Introduction
Renal cell carcinoma (RCC), arising from the renal
cortex, is the most common type of malignant tumor in the adult
kidney (1). Clear cell RCC is the
most common histological type of RCC, and is more aggressive than
other types (2). Novel target
therapeutic strategies have improved the treatment of patients with
RCC, although radical nephrectomy remains the predominant treatment
strategy for patients with RCC due to the resistance of the tumor
to radiation and chemotherapy (3,4).
However, early detection and diagnosis of RCC is difficult due to
the lack of clinical signs and manifestations (3). Additionally, RCC is highly vascular,
invasive and metastatic. Once metastasis occurs, patients with RCC
have a poorer prognosis. A third of patients with RCC develop
distant metastasis at initial diagnosis, and ~50% of these patients
develop disease recurrence, of which two thirds recur within the
first year (4,5). Thus, it is crucial to investigate the
mechanism underlying RCC tumorigenesis.
MicroRNAs (miRNAs) are a class of noncoding RNA,
typically 20–23 nt in length, which are cleaved from 70–100 nt
hairpin-shaped precursors (pre-miRNA) (6). Several studies have demonstrated that
miRNAs are important in a wide range of biological and pathological
processes, including cell differentiation, migration, growth,
proliferation, apoptosis and metabolism (6–9). A
link between miRNA function and cancer pathogenesis has been
supported by studies examining the signatures and functions of
miRNA in clinical samples (10).
In urological cancer, including RCC, the aberrant expression of
miRNAs has been reported between malignant and normal renal
tissues, and a specific miRNAs have been found to exert novel
oncogenic or tumor suppressive functions though target genes
(11,12).
Previous studies have identified that downregulated
miR-30a and miR-30a-3p increase angiogenesis-specific Delta-like
ligand 4 (DLL4) and hypoxia-inducible factor 2α (HIF2α) expression,
and promote tumor metastasis and growth, in clear cell RCC
(13,14). Previous studies on expression have
shown that miR-30a-5p is downregulated in malignancies of the lung
(14), blood (15), liver (16), nasopharynx (17), bone (18), breast (19) and bladder (20). However, miR-30a-5p has been
reported to be upregulated in glioma (21,22)
cell lines and specimens, compared with normal tissues and cell
lines. In RCC, several microarray chip assays have revealed
consistent results, that the expression levels of miR-30a-5p are
lower in RCC tissues and metastatic RCC tissues, compared with
adjacent normal tissues and paired primary RCC tissues (24–28).
These data suggest that miR-30a-5p may offer potential as a useful
diagnostic, prognostic and predictive biomarker in the treatment of
RCC. However, the expression, function and molecular mechanism of
miR-30a-5p in RCC remain to be fully elucidated.
In the present study, the expression levels of
miR-30a-5p in RCC specimens and cell lines were examined, and the
function of miR-30a-5p in RCC cells was investigated using cell
migration, proliferation and apoptotic assays. To further examine
the miR-30a-5p-mediated molecular pathway, polypeptide N-ac
etylgalactosaminyltransferase 7 (GALNT7) was identified as a
potential target of miR-30a-5p. Collectively, the results of the
present study may determine whether miR-30a-5p has an inhibitory
effect in the carcinogenesis of RCC though changes in the
expression of GALNT7.
Materials and methods
Cell lines and transfection
The human RCC cell lines (786-O and ACHN), human
cervical cancer cell line (Hela) and human embryo kidney cell line
(293T) were obtained from American Type Culture Collection
(Manassas, VA, USA), and were routinely cultured in Dulbecco′s
modified Eagle′s medium (DMEM)/high glucose medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 1%
antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin; Gibco;
Thermo Fisher Scientific, Inc.) and 1% glutamate (Gibco; Thermo
Fisher Scientific, Inc.). All cells were cultured at 37°C in a
humidified chamber containing 5% CO2.
To induce the upregulation of miR-30a-5p in the
cells, chemically synthesized miR-30a-5p mimics (GenePharma, Inc.,
Shanghai, China) were transfected into the cells at a confluence of
60–70% using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), which were mixed in Opti-MEM® I
reduced serum medium (Gibco; Thermo Fisher Scientific, Inc.)
following culture for 24 h. To validate the transient transfection
efficiency, fluorescence microscopy (using a DMIRB fluorescence
microscope; Leica Microsystems, Wetzlar, Germany) was performed
after transfection of Negative Control-Fluorescein amidite
(NC-FAM), and real-time quantitative polymerase chain reaction
(RT-qPCR) was performed after the transfection of miR-30a-5p mimics
and NC. The sequences of the miRNAs (NC-FAM, NC and miR-30a-5p
mimic) are summarized in Table
I.
 | Table ISequences of primers and
microRNAs. |
Table I
Sequences of primers and
microRNAs.
|
Primer/microRNA | Sequence |
|---|
| GALNT7 | Forward
5′-GGGATTATTTGCCATTGAACGA-3′
Reverse 5′-AGACGGTAGATATGTCCAACAC-3′ |
| GAPDH | Forward
5′-GGAGCGAGATCCCTCCAAAAT-3′
Reverse 5′-GGCTGTTGTCATACTTCTCATGG-3′ |
| miR-30a-5p | Forward
5′-TGTAAACATCCTCGACTGGAAG-3′
Reverse provided by the miScript SYBR® Green PCR kit
(Qiagen) |
| U6 | Forward
5′-CTCGCTTCGGCAGCACA-3′
Reverse 5′-ACGCTTCACGAATTTGCGT-3′ |
| miR-30a-5p
mimic | Forward
5′-UGUAAACAUCCUCGACUGGAAG-3′
Reverse 5′-UCCAGUCGAGGAUGUUUACAUU-3′ |
| NC | Forward
5′-UUCUCCGAACGUGUCACGUTT-3′
Reverse 5′-ACGUGACACGUUCGGAGAATT-3′ |
| NC-FAM | Forward
5′-UUCUCCGAACGUGUCACGUTT-3′
Reverse 5′-ACGUGACACGUUCGGAGAATT-3′ |
Tissue collection
The collection and use of tissue samples in the
present study were reviewed and approved by the ethics committee of
Peking University Shenzhen Hospital (Shenzhen, Guangdong, China).
Written informed consent were signed and obtained from all 32
patients. In the present study, a total of 32 paired fresh RCC and
adjacent normal tissue samples (located 5.0 cm from the visible RCC
lesions) were obtained from Peking University Shenzhen Hospital
(Shenzhen, China). Once dissected, all fresh tissue samples were
immediately immersed in RNAlater (Qiagen, Hilden, Germany) and
frozen in liquid nitrogen for total RNA extraction. The
clinicopatho-logical information obtained from the patients is
shown in Table II. Stage
classification was performed using the 2010 American Joint
Committee on Cancer staging system (29).
 | Table IIClinicopathological features of
patients with renal cell carcinoma. |
Table II
Clinicopathological features of
patients with renal cell carcinoma.
| Characteristic | Cases (n) |
|---|
| Mean age; range
(years) | 52; 29–71 |
| Gender |
| Male/female | 20/12 |
| Histological
type |
| Clear
cell/papillary | 29/3 |
| Primary tumor
stage |
| T1/T2/T3+T4 | 18/13/1 |
| Fuhrman grade |
| I/II/III/IV | 11/15/4/2 |
| AJCC clinical
stage |
| I/II/III+IV | 17/14/1 |
Total RNA extraction, reverse
transcription and RT-Qpcr
The 786-O, ACHN and 293T cells (4×105
cells/well) were plated into 6-well plates (BD Biosciences, San
Jose, CA, USA) with three replicate wells, respectively. After 24 h
at 37°C, the cells were trypsinized to extract the total RNA using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was extracted from the 32 paired RCC tissues and
normal tissues using TRIzol® reagents and were purified
using the RNeasy® Maxi kit (Qiagen), according to the
manufacturer′s protocol. For the homogenization step, 50–100 mg
tissue or ~1.5×106 cells were added to 1 ml RNAiso Plus
reagent and homogenized using a motor-driven tissue grinder
(OSE-Y10; Tiangen Biotech (Beijing) Co., Ltd., Beijing, China).
