Introduction
Periodontitis is a highly prevalent inflammatory
disease that causes the destruction of connective tissue,
resorption of surrounding bone tissue and eventually tooth loss.
Therapy for periodontitis consists of controlling inflammation and
promoting healing. The remaining healthy periodontal ligament (PDL)
has an essential role by retaining its potential for regeneration
to a certain extent throughout adulthood. The PDL is composed of
several different cell populations that are characterized by their
ability to remodel and renew periodontium (1). In particular, PDL fibroblasts are the
key cells involved in these processes; during periodontal wound
healing, fibroblasts are activated, migrate to the wound site and
repair damaged tissue by remodeling the extracellular matrix
(2).
From a biological perspective, the wound healing
process involves a complexity of cellular biochemical events. It is
necessary to maintain a delicate balance between the positive and
deleterious effects of reactive oxygen species (ROS) during wound
healing and successful tissue regeneration. ROS are chemically
reactive molecules containing oxygen. High ROS levels were
originally considered to be closely linked to aging and various
degenerative diseases, including stroke and cardiovascular disease.
However, a number of studies have shown that ROS have a
physiological role in several non-phagocytic cells. It is now
widely recognized that at low concentrations, ROS act as ubiquitous
intracellular messengers that support wound healing (3). Increased ROS generation has been
associated with the mitogenic stimulation of growth factors
(4).
Glutathione is a non-protein thiol found in
mammalian cells, and is essential for maintaining intracellular ROS
balance and the thiol status of proteins (5).
Gamma-glutamyl transferase (GGT) is the only
cell-surface enzyme that catalyzes the initial step of
extracellular glutathione degradation, thereby providing additional
cysteine, which is the rate-limiting substrate for intracellular
de novo glutathione synthesis (6). Therefore, catabolism of glutathione
by GGT affects intracellular ROS levels and cysteine homoeostasis
(7). GGsTop is a novel
phosphonate-based, irreversible inhibitor of GGT, and exhibits a
much higher inhibitory activity than the traditional GGT inhibitor,
acivicin. Furthermore, GGsTOP inhibits only GGT, and does not have
any influence on glutamine amidotransferases (8).
Based on the above evidence, the present study
hypothesized that GGT may be produced in the PDL. In addition, the
inhibition of GGT activity by GGsTOP may affect intracellular ROS
levels, and thereby influence the remodeling and renewal of human
periodontal ligament cells (hPLCs) to induce the fiber synthesis
necessary to repair PDL tissues. Hence, the objective of the
present study was to investigate the levels of GGT in PDL tissues,
and to evaluate the effects of the inhibition of GGT activity on
hPLCs.
Materials and methods
Reagents
The reagen1ts dichlorodihydrofluorescein diacetate
(DCFH-DA), 4′,6-diamidino-2-phenylindole dihydrochlorid-edimethyl
sulfoxide (DAPI), N-acetyl-l-cysteine (NAC) were purchased
from Sigma-Aldrich (St. Louis, MO, USA). GGsTop (Wako Pure Chemical
Industries, Osaka, Japan) is freely soluble in distilled water;
therefore, a 10-mM stock solution was prepared, stored at −20°C,
and for subsequent experiments diluted to different concentrations
with Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Isolation and cell culture of hPLCs
Human tissue sample collection was approved by the
ethics committee of the Second Affiliated Hospital of Harbin
Medical University (Harbin, China), and informed consent was
obtained from all patients or their advisers prior to teeth
extraction, according to the ethics committee guidelines. A total
of 8 healthy premolar teeth were collected from four 12–16
adolescents (two males and two females) who had extracted teeth for
orthodontic reasons with no evidence of caries, periodontitis or
gingivitis. The periodontal ligament tissues were scraped from the
middle one-third of the tooth roots, minced and then placed in
25-cm2 culture flasks containing DMEM with 10% (v/v)
fetal bovine serum (GE Healthcare Life Sciences, Logan, UT, USA),
100 U/ml penicillin and 100 μg/ml streptomycin (Gibco;
Thermo Fisher Scientific, Inc.). The tissues were incubated at 37°C
in 5% CO2 and 100% relative humidity. Cells were
passaged until 80% confluent, and cells from the third to fifth
passages were used for the experiment.
Immunohistochemistry
Male C57BL/6 mice (n=4; age, four weeks; weight,
20–30 g) were purchased from the experimental animal center of the
Second Affiliated Hospital of Harbin Medical University. The animal
experiments were approved by the ethics committee of the Second
Affiliated Hospital of Harbin Medical University. The mice were
anesthetized with 10% chloral hydrate (250 mg/kg; Wuhan Hechang
Chemical Co., Ltd., Wuhan, China) by intraperitoneal injection and
perfused transcardially with 4% paraformaldehyde (Tianjin Guangfu
Chemical Co., Ltd., Tianjin, China) in phosphate-buffered saline
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China). The mandibles were removed and immersed in 4%
paraformaldehyde at 4°C for 48 h. The tissues were subsequently
decalcified and embedded in paraffin (Shanghai Hushi Laboratorial
Equipment Co., Ltd, Shanghai, China). Five-micron-thick sections
were analyzed by immunohistochemistry using a mouse monoclonal
antibody against gamma-glutamyl transferase (1:400 dilution; cat.
no. ab55138; Abcam, Cambridge, MA, USA). The sections were then
incubated with a biotinylated secondary antibody using a
Histostain-Plus kit (ZSGB-BIO, Beijing, China). The color reaction
was finally developed using 3,3′-diaminobenzidine (Nichirei, Tokyo,
Japan). The sections were observed using a light microscope
(Eclipse 80i; Nikon, Tokyo, Japan).
Determination of GGT activity
Determination of GGT activity was performed as
described by Huseby and Srömme (9)
using 22.5 mmol/l γ-glutamyl-p-nitroanilide (Sigma-Aldrich) as the
donor and 123 mmol/l glycylglycine (Sigma-Aldrich) as the glutamate
acceptor, with a volume fraction of 1:11. Confluent cell monolayers
of human hepatoma HepG2 cells (American Type Culture Collection,
Manassas, VA, USA) and hPLCs were pre-treated with GGsTOP for 2 h
followed by further treatment with hypotonic lysis buffer (10 mM
Tris-HCl, pH 7.8), and ground using a glass Dounce homogenizer
(3432N75; Thomas Scientific, Swedesboro, NJ, USA) (30 strokes;
4°C). The cells were collected separately and amounts of
p-nitroaniline formed were measured by spectrophotometry at 405 nm
using a 722S Visible spectrophotometer (Shanghai Inesa Scientific
Instrument, Shanghai, China). The units of enzyme activity were
calculated using a molar extinction coefficient of 9.9 for
p-nitroanilide. One unit of GGT activity was defined as the amount
of enzyme required to release 1 μmol of substrate
transformed/ml/min. The results were expressed as U/g cell protein.
