Introduction
Acute pro-myelocytic leukemia (APL), which accounts
for ~10% of all acute myeloid leukemia (AML) cases, is classified
as the M3 subtype of AML within the French-American-British
morphological classification system (1), A reciprocal chromosomal translocation
affects the long arm of chromosomes 17 and 15 at the site of the
retinoic acid receptor alpha (RARα) gene and the pro-myelocyte
(PML) gene, respectively, resulting in a PML-RARA fusion gene,
which leads to formation of the PML-RARα chimeric protein in 95% of
APL cases (2–7). The PML and RARα oncoproteins, whose
roles in leukemogenesis have been elucidated, are known to be
targeted by specific biomolecules that are active in the disease
(8).
Neutrophil elastase (NE), also known as human
leukocyte elastase, is a neutrophil-derived serine proteinase with
specificity for a broad range of substrates and is encoded by
Elane, formerly known as Ela2 (9). The major physiological role of NE is
to kill engulfed microorganisms within neutrophil phagolysosomes
(10–13). NE has been reported to be
associated with the pathogenesis of several diseases, including
acute lung injury, cystic fibrosis, emphysema and leukemia
(14–17). NE has also been characterized by
its ability to cleave extracellular matrix proteins (18). NE is able to cleave the fusion
protein PML-RARα into two different protein variants (16,18,19).
This type of cleavage action has a role in the development of APL
(20,21). A previous study by our group showed
that NE has a role in the development of leukemia (22). However, the mechanism underlying
the effect of NE in APL remain to be fully elucidated.
In order to explore the biological functions of NE
in APL, NE was overexpressed using a constructed lentiviral vector.
The effects of NE on NB4 cell proliferation and apoptosis were
assessed and the possible mechanistic functions of NE were explored
in APL cell lines. In addition, NE was silenced using small hairpin
(sh)RNA to clarify its effect on the PI3K/Akt pathway in U937
cells.
Materials and methods
Cell lines and culture
Human 293T cells (Chinese Academy Cell Bank;
Shanghai, China) were maintained in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Waltham, MA, USA)
with 10% fetal bovine serum (FBS; Gibco). Human NB4 cells (Chinese
Academy Cell Bank) were maintained in RPMI 1640 medium (Gibco) with
10% FBS. Human U937 cells (Chinese Academy Cell Bank) were
maintained in RPMI-1640 medium (Gibco) with 10% FBS. Cells were
cultured at 37°C in a humidified atmosphere containing 5%
CO2, and the culture medium was replaced at two-day
intervals.
Construction of lentivirus
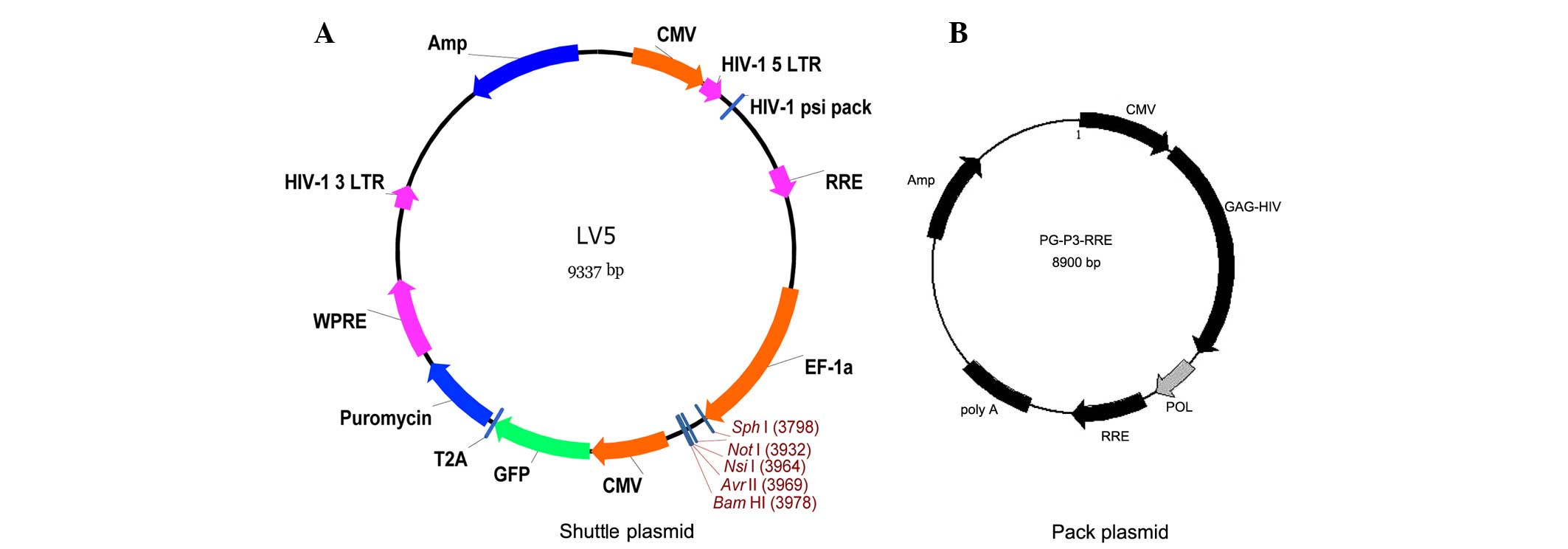
Shuttle plasmid and packing plasmid vector maps
(Genepharma, Shanghai, China) are shown in Fig. 1A and B, respectively. A recombinant
plasmid containing a lentiviral vector and three original auxiliary
packaging vectors was constructed. NE was inserted into the LV5
plasmid but was not inserted for the negative control group. The
three different plasmids were extracted with endo-free and high
purity kits (Takara Bio, Inc., Otsu, Japan). The restriction
enzymes used were NotI and BamHI (Takara Bio, Inc.).
The recombinant plasmid and vectors were co-transfected into 293T
cells using RNAi-Mate transfection reagent (Genepharma). The medium
was replaced with complete medium after transfection for 6 h. After
culture for 72 h, the cell supernatant was collected, which
contained the lentivirus. Following concentration of the
supernatant, the viral titer was determined.
Titer detection
293T cells were cultured in 96-well plates at a
density of 0.5–10×103 cells/100 µl per well for
24 h. Then 90 µl complete medium (DMEM+10% FBS) was added to
7–10 EP tube. Original lentivirus (10 µl) was added to the
first tube. Then 10 µl of the mixed suspension from the
first tube was added to the second tube. This was repeated until
each tube had been filled. Then, 90 µl of culture medium was
discarded and the mixed suspension by concentration gradient
(lowest to highest) was added to the cells. When cells had been
cultured at 37°C in a humidified atmosphere containing 5%
CO2 for 48 h, 100 µl of culture medium was added
to each well. The cells were cultured for a further 72 h and
subsequently counted based on the expression of green fluorescent
protein (GFP) under an Eclipse 80i, Nikon fluorescence microscope
(Nikon, Tokyo, Japan). The titer was calculated using the following
formula: TU/µl = (PxN/100xV)/DF, where P is the percentage
of GFP-positive cells, N is the number of cells, V is the virus
dilution volume and DF is the dilution factor.
Lentiviral transfection
NB4 cells in the logarithmic growth phase
(1×105 cells/well) were seeded in a 24-well plate. These
cells were transfected with the GFP-expressing lentiviral vector
LV5-NC and the NE-expressing recombinant lentivirus LV5-NE, and 1
µg/ml polybrene (Genepharma) was added. After culture for 24
h, the medium was refreshed. Fluorescence was detected following
48–72 h of incubation using the fluorescence microscope. The
LV5-NE- and LV5-NC-transfected NB4 cells were screened with
puromycin (Sigma-Aldrich, St. Louis, MO, USA) and successful
transfectants were used for subsequent experiments. Experiments
were performed on untransfected NB4 cells as well as on LV5-NE- and
LV5-NC-transfected NB4 cells.
Transient transfection
U937 cells (2.5×105/ml) in the
logarithmic growth phase were seeded in six-well plates. For
transfection, 5 µl siRNA and 5 µl Lipofectamine 2000
(Invitrogen; Thermo Fisher Scentific, Inc.) were diluted in 100 μl
Opti-MEM (Invitrogen) separately. The siRNA and Lipofectamine 2000
were then gently mixed and incubated for 25 min at room
temperature. The siRNA-Lipofectamine 2000 complexes were
subsequently added to each well and mixed by gentle agitation. The
siRNA was purchased from RiboBio (Guangzhou, China) and the
sequences of the NE-specific siRNAs were as follows:
5′-GAUCGACUCUAUCAUCCAAdTdT-3′ and 3′-dTdTCUAGCUGAGAUAGUAGGUU-5′.
The resulting solution was added to each well, followed by
incubation for 6 h. Subsequently, 1 ml fresh complete medium was
added. The transfected cells were named as U937/NE-shRNA. In the
negative control group, U937/shRNA-NC cells were processed
similarly. Following 48 h of transfection, the successfully
transfected cells were verified by fluorescent microscopy. The
transfection efficiency was expressed as the percentage of red
fluorescence protein-positive cells. In subsequent experiments,
native U937 cells as well as U937/shRNA-NC and U937/NE-shRNA cells
were used.
RNA isolation and reverse-transcription
quantitative polymerase chain reaction (RT-qPCR) analysis
In each group of cells, TRIzol (Invitrogen, Thermo
Fisher Scientific, Inc.) was used to extract total RNA, which was
reverse transcribed into complementary DNA. In brief, 10 µl
RNA solution (Takara Bio, Inc.) was subjected to RT. PCR
amplification was then performed in a ThermoScript™ RT-PCR system
(Invitrogen) using a PreTaq kit (Takara Bio, Inc.). All the primers
were synthesized from Sangon Biotech, Co., Ltd. (Shanghai, China).
The specific primers for NE of RT-PCR were 5′-CGG AAT TCA TGA CCC
TCG GCC GCC GA-3′ (forward) and 5′-CCGCTCGAGTCAGTGGGTCCTGCTGGC-3′
(reverse). The reaction mixture contained 1 µl PrimeScriptRT
Enzyme mix I (Takara Bio, Inc.), 1 µl (10 µM of each
primer and 500 ng RNA in a final volume of 20 µl).
Thermocycling conditions were as follows: Reverse transcription at
37°C for 15 min, then reverse transcriptase inactivation reaction
at 85°C for 5 sec. The RT-PCR products were verified by agarose gel
(Invitrogen; Thermo Fisher Scentific, Inc.). The specific primers
for NE used for qPCR were 5′-ACTGCGTGGCGAATGTAA-3′ (forward) and
5′-CGATGTCGTTGAGCAAGTT-3′ (reverse). Primers for GAPDH were
5′-GAAGGTGAAGGTCGGAGTC-3′ (forward) and 5′-GAAGATGGTGATGGGATTTC-3′
(reverse). The reaction mixture contained 5 µl PreTaq
Enzyme, 0.5 µl (10 µM) of each primer and 100 ng cDNA
in a final volume of 10 µl. Thermocycling conditions were as
follows: Pre-denaturation at 95°C for 5 min, 39 cycles of
denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and
extension at 72°C for 30 sec, and a final extension at 95°C for 5
min. GAPDH was used as a reference gene and the RT-PCR products
were verified by agarose gel with the DL 2,000 DNA maker (Takara
Bio, Inc.). Quantity One Software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used for quantification of mRNA levels.
Expression of NE, relative to GAPDH was determined using the
2−ΔΔCq method (23).
Western blot assay
For extraction of total protein, cells in each group
were washed with ice-cold phosphate-buffered saline and lysed in
radioimmunoprecipitation solu tion containing a protease inhibitor
cocktail (Roche, Los Angeles, CA, USA). Following centrifugation at
13,000 × g per minute for 30 min, protein was stored at -80°C
following determination of the concentration was via the
bicinchoninic acid method (Beyotime Institute of Biotechnology,
Shanghai, China). A total of 100 µg protein per group was
subjected to 8% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and then transferred onto a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA). Membranes
were then blocked for 2 h at room temperature in 5% skimmed milk,
followed by then incubation with primary antibody overnight at 4°C.
The following primary antibodies were used: Mouse anti-β-actin
monoclonal antibody (1:1,000 dilution; cat. no. 3700s; Cell
Signaling Technology, Inc., Danvers, MA, USA); rabbit anti-NE
polyclonal antibody (pAb; cat. no. sc-9520), rabbit anti-human
polyclonal B-cell lymphoma 2 (Bcl-2; cat. no. sc-492) and rabbit
anti-human polyclonal Bcl-2-associated X protein (Bax; cat. no.
sc-6236) antibodies (all 1:1,000 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA); and rabbit anti-Akt pAb
(pAb; cat.no. ab8805) and rabbit anti-p-Akt (308; cat. no. ab38449)
(both from 1:1,000 dilution; Abcam, Cambridge, MA, USA). Membranes
were then incubated with secondary antibodies [goat anti-mouse IgG
antibody (cat. no. ZM-0491) or goat anti-rabbit IgG antibody (cat.
no. ZA-0448); 1:1,000 dilution; Zhongshan Goldenbridge
Biotechnology Co., Ltd., Beijing, China] for 1 h at 37°C. After
washing three times in Tris-buffered-saline with Tween 20 (TBST),
immunoreactive complexes were visualized using an enhanced
chemiluminescence system (Bio-Rad Laboratories, Inc.). β-actin
served as an internal positive control. Protein bands were
quantitatively analyzed using Quantity One Software 4.5.2 (Bio-Rad
Laboratories, Inc.).
Cell viability assay
The Cell Counting Kit-8 (CCK-8) assay (7Sea Biotech,
Shanghai, China) was used to assess cell viability. Cells in each
group (1.0×104/well) were seeded in 96-well plates and
incubated for 1–4 days. Subsequently, 10 µl CCK-8 solution
(5 mg/ml) was added to each well, and cells were incubated for 2 h
at 37°C. The absorbance at 450 nm was measured using an Eon
spectrophotometer (Bio-Rad Laboratories, Inc.) and plotted to
obtain cell growth curves. The experiment was repeated three times
in triplicate wells for each condition.
Flow cytometric analysis
The cells were routinely collected and centrifuged
at 500 × g for 5 min at room temperature. After the cells had been
washed twice with phosphate-buffered saline (PBS), a staining
mixture was prepared containing 5 µl Annexin-V-fluorescein
isothiocyanate fluorescent dye (Sigma-Aldrich) and 5 µl
propidium iodide (Sigma-Aldrich). The rate of cell apoptosis was
analyzed using a FACsorter (BD Biosciences, San Jose, CA, USA)
following a 15 min incubation at room temperature. Furthermore,
5×105 cells were collected, centrifuged at 500 × g for 5
min and washed with pre-cooled PBS twice. The supernatant was
discarded, precooled 70% ethanol (1 ml) was added and the samples
were fixed overnight at 4°C. Following fixation, the samples were
centrifuged at 500 × g for 5 min and washed with 1 ml PBS twice.
