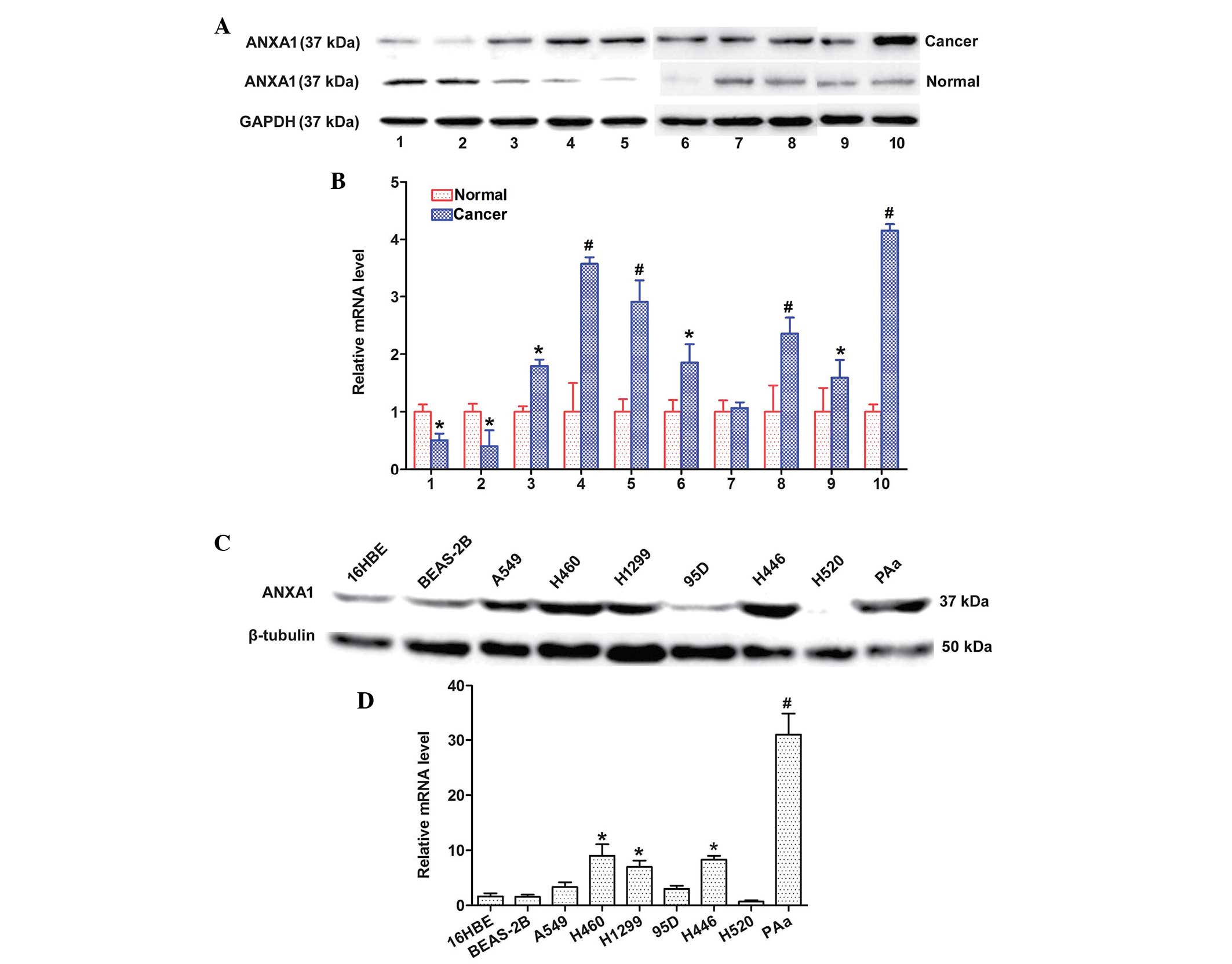
ANXA1 expression is upregulated in NSCLC
tissues and cell lines
The expression levels of ANXA1 were detected in 10
matched clinical tissue samples and seven NSCLC cell lines by
western blotting and qPCR. The protein expression levels of ANXA1
were increased in the 8 matched cancer tissues, as compared with in
the normal tissues (Fig. 1A). In
addition, the mRNA expression levels of ANXA1 were markedly
upregulated in the eight matched cancer tissues, as compared with
in the normal tissues (Fig. 1B).
The protein expression levels of ANXA1 were also upregulated in the
five NSCLC cell lines (Fig. 1C).
Similarly, ANXA1 mRNA expression was increased in the five NSCLC
cell lines (Fig. 1D) as compared
with in the normal cell line. The cancer and normal tissues were
obtained from the same patients. These results indicate that ANXA1
expression may be upregulated at both the mRNA and protein level in
NSCLC.