Protein content was measured using the bicinchoninic acid (BCA)
method (Beyotime Institute of Biotechnology, Haimen, China).
Cell proliferation
Cell viability was measured in hPLCs using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. In brief, hPLCs (2.5×103 cells/well) were seeded
into 96-well plates and allowed to attach and grow for 24 h. The
cells were starved overnight and then incubated with 0, 5, 10, 20
or 50 μM GGsTOP for 1, 3, 5 and 7 days. The medium was
replaced every other day. Then, 20 μl MTT (Sigma-Aldrich)
was added to each well at the indicated time point and the cells
were incubated for 4 h. The medium was subsequently removed and 150
μl dimethyl sulfoxide (Sigma-Aldrich) was added to each well
for 10 min. The absorbance was determined at 490 nm with a
microplate reader (Model 550; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Generation of ROS
Cellular levels of ROS were measured by means of the
previously described semi-quantitative DCFH-DA fluorescence
technique, which can be used to track changes in the ROS
concentration over time (10). The
hPLCs were seeded into six-well plates at a density of
7×105 cells/well and starved overnight. The cells were
then pre-incubated with the fluorescent dye DCFH-DA (10 mM/l in
DMEM) for 20 min at 37°C and 5% CO2 in the dark. After
incubation with the dye, the cells were rinsed twice in
phosphate-buffered saline prior to treatment with GGsTOP (10
μM) or GGsTOP (10 μM)+NAC (3 mM); cells without any
treatment were used as controls. Cells from all groups were
subjected to fluorescence microscopy at 0, 5, 10, 15, 30, 60, 120
and 180 min. Six random fields at the center of each well (border
of 4×4 mm) were analyzed at excitation and emission wavelengths of
485 and 525 nm using a fluorescence microscope (Eclipse Ti-S,
Nikon). The results were analyzed by NIS-Elements Basic Research
software 4.10 (Nikon). Values are expressed as the percentage
change in fluorescence at the pixel level compared with that at
time 0 (baseline).
Wound healing assay
To test the effects of GGsTOP on cell migration,
hPLCs (5×105 cells/well) were seeded into six-well
plates and cultured until 80–90% confluent for the in vitro
scratch-wound assay (11).
Briefly, a mark was made on the outside of each well before the
experiment, so as to capture the same image area at each time
point, using NIS-Elements Basic Research software. A uniform
scratch wound was created by dragging a plastic pipette tip across
the culture dish. The cells were incubated with medium containing
10 μM GGsTOP or 10 μM GGsTOP+3 mM NAC, while control
cells did not receive any treatment. At 0, 24 and 48 h after the
wounding procedure, images were taken in each group under light
microscopy (Eclipse 80i; Nikon). To calculate the number of cells
migrated into the wound area, cells were fixed with 4%
paraformaldehyde for 30 min and stained with DAPI at 48 h, then
were observed under a fluorescence microscope (Eclipse Ti-S;
Nikon).
Western blot analysis
hPLCs (1×106 cells/well) were incubated
in medium containing 10 μM GGsTOP, 20 μM GGsTOP or 10
μM GGsTOP+3 mM NAC for 72 h in a six-well plate, while the
control cells did not undergo any treatment. Cell protein was
extracted using radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology), and quantified using the BCA method
(Beyotime Institute of Biotechnology). The protein extracts were
resolved on a 10% tris-glycine acrylamide gel (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) under denaturing
conditions and then electrotransferred onto polyvinylidene
difluoride membranes (Immobilon-P; Millipore, Billerica, MA, USA).
The membranes were serially incubated with specific primary
antibodies: Rabbit anti-α-smooth muscle actin (SMA; cat. no.
ab5694; 1:1,000 dilution; Abcam) and mouse anti-β-actin (cat. no.
sc-47778; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
overnight at 4°C. The membranes were washed and incubated with the
following secondary antibodies: Peroxidase-Conjugated AffiniPure
Goat Anti-Rabbit IgG (cat. no. ZB-5301; 1:20,000; ZSGB-BIO) and
Peroxidase-Conjugated AffiniPureGoat Anti-Mouse IgG (cat. no.
ZB-5305; 1:20,000; ZSGB-BIO). Signals were visualized with an
enhanced chemiluminescence kit (ECL Plus; GE Healthcare, Little
Chalfont, UK). The gray density of protein was analyzed by the
Chemiluminescence imaging system Smart ChemiTM420 (IV) (Beijing
Sage Creation Science Co., Ltd., Beijing, China).
Immunofluorescence analysis
hPLCs (5×105 cells/well) were incubated
with a medium containing 10 μM GGsTOP, 20 μM GGsTOP
or 10 μM GGsTOP+3 mM NAC for 72 h in a six-well plate. The
control cells and negative control cells did not undergo any
treatment. The cells were fixed with 4% paraformaldehyde for 30 min
and blocked with 3% bovine serum albumin (Beyotime Institute of
Biotechnology) for 1 h at room temperature. The samples were
incubated with 1:500 diluted rabbit anti-human collagen-I
polyclonal antibody (cat. no. ab34710; Abcam) overnight at 4°C.
Rabbit immunoglobulin (Ig)G (cat. no. sc-66928; 1:40 dilution;
Santa Cruz Biotechnology, Inc.) was applied for the negative
control. A fluorescein isothiocyanate (FITC)-conjugated goat
polyclonal anti-rabbit IgG was used as the secondary antibody
(1:160; cat. no. ZF-0314; ZSGB-BIO) for 1 h at room temperature.
For nuclear detection, the samples were reacted with DAPI for 3
min. Immunofluorescence was observed under a fluorescence
microscope (Nikon).