The cells were resuspended in 100 µl of 1 mg/ml RNase
solution (Takara Bio, Inc.). in a 37°C water bath for 30 min.
Following incubation, 150 µl of 50 µg/ml propidium
iodide staining solution was added and incubated for 30 min in dark
at room temperature. The cell cycle distribution was detected using
a FACsorter. Experiments were repeated three times.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). An independent samples
t-test was employed for comparing the means between two groups.
P<0.05 was considered to indicate a statistically significant
difference. Each experiment was repeated at least three times.
Results
Vector-mediated expression of NE in NB4
cells
The recombinant lentivirus LV5-NE was transfected
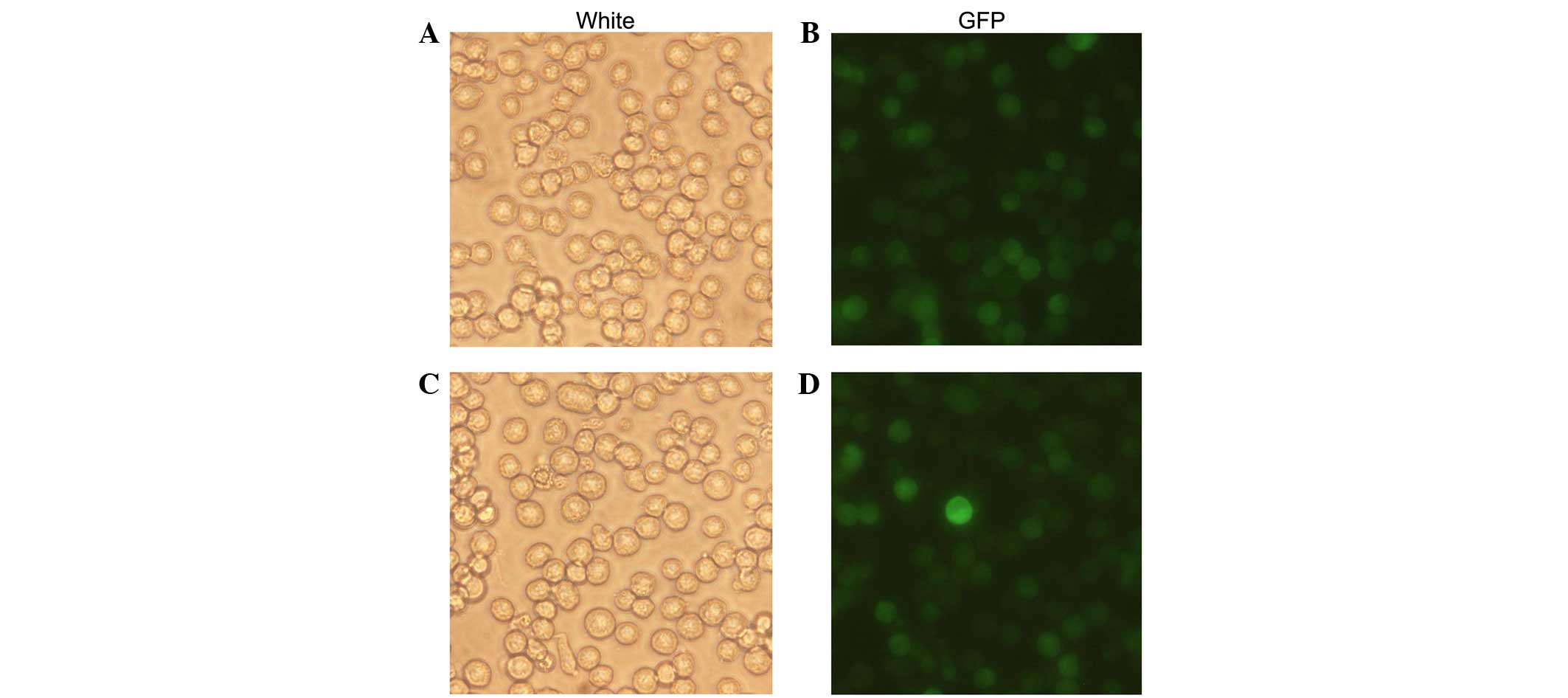
into NB4 cells to induce NE overexpression. The percentage of
GFP-positive cells was ~100%, demonstrating that after screening
with puromycin, all cells were positive transfectants (Fig. 2). RT-PCR and qPCR assays showed
that NE mRNA was expressed in the LV5-NE-transfected cells
(P<0.05 vs. control), whereas native NB4 cells or
LV5-NC-transfected cells showed no expression of NE (Fig. 3A). Furthermore, western blot
analysis also showed that NE protein was expressed in the
LV5-NE-transfected cells (P<0.05 vs. control), while NE protein
expression was low in native NB4 cells and LV5-NC-transfected cells
(Fig. 3B).
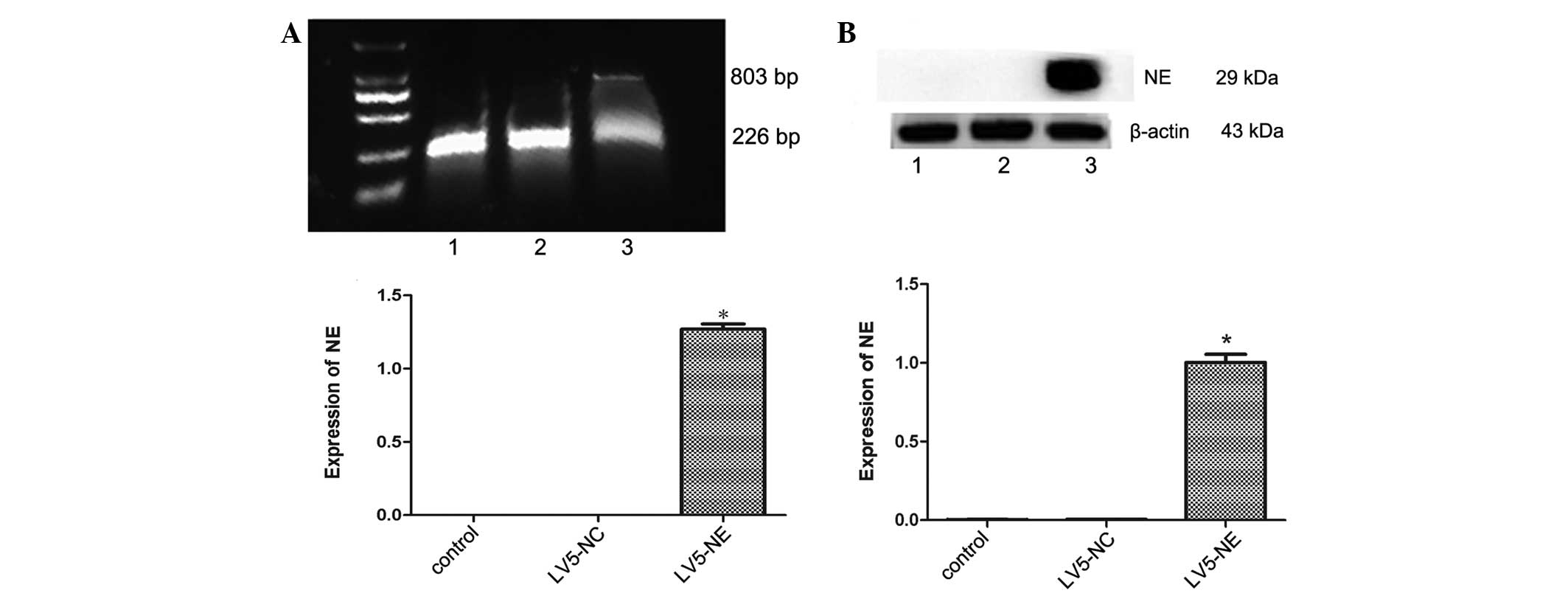 | Figure 3Effects of transfection with LV5-NC
or LV5-NE on NE expression in NB4 cells. (A) The mRNA expression of
NE was assessed by reverse-transcription semi-quantitative PCR
analysis. The upper panel shows an electrophoretic gel containing
the PCR products, which were quantified by densitometry (lower
panel). The mRNA of NE was expressed in LV5-NE-transfected NB4
cells, but not in untreated or LV5-NC-transfected cells. The 803 bp
band was mRNA of NE and 226 bp was mRNA of glyceraldehyde
3-phosphate dehydrogenase. (B) The protein expression of NE was
assessed by western blot analysis. The upper panel shows a
representative blot, and quantitative analysis of protein levels
was performed by densitometric analysis. The NE protein was highly
expressed in LV5-NE-transfected NB4 cells, while expression was low
in untreated or LV5-NC-transfected cells. Lanes: M, marker; 1, NB4
cells; 2, NB4 cells transfected with LV5-NCs; 3, NB4 cells
transfected with LV5-NE. Values are expressed as the mean ±
standard deviation. *P<0.05 vs. control/LV5-NC
groups. LV5-NC, negative control vector; LV5-NE, lentiviral vector
overexpressing neutrophil elastase; PCR, polymerase chain
reaction. |
NE enhances the proliferation of NB4
cells
The CCK-8 assay showed that overexpression of NE
promoted the proliferation of NB4 cells when compared with that of
the control cells (P<0.05) (Fig.
4A). Furthermore, flow cytometric analysis showed that the
percentage of LV5-NE-transfected NB4 cells in S phase was
significantly higher than that of native or LV5-NC-transfected NB4
cells (P<0.05) (Fig. 4B–D).
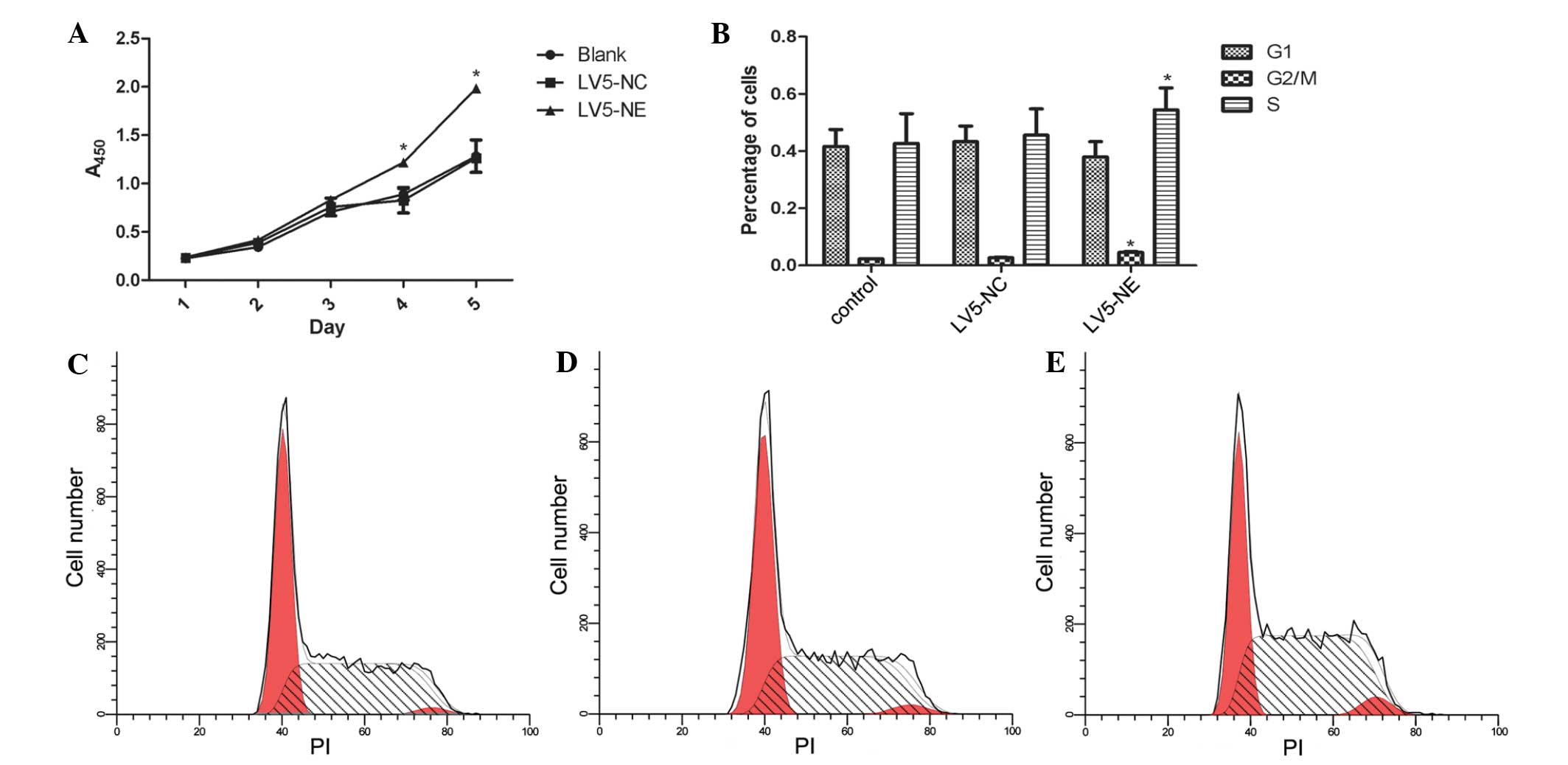 | Figure 4Effects of NE on the proliferation
and the cell cycle of NB4 cells. (A) The Cell Counting Kit-8 assay
was used to assess cell proliferation on days 1, 2, 3, 4 and 5
post-transfection. Absorbance was measured at 450 nm. At days 3 and
4, the proliferation of NB4 cells was significantly increased
following transfection with LV5-NE, while not being affected by
transfection with LV5-NC. (B) Flow cytometric analysis showed that
transfection with LV5-NE significantly increased the S-phase
population of NB4 cells, while the cell cycle was not significantly
affected by transfection with LV5-NC. Values are expressed as the
mean ± standard deviation. *P<0.05 vs. control. Cell
cycle distribution profiles of (C) NB4 cells, (D) NB4 cells
transfected with LV5-NC and (E) NB4 cells transfected with LV5-NE.
A, absorbance; PI, propidium iodide; LV5-NC, negative control
vector; LV5-NE, lentiviral vector overexpressing neutrophil
elastase. |
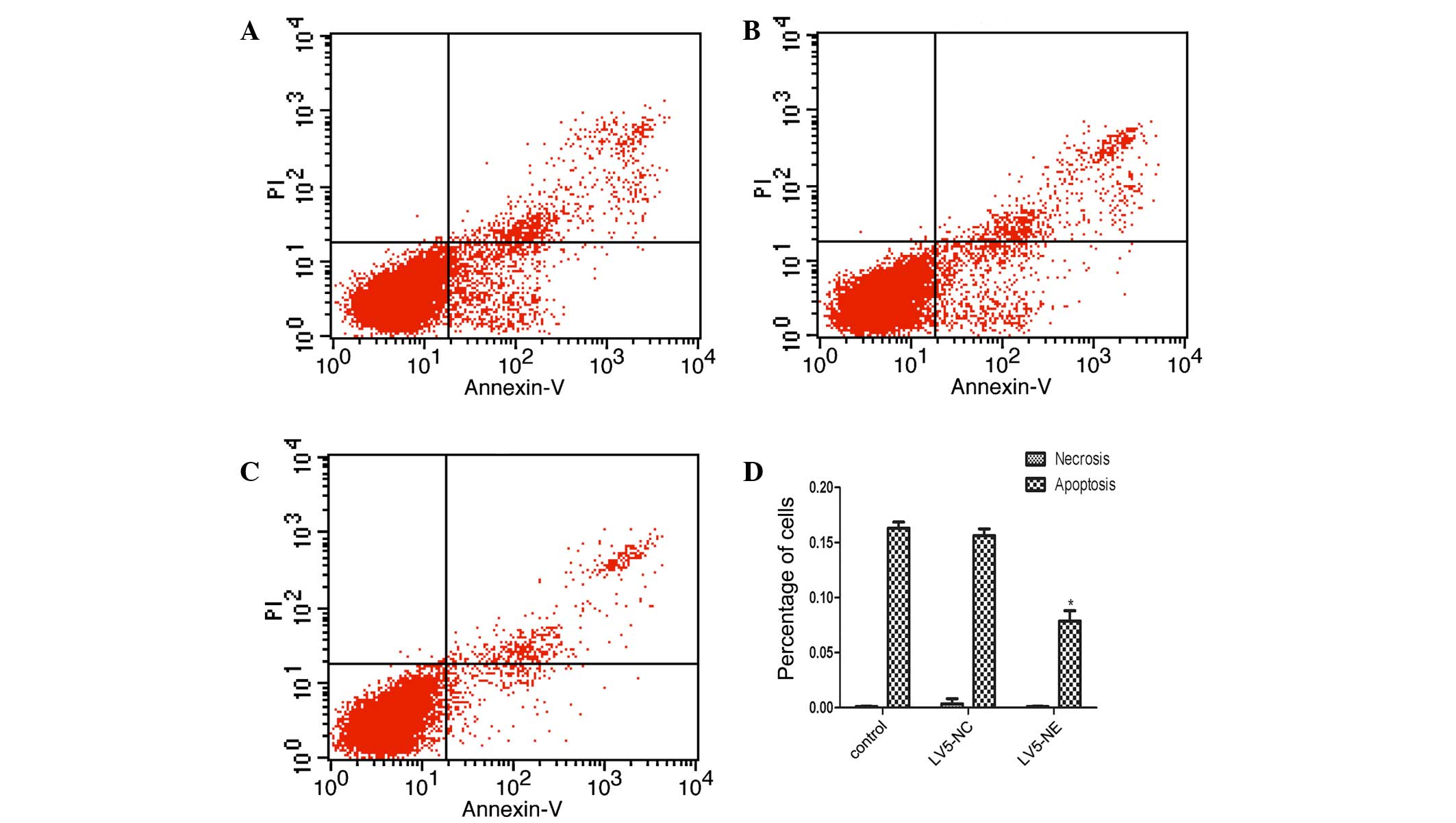
NE inhibits apoptosis in NB4 cells
Flow cytometric analysis showed that the apoptotic
rate of NB4 cells was significantly decreased following LV5-NE
transfection, while LV5-NC had no significant effect (Fig. 5). Furthermore, western blot
analysis demonstrated that the expression of anti-apoptotic protein
B-cell lymphoma 2 (Bcl-2) was upregulated in NB4 cells following
LV5-NE transfection (P<0.05), while LV5-NC had no effect on
Bcl-2 levels (Fig. 6). The
apoptotic protein Bcl-2-associated X (Bax) was not affected by any
of the vectors. These results indicated that NE inhibits apoptosis
of NB4 cells by upregulating the expression of the cell survival
protein Bcl-2.
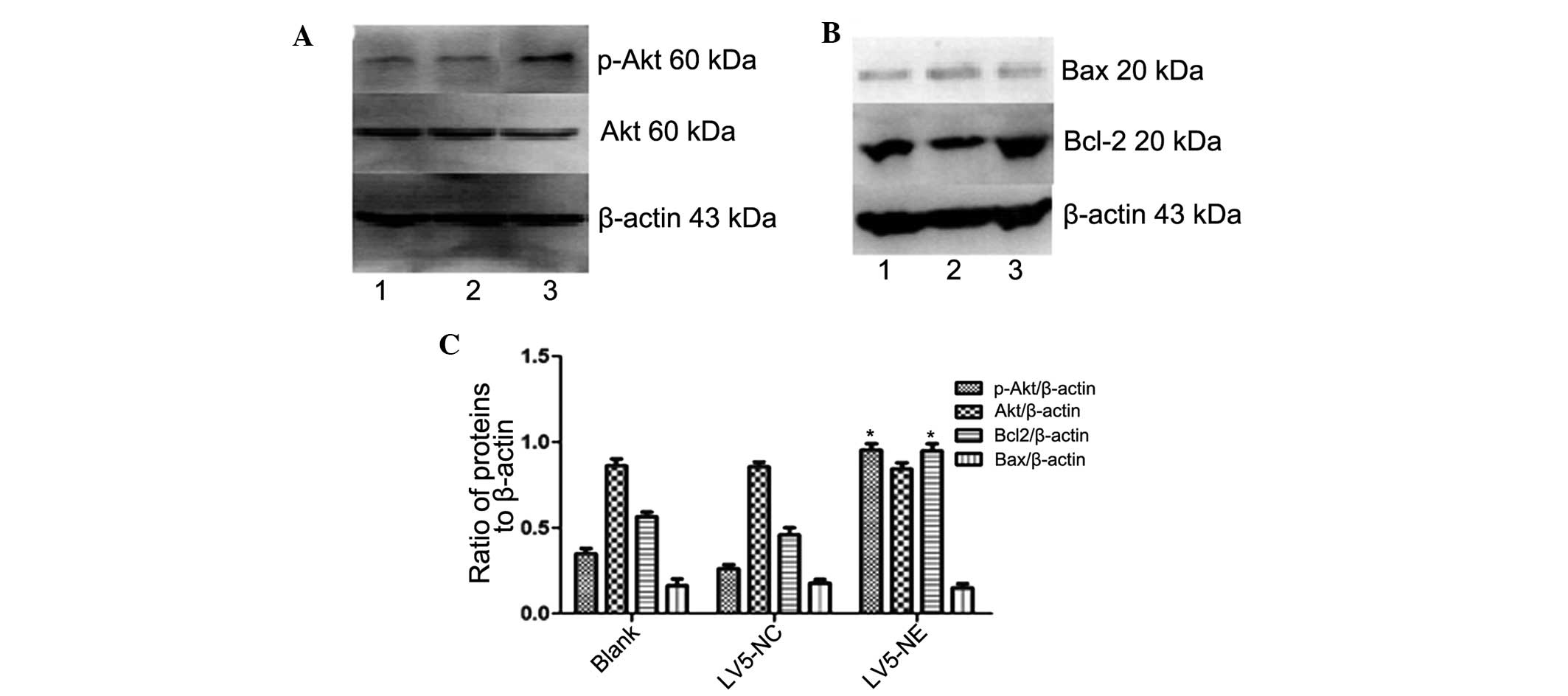 | Figure 6Effects of NE on Akt and apoptotic
signaling in NB4 cells. (A and B) The protein expression of Akt,
p-Akt, Bax and Bcl-2 in NB4 cells following transfection with
LV5-NC or LV5-NE was assessed by western blot analysis. Lanes: 1,
NB4 cells; 2, NB4 cells transfected with LV5-NCs; 3, NB4 cells
transfected with LV5-NE. (C) Densitometric quantification of
protein expression. Overexpression of NE led to a significant
increase in Akt phosphorylation and Bcl-2 expression, while total
Akt and Bax were not significantly affected. Values are expressed
as the mean ± standard deviation. *P<0.05 vs.
control. LV5-NC, negative control vector; LV5-NE, lentiviral vector
overexpressing neutrophil elastase; p-Akt, phosphorylated Akt;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein. |
NE activates the phosphoinositide-3
kinase (PI3K)/Akt pathway in NB4 cells
Western blot analysis showed increased expression of
phosphorylated (activated) Akt in NB4 cells following transfection
with LV5-NE (P<0.05), while LV5-NC had no obvious effect on Akt
phosphorylation and levels of total Akt were not affected by any of
the vectors (Fig. 6A). This result
indicated that NE may promote the proliferation of NB4 cells by
activation of the PI3K/Akt pathway.
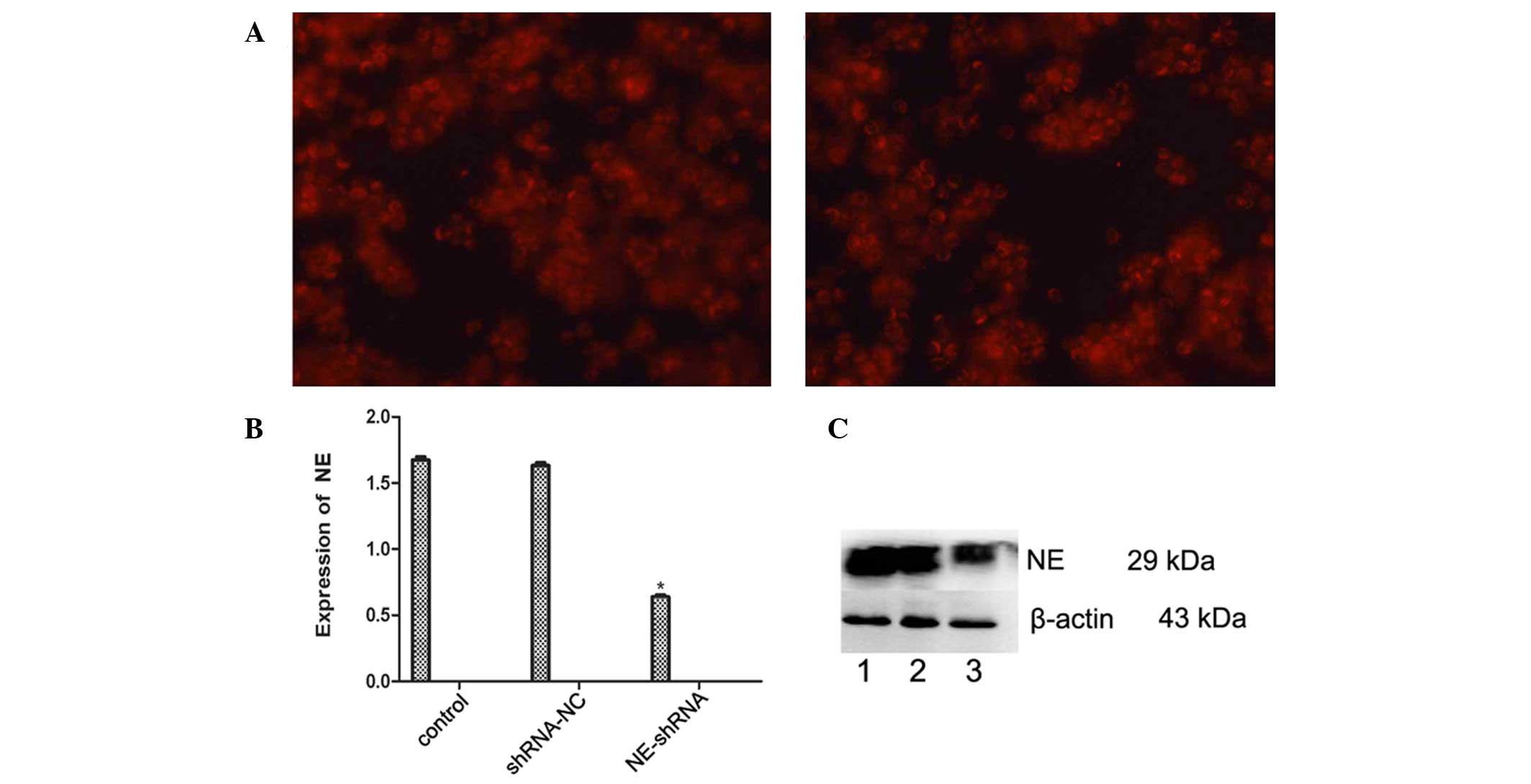
Knockdown of NE in U937 cells
NE-shRNA was used to silence NE in U937 cells.
Transfection with NE-shRNA and shRNA-NC vectors was confirmed by
fluorescence microscopy (Fig. 7A),
and RT-qPCR demonstrated that the mRNA expression of NE was
efficiently reduced in the U937/NE-shRNA cells (P<0.05), while
shRNA-NC did not affect the mRNA levels of NE (Fig. 7B). Western blot analysis showed
that the protein expression of NE in U937 cells was also decreased
by NE-shRNA (P<0.05), while not being affected by shRNA-NC
(Fig 7C).
NE knockdown deactivates the PI3K/Akt
pathway and induces apoptotic signaling in U937 cells
Western blot analysis indicated that the expression
of phosphorylated (activated) Akt and Bcl-2 in U937/NE-shRNA cells
were decreased compared with those in U937 or U937/shRNA-NC cells
(P<0.05) (Fig. 8). However, the
protein expression levels of total Akt and Bax were in U937 cells
were not significantly affected by shRNA-NE (P>0.05) (Fig 8). Collectively, these results
further confirmed that NE has an oncogenic role in leukemia cells,
and that its knockdown leads to deactivation of the PI3K/Akt
pathway and induction of apoptosis.
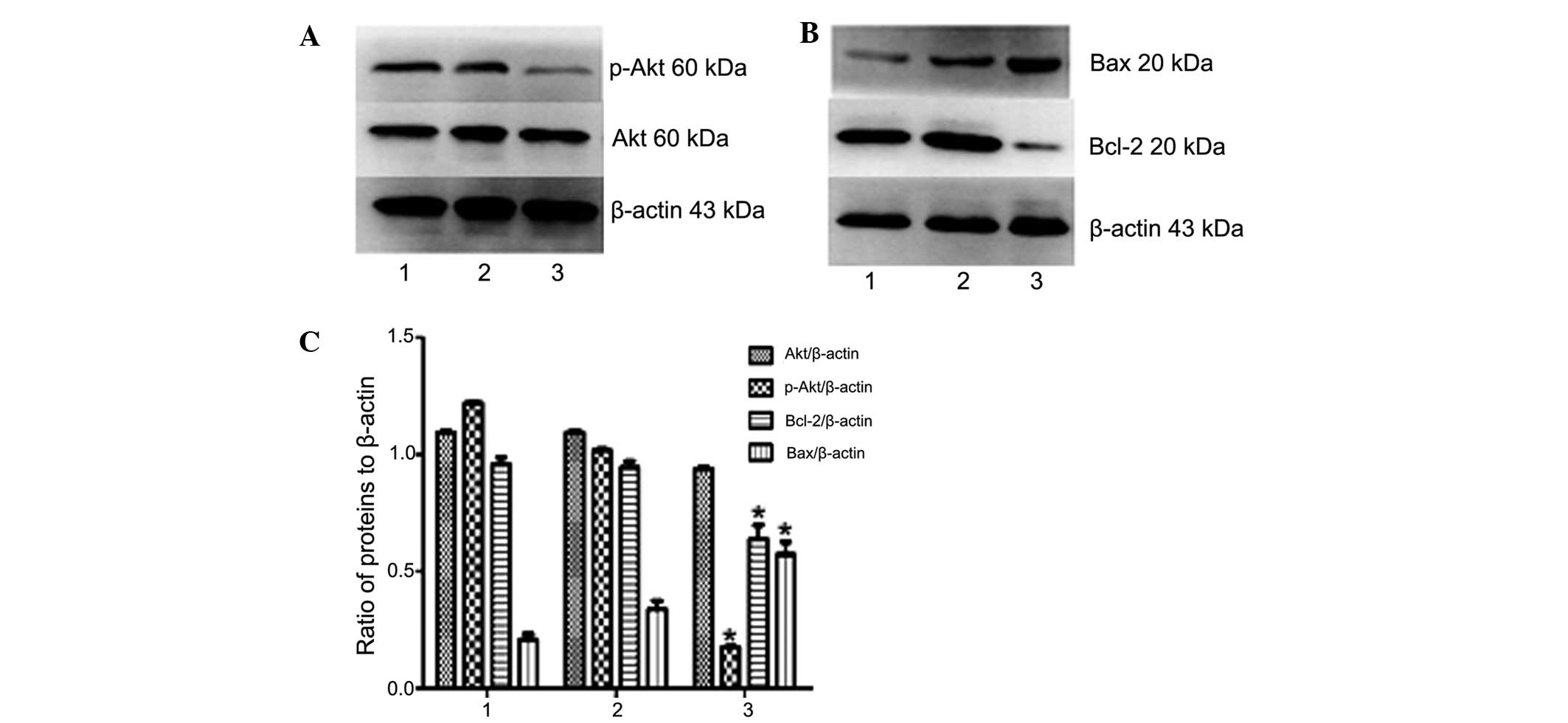 | Figure 8Effects of NE knockdown on Akt and
apoptotic signaling in U937 cells. (A and B) The protein expression
of Akt, p-Akt, Bax and Bcl-2 in U937 cells following transfection
with shRNA-NC or shRNA-NE was assessed by western blot analysis.
Lanes: 1, NB4 cells; 2, NB4 cells transfected with shRNA-NC; 3, NB4
cells transfected with shRNA-NE. (C) Densitometric quantification
of protein expression. Following knockdown of NE, the protein
expression of Bax in U937 cells was significantly increased, while
the expression of Bcl-2 and levels of p-Akt were significantly
increased, and total Akt was not affected. Values are expressed as
the mean ± standard deviation. *P<0.05 vs. control.
shRNA-NC, negative control small hairpin RNA; shRNA-NE, small
hairpin RNA targeting neutrophil elastase; p-Akt, phosphorylated
Akt; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein. |
Discussion
NE belongs to the chymotrypsin family of serine
proteases and has been reported to be associated with numerous
pathological conditions. Previous studies have shown that NE can
promote the proliferation, diffusion and metastasis of tumor cells,
and is closely associated with tumor occurrence, development and
prognosis. NE is also crucially involved in the defense against
bacterial pathogens through various mechanisms, including cell
lysis (24), degradation of
virulence factors (12), or
regulation of the inflammatory response by targeting chemokines,
cytokines and their receptors (25,26).
The underlying mechanisms of the roles of NE in diseases have been
elucidated in pulmonary conditions in particular, and it is known
that NE causes tissue damage to decrease the tolerance of the host
towards pulmonary infection with Burkholderia species
(27). NE was first reported to
promote cellular proliferation in psoriasis, a benign neoplastic
disorder of keratinocytes (28).
NE has been reported to promote lung cancer cell proliferation
in vitro and in vivo by degrading a novel target
substrate, insulin receptor substrate-1, leading to hyperactivity
of PI3K and ultimately resulting in enhanced tumor cell
proliferation (29,30). NE can also selectively bind to the
cancer cell surface and undergo classic clathrin pit-mediated
endocytosis (31). The ability of
NE to enter cancer cells while inducing their proliferation has
been demonstrated in a number of cell types, most notably in breast
cancer cells (32); however, its
role in acute pro-myelocytic leukemia has not been reported.
APL is a rare form of cancer, and targeted therapy
has proven to successfully eradicate leukemia stem cells in the
majority of affected patients (33,34).
Exploration of the possible mechanism of the roles of NE in APL may
provide a foundation for further developing targeted therapies for
this disease. NE inhibitors have been used for the clinical
treatment of a variety of conditions. Sirtinol inhibits NE activity
and was shown to attenuate lipopolysaccharide-mediated acute lung
injury in mice (35). NE
inhibitors were further shown to prevent liver injury induced by
lipopolysaccharide and the inhibition of NE induced hepatic
microvascular responses (36,37).
A previous study by our group showed that NE
promoted the proliferation and inhibited apoptosis of K562 cells
(38). In the present study, a
lentivirus expressing the NE gene was constructed and transfected
into NB4 cells, which promoted proliferation and inhibited
apoptosis; furthermore, the S-phase population of the cell cycle
was increased. This was paralleled by increased activation of Akt,
suggesting that NE may promote NB4 cell proliferation by activation
of the PI3K/Akt pathway.
Acknowledgments
The current study was supported by the National
Natural Science Foundation of China (no. 81171658) and the Natural
Science Foundation Project of CQ CSTC (no. 2011BA5037).
References
|
1
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposed revised
criteria for the classification of acute myeloid leukemia. Ann
Intern Med. 103:620–625. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lo Coco F, Diverio D, D'Adamo F, Avvisati
G, Alimena G, Nanni M, Alcalay M, Pandolfi PP and Pelicci PG:
PML/RAR-alpha rearrangement in acute promyelocytic leukaemias
apparently lacking the t(15; 17) translocation. Eur J Haematol.
48:173–176. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berger R, Bernheim A, Daniel MT, Valensi F
and Flandrin G: Cytological types of mitoses and chromosomal
abnormalities in acute leukemia. Leuk Res. 7:221–236. 1983.
View Article : Google Scholar
|
|
4
|
de Thé H, Chomienne C, Lanotte M, Degos L
and Dejean A: The t(15; 17) translocation of acute promyelocytic
leukaemia fuses the retinoic acid receptor alpha gene to a novel
transcribed locus. Nature. 347:558–561. 1990. View Article : Google Scholar
|
|
5
|
Borrow J, Goddard AD, Sheer D and Solomon
E: Molecular analysis of acute promyeloeytic leukemia breakpoint
cluster region on chromosome 17. Science. 249:1577–1580. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rowley JD, Golomb HM and Dougherty C:
15/17 translocation, a consistent chromosomal change in acute
promyelocytic leukaemia. Lancet. 1:549–550. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kakizuka A, Miller WH Jr, Umesono K,
Warrell RP Jr, Frankel SR, Murty W, Dmitrovsky E and Evans RM:
Chromosomal translocation t(15; 17) in human acute promyelocytic
leukemia fuses RAR alpha with a novel putative transcription
factor, PML. Cell. 66:663–674. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Thé H and Chen Z: Acute promyelocytic
leukaemia: Novel insights into the mechanisms of cure. Nat Rev
Cancer. 10:775–783. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baugh RJ and Travis J: Human leukocyte
granule elastase: Rapid isolation and characterization.
Biochemistry. 15:836–841. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Belaaouaj A, McCarthy R, Baumann M, Gao Z,
Ley TJ, Abraham SN and Shapiro SD: Mice lacking neutrophil elastase
reveal impaired host defense against gram-negative bacterial
sepsis. Nat Med. 4:615–618. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Belaaouaj A, Kim KS and Shapiro SD:
Degradation of outer membrane protein A in Escherichia coli killing
by neutrophil elastase. Science. 289:1185–1188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weinrauch Y, Drujan D, Shapiro SD, Weiss J
and Zychlinsky A: Neutrophil elastase targets virulence factors of
enterobacteria. Nature. 417:91–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gabay JE, Scott RW, Campanelli D, Griffith
J, Wilde C, Marra MN, Seeger M and Nathan CF: Antibiotic proteins
of human polymorphonuclear leukocytes. Proc Natl Acad Sci USA.
86:5610–5614. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaynar AM, Houghton AM, Lum EH, Pitt BR
and Shapiro SD: Neutrophil elastase is needed for neutrophil
emigration into lungs in ventilator-induced lung injury. Am J
Respir Cell Mol Biol. 39:53–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shapiro SD, Goldstein NM, Houghton AM,
Kobayashi DK, Kelley D and Belaaouaj A: Neutrophil elastase
contributes to cigarette smoke-induced emphysema in mice. Am J
Pathol. 163:2329–2335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Senior RM, Tegner H, Kuhn C, Ohlsson K,
Starcher BC and Pierce JA: The induction of pulmonary emphysema
with human leukocyte elastase. Am Rev Respir Dis. 116:469–475.
1997. View Article : Google Scholar
|
|
17
|
Lane AA and Ley TJ: Neutrophil elastase
cleaves PML-RAR and is important for the development of acute
promyelocytic leukemia in mice. Cell. 115:305–318. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hedstrom L: Serine protease mechanism and
specificity. Chem Rev. 102:4501–4524. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lane AA and Ley TJ: Neutrophil elastase is
important for PML-retinoic acid receptor activities in early
myeloid cells. Mol Cell Biol. 25:23–33. 2005. View Article : Google Scholar :
|
|
20
|
Gao YM, Zhong L, Zhang X, Hu XX and Liu
BZ: PML (NLS(−)) inhibits cell apoptosis and promotes proliferation
in HL-60 cells. Int J Med Sci. 10:498–507. 2013. View Article : Google Scholar
|
|
21
|
Hu XX, Zhong L, Zhang X, Gao YM and Liu
BZ: NLS-RARα promotes proliferation and inhibits differentiation in
HL-60 cells. Int J Med Sci. 11:247–254. 2014. View Article : Google Scholar
|
|
22
|
Jiang KL, Ma PP, Yang XQ, Zhong L, Wang H,
Zhu XY and Liu BZ: Neutrophil elastase and its therapeutic effect
on leukemia cells. Mol Med Rep. 12:4165–4172. 2015.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Belaaouaj A: Neutrophil elastase-mediated
killing of bacteria: Lessons from targeted mutagenesis. Microbes
Infect. 4:1259–1264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kessenbrock K, Dau T and Jenne DE:
Tailor-made inflammation: How neutrophil serine proteases modulate
the inflammatory response. J Mol Med (Berl). 89:23–28. 2011.
View Article : Google Scholar
|
|
26
|
Pham CT: Neutrophil serine proteases:
Specific regulators of inflammation. Nat Rev Immunol. 6:541–550.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sahoo M, Del Barrio L, Miller MA and Re F:
Neutrophil elastase causes tissue damage that decreases host
tolerance to lung infection with burkholderia species. PLoS Pathog.
10:e10043272014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wiedow O, Wiese F, Streit V, Kalm C and
Christophers E: Lesional elastase activity in psoriasis, contact
dermatitis and atopic dermatitis. J Invest Dermatol. 99:306–309.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Houghton AM, Rzymkiewicz DM, Ji H, Gregory
AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR,
et al: Neutrophil elastase-mediated degradation of IRS-1
accelerates lung tumor growth. Nat Med. 16:219–223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Metz HE and Houghton A: Insulin receptor
substrate regulation of phosphoinositide 3-kinase. Clin Cancer Res.
17:206–211. 2011. View Article : Google Scholar
|
|
31
|
Gregory AD, Hale P, Perlmutter DH and
Houghton AM: Clathrin pit-mediated endocytosis of neutrophil
elastase and cathepsin G by cancer cells. J Biol Chem.
287:35341–35350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mittendorf EA, Alatrash G, Qiao N, Wu Y,
Sukhumalchandra P, St John LS, Philips AV, Xiao H, Zhang M,
Ruisaard K, et al: Breast cancer cell uptake of the inflammatory
mediator neutrophil elastase triggers an anticancer adaptive immune
response. Cancer Res. 72:3153–3162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nasr R, Guillemin MC, Ferhi O, Soilihi H,
Peres L, Berthier C, Rousselot P, Robledo-Sarmiento M,
Lallemand-Breitenbach V, Gourmel B, et al: Eradication of acute
promyelocytic leukemia-initiating cells through PML-RARA
degradation. Nat Med. 14:1333–1342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de Thé H, Le Bras M and
Lallemand-Breitenbach V: The cell biology of disease: Acute
promyelocytic leukemia, arsenic and PML bodies. J Cell Biol.
198:11–21. 2012. View Article : Google Scholar
|
|
35
|
Tsai YF, Yu HP, Chang WY, Liu FC, Huang ZC
and Hwang TL: Sirtinol inhibits neutrophil elastase activity and
attenuates lipopolysaccharide-mediated acute lung injury in mice.
Sci Rep. 5:83472015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kwon AH and Qiu Z: Neutrophil elastase
inhibitor prevents endotoxin-induced liver injury following
experimental partial hepatectomy. Br J Surg. 94:609–619. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishii K, Ito Y, Katagiri H, Matsumoto Y,
Kakita A and Majima M: Neutrophil elastase inhibitor at tenuates
lipopolysaccharide-induced hepatic micro vascular dysfunction in
mice. Shock. 18:163–168. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang K, Ma P, Yang X, Zhong L, Wang H,
Zhu X and Liu B: Over-expression of neutrophil elastase promotes
proliferation and inhibits apoptosis in K562 cells. Chin J Cell Mol
Immunol. 31:159–162. 1672015.In Chinese.
|