Subsequently, the homogenized samples were incubated with RNAiso
Plus reagent for 5 min at room temperature to permit the complete
dissociation of nucleoprotein complexes. The tubes were agitated
vigorously by hand for 15 sec, and then the samples were
centrifuged at 12,000 × g for 15 min at 4°C.
The RNA samples with 260/280 ratios of 1.8-2.0 were
used in the subsequent experiments. The total RNA was converted
into a cDNA template using an miScript II RT kit (Qiagen) for
quantification of miR-30a-5p, or a PrimeScript™ RT reagent kit
(Takara Bio., Inc., Otsu, Japan) for quantification of GALNT7
mRNA.
The expression of miR-30a-5p was confirmed using the
miScriptSYBR® green PCR kit (Qiagen), and the mRNA
expression of GALNT7 was validated using SYBR® Premix Ex
Taq™ II (Takara Bio, Inc.), respectively, on a Roche lightcycler
480 Real-Time PCR system (Roche Applied Science, Mannheim,
Germany). The 20 µl reaction mixture contained 10 µl
2X QuantiTect SYBR Green PCR Master mix, 2 µl 10X miScript
Universal Primer, 0.4 µl specific miRNA primer, 1 µl
cDNA template and 6.6 µl RNase-free water. The thermocycling
steps were 95°C for 15 min, followed by 40 cycles at 94°C for 15
sec, 55°C for 30 sec and 72°C for 30 sec. U6 and GAPDH were used as
internal controls. The expression levels were calculated as fold
changes relative to the U6 and GAPDH controls, which was based on
the following equation: Relative expression = 2−ΔΔCq
(30). The primers used are shown
in Table I.
Wound scratch assay
Cell migration ability was examined using a wound
scratch assay. The cells (~3×105 cells/well) were seeded
into a 12-well dish and, on reaching 80-90% confluence, the
miR-30a-5p mimic or negative control (NC) were introduced into the
786-O and ACHN cells using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). The NC (GenePharma, Inc., Shanghai,
China) comprised miRNA oligonucleotides that were chemically
synthesized in order to eliminate the non-sequence binding effects.
The sequences of the miRNAs (NC, NC-FAM and miR-30a-5p mimic) were
all summarized in Table I.
Following 6 h of transfection, a sterile 200 µl pipette tip
and markers were used to introduce a scratch into the cell
monolayer. The cells were then rinsed with phosphate-buffered
saline (PBS) three times, cultured in DMEM, and incubated at 37°C.
Images of the scratches were acquired using a digital camera system
(model DMIRB; Leica Microsystems, Wetzlar, Germany) at 0 and 24 h
following introduction of the scratches at the same points. The
experiments were performed in three independent repeats in
triplicate, and analyzed in a double-blinded manner by at least two
observers.
Cell proliferation assay
A
3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazoliumbromide (MTT;
5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) assay was performed to
determine the proliferation abilities of the 786-O and ACHN cells.
The 786-O and ACHN cells were seeded into 96-well plates at a cell
density of 5×103 cells/well, with five replicate wells
for each condition. After 24 h, the cells in each wells were
transfected with either 5 pmol miR-30a-5p mimic or NC. MTT (20
µl) was added to each well and the plate was incubated at
37°C for ~5 h prior to measurement. The MTT medium mixtures were
then discarded and the reaction was terminated by the addition of
120 µl dimethylsulphoxide (Sigma-Aldrich). Following
agitation for 15 min at room temperature, the optical density was
measured at the wavelength of 490 nm, with 630 nm as the reference
wavelength, using an enzyme immunoassay instrument (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). All assays were performed
in triplicate.
Flow cytometric analysis
The early apoptotic rates of the 786-O and ACHN
cells were stained with fluorescein isothiocyanate
(FITC)-conjugated Annexin V (Annexin V-FITC) and propidium iodide
(PI; Invitrogen; Thermo Fisher Scientific, Inc.) and quantified
using flow cytometry (EPICS, Xl-4, Beckman Coulter, Brea, CA, USA).
The 786-O and ACHN cells (3×105) were seeded into 6-well
plates, and 200 pmol of either miR-30a-5p mimic or NC were
introduced to the cells via transfection for 6 h. Subsequently, the
cells were incubated at 37°C in a humidified chamber containing 5%
CO2 for 48 h following transfection. The cells,
including floating cells, were collected and washed twice with
pre-chilled PBS. The cells were resuspended in 100 µl 1X
binding buffer (comprising 10 mM HEPES, 140 mM NaCl, 2.5 mM
CaCl2, pH 7.4; Invitrogen; Thermo Fisher Scientific,
Inc.) and stained with 5 µl Annexin V-FITC and PI for 15 min
at room temperature, using an Annexin V-FITC/PI detection kit
(Invitrogen; Thermo Fisher Scientific, Inc.). The flow cytometric
measurements were obtained within 30 min of staining, and 400
µl of 1X binding buffer was added to each sample upon
measurement. Each experiment was performed at least three
times.
Bioinformatics analysis
Computational algorithms have been confirmed as an
effective method for predicting the candidate target genes of a
specific miRNA. In the present study, the following target
prediction algorithms were used: miRanda (http://mirdb.org/miRDB/index.html), TargetScan Release
6.2 (http://www.targetscan.org), microRNA
(http://www.microrna.org) and miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk).
These approaches are based on the identification of elements in the
3′-untranslated region (UTR) of target genes, which are
complementary to the seed sequence of the microRNA of interest.
Putative genes predicted by at least three algorithms were
accepted, and candidates were selected based on the gene
function.
Luciferase reporter assay
The 3′-UTR of GALNT7 (519 bp), containing the
putative binding site (5′-TGTTTAC-3′) for miR-30a-5p, was cloned
into an empty psiCHECK-2 Vector (Promega, Madison, WI, USA),
generating the wild-type psiCHECK2-3′-UTR. The primer sequences for
the 3′-UTR of GALNT7 containing the miR-30a-5p binding site
(forward primer, 5′-CCG CTC GAG CTA CTG A CAA GTA AAT TTA TAC
AGG-3′ and reverse primer, 5′-AAG GAA AAA AGC GGC CGC AGA GGC ACT
AAA TGT GTT GA-3′) were designed. The mutant type was generated by
changing the putative binding site to 5′-ACAAATG-3′ in the
complementary site for seed region of miR-30a-5p. All constructed
plasmids were verified by DNA sequencing analysis. The DNA
sequencing analysis was performed on an Applied Biosystems 3730XL
DNA Analyzer (Invitrogen; Thermo Fisher Scientific, Inc.) using the
dideoxy-mediated chain-termination method.
Sequencing primers and the bacteria which contained
the wild/mutant types of constructed plasmids were obtained from
Thermo Fisher Scientific, Inc. The detected DNA sequences were also
obtained from Thermo Fisher Scientific, Inc., and the DNA sequences
were compared with the sequences designed for the present study to
check whether the sequences, including the 3′-UTR of GALNT7 (wide
or mutant type), had been incorporated into the psi-CHECK2
vector.
For the luciferase reporter assay, 293T and Hela
cells were seeded into 24-well plates, and were co-transfected with
0.5 µg of the constructed plasmids and 40 pmol of the
miR-30a-5p mimic or NC using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). At 24 h post-trans-fection, firefly and
Renilla luciferase in the cells were detected using a
Dual-Luciferase Reporter Assay system (Promega) on a Modulus™
Single Tube Multimode Reader (Turner BioSystems, Madison, WI, USA).
All experiments were performed in triplicate wells and repeated at
least three times.
Western blot analysis
The 786-O and ACHN cells (3×105) were
seeded into 6-well plates and transfected with 200 pmol of either
the miR-30a-5p mimic or NC using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) for 6 h. At 48 h post-transfection,
radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich) was
used to lyse the cells on ice, and protein concentrations were
quantified using a Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.). A total of 60 µg of protein sample was
loaded in each well and separated using 10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE), with gels that were
made prior to use: The formulation of the lower 10% separating gel
was 4.0 ml H2O, 3.3 ml 30% acrylamide, 2.5 ml 1.5 M Tris
(pH 8.8), 0.1 ml 10% SDS, 0.1 ml 10% AP, 4 µl
tetramethyle-thylenediamine (TEMED), and that of the upper SDS-PAGE
gel (5% stacking gel) was: 2.7 ml H2O, 0.67 ml 30%
acryl-amide, 0.5 ml 1 M Tris (pH 6.8), 0.04 ml 10% SDS, 0.04 ml 10%
AP and 4 µl TEMED. Subsequently, the proteins were
transferred onto polyvinylidene difluoride (PVDF) membranes (EMD
Millipore, Billerica, MA, USA) via wet blotting. The PVDF membranes
were blocked with 5% nonfat milk at room temperature for 2 h, and
incubated with primary antibodies overnight at 4°C. The primary
antibodies against GALNT7 and β-actin (internal control) were
rabbit polyclonal anti-GALNT7 (1:1,000; cat. no. ab97645; Abcam,
Cambridge, MA, USA) and rabbit polyclonal anti-β-actin (1:10,000;
cat. no. NB600-532; Novus Biologicals, Littleton, CO, USA),
respectively. The second day, following washing three times with
Tris-buffered saline with 0.05% Tween (TBST), the membranes were
treated with horesradish peroxidase (HRP)-linked goat anti-rabbit
IgG (1:10,000; cat. no. E031120-01, EarthOx, Life Sciences,
Millbrae, CA, USA) for 2 h, followed by the detection of protein
bands using an Immun-Star™ HRP Chemiluminescence kit (Bio-Rad
Laboratories, Inc.) Each assay was repeated at least three
times.
Statistical analysis
The data are presented as the mean ± standard
deviation from three independent experiments. All data were
analyzed using SPSS 19.0 statistical software (IBM SPSS, Inc.,
Armonk, NY, USA). The MTT data were analyzed using one-way analysis
of variance. The clinicopathological information of the patients
were analyzed using a χ2 test, whereas other data were
analyzed using Student′s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
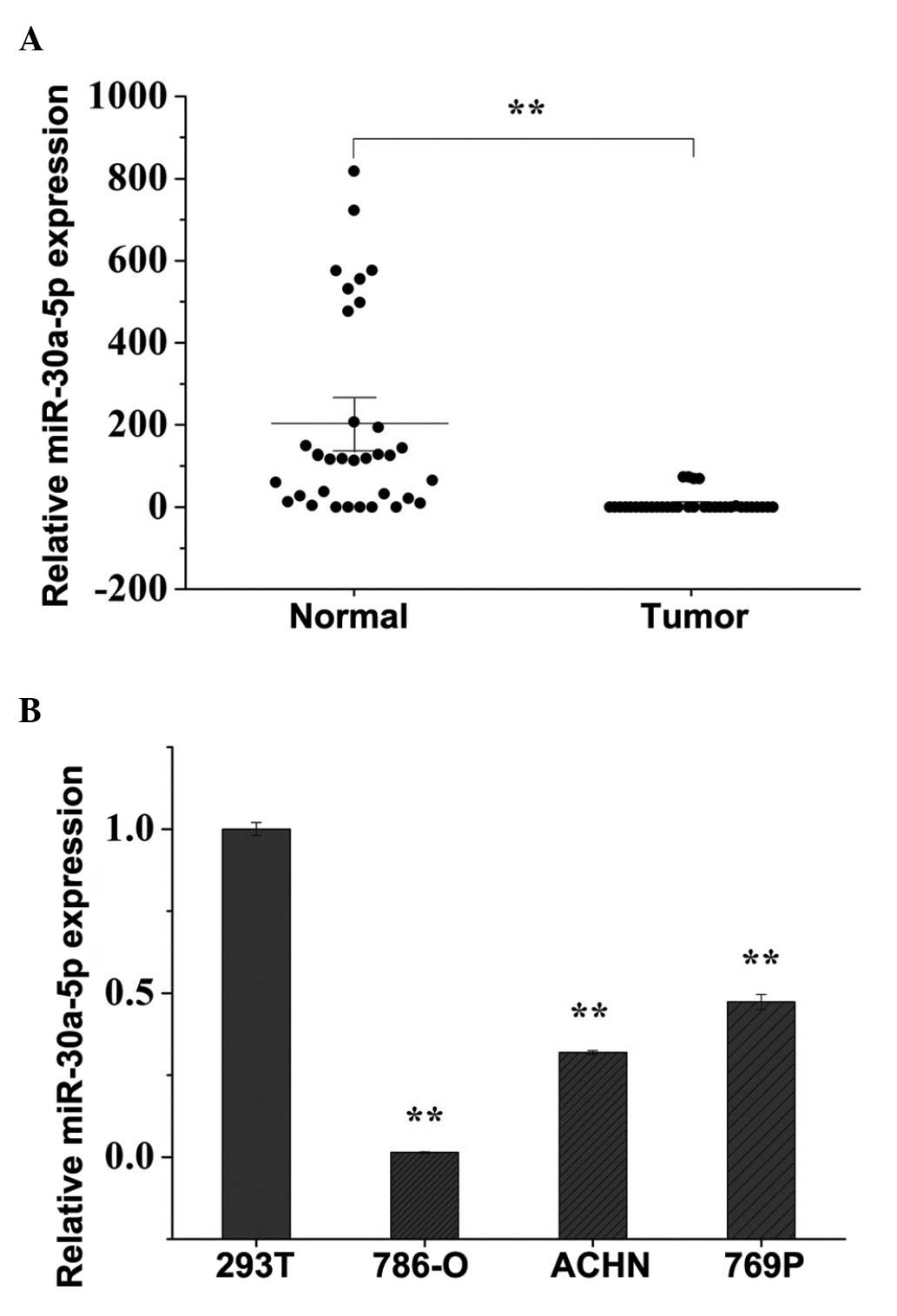
Expression levels of miR-30a-5p are
downregulated in human RCC clinical specimens and cell lines
The expression levels of miR-30a-5p were examined in
32 paired RCC tissues and adjacent normal tissues, and the
expression of miR-30a-5p was found to be significantly
downregulated in the RCC tissues, compared with the adjacent normal
tissues, as shown in Fig. 1A. The
expression levels of miR-30a-5p in 293T cells and the three RCC
cell lines were also quantified using RT-qPCR. As shown in Fig. 1B, the expression levels of
miR-30a-5p were lower in the 786-O, ACHN and 769P cells
(P<0.01), compared with the level in the 293T cells, which was
in accordance with the expression pattern of miR-30a-5p in the
clinical specimens. The present study selected 786-O and ACHN cells
to perform the subsequent functional experiments.
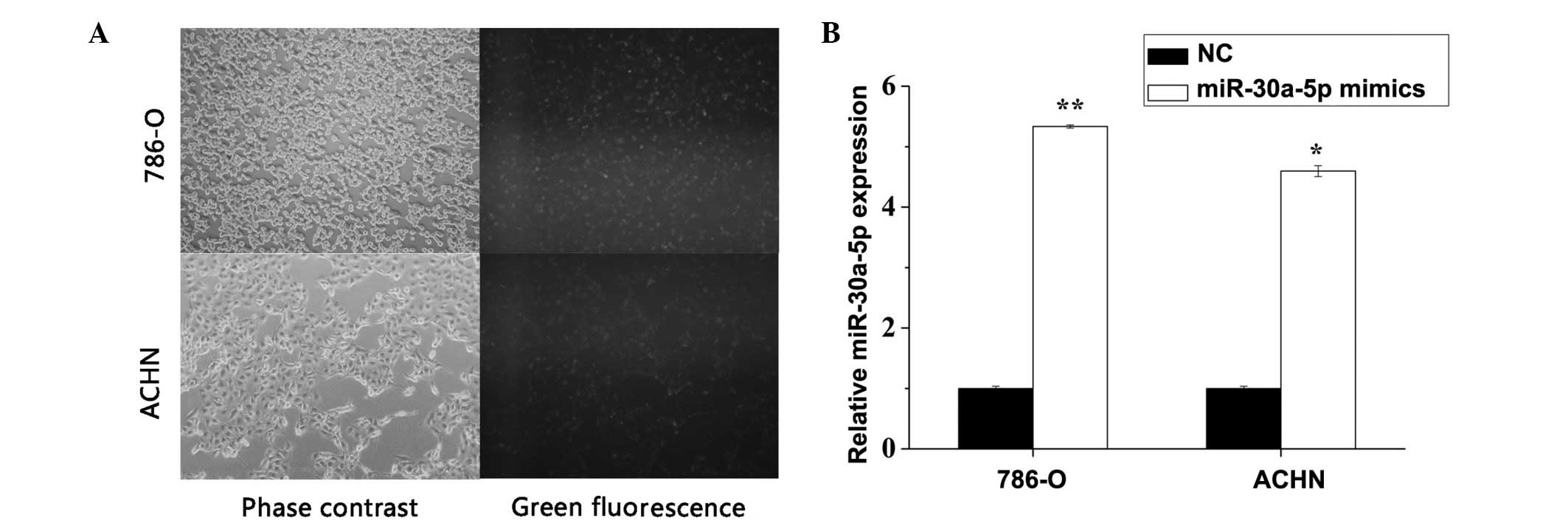
Transfection efficiency validation
The NC-fluorescein amino-modified oligonucleotide
(NC-FAM), miR-30a-5p mimic or NC were transfected into 786-O and
ACHN to validate the transfection efficiency. As seen in Fig. 2A, transfection efficiency was
>90% when the cells were transfected with NC-FAM. RT-qPCR was
also used to verify transfection efficiency, and it was revealed
that miR-30a-5p was significantly overexpressed following
transfection with the miR-30a-5p mimic in the 786-O (P<0.01) and
ACHN (P=0.015) cells (Fig.
2B).
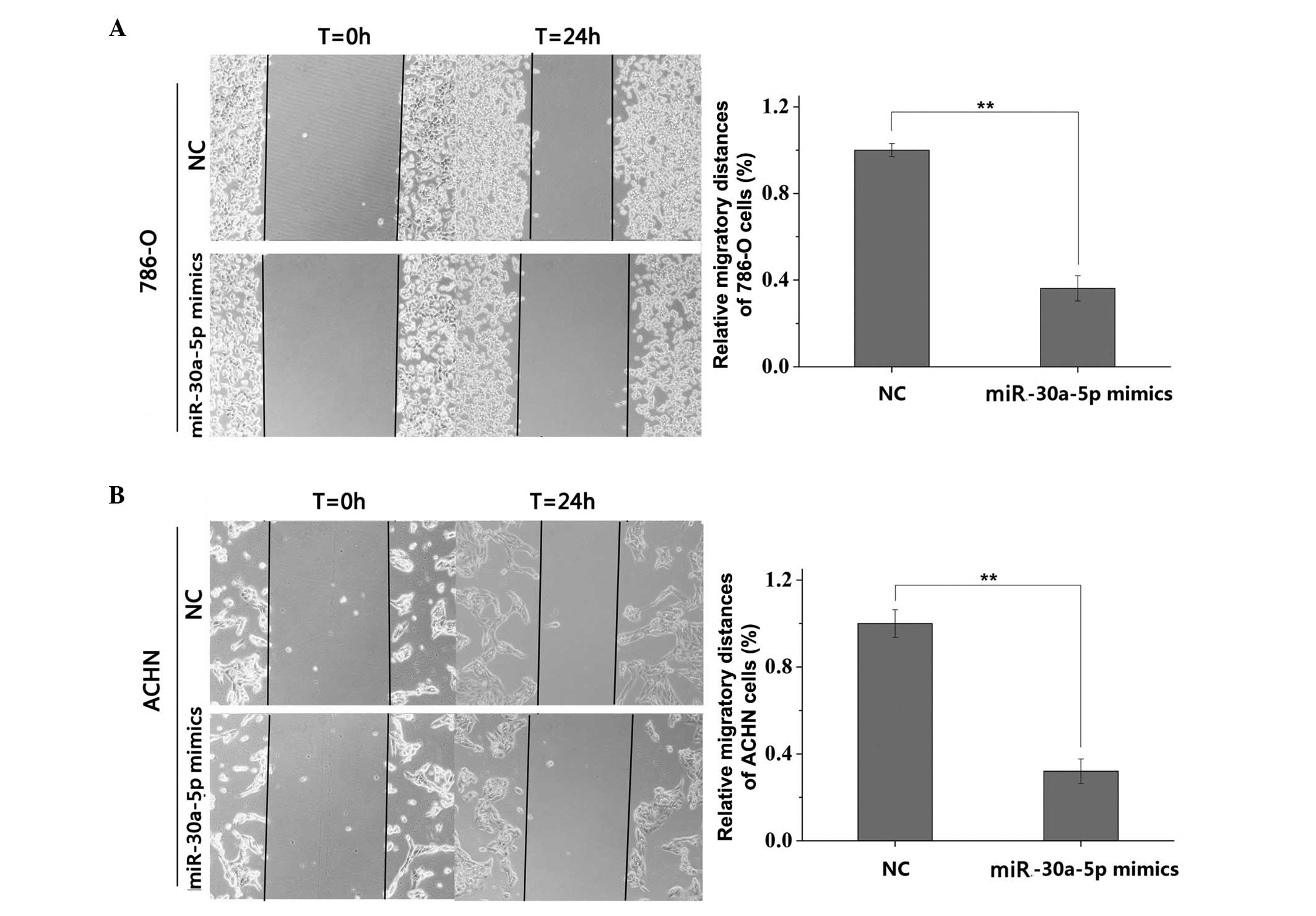
miR-30a-5p suppresses RCC cell
migration
The wound scratch assay showed that cell migration
was significantly inhibited in the groups transfected with
miR-30a-5p, compared with the NC groups. The inhibition rates of
migration were 63.80% for the 786-O cells (Fig. 3A) and 67.95% for the ACHN cells
(Fig. 3B), indicating that
miR-30a-5p had a significant negative effect on RCC cell
migration.
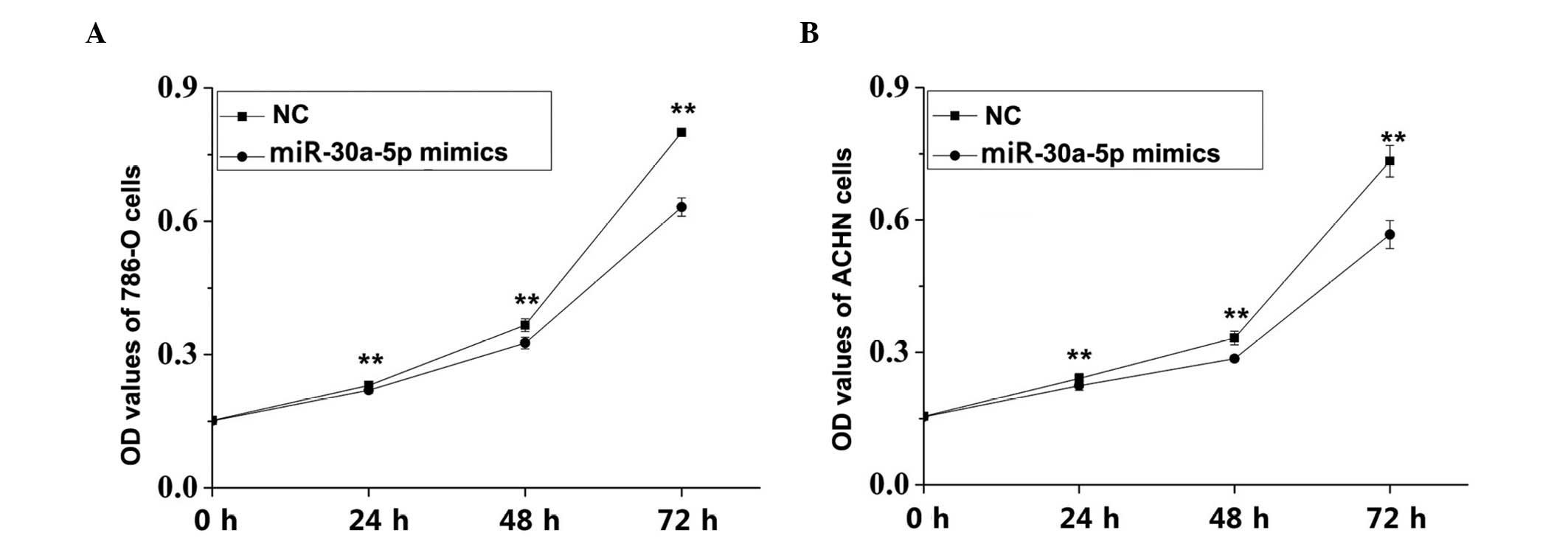
Proliferation of RCC cells is inhibited
by miR-30a-5p mimic transfection
An MTT assay was performed to observe whether
differential expression of miR-30a-5p affected the proliferation
ability of the RCC cells. The results revealed that cell
proliferation rates in the miR-30a-5p transfectants were
significantly decreased by 4.66% (24 h; P<0.01), 10.99% (48 h;
P<0.01) and 21.02% (72 h; P<0.01) in the 786-O cells
(Fig. 4A). In the ACHN cells, the
rates of proliferation inhibition were 6.84, 14.05 and 22.67% at
24, 48 and 72 h, respectively (P<0.01; Fig. 4B).
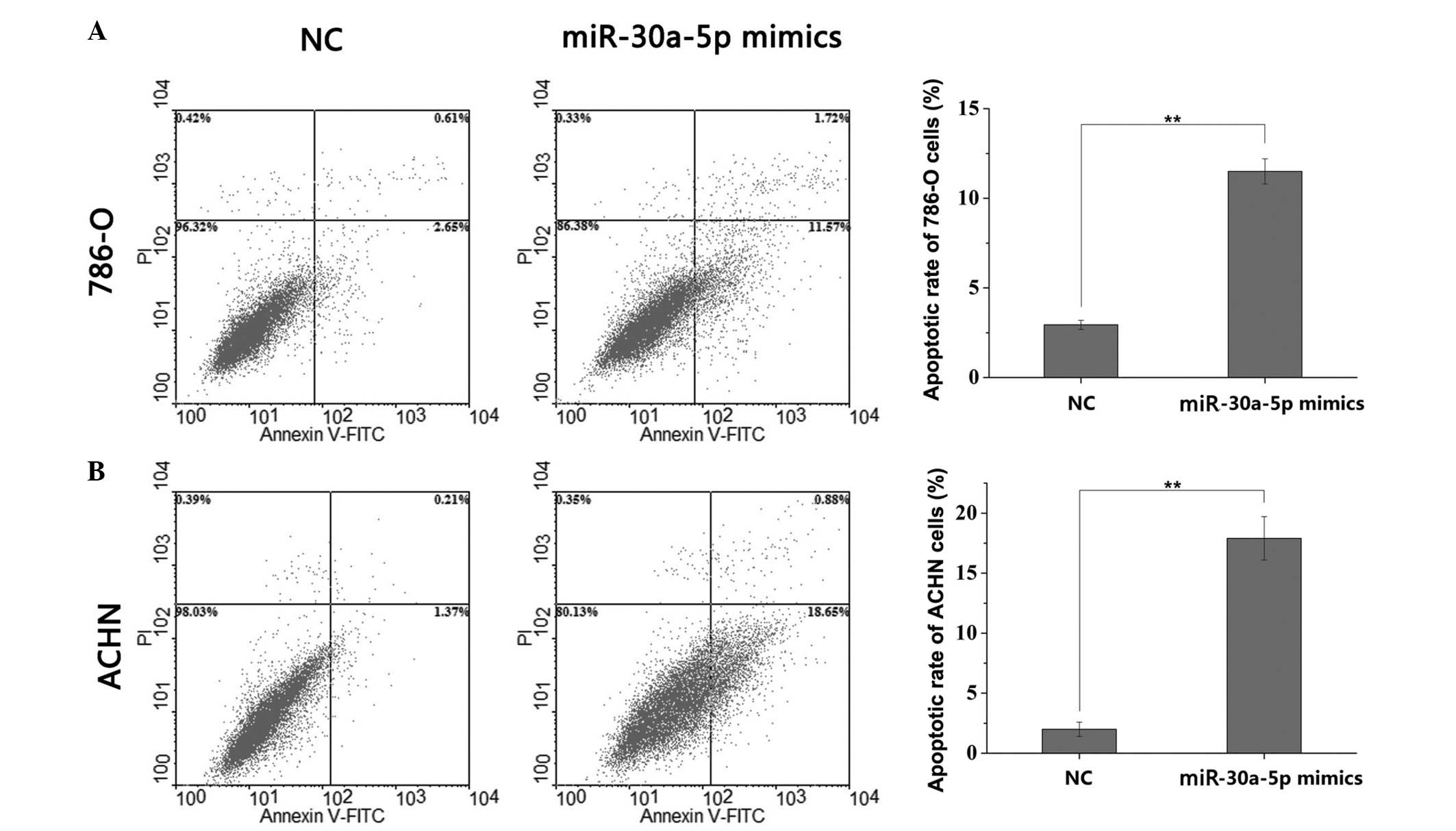
Cell apoptosis is induced by miR-30a-5p
mimic
To investigate the effect of miR-30a-5p on the
apoptosis of RCC cells, flow cytometry was performed to detect the
early apoptotic rate. The results showed that, compared with the
cells transfected with NC, the apoptotic rate was significantly
increased in the miR-30a-5p mimic-transfected 786-O cells (2.94,
vs. 11.50%, respectively; P<0.01; Fig. 5A) and the miR-30a-5p
mimic-transfected ACHN cells (2.00, vs. 17.90%, respectively;
P<0.01, Fig. 5B). These data
suggested that miR-30a-5p was important in RCC cell apoptosis.
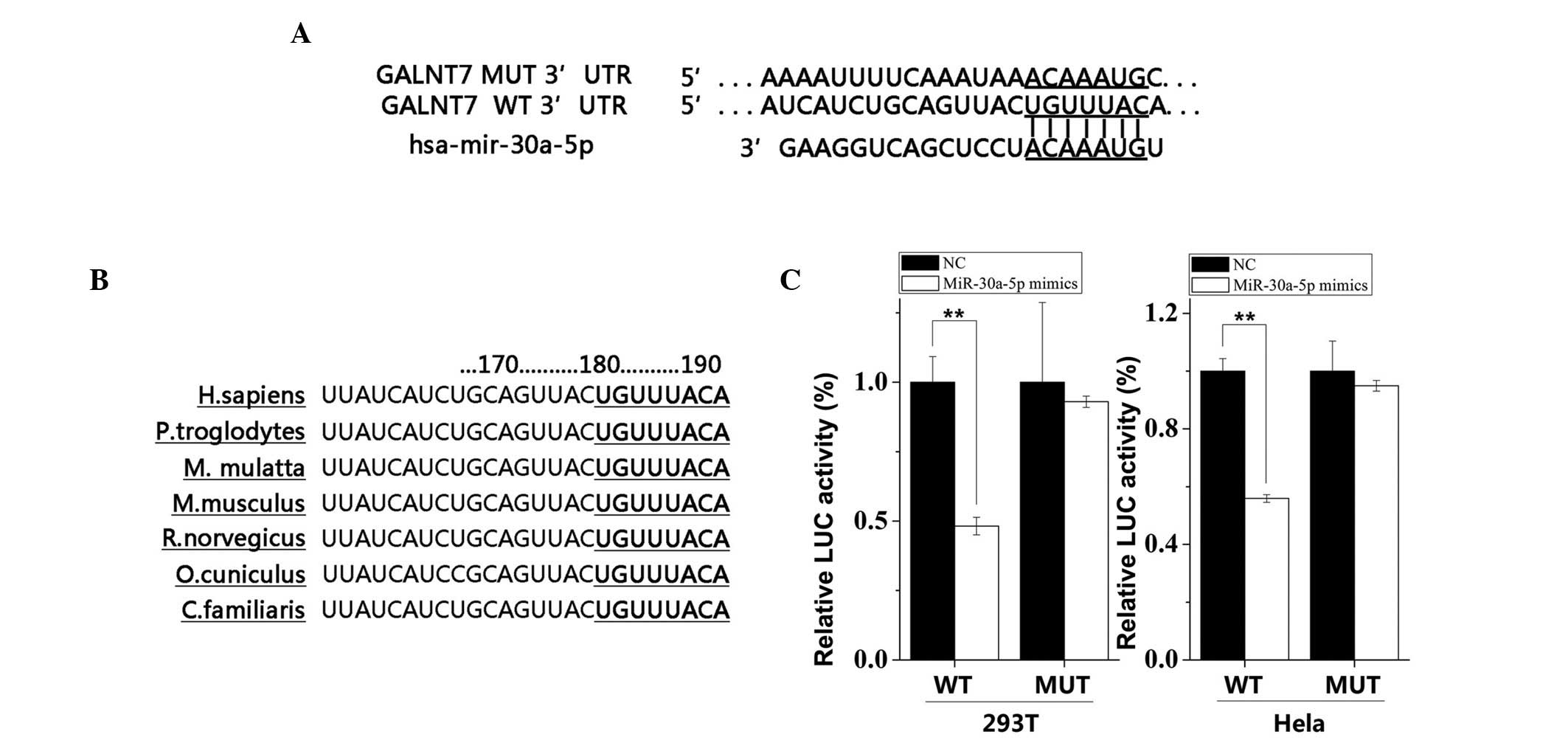
GALNT7 is a direct target of
miR-30a-5p
miRNAs exert their functions by regulating the
expression of their downstream target gene/s. Multiple
computational algorithms using miRanda, TargetScan, microRNA and
miRWalk predicted that the 3′-UTR of the human GALNT7 gene is a
target for miR-30a-5p. The 3′-UTR of GALNT7 mRNA contained a
complementary nucleotide sequence for the seed region of miR-30a-5p
(Fig. 6A). This seed region of
miR-30a-5p was highly conserved among species, indicating its
regulatory importance (Fig. 6B).
As shown in Fig. 6C, the
luciferase reporter assay confirmed that the activity of the
reporter containing the 3′-UTR of GALNT7 was decreased
significantly when the cells were transfected with the miR-30a-5p
mimic in the 293T cells (P<0.01) and Hela cells (P<0.01),
compared with those transfected with the NC. However, no
significant changes were observed in the activity of the reporter
containing the mutated seed sequence following transfection of the
cells with either the miR-494 mimic or the NC.
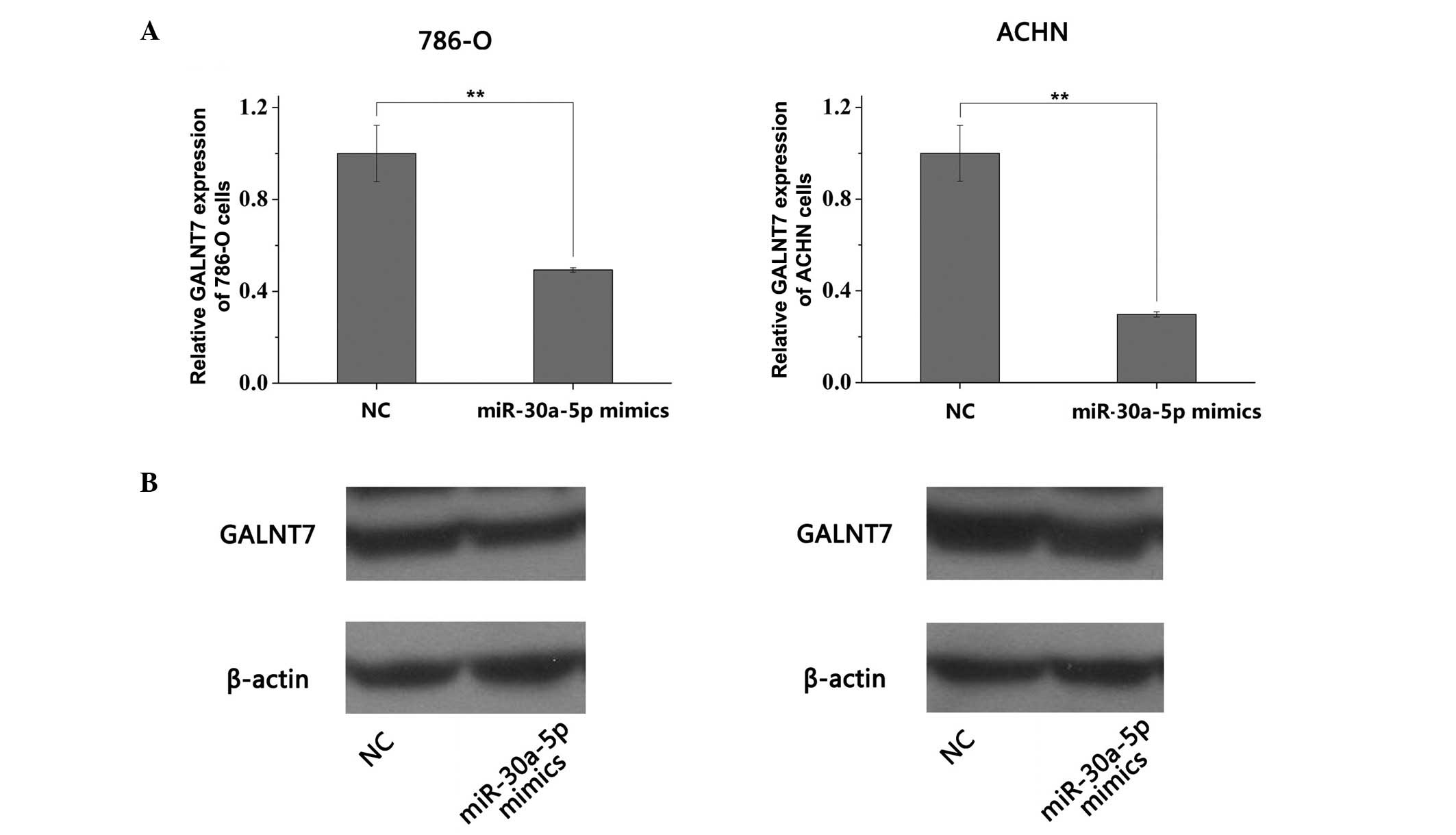
miR-30a-5p downregulates the mRNA and
protein levels of GALNT7 in RCC cell lines
To validate the results of the luciferase reporter
assay and evaluate the association between miR-30a-5p and GALNT7,
the present study performed RT-qPCR and Western blot analyses to
quantify the mRNA and protein level of GALNT7 in the 786-O and ACHN
cells 48 h following transfection with the miR-30a-5p mimic or NC.
The mRNA levels of GALNT7 in the 786-O and ACHN cells were
significantly decreased following transfection with the miR-30a-5p
mimic, compared with the NC group (P<0.01; Fig. 7A). In accordance with the
downregulation in the mRNA levels of GALNT7, the protein levels of
GALNT7 were significantly reduced when the 786-O and ACHN cells
were transfected with the miR-30a-5p mimic (P<0.01; Fig. 7B). These results suggested that
miR-30a-5p regulated the mRNA and protein expression levels of
GALNT7, and exerted regulation at transcriptional and
post-transcriptional levels.
Discussion
miRNAs have multiple roles in various types of
cancer, and the functions of miRNAs depend predominantly on their
target genes, which include ~50% of human protein-coding genes
(9). Generally, the expression of
functional miRNAs is dysregulated in various types of cancer,
acting either as oncogenes when overexpressed, or tumor suppressors
when downregulated (31,32). The present study screened for
differentially expressed miRNAs in RCC and confirmed that
miR-30a-5p was markedly downregulated in RCC tissues and cell
lines. The reduced expression levels of miR-30a-5p in different
types of cancer suggests that it may have anticancer effects. In
the present study, the role of miR-30a-5p was investigated
extensively, and its target gene in RCC cells was identified.
It has been reported that miR-30a-5p is important in
the initiation and development of cancer. For example, miR-30a has
tumor suppressive effects towards T-ALL and DLBCL cell lines by
cell growth suppression, cell cycle arrest and the induction of
apoptosis, whereas depletion of miR-30a restores tumorigenesis
(33). In chronic myeloid
leukemia, miR-30a decreases proliferation and arrests cell cycle
progression between the G1 and S phases, acting as a tumor
suppressor by downregulating the expression levels of abelson
murine leukemia viral oncogene homolog 1 (ABL1) and breakpoint
cluster protein-ABL1 (34). The
dowregulation of miR-30a promotes cell survival and proliferation
in T-cell lymphoblastic lymphomas via activation of GLI/Hedgehog
signaling (35). Low expression of
miR-30a in hepatocellular carcinoma facilitate migration, invasion
and epithelial-mesenchymal transition by targeting SNAI1 (17). In addition, miR-30a promotes
invasiveness and metastasis in vitro and in vivo
through epithelial-mesenchymal transition, and results in poor
survival rates in patients with nasopharyngeal carcinoma (18). All the above studies indicate that
downregulation of miR-30a occurs frequently in human cancer, and
has functions in tumor onset and progression. However, its
relevance in the development of RCC remains to be fully elucidated.
Accordingly, the present study revealed that the upregulation of
miR-30a-5p inhibited the migration and proliferation abilities, and
promoted the apoptosis of RCC cells.
GALNT7 is a member of the GalNAc-transferase family,
which is important in initiating mucin-type O-glycosylation in the
Golgi apparatus by transferring GalNAc from UDP-GalNAc to the Ser
and Thr residues of polypeptide acceptors (36). Mucin is a cell-surface
glycoprotein, which establishes a molecular barrier at the
epithelial surface and engages in morphogenetic signal
transduction. Alterations in mucin glycosylation accompany the
development of cancer and affect cellular growth, differentiation,
transformation, adhesion, invasion and immune surveillance
(37,38). According to previous studies,
GALNT7 is upregulated in several types of cancer, including
laryngeal carcinoma, cervical cancer and pancreatic cancer
(39-41), and is targeted by tumor suppressive
miRNAs. Additionally, GALNT7, as a target of upregulated
miR-30b/30d is important in the metastatic behavior of melanoma
cells (42). Previously, the
expression of GALNT7 in thyroid tissues, in relation to iodine-131
doses received from the Chernobyl accident, were investigated, and
a statistically significant dose-expression association was
confirmed, suggesting that GALNT7 is important in radiation
carcinogenesis (43). In the
present study, GALNT7 was identified as a downstream gene of
miR-30a-5p following bioinformatics and experimental verification.
However, the expression and function of GALNT7 in RCC remains to be
fully elucidated, and its role warrants confirmation in future
investigations.
It appears controversial that miR-30a-5p functions
as a tumor suppressor in certain types of cancer, and an oncogene
in others. Unlike small interfering RNA, the interactions between
miRNAs and the 3′-UTRs of target genes are not entirely
complementary (44). Due to the
fact that the seed region between miRNAs and genes requires only ≥7
base pairs, the network between miRNAs and target genes is not
one-to-one, but one-to-multiple and multiple-to-one. Additionally,
the expression of miRNAs was various in different tissues and
stages of development, thus the functions of miRNAs are tissue-and
temporally-specific.
In conclusion, the present study provided evidence
indicating that miR-30a-5p may be an important tumor suppressor in
RCC tumorigenesis by targeting GALNT7.
Acknowledgments
This work was supported by the National Natural
Science Foundation of China (no. 81101922), the Guangdong Natural
Science Foundation (no. 2015A030313889), Science and Technology
Development Fund Project of Shenzhen (nos. JCYJ20120616144352139,
JCYJ20130402114702124, JCYJ20140415162542975 and
JCYJ20150403091443329) and the Fund of Guangdong Key Medical
Subject.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Motzer RJ, Bander NH and Nanus DM:
Renal-cell carcinoma. N Engl J Med. 335:865–875. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Janzen NK, Kim HL, Figlin RA and
Belldegrun AS: Surveillance after radical or partial nephrectomy
for localized renal cell carcinoma and management of recurrent
disease. Urol Clin North Am. 30:843–852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rouvière O, Bouvier R, Négrier S, Badet L
and Lyonnet D: Nonmetastatic renal-cell carcinoma: Is it really
possible to define rational guidelines for post-treatment
follow-up? Nat Clin Pract Oncol. 3:200–213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huntzinger E and Izaurralde E: Gene
silencing by microRNAs: Contributions of translational repression
and mRNA decay. Nat Rev Genet. 12:99–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI
|
|
10
|
Ichimi T, Enokida H, Okuno Y, Kunimoto R,
Chiyomaru T, Kawamoto K, Kawahara K, Toki K, Kawakami K, Nishiyama
K, et al: Identification of novel microRNA targets based on
microRNA signatures in bladder cancer. Int J Cancer. 125:345–352.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rydzanicz M, Wrzesiński T, Bluyssen HA and
Wesoly J: Genomics and epigenomics of clear cell renal cell
carcinoma: Recent developments and potential applications. Cancer
Lett. 341:111–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fendler A, Stephan C, Yousef GM and Jung
K: MicroRNAs as regulators of signal transduction in urological
tumors. Clin Chem. 57:954–968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang QB, Ma X, Zhang X, Liu SW, Ai Q, Shi
TP, Zhang Y, Gao Y, Fan Y, Ni D, et al: Down-Regulated miR-30a in
Clear Cell Renal Cell Carcinoma Correlated with Tumor Hematogenous
Metastasis by Targeting Angiogenesis-Specific DLL4. PLoS One.
82013.
|
|
14
|
Mathew LK, Lee SS, Skuli N, Rao S, Keith
B, Nathanson KL, Lal P and Simon MC: Restricted expression of
miR-30c-2-3p and miR-30a-3p in clear cell renal cell carcinomas
enhances HIF2alpha activity. Cancer Discov. 4:53–60. 2014.
View Article : Google Scholar :
|
|
15
|
Tan X, Qin W, Zhang L, Hang J, Li B, Zhang
C, Wan J, Zhou F, Shao K, Sun Y, et al: A 5-microRNA signature for
lung squamous cell carcinoma diagnosis and hsa-miR-31 for
prognosis. Clin Cancer Res. 17:6802–6811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fuster O, Llop M, Dolz S, García P, Such
E, Ibáñez M, Luna I, Gómez I, López M, Cervera J, et al: Adverse
prognostic value of MYBL2 overexpression and association with
microRNA-30 family in acute myeloid leukemia patients. Leuk Res.
37:1690–1696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Tu K and Liu Q: Effects of
microRNA-30a on migration, invasion and prognosis of hepatocellular
carcinoma. FEBS Lett. 588:3089–3097. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang HY, Li YY, Fu S, Wang XP, Huang MY,
Zhang X, Shao Q, Deng L, Zeng MS, Zeng YX and Shao JY: MicroRNA-30a
promotes invasiveness and metastasis in vitro and in vivo through
epithelial-mesenchymal transition and results in poor survival of
nasopharyngeal carcinoma patients. Exp Biol Med. 239:891–898. 2014.
View Article : Google Scholar
|
|
19
|
Huang Q, Jiang Z, Meng T, Yin H, Wang J,
Wan W, Cheng M, Yan W, Liu T, Song D, et al: MiR-30a inhibits
osteolysis by targeting RunX2 in giant cell tumor of bone. Biochem
Biophys Res Commun. 453:160–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fu J, Xu X, Kang L, Zhou L, Wang S, Lu J,
Cheng L, Fan Z, Yuan B, Tian P, et al: miR-30a suppresses breast
cancer cell proliferation and migration by targeting Eya2. Biochem
Biophys Res Commun. 445:314–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang X, Du L, Wang L, Li J, Liu Y, Zheng
G, Qu A, Zhang X, Pan H, Yang Y and Wang C: Serum microRNA
expression signatures identified from genome-wide microRNA
profiling serve as novel noninvasive biomarkers for diagnosis and
recurrence of bladder cancer. Int J Cancer. 136:854–862. 2015.
View Article : Google Scholar
|
|
22
|
Jia Z, Wang K, Wang G, Zhang A and Pu P:
MiR-30a-5p antisense oligonucleotide suppresses glioma cell growth
by targeting SEPT7. PLoS One. 8:e550082013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang K, Jia Z, Zou J, Zhang A, Wang G, Hao
J, Wang Y, Yang S and Pu P: Analysis of hsa-miR-30a-5p expression
in human gliomas. Pathol Oncol Res. 19:405–411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu H, Brannon AR, Reddy AR, Alexe G,
Seiler MW, Arreola A, Oza JH, Yao M, Juan D, Liou LS, et al:
Identifying mRNA targets of microRNA dysregulated in cancer: With
application to clear cell renal cell carcinoma. BMC Syst Biol.
4:512010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Juan D, Alexe G, Antes T, Liu H,
Madabhushi A, Delisi C, Ganesan S, Bhanot G and Liou LS:
Identification of a microRNA panel for clear-cell kidney cancer.
Urology. 75:835–841. 2010. View Article : Google Scholar
|
|
26
|
Yi Z, Fu Y, Zhao S, Zhang X and Ma C:
Differential expression of miRNA patterns in renal cell carcinoma
and nontumorous tissues. J Cancer Res Clin Oncol. 136:855–862.
2010. View Article : Google Scholar
|
|
27
|
White NM, Khella HW, Grigull J, Adzovic S,
Youssef YM, Honey RJ, Stewart R, Pace KT, Bjarnason GA, Jewett MA,
et al: miRNA profiling in metastatic renal cell carcinoma reveals a
tumour-suppressor effect for miR-215. Br J Cancer. 105:1741–1749.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Müller S and Nowak K: Exploring the
miRNA-mRNA regulatory network in clear cell renal cell carcinomas
by next-generation sequencing expression profiles. Biomed Res Int.
2014:9484082014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th ed. New
York, NY: Springer; pp. 479–489. 2010
|
|
30
|
Duan HF, Li XQ, Hu HY, Li YC, Cai Z, Mei
XS, Yu P, Nie LP, Zhang W, Yu ZD and Nie GH: Functional elucidation
of miR-494 in the tumorigenesis of nasopharyngeal carcinoma. Tumour
Biol. 36:6679–6689. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: Oncogene or a tumor suppressor? Cancer
Metastasis Rev. 28:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in Cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ortega M, Bhatnagar H, Lin AP, Wang L,
Aster JC, Sill H and Aguiar RC: A microRNA-mediated regulatory loop
modulates NOTCH and MYC oncogenic signals in B-and T-cell
malignancies. Leukemia. 29:968–976. 2015. View Article : Google Scholar :
|
|
34
|
Liu Y, Song Y, Ma W, Zheng W and Yin H:
Decreased microRNA-30a levels are associated with enhanced ABL1 and
BCR-ABL1 expression in chronic myeloid leukemia. Leuk Res.
37:349–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
González-Gugel E, Villa-Morales M, Santos
J, Bueno MJ, Malumbres M, Rodríguez-Pinilla SM, Piris MÁ and
Fernández-Piqueras J: Down-regulation of specific miRNAs enhances
the expression of the gene Smoothened and contributes to T-cell
lymphoblastic lymphoma development. Carcinogenesis. 34:902–908.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perrine CL, Ganguli A, Wu P, Bertozzi CR,
Fritz TA, Raman J, Tabak LA and Gerken TA: Glycopeptide-preferring
polypeptide GalNAc transferase 10 (ppGalNAc T10), involved in
mucin-type O-glycosylation, has a unique GalNAc-O-Ser/Thr-binding
site in its catalytic domain not found in ppGalNAc T1 or T2. J Biol
Chem. 284:20387–20397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pegram MD, Borges VF, Ibrahim N, Fuloria
J, Shapiro C, Perez S, Wang K, Schaedli Stark F and Courtenay Luck
N: Phase I dose escalation pharmacokinetic assessment of
intravenous humanized anti-MUC1 antibody AS1402 in patients with
advanced breast cancer. Breast Cancer Res. 11:R732009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hollingsworth MA and Swanson BJ: Mucins in
cancer: Protection and control of the cell surface. Nat Rev Cancer.
4:45–60. 2004. View Article : Google Scholar
|
|
39
|
Peng RQ, Wan HY, Li HF, Liu M, Li X and
Tang H: MicroRNA-214 suppresses growth and invasiveness of cervical
cancer cells by targeting UDP-N-acetyl-α-D-galactosamine:
Polypeptide N-acetylgalactosaminyltransferase 7. J Biol Chem.
287:14301–14309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Taniuchi K, Cerny RL, Tanouchi A, Kohno K,
Kotani N, Honke K, Saibara T and Hollingsworth MA: Overexpression
of GalNAc-transferase GalNAc-T3 promotes pancreatic cancer cell
growth. Oncogene. 30:4843–4854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li W, Ma H and Sun J: MicroRNA34a/c
function as tumor suppressors in Hep2 laryngeal carcinoma cells and
may reduce GALNT7 expression. Mol Med Rep. 9:1293–1298.
2014.PubMed/NCBI
|
|
42
|
Gaziel-Sovran A, Segura MF, Di Micco R,
Collins MK, Hanniford D, Vega-Saenz de Miera E, Rakus JF, Dankert
JF, Shang S, Kerbel RS, et al: miR-30b/30d regulation of GalNAc
transferases enhances invasion and immunosuppression during
metastasis. Cancer Cell. 20:104–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Abend M, Pfeiffer RM, Ruf C, Hatch M,
Bogdanova TI, Tronko MD, Riecke A, Hartmann J, Meineke V, Boukheris
H, et al: Iodine-131 dose dependent gene expression in thyroid
cancers and corresponding normal tissues following the Chernobyl
accident. PLoS One. 7:e391032012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim VN: MicroRNA biogenesis: Coordinated
cropping and dicing. Nat Rev Mol Cell Biol. 6:376–385. 2005.
View Article : Google Scholar : PubMed/NCBI
|