Statistical analysis
All values are expressed as the mean ± standard
deviation. Statistical analysis was performed by one-way analysis
of variance followed by Dunnett's test. The processing of the data
was performed using SPSS version 19.0.1 for Windows (SPSS Inc.,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Localization of GGT in PDL tissues from
mice and hPLCs
Immunohistochemical analysis revealed the presence
of GGT-positive cells throughout the mandibular tissues, including
the PDL and pulp. The amount of GGT in PDL tissues was greater than
that in the pulp, whereas no staining was observed in the alveolar
bone (Fig. 1A–C). Positive
staining was distinct in the plasma membrane but not in the nucleus
of the cells (Fig. 1D–F).
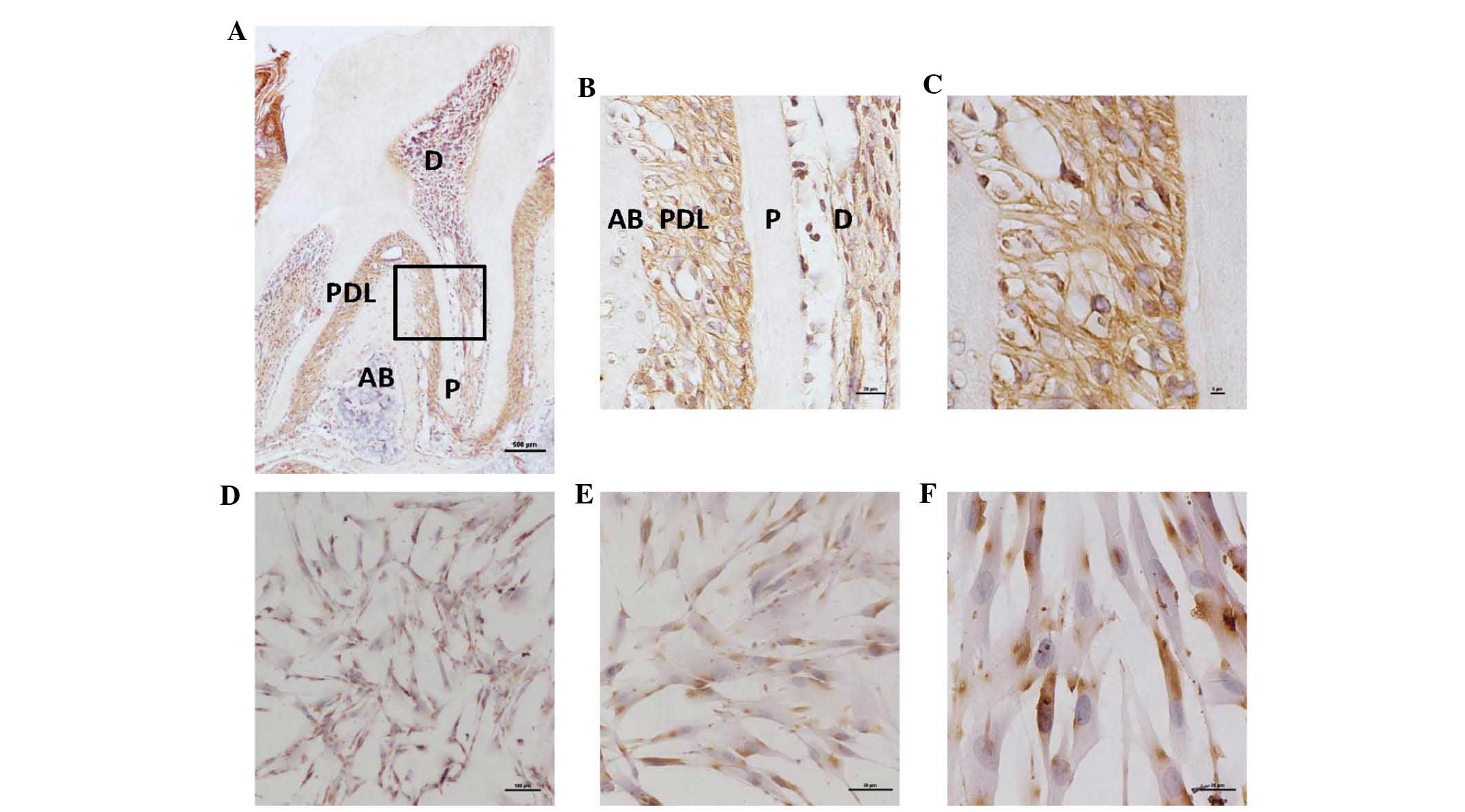 | Figure 1GGT in PDL tissues of mice and hPLCs.
(A) Immunohistochemical analysis of GGT in PDL tissues of the lower
second molars of four-week-old C57BL/6 mice (scale bar, 500
μm; diaminobenzidine and hematoxylin staining). (B)
Magnified area within the black rectangle in A (magnification, ×20;
scale bar, 20 μm). (C) The PDL tissue area within the black
rectangle in A (magnification, ×40; scale bar, 5 μm). (D)
Immunohistological localization of GGT in hPLCs at passage three
(magnification, ×4; scale bar, 100 μm). (E and F) Magnified
areas from D (magnification, ×10 and ×20, respectively; scale bars,
50 and 20 μm, respectively. AB, alveolar bone; P, pulp; D,
dentin; GGT, gamma-glutamyl transferase; hPLC, human periodontal
ligament cell; PDL, periodontal ligament. |
GGsTOP inhibits GGT activity in
hPLCs
To explore possible mechanisms underlying the
effects of GGT inhibition on PDL tissue, the effect of GGsTOP on
GGT activity in hPLCs was evaluated. A biochemical assay of the
homogenates showed that the GGT activity in hPLCs was 2.93±0.7 U/g
protein, while that in HepG2 cells was 23.05±1.5 U/g protein
(Table I). A significant decrease
in GGT activity was noted in the hPLCs within 2 h of treatment with
GGsTOP (10 μM).
 | Table IGGT activity (U/g protein) in
different groups. |
Table I
GGT activity (U/g protein) in
different groups.
| Group | GGT activity |
|---|
| hPLCs | 2.93±0.70 |
| GGsTOP+hPLCs | 0.76±0.40 |
| HepG2 | 23.05±1.50 |
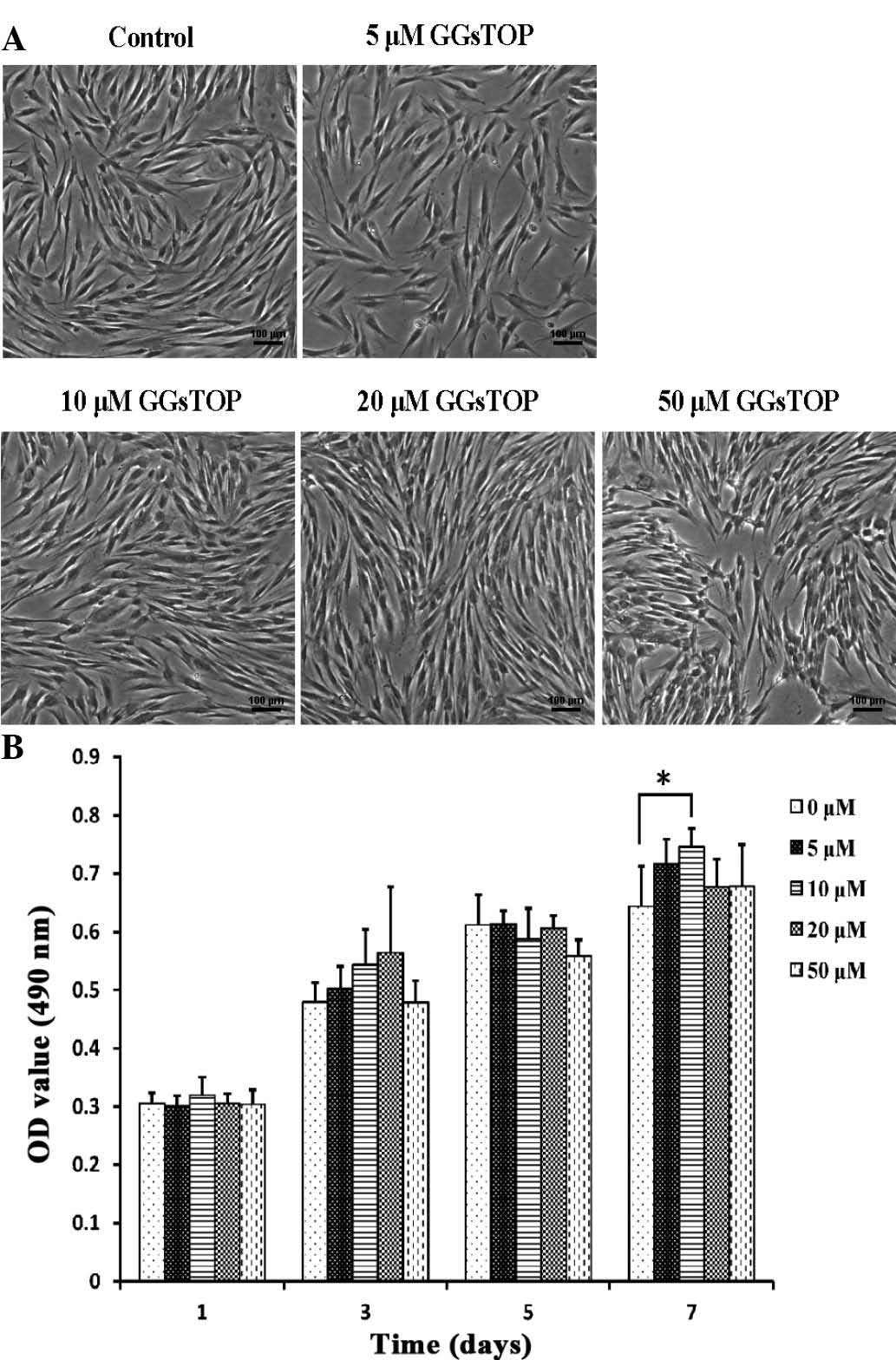
GGsTOP does not affect the viability of
hPLCs
Light microscopic examination indicated that GGsTOP
treatment did not produce any cytotoxic effects in hPLCs relative
to cells in the untreated group (Fig.
2A). The hPLCs appeared to be elongated, without any
interspersed non-viable cells. Cell survival was further assessed
using the MTT method, which revealed that cell viability was not
significantly affected by GGsTOP treatment (5, 10, 20 and 50
μM for 1, 3 and 5 days) (Fig.
2B). It was noted that treatment with 10 μM GGsTOP
resulted in enhanced proliferation of hPLCs when compared with the
control cells after 7 days (P<0.05). These results suggested
that application of GGsTOP in the 5–50 μM range was safe for
hPLCs under the conditions used in the present study.
GGsTOP induces the generation of ROS in
hPLCs
Intracellular ROS were measured by pre-incubating
the cells with DCF-DA to monitor the ROS-sensitive fluorescent
product of DCFH (Fig. 3).
Incubation with GGsTOP (10 μM) resulted in elevated levels
of ROS at 120 min of incubation, as demonstrated by fluorescence
microscopy (Fig. 3A).
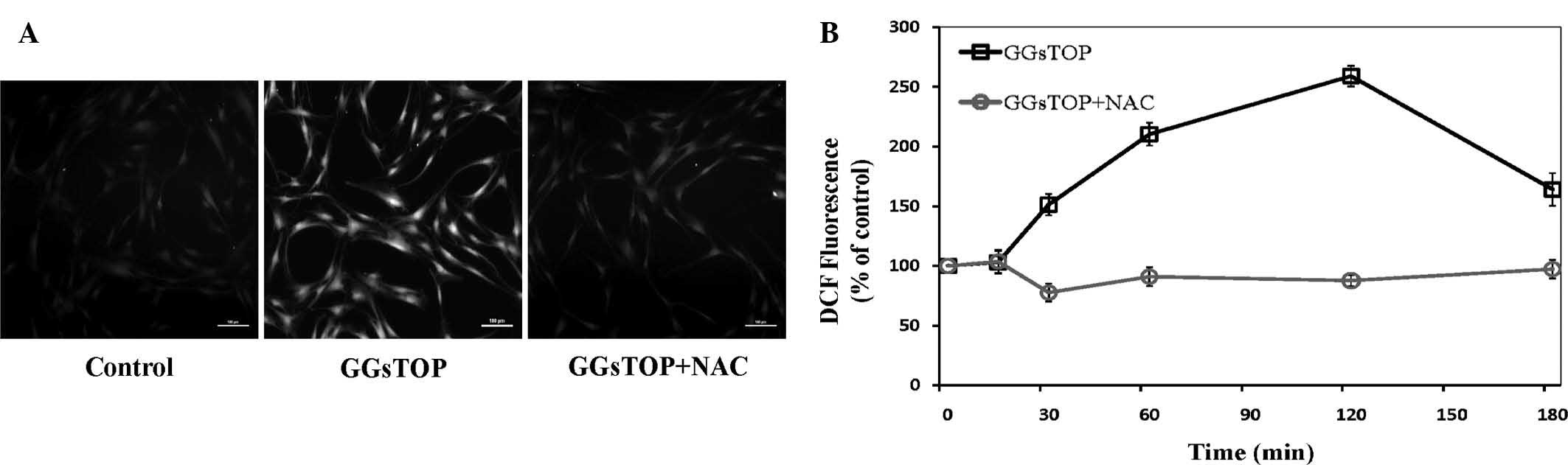 | Figure 3GGsTOP induces ROS generation in
hPLCs. (A) hPLCs were pre-incubated with DCFH-DA (10 mM) to detect
ROS-induced fluorescence. Unstimulated (control), GGsTOP (10
μM)-stimulated, and NAC (10 mM)+GGsTOP (10
μM)-stimulated cells observed at 120 min (scale bar, 100
μm). (B) Graph depicting the time course of ROS generation
in cells stimulated with GGsTOP at 0, 15, 30, 60, 120 and 180 min,
with (○) or without (□) NAC. DCF fluorescence was expressed as the
percentage of the control. Values are expressed as the mean ±
standard deviation (n=3). ROS, reactive oxygen species; NAC,
N-acetylcysteine; DCFH-DA, dichlorodihydrofluorescein
diacetate; hPLC, human periodontal ligament cell. |
Quantitative analysis of fluorescence microscopy
images at various time-points allowed for assessment of the
time-dependency of ROS induction by GGsTOP in hPLCs (Fig. 3B). hPLCs exposed to GGsTOP were
found to exhibit a transient increase in intracellular ROS over
time; intracellular ROS were significantly elevated at 15 min of
stimulation, peaked at 120 min and declined at 180 min. However,
co-treatment with NAC completely inhibited the GGsTOP-induced ROS
production.
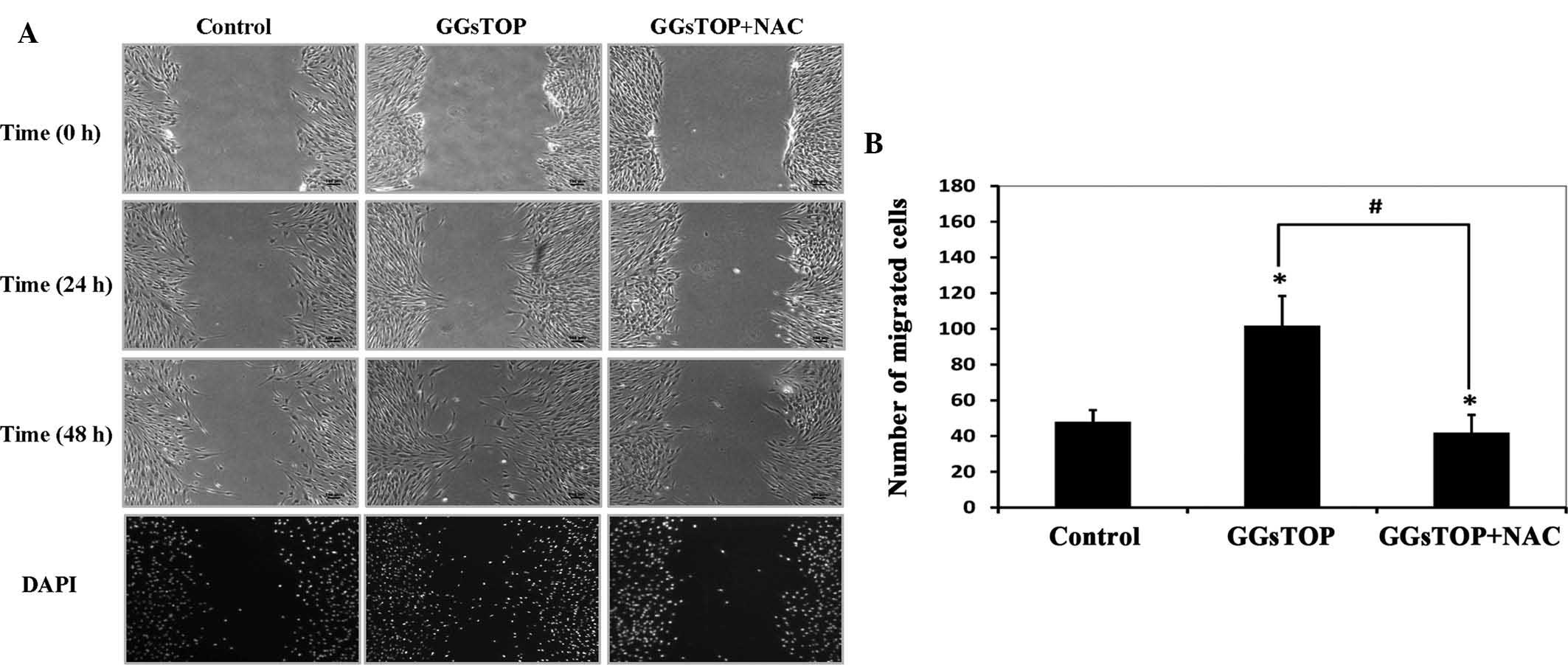
GGsTOP is associated with altered
migration of hPLCs via the ROS pathway
To further determine whether GGsTOP treatment has a
beneficial effect on the migration of cultured hPLCs, A
scratch-wound assay was performed as described by Gao et al
(11). GGsTOP was found to
facilitate the migration of cells when compared with the control
cells (Fig. 4A). Migration of the
control cells was slow at 24 h and was not much different at 48 h
post-wounding. However, increased cell migration was observed in
the GGsTOP group, in which the wounded area was reduced by ~50% at
48 h (Fig. 4B). By contrast, in
the presence of NAC, the wound closure stimulated by GGsTOP was
completely inhibited at 24 and 48 h post-wounding.
GGsTOP increases COL-I and α-SMA
synthesis in hPLCs
The expression of α-SMA protein in hPLCs stimulated
by GGsTOP was analyzed by western blot analysis (Fig. 5A). Notably, at 55 kDa an extra band
was observed, representing vimentin with which the antibody had
cross-reacted. Vimentin is predominantly expressed in mesenchymal
cells. The results showed significantly elevated levels of α-SMA in
the cells treated with 10 or 20 μM GGsTOP for 72 h when
compared with the control (P<0.01), while this effect was
completely inhibited by co-treatment with NAC (Fig. 5A). Immunofluorescent staining
revealed low levels of COL-I in the hPLCs under normal conditions
(Fig. 5B). By contrast, cells
stimulated with 10 or 20 μM GGsTOP contained elevated levels
of COL-I, while co-incubation with the anti-oxidant NAC completely
inhibited these increases. Staining with a control IgG used as the
primary antibody did not reveal any positive reaction. It is well
known that NAC is an antioxidant which reduces intracellular ROS
production. Our results (Fig. 3)
also demonstrated that GGsTOP-stimulated ROS was totally eliminated
when NAC was present in the cells. These findings suggested that
treatment with GGsTOP significantly increased COL-I and α-SMA in
hPLCs, and that the generation of ROS was essential in this
process.
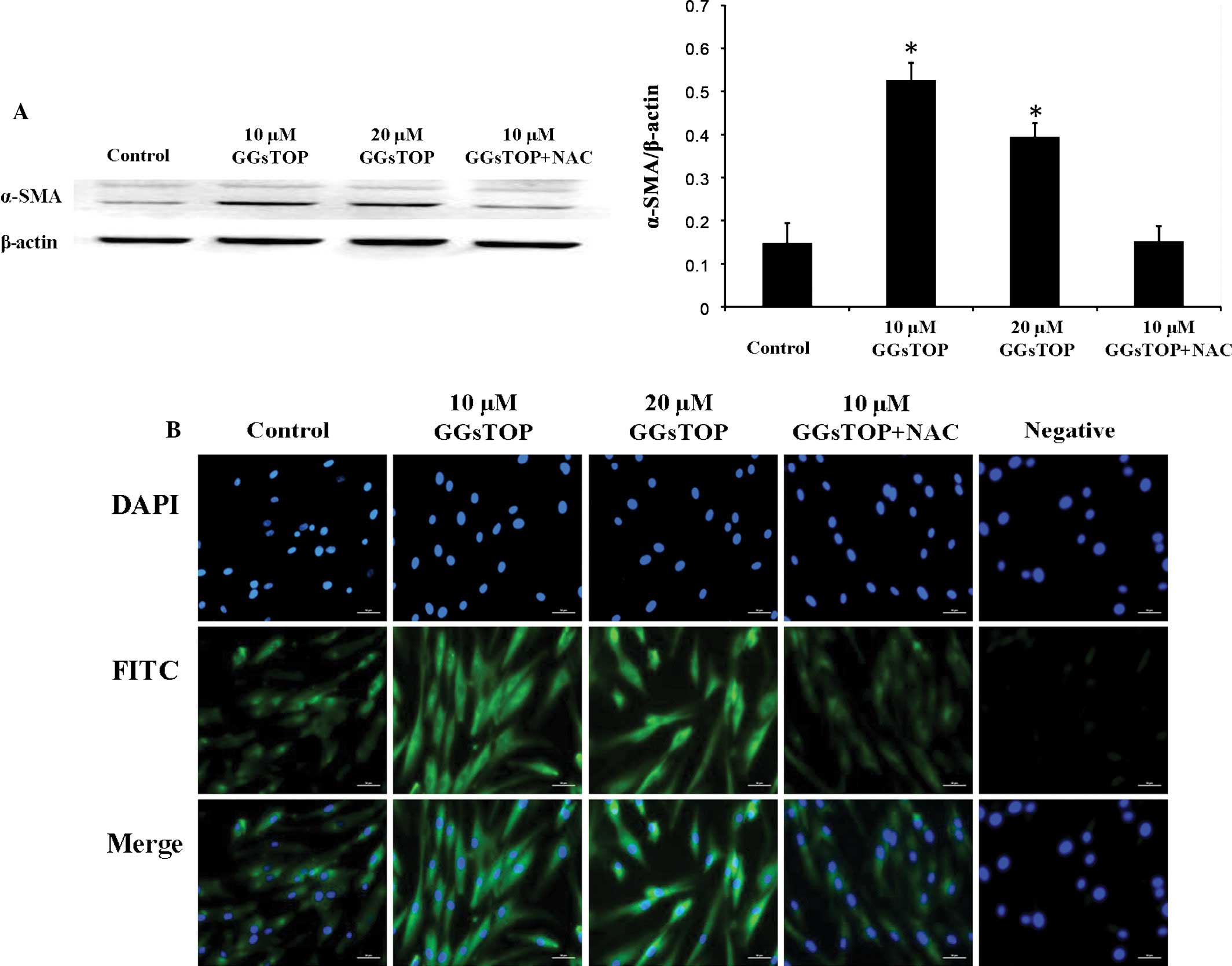 | Figure 5Expression of COL-I and α-SMA in
GGsTOP-treated hPLCs. (A) GGsTOP increased α-SMA protein levels in
hPLCs. Western blots revealed clear bands at 42 kDa, representing
α-SMA. Additional bands representing vimentin, with which the
antibody had cross-reacted, were observed at 55 kDa. Quantitative
representation of the western blots in A. Values are expressed as
the mean ± standard deviation (n=3; *P<0.05 vs.
control). (B) Immunofluorescent analysis of COL-I (FITC) in GGsTOP
(10, 20 μM)-treated hPLCs cultured with or without NAC (3
mM). After three days of culture, the hPLCs were incubated with a
control mouse immunoglobulin G (Negative group) or an anti-COL-I
antibody (green). Nuclei were stained with DAPI (blue). Scale bars,
50 μm. COL, collagen; SMA, smooth muscle actin; FITC,
fluorescein isothiocyanate; hPLC, human periodontal ligament cell;
NAC, N-acetylcysteine. |
Discussion
GGT is an important enzyme required for the
maintenance of steady-state glutathione concentration inside as
well as outside of the cells (extracellular fluids), and is used as
a diagnostic marker for liver disease in the clinic. However, to
the best of our knowledge, the localization and functions of GGT in
intact PDL tissues have not been previously reported. Levels of GGT
vary considerably among normal tissues and organs. Strong
immunoreactivity for this enzyme has been reported in the renal
proximal convoluted tubule, hepatic bile canaliculi and brain
capillaries (12). The present
study was the first to report the presence of GGT-positive cells in
mandibular tissues, including PDL and pulp tissues. The activity of
GGT was found to be eight times greater in HepG2 cells compared
with that in hPLCs, indicating a great variability among the two
cell types. In addition, it was demonstrated that GGsTOP markedly
inhibited GGT activity in hPLCs after 2 h. The present study
further investigated whether intervention of GGT activity had any
effect on PDL cells under physiological conditions.
In the present study, an MTT assay demonstrated that
the viability of hPLCs was not affected by GGsTOP, indicating its
suitability for the treatment of these cells. Furthermore,
microscopic observation revealed that hPLCs appeared elongated,
without the presence of any interspersed non-viable cells. This
morphology conformed to that of a healthy fibroblastic phenotype
(13). GGT inhibition by acivicin
has been shown to arrest cell growth (14). However, a previous study has
reported that acivicin increases the lifespan, and may therefore be
applicable for the development of anti-aging therapies (15). It is therefore likely that the
effects of GGsTOP on proliferation may depend on the cell type,
stage of differentiation and culture conditions.
The observation of the present study that GGsTOP
induced a transient elevation of intracellular ROS in hPLCs agrees
with the results reported by Zalata et al (16), according to which GGT activity was
inversely correlated with ROS production in the spermatozoa, an
observation also consistent with that of Johnsen et al
(17). These results also
indicated that hPLCs, analogous to other non-phagocytic cells, can
produce intracellular ROS during stimulation (18).
ROS are thought to act as signaling molecules that
direct the changes necessary for cell movement. To clarify whether
GGsTOP promotes cell migration in cultured hPLCs via ROS, the
antioxidant NAC was added to the medium to eradicate intracellular
ROS generation. The results indicated that GGsTOP facilitates cell
migration at 48 h. However, the function of GGsTOP was blocked when
NAC was also present in the medium. To determine whether GGsTOP
promotes cell migration in cultured hPLCs, a scratch-wound test was
performed. The results indicated that GGsTOP facilitates cell
migration, which may be attributed to the rise in intracellular ROS
levels. However, the underlying mechanisms involved in these
processes remain elusive. Previous studies suggested that the ROS
that form in the superficial cell layers next to the wound
interface are susceptible to mechanical damage, which can directly
lead to the activation of the mitogen-activated protein kinase
signaling pathway. Evidence also suggested that ROS-mediated
enhancement of fibroblast migration is associated with the bone
morphogenetic protein/SMAD signaling pathway (19). The level of ROS is a key factor in
the wound healing process. Therefore, it requires a
well-orchestrated and dynamic response to maintain the cellular
redox homeostasis, which is achieved by various oxidordeuctases and
small molecules. GSH is of great importance as it serves as a
cellular redox buffer. A role for GSH in wound repair has been
suggested, since the GSH level was reduced in skin wounds, and
topical treatments in diabetic mice with GSH accelerated the repair
processes (20). GGT, as a
rate-limiting enzyme, can influence intracellular de novo
glutathione synthesis. Thus, GGT may be crucial for efficient wound
healing. However, the exact mechanism by which activation of cell
surface receptors activates GGsTOP and generates ROS during cell
migration remains elusive. In addition, the identities of the
redox-regulated proteins that are oxidized remain unknown. However,
GGT activity is known to mediate nuclear factor-kappa B activation,
which can modulate their function and influence cell migration
(21).
In the present study, the effects of GGT activity in
hPLCs under physiological conditions were assessed, as it has been
reported that numerous growth factors and chemoattractants regulate
tissue regeneration, accompanied by the production of intracellular
ROS in various cell types. Transforming growth factor β1 (TGFβ1)
can stimulate ROS in fibroblasts, while platelet-derived growth
factor (PDGF) induces ROS production in lens epithelial cells and
ROS are also essential factors associated with epidermal growth
factor (EGF)-stimulated corneal epithelial cell migration (4,18,22).
Choe et al (23)
demonstrated that ROS produced by glucose oxidase increased the
viability, proliferation and osteoblastic differentiation of hPLCs.
In addition, it is known that inhibition of GGT activity at the
surface of human myeloid cells correlates with macrophage
maturation and transforming growth factor-β production in several
cell types (24). TGF-β1 is
considered to be a key regulator of COL I and α-SMA expression, as
well as extracellular matrix components in hPLCs (25). Collagen fibers have a pivotal role
in anchoring the tooth to the alveolar bone socket and are
primarily composed of type I collagen (26). Expression of α-SMA is generally
associated with high remodeling capacity as seen in the periodontal
ligament during conditions of increased remodeling (27). For these reasons, the present study
analyzed the effects of GGsTOP on the protein levels of COL-I and
α-SMA. The results indicated that inhibition of GGT activity using
various concentrations of GGsTOP obviously increased the expression
of COL-I and α-SMA (Fig. 5). These
findings suggested that GGsTOP induces the differentiation of
immature hPLCs into functional PDL fibroblasts through the
upregulation of COL-I and α-SMA. However, the underlying mechanisms
warrant further evaluation.
In conclusion, the present study demonstrated for
the first time, to the best of our knowledge, that GGT is present
in PDL tissues. Treatment of hPLCs with GGT inhibitor GGsTOP
increased cell migration and ROS levels, and enhanced the
expression levels of COL-I and α-SMA, which have vital roles during
wound healing. Of note, the anti-oxidant NAC inhibited these
effects of GGsTOP, possibly through inhibition of ROS. In the light
of the absence of any cytotoxic effects of GGsTOP, these results
indicated that GGsTOP may be used for the treatment of periodontal
ligament wound healing.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81170960) and the
Special Foundation for Sino-Russian Translational Medicine Research
Center of Harbin Medical University (grant no. CR201412).
References
|
1
|
Nomura Y, Ishikawa M, Yashiro Y,
Sanggarnjanavanich S, Yamaguchi T, Arai C, Noda K, Takano Y,
Nakamura Y and Hanada N: Human periodontal ligament fibroblasts are
the optimal cell source for induced pluripotent stem cells.
Histochem Cell Biol. 137:719–732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cáceres M, Martínez C, Martínez J and
Smith PC: Effects of platelet-rich and -poor plasma on the
reparative response of gingival fibroblasts. Clin Oral Implants
Res. 23:1104–1111. 2012. View Article : Google Scholar
|
|
3
|
Fu XJ, Peng YB, Hu YP, Shi YZ, Yao M and
Zhang X: NADPH oxidase 1 and its derived reactive oxygen species
mediated tissue injury and repair. Oxid Med Cell Longev.
2014(282854)2014. View Article : Google Scholar
|
|
4
|
Chen KC, Zhou Y, Xing K, Krysan K and Lou
MF: Platelet derived growth factor (PDGF)-induced reactive oxygen
species in the lens epithelial cells: The redox signaling. Exp Eye
Res. 78:1057–1067. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu SC: Regulation of hepatic glutathione
synthesis: Current concepts and controversies. FASEB J.
13:1169–1183. 1999.PubMed/NCBI
|
|
6
|
Wickham S, West MB, Cook PF and Hanigan
MH: Gamma-glutamyl compounds: Substrate specificity of
gamma-glutamyl transpeptidase enzymes. Anal Biochem. 414:208–214.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rojas E, Valverde M, Kala SV, Kala G and
Lieberman MW: Accumulation of DNA damage in the organs of mice
deficient in gamma-glutamyltranspeptidase. Mutat Res. 447:305–316.
2000. View Article : Google Scholar
|
|
8
|
Yamamoto S, Watanabe B, Hiratake J, Tanaka
R, Ohkita M and Matsumura Y: Preventive effect of GGs Top, a novel
and selective γ-glutamyl transpeptidase inhibitor, on
ischemia/reperfusion-induced renal injury in rats. J Pharmacol Exp
Ther. 339:945–951. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huseby NE and Strömme JH: Practical points
regarding routine determination of gamma-glutamyl transferase
(gamma-GT) in serum with a kinetic method at 37 degrees C. Scand J
Clin Lab Invest. 34:357–363. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cannito S, Novo E, Compagnone A, Valfrè di
Bonzo L, Busletta C, Zamara E, Paternostro C, Povero D, Bandino A,
Bozzo F, et al: Redox mechanisms switch on hypoxia-dependent
epithelial-mesenchymal transition in cancer cells. Carcinogenesis.
29:2267–2278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao C, Negash S, Guo HT, Ledee D, Wang HS
and Zelenka P: CDK5 regulates cell adhesion and migration in
corneal epithelial cells. Mol Cancer Res. 1:12–24. 2002.PubMed/NCBI
|
|
12
|
Hanigan MH and Frierson HF Jr:
Immunohistochemical detection of gamma-glutamyl transpeptidase in
normal human tissue. J Histochem Cytochem. 44:1101–1108. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eckes B, Zweers MC, Zhang ZG, et al:
Mechanical tension and integrin α2β1 regulate fibroblast functions.
J Invest Dermatol. 11:66–72. 2006. View Article : Google Scholar
|
|
14
|
del Bello B, Paolicchi A, Comporti M,
Pompella A and Maellaro E: Hydrogen peroxide produced during
gamma-glutamyl transpeptidase activity is involved in prevention of
apoptosis and maintainance of proliferation in U937 cells. FASEB J.
13:69–79. 1999.PubMed/NCBI
|
|
15
|
Stephan J, Franke J and Ehrenhofer-Murray
AE: Chemical genetic screen in fission yeast reveals roles for
vacuolar acidification, mitochondrial fission, and cellular GMP
levels in lifespan extension. Aging Cell. 12:574–583. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zalata A, Hafez T, Mahmoud A and Comhaire
F: Relationship between resazurin reduction test, reactive oxygen
species generation, and gamma-glutamyltransferase. Hum Reprod.
10:1136–1140. 1995.PubMed/NCBI
|
|
17
|
Johnsen O, Eliasson R and Samuelson U:
Conditioning effect of seminal plasma on the lipid peroxide
potential of washed human spermatozoa. Acta Physiol Scand.
116:305–307. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu RM, Liu Y, Forman HJ, Olman M and
Tarpey MM: Glutathione regulates transforming growth
factor-beta-stimulated collagen production in fibroblasts. Am J
Physiol Lung Cell Mol Physiol. 286:L121–L128. 2004. View Article : Google Scholar
|
|
19
|
Tandon N, Cimetta E, Villasante A,
Kupferstein N, Southall MD, Fassih A, Xie J, Sun Y and
Vunjak-Novakovic G: Galvanic microparticles increase migration of
human dermal fibroblasts in a wound-healing model via reactive
oxygen species pathway. Exp Cell Res. 320:79–91. 2014. View Article : Google Scholar
|
|
20
|
Mudge BP, Harris C, Gilmont RR, Adamson BS
and Rees RS: Role of glutathione redox dysfunction in diabetic
wounds. Wound Repair Regen. 10:52–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Djavaheri-Mergny M, Accaoui MJ, Rouillard
D and Wietzerbin J: Gamma-glutamyl transpeptidase activity mediates
NF-kappaB activation through lipid peroxidation in human leukemia
U937 cells. Mol Cell Biochem. 232:103–111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Svegliati S, Cancello R, Sambo P, Luchetti
M, Paroncini P, Orlandini G, Discepoli G, Paterno R, Santillo M,
Cuozzo C, et al: Platelet-derived growth factor and reactive oxygen
species (ROS) regulate Ras protein levels in primary human
fibroblasts via ERK1/2. Amplification of ROS and Ras in systemic
sclerosis fibroblasts. J Biol Chem. 280:36474–36482. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choe Y, Yu JY, Son YO, Park SM, Kim JG,
Shi X and Lee JC: Continuously generated H2O2
stimulates the proliferation and osteoblastic differentiation of
human periodontal ligament fibroblasts. J Cell Biochem.
113:1426–1436. 2012. View Article : Google Scholar :
|
|
24
|
Bauvois B, Laouar A, Rouillard D and
Wietzerbin J: Inhibition of gamma-glutamyl transpeptidase activity
at the surface of human myeloid cells is correlated with macrophage
maturation and transforming growth factor beta production. Cell
Growth Differ. 6:1163–1170. 1995.PubMed/NCBI
|
|
25
|
Fujita T, Shiba H, Van Dyke TE and
Kurihara H: Differential effects of growth factors and cytokines on
the synthesis of SPARC, DNA, fibronectin and alkaline phosphatase
activity in human periodontal ligament cells. Cell Biol Int.
28:281–286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Narayanan AS and Page RC: Connective
tissues of the periodontium: A summary of current work. Coll Relat
Res. 3:33–64. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|