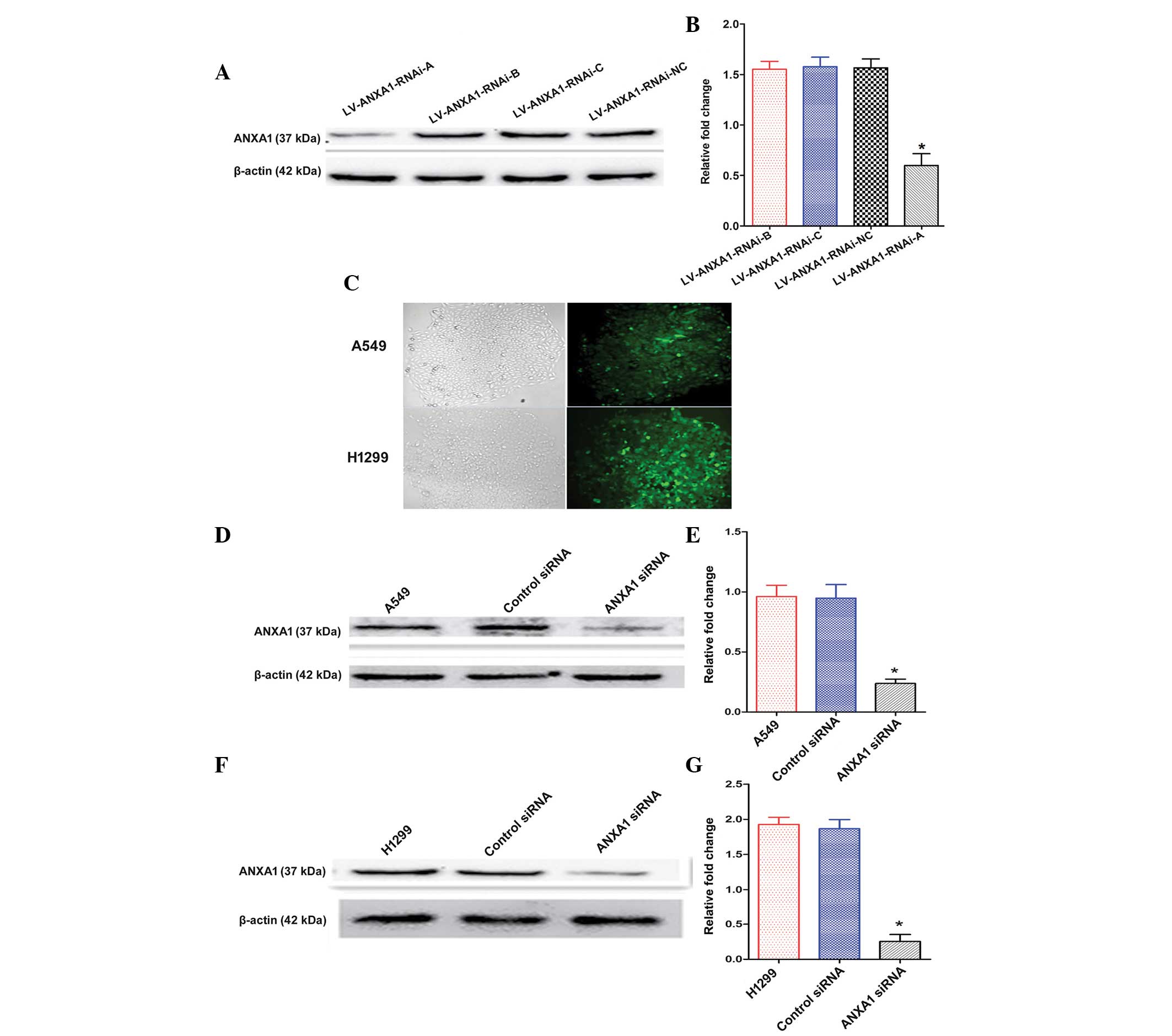
Construction and selection of the most
effective ANXA1 siRNA expression vector
The expression levels of ANXA1 were detected by
western blotting 72 h post-transfection of A549 cells with the
three siRNAs. The ANXA1 siRNA sequences are presented in Table I. The transfection of
LV-ANXA1-RNAi-A inhibited the expression of ANXA1 in A549 cells
(Fig. 2A). In particular, the most
obvious gene-silencing effect was observed following transfection
with LV-ANXA1-RNAi-A, which reduced ANXA1 expression by 90.1%
(Fig. 2B). Therefore,
LV-ANXA1-RNAi-A was selected for further experimentation; its viral
titer was 3.2×109 TU/ml.
Knockdown of ANXA1 expression with ANXA1
siRNA
The stably transduced cells were selected and
observed under a fluorescence microscope, in order to detect green
fluorescence. No alterations in cellular morphology were detected
post-transduction (Fig. 2C). The
A549 and H1299 cells were shown to exhibit a higher ANXA1 gene
expression in preliminary experiments (Fig. 1C and D). The present study aimed to
examine the effects of ANXA1 siRNA on A549 and H1299 cells. Western
blot analysis demonstrated that the protein expression levels of
ANXA1 in A549 cells of the ANXA1 siRNA group were significantly
decreased, as compared with in the cells of the siRNA NC and
untreated groups (Fig. 2D and E).
Similar results were obtained from the H1299 cells (Fig. 2F and G). Statistical analysis
revealed that the protein expression levels of ANXA1 were markedly
downregulated by ANXA1 siRNA, as compared with in the NC siRNA
group (P<0.05). In addition, no significant difference was
detected between the NC siRNA and untreated A549 cell groups
(Fig. 2E). Similar results were
obtained from the H1299 cells (Fig.
2G). These results indicate that ANXA1 siRNA was successfully
constructed.
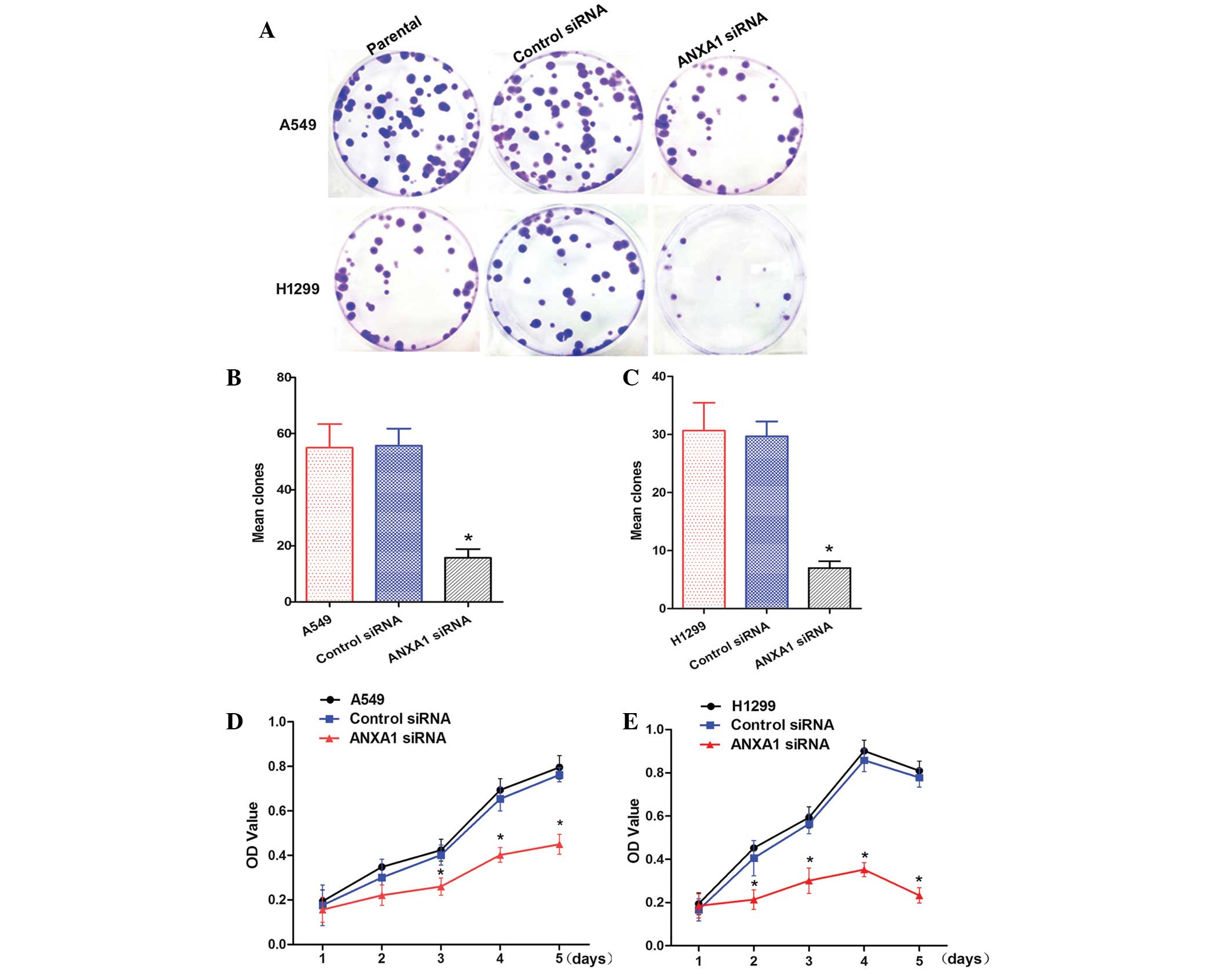
ANXA1 siRNA inhibits clone formation and
cell proliferation
To determine whether knockdown of ANXA1 expression
affected biological function of NSCLC cells, cellular activities
were analyzed using clone formation and CCK-8 assays. ANXA1
knockdown inhibited the clone forming ability of A549 and H1299
cells (Fig. 3A). Statistical
analysis demonstrated that clone formation was inhibited by ANXA1
siRNA, as compared with in the NC siRNA group (P<0.05). In
addition, there was no significant difference between the NC siRNA
and untreated cell groups (Fig. 3B and
C). Cells from the ANXA1 siRNA, NC siRNA and untreated groups
were cultured for 5 days. The CCK-8 assay indicated that A549 cell
growth in the ANXA1 siRNA group was significantly decreased
(P<0.05). Statistical analysis revealed that the proliferation
rate was significantly decreased, and no significant differences
were detected between the NC siRNA and untreated groups (Fig. 3D). Similarly, ANXA1 knockdown
inhibited H1299 cell proliferation (Fig. 3E; P<0.05). These data suggest
that ANXA1 knockdown inhibits NSCLC cell proliferation, and may
have a critical role in NSCLC.
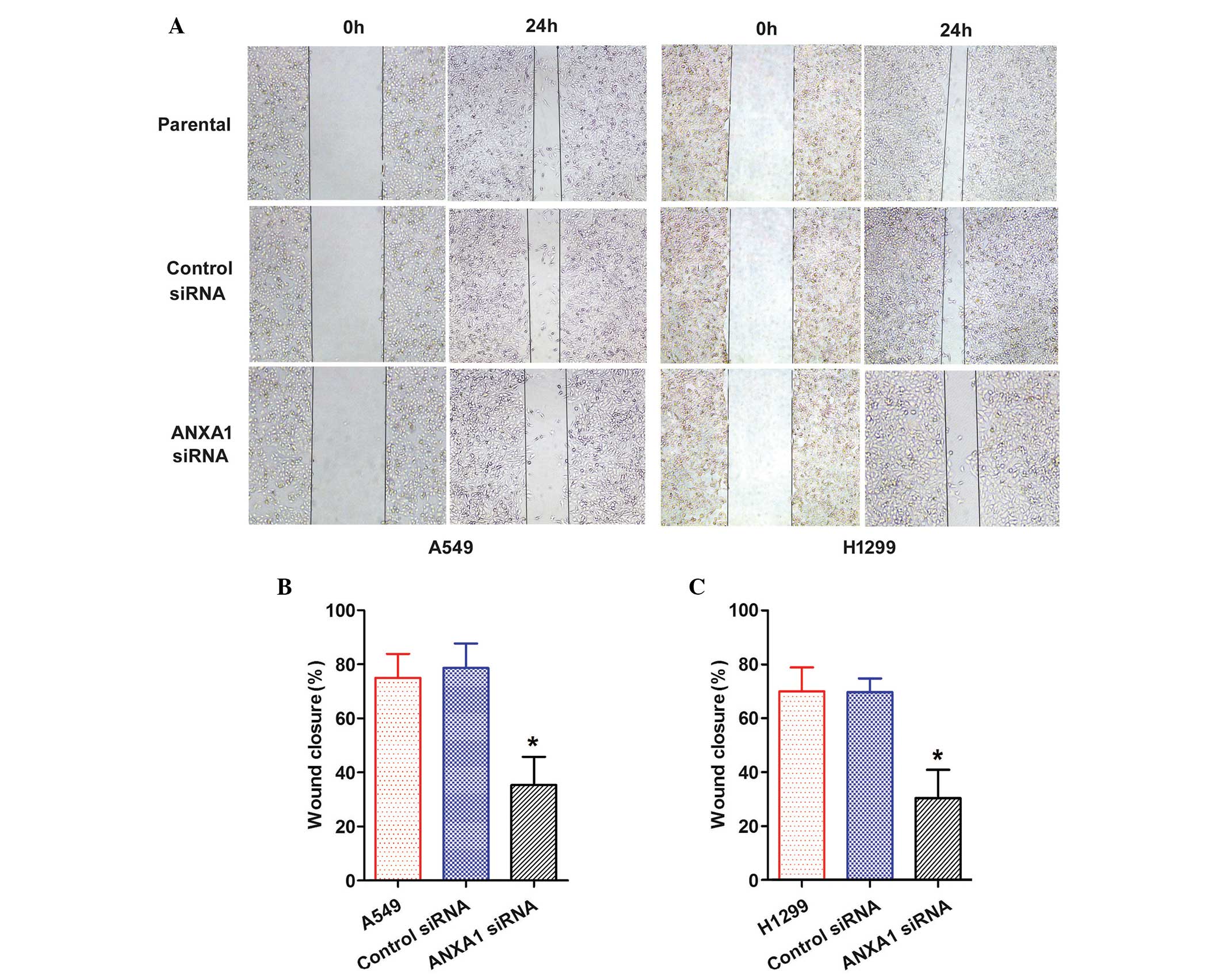
ANXA1 knockdown suppresses the migration
and invasion of NSCLC cells
The role of ANXA1 in the migration of NSCLC cells
was evaluated by wound healing and Transwell chamber assays. A
scratch was made in the cell layer, and wound closure was monitored
over the course of 24 h. A gradual decrease in wound closure was
recorded in both the ANXA1 siRNA groups (Fig. 4A). Statistical analysis revealed
that ANXA1 knockdown significantly suppressed migration of the
cells, as compared with in the NC siRNA group (P<0.05), and
there was no significant difference between the NC siRNA and
untreated groups (Fig. 4B and
C).
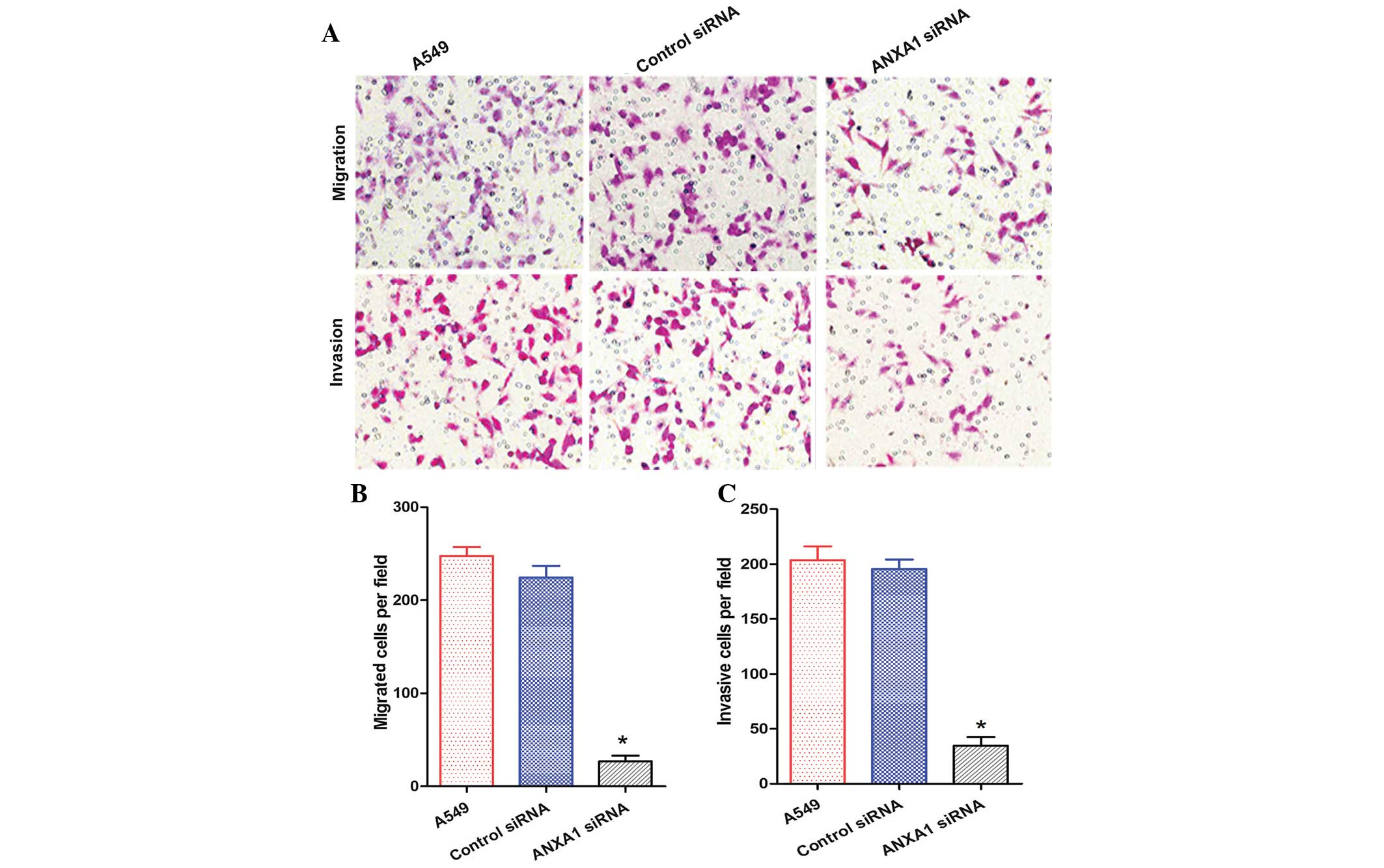
The migration and invasion of A549 cells in the
ANXA1 siRNA group was decreased, as compared with that of the NC
siRNA and untreated groups (Fig.
5A). Statistical analysis revealed that ANXA1 siRNA
significantly suppressed the metastasis and invasion of A549 cells
(P<0.05), and there was no significant difference between the NC
siRNA and untreated groups (Fig. 5B
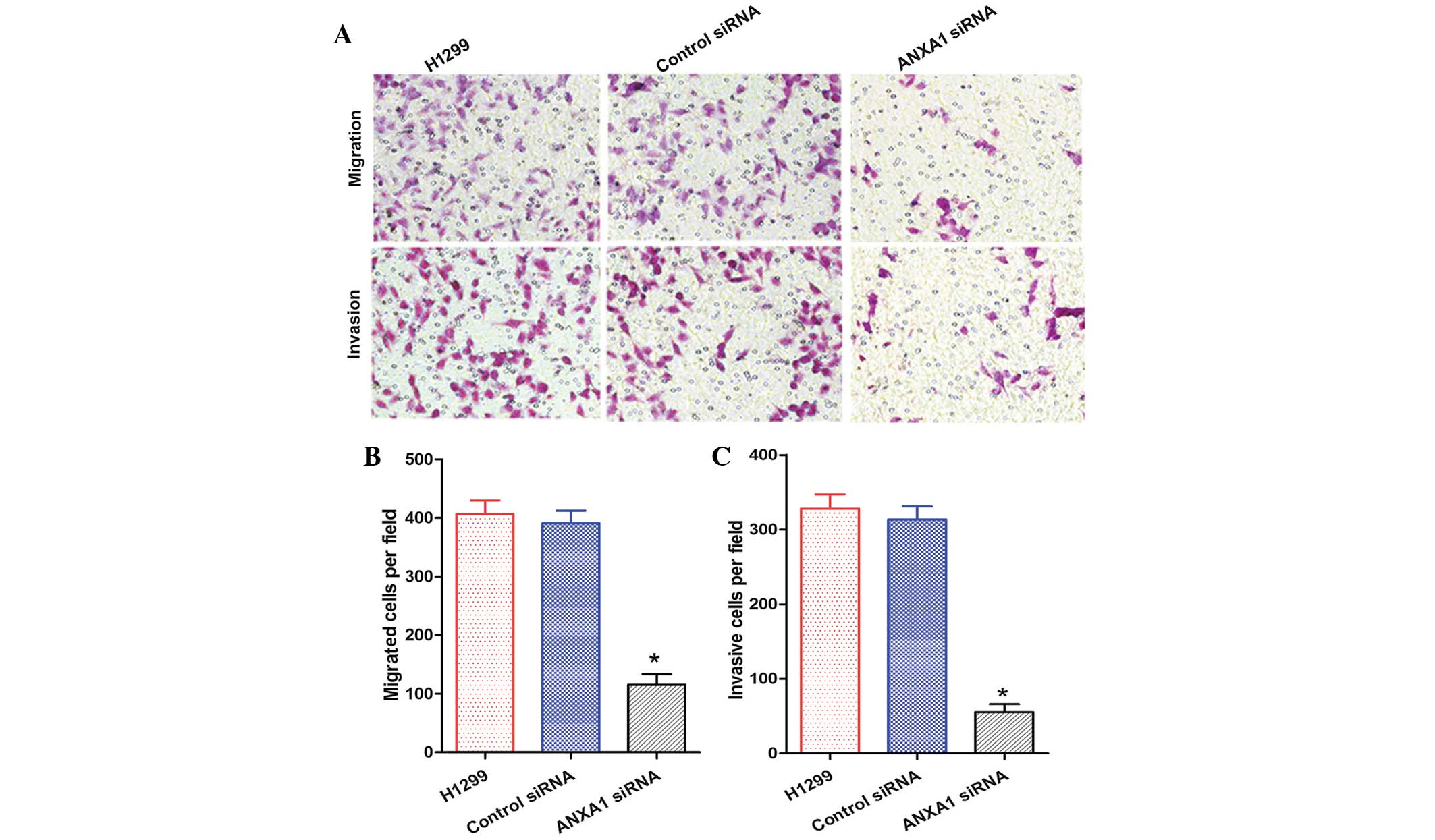
and C). In addition, the migration and invasion of H1299 cells
in the ANXA1 siRNA group was decreased, as compared with in the NC
siRNA and untreated groups (Fig.
6A). Statistical analysis revealed that ANXA1 siRNA
significantly inhibited the metastasis and invasion of H1299 cells
(Fig. 6B and C; P<0.05). Cell
migration is a critical step in metastasis, and these results
suggest that ANXA1 may have a critical role in the metastatic
behavior of cancer cells.
Discussion
Human lung cancer is a major cause of
cancer-associated mortality worldwide, and is associated with a low
5-year survival rate. Lung cancer-associated mortality is often
caused by extensive metastasis (16,17).
Tumor metastasis is a complex multi-step and multistage process
(18,19); therefore, the identification of
novel diagnostic methods and treatment biomarkers is required. A
previous study demonstrated that ANXA1 expression was significantly
higher in patients with NSCLC, as compared with in control subjects
(20). Increased ANXA1 expression
has also been detected in lung squamous carcinoma cell (21). Previous 2D-PAGE results indicated
that ANXA1 protein is upregulated in lung cancer and lung
cancer/chronic obstructive pulmonary disorder groups, as compared
with in the control group (22).
In addition, ANXA1 expression is significantly upregulated in tumor
tissues, as compared with in the normal tissues of patients with
lung cancer (23,24). The present study demonstrated that
ANXA1 was upregulated in 10 matched cancer tissues, thus indicating
that ANXA1 may exhibit increased expression in NSCLC. However, the
possible biological function of ANXA1 in NSCLC remains to be
elucidated.
The present study revealed that ANXA1 was
functionally involved in NSCLC progression and metastatic
formation, and ANXA1 was shown to be upregulated in NSCLC tissues
and cell lines. In addition, knockdown of ANXA1 suppressed the
proliferation of NSCLC cell lines, and inhibited invasion and
migration. These results indicated that silencing ANXA1 expression
in A549 and H1299 cells may inhibit metastasis in vitro.
The present study was designed to estimate the
effects of ANXA1 on the migration of cancer cells, and to
investigate the underlying mechanism. It has previously been
suggested that ANXA1 may regulate miR-26b and miR-562 via
down-regulation of nuclear factor (NF)-κB activity, which may lead
to higher endothelial cell tube formation and inhibit wound healing
capacity (25). Furthermore, ANXA1
has been shown to regulate tumor necrosis factor-β-induced
proliferation and inflammation in lung fibroblasts, via effects on
the extracellular signal-regulated kinases and NF-κB pathways
(26). ANXA1 has also been
reported to promote metastasis via activation of the transforming
growth factor-β/Smad signaling pathway (27).
In conclusion, the present study aimed to
investigate the role of ANXA1 in NSCLC, and to evaluate the
potential of using ANXA1 as a marker for NSCLC diagnosis and
treatment. However, further investigations are required regarding
the mechanism by which ANXA1 is associated with NSCLC
metastasis.
Acknowledgments
The present study was funded by the National Nature
Science Foundation of China (grant no. 81401391); the Ph.D.
Programs Foundation of Ministry of Education of China (grant no.
20134423110001); the National Nature Science Foundation of
Guangdong Province (grant no. S2012010010181); the Science and
Technology Project of Guangzhou City (grant no. 2014Y2-00171); and
the Education System Innovative Academic Team of Guangzhou City
(grant no. 13C06).
References
|
1
|
Ni L, Zhu X, Gong C, Luo Y, Wang L, Zhou
W, Zhu S and Li Y: Trichosanthes kirilowii fruits inhibit non-small
cell lung cancer cell growth through mitotic cell-cycle arrest. Am
J Chin Med. 43:349–364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gerke V and Moss SE: Annexins: From
structure to function. Physiol Rev. 82:331–371. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo C, Liu S and Sun MZ: Potential role of
Anxa1 in cancer. Future Oncol. 9:1773–1793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bist P, Shu S, Lee H, Arora S, Nair S, Lim
JY, Dayalan J, Gasser S, Biswas SK, Fairhurst AM and Lim LH:
Annexin-A1 regulates TLR-mediated IFN-β production through an
interaction with TANK-binding kinase 1. J Immunol. 191:4375–4382.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin Y, Lin G, Fang W, Zhu H and Chu K:
Increased expression of annexin A1 predicts poor prognosis in human
hepatocellular carcinoma and enhances cell malignant phenotype. Med
Oncol. 31(327)2014. View Article : Google Scholar
|
|
6
|
Huang Y, Zhang C, Chen C, Sun S, Zheng H,
Wan S, Meng Q, Chen Y and Wei J: Investigation of circulating
antibodies to ANXA1 in breast cancer. Tumour Biol. 36:1233–1236.
2015. View Article : Google Scholar
|
|
7
|
Okano M, Kumamoto K, Saito M, Onozawa H,
Saito K, Abe N, Ohtake T and Takenoshita S: Upregulated Annexin A1
promotes cellular invasion in triple-negative breast cancer. Oncol
Rep. 33:1064–1070. 2015.PubMed/NCBI
|
|
8
|
Boudhraa Z, Merle C, Mazzocut D, Chezal
JM, Chambon C, Miot-Noirault E, Theisen M, Bouchon B and Degoul F:
Characterization of pro-invasive mechanisms and N-terminal cleavage
of ANXA1 in melanoma. Arch Dermatol Res. 306:903–914. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boudhraa Z, Rondepierre F, Ouchchane L,
Kintossou R, Trzeciakiewicz A, Franck F, Kanitakis J, Labeille B,
Joubert-Zakeyh J, Bouchon B, et al: Annexin A1 in primary tumors
promotes melanoma dissemination. Clin Exp Metastasis. 31:749–760.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han G, Tian Y, Duan B, Sheng H, Gao H and
Huang J: Association of nuclear annexin A1 with prognosis of
patients with esophageal squamous cell carcinoma. Int J Clin Exp
Pathol. 7:751–759. 2014.PubMed/NCBI
|
|
11
|
Luthra R, Singh RR, Luthra MG, Li YX,
Hannah C, Romans AM, Barkoh BA, Chen SS, Ensor J, Maru DM, et al:
MicroRNA-196a targets annexin A1: A microRNA-mediated mechanism of
annexin A1 downregulation in cancers. Oncogene. 27:6667–6678. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang KL, Wu TT, Resetkova E, Wang H,
Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A, Hamilton
SR and Albarracin CT: Expression of annexin A1 in esophageal and
esophagogastric junction adenocarcinomas: Association with poor
outcome. Clin Cancer Res. 12:4598–4604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inokuchi J, Lau A, Tyson DR and Ornstein
DK: Loss of annexin A1 disrupts normal prostate glandular structure
by inducing autocrine IL-6 signaling. Carcinogenesis. 30:1082–1088.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Z, Zhu JS, Xu ZP and Zhang Q:
Lentiviral vector-mediated siRNA knockdown of the YAP gene inhibits
growth and induces apoptosis in the SGC7901 gastric cancer cell
line. Mol Med Rep. 4:1075–1082. 2011.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Bonomi M, Pilotto S, Milella M, Massari F,
Cingarlini S, Brunelli M, Chilosi M, Tortora G and Bria E: Adjuvant
chemotherapy for resected non-small-cell lung cancer: Future
perspectives for clinical research. J Exp Clin Cancer Res.
30(115)2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rothwell PM, Wilson M, Price JF, Belch JF,
Meade TW and Mehta Z: Effect of daily aspirin on risk of cancer
metastasis: A study of incident cancers during randomised
controlled trials. Lancet. 379:1591–1601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soda K: The mechanisms by which polyamines
accelerate tumor spread. J Exp Clin Cancer Res. 30:952011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang JD, Nakamura I and Roberts LR: The
tumor microenvironment in hepatocellular carcinoma: Current status
and therapeutic targets. Semin Cancer Biol. 21:35–43. 2011.
View Article : Google Scholar :
|
|
20
|
Wang W, Guan S, Sun S, Jin Y, Lee KH, Chen
Y and Wei J: Detection of circulating antibodies to linear peptide
antigens derived from ANXA1 and DDX53 in lung cancer. Tumour Biol.
35:4901–4905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nan Y, Yang S, Tian Y, Zhang W, Zhou B, Bu
L and Huo S: Analysis of the expression protein profiles of lung
squamous carcinoma cell using shot-gun proteomics strategy. Med
Oncol. 26:215–221. 2009. View Article : Google Scholar
|
|
22
|
Pastor MD, Nogal A, Molina-Pinelo S,
Meléndez R, Salinas A, González De la Peña M, Martín-Juan J, Corral
J, García-Carbonero R, Carnero A and Paz-Ares L: Identification of
proteomic signatures associated with lung cancer and COPD. J
Proteomics. 89:227–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rho JH, Roehrl MH and Wang JY:
Glycoproteomic analysis of human lung adenocarcinomas using
glycoarrays and tandem mass spectrometry: Differential expression
and glycosylation patterns of vimentin and fetuin A isoforms.
Protein J. 28:148–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu QY, Gao Y, Liu Y, Yang WZ and Xu XY:
Identification of differential gene expression profiles of
radioresistant lung cancer cell line established by fractionated
ionizing radiation in vitro. Chin Med J (Engl). 121:1830–1837.
2008.
|
|
25
|
Anbalagan D, Yap G, Yuan Y, Pandey VK, Lau
WH, Arora S, Bist P, Wong JS, Sethi G, Nissom PM, et al: Annexin-A1
regulates microRNA-26b* and microRNA-562 to directly target NF-κB
and angiogenesis in breast cancer cells. PloS One. 9:e1145072014.
View Article : Google Scholar
|
|
26
|
Jia Y, Morand EF, Song W, Cheng Q, Stewart
A and Yang YH: Regulation of lung fibroblast activation by annexin
A1. J Cell Physiol. 228:476–484. 2013. View Article : Google Scholar
|
|
27
|
de Graauw M, van Miltenburg MH, Schmidt
MK, Pont C, Lalai R, Kartopawiro J, Pardali E, Le Dévédec SE, Smit
VT, van der Wal A, et al: Annexin A1 regulates TGF-beta signaling
and promotes metastasis formation of basal-like breast cancer
cells. Proc Natl Acad Sci USA. 107:6340–6345. 2010. View Article : Google Scholar : PubMed/NCBI
|