Introduction
Stroke is the most common acute cerebrovascular
disease and has high rates of morbidity and mortality (1,2).
Despite extensive investigations into the mechanisms of and
treatments for stroke, effective therapeutic strategies remain
limited. Cerebral ischemia is the major insult caused by stroke,
leading to acute neuronal death via a reduction or complete
blockade of blood flow to the brain, resulting in oxygen and
nutrient deficiency (3).
Additionally, cerebral ischemia is associated with changes in
several other factors, including vasopressin and endothelin levels,
inflammation and intracellular pH (pHi) homeostasis (4). It is understood that pHi is important
in modulating neuronal survival following hypoxia injury (5). Under physiologic conditions, the pHi
of the brain is maintained at ~7.03. However, mild acidosis during
reperfusion injury following transient hypoxia has previously been
demonstrated to have a protective effect against ischemic injury
(6-8), while mild alkalosis has the opposite
effect. Hypoxia significantly effects pH homeostasis via lactate
production and the upregulation of glycolysis to maintain ATP
production, which facilitates a reduction in pHi (9).
Na+/H+ exchanger isoform 1
(NHE1) regulates pHi homeostasis and cell volume (10,11)
by mediating an electroneutral (1:1) exchange of intracellular
H+ for extracellular Na+ (12). Previous studies have indicated that
NHE1 is important during ischemia and reperfusion, and inhibitors
of NHE1 have been clinically evaluated for their ability to
alleviate ischemia-reperfusion injury (9,13,14).
However, it remains unknown whether NHE1 is activated during
ischemia, and whether a lower pHi and cellular ATP depletion
preclude NHE1 activity (15–17).
It has been suggested that the controversies regarding the extent
of ATP depletion may be due to differences in the experimental
protocols and time courses used (15–17).
Additionally, certain evidence has suggested that the pHi and NHE1
activity decline when O2 tension is reduced to <2%
O2 (18). However,
previous studies demonstrated that brain pHi is not acidic but
alkaline (pHi 7.1–7.4) following stroke (19). Excessive activation of NHE1 was
considered to be responsible for this brain intracellular
alkalosis, which was demonstrated to correlate with the severity of
brain injury (8,20). Furthermore, excess intracellular
Na+ resulted in increased intracellular Ca2+
via the Na+/Ca2+ exchanger, which may
accelerate the Ca2+-mediated signaling cascades and lead
to secondary ischemic brain injury (21,22).
Hypoxia-inducible factor-1α (HIF-1α), a key
regulator of the cellular response to oxygen deprivation (18), is a master transcription factor for
several genes involved in glucose uptake, angiogenesis, glycolysis,
pH balance and metastasis (23).
CoCl2, an established hypoxia-mimetic, can produce a
chemical hypoxic-like environment, induce mitochondrial damage and
increase the generation of reactive oxygen species (24,25).
It is well-established that CoCl2 exposure activates
HIF-1α under normoxic conditions in vitro and in vivo
(26). In addition, it has been
reported reduced pHi in solutions supplemented with 100 µM
CoCl2 is associated with solutions pre-equilibrated at
1% O2 (18). These
findings suggest that the model of hypoxic damage induced by
CoCl2 is a suitable tool to investigate the mechanisms
of cell injury caused by ischemia (27).
Astrocytes have been intensively investigated in the
field of ischemic stroke research, as ischemic stroke causes
neuronal injury and also damages astrocytes (28–30).
Previous studies have demonstrated that, in ischemic infarcts,
neurons will not survive if their neighboring astrocytes are not
viable (31,32). Greater understanding of NHE
regulation and the pattern of pHi changes in astrocytes in
responding to CoCl2 treatment may aid the understanding
of the mechanisms of hypoxia-induced neural injury, and facilitate
the design of more effective treatment strategies. Additionally, as
numerous physiological pathways, each with a characteristic
reaction time, are spontaneously activated following the onset of
stroke (33), the aim of the
current study was to investigate whether NHE activity and pHi
homeostasis are altered in a time-dependent manner under
hypoxia-mimicking conditions induced by CoCl2 treatment
of astrocytes.
Materials and methods
Materials
All cell culture reagents, including Dulbecco's
modified Eagle medium (DMEM), antibiotics, fetal bovine serum (FBS)
and phosphate-buffered saline (PBS) were purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA).
2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester
(BCECF-AM), cariporide, 5-(N-ethyl-N-isopropyl) amiloride (EIPA)
and antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Cell culture plastics were purchased from
Corning Incorporated (Corning, New York, USA). TRIzol reagent,
Super Script III reverse transcriptase and Platinum SYBR Green qPCR
Super Mix were purchased from Invitrogen (Thermo Fisher Scientific,
Inc.). The Annexin V-fluorescein isothiocyanate (FITC) apoptosis
detection kit was purchased from Beyotime Institute of
Biotechnology (Haimen, China). All other chemicals were purchased
from Sigma-Aldrich (St. Louis, MO, USA).
Animals
BALB/c (n=22; 1-day old; 2±0.3 g) mice were obtained
from the Experimental Animal Centre of Zhengzhou University
(Zhengzhou, China), housed in a specific-pathogen-free environment
and fed with commercial pellets. All experiments were approved by
the Ethics Committee of Life Sciences of Zhengzhou University.
Primary astrocyte culture
Primary astrocytic cultures were prepared as
described previously (34).
Neonatal mice (1-day-old) were sacrificed by decapitation following
anesthesia with Zoletil 100 (30 mg/kg, Virbac Corporation, France).
The brains were rapidly dissected, as follows: The cerebellum and
olfactory bulbs were removed and the meninges and blood vessels
were carefully stripped off using a XYH-4A stereo-microscope
(Shanghai Optical Instrument Factory Co., Ltd., Shanghai, China).
The cerebral cortices were removed and the tissue was carefully
transferred to complete DMEM (supplemented with 10% FBS, 50 U/ml
penicillin and 50 mg/ml streptomycin-sulfate) containing 0.0025%
trypsin/EDTA to dissociate. Subsequently, the cell suspension was
filtered through a 75-µm pore-size mesh filter (XBSW-200,
Shanghai Junsheng Biotechnology Co, Ltd., Shanghai, China).
Following centrifugation at 900 × g for 10 min, the cells were
seeded (3×104 cells/cm2) in
poly-L-lysine-coated tissue culture flasks (Corning BioCoat;
Corning Inc., New York, USA) and maintained in complete DMEM at
37°C in a humidified atmosphere with 5% CO2. The medium
was renewed on day in vitro 1 (DIV1), DIV5 and DIV7. On
DIV9, the microglia were discarded from the astrocytic cultures
using the shake-off method (35).
Astrocytes were harvested from the flasks with
trypsin and reseeded at a density of 3×104
cells/cm2 in complete DMEM/F12 medium containing 10%
heat-inactivated FBS, 50 U/ml penicillin and 50 mg/ml streptomycin.
The purity of the cultures was monitored by immunocytochemical
staining using an antibody against the astrocyte marker glial
fibrillary acidic protein (GFAP; 1:1,000; Sigma-Aldrich; cat. no.
SAB5500113) and the absence of microglial cells was confirmed using
isolectin B4 (1:100; Sigma-Aldrich; cat. no. L5391). The
homogeneity of astrocytes was ~90–95% and <5–10% of the cells
were isolectin B4 (1:100; Sigma-Aldrich; cat. no. L5391) (36).
Astrocytes were reseeded at a density of
5×104 cells/cm2 and incubated at 37°C with 5%
CO2. The culture medium was changed every 3 days and
experiments were carried out on DIV19-22 (10–13 days after plating
at which point they formed an incomplete monolayer of both
stellate- and flat-shaped astrocytes). The cells were then exposed
to 100 µM CoCl2 and analyzed at distinct time
points over 24 h.
Cell viability assay
To determine the effects of hypoxia on the viability
of astrocytes, cells were treated with 100 µM
CoCl2 in serum-free DMEM/F12 medium prior to the
measurement of cell viability every 4 h using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
assay, according to manufacturer's instructions.
The assay was performed by adding MTT solution (5
mg/ml) to each well and incubating at 37°C for 4 h. Following
removal of the culture medium, 1 ml dimethyl sulfoxide was added
and thoroughly mixed for 10 min. MTT absorbance was measured at 570
and 630 nm by using an SP-Max 2300A2 microplate reader (Shanghai
Flash Spectrum Biological Technology Co., Ltd., Shanghai,
China).
Flow cytometry
To confirm the results of the MTT assay, cells were
harvested using trypsin/EDTA solution every 4 h following
CoCl2 treatment. The cells were rinsed with binding
buffer (0.01 M HEPES; 0.14 M NaCl; and 2.5 mM CaCl2, pH
7.4) and collected by centrifugation at 1,500 × g for 5 min. Cell
pellets were re-suspended in binding buffer at a concentration of
1×106 cells/ml, and incubated with Annexin V-FITC and
propidium iodide for 15 min at room temperature in the dark,
according to the manufacturer's protocol. Cell injury analysis was
performed using a FACSCalibur (BD Biosciences, Franklin Lakes, NJ,
USA), analyzing 10,000 cells per sample.
pHi measurement
Astrocytes were cultured on glass coverslips until
confluent and pHi was determined at distinct time points following
CoCl2 treatment. Cellular NHE activity was determined
fluorometrically using the pHi-sensitive dye BCECF-AM, as described
previously (37), with
modification.
Briefly, cells were exposed to Na+
solution (138 mM NaCl; 5 mM KCl; 2 mM CaCl2; 1 mM
MgSO4; 1 mM NaH2PO4; 25 mM
glucose; and 20 mM HEPES, pH 7.4) with 5 µM BCECF-AM for 1 h
at 37°C. During dye loading, cells of the CoCl2
treatment group were treated with Na+ solution with or
without 100 µM CoCl2. Cells were then washed with
the Na+ solution to remove extracellular dye. Filters
were mounted in a cuvette, placed in a fluorometer (Photon
Technology International, Inc., South Brunswick, NJ, USA), perfused
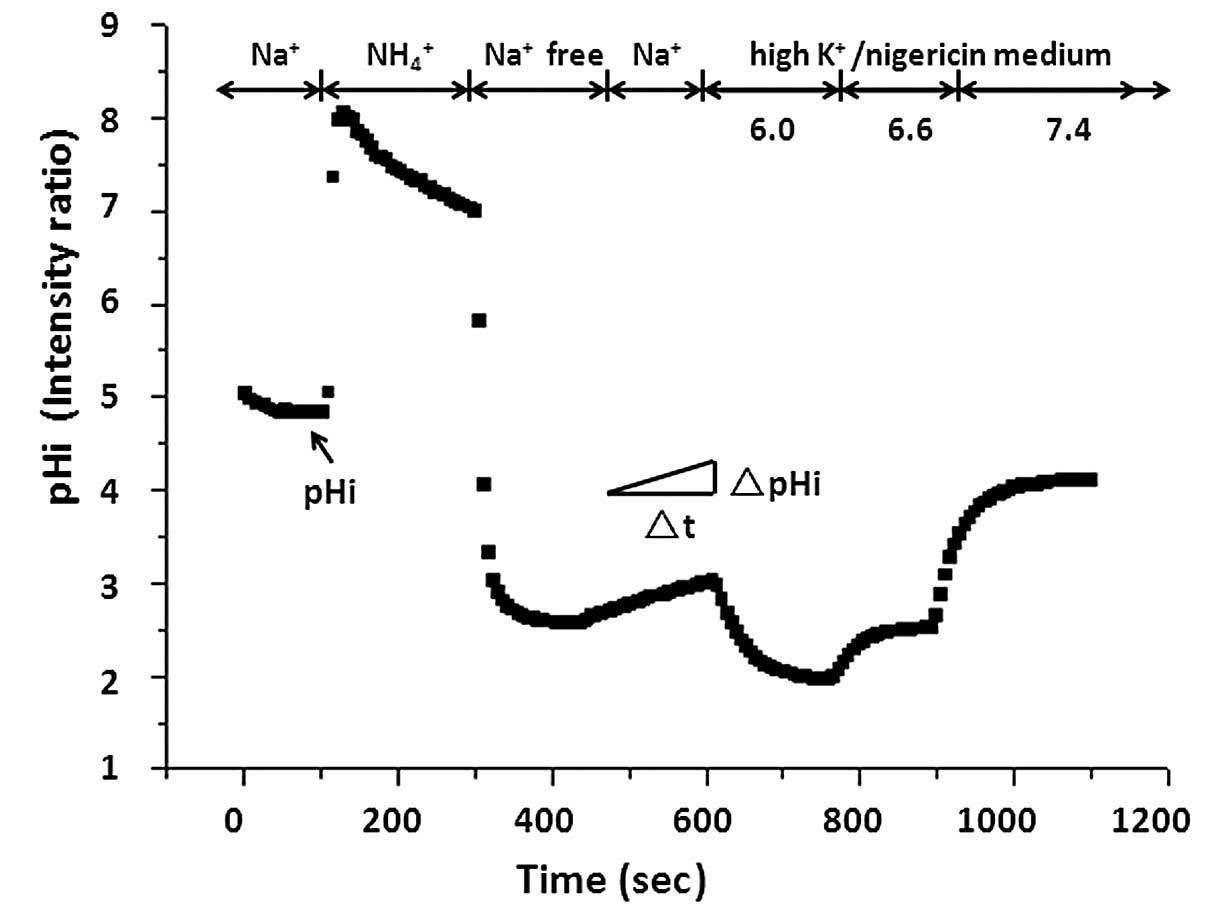
with Na+ solution and the baseline pHi was measured.
Following measurement of the baseline pHi, a standard ammonia pulse
technique was used to measure Na+/H+ exchange
activity (Fig. 1). Cells were
briefly exposed to 40 mM NH4Cl (pH 7.4), which caused
alkalization. Then, cells were perfused with Na+-free
medium (130 mM tetramethyl ammonium chloride; 5 mM KCl; 2 mM
CaCl2, 1 mM MgSO4; 1 mM
NaH2PO4; 25 mM glucose; and 20 mM HEPES, pH
7.4), resulting in acidification due to the rapid diffusion and
washout of NH3, leaving behind H+ ions.
Subsequently, the external solution was replaced with the
Na+ solution. Re-addition of extracellular
Na+ allowed activation of Na+/H+
exchange and recovery from acidification to basal levels. At the
end of each experiment, the fluorescence ratio was calibrated to
pHi using nigericin (2 µM; Sigma-Alrich)/high K+
solutions of different pH values (38).
The initial rate of Na+-dependent
recovery from intracellular acidification corresponding to NHE
activity was almost linear during the first 1 min of the reaction.
NHE activity was measured by calculating the pHi change over the
first 1 min using Origin 6.0 (OriginLab, Northampton, MA, USA) and
expressed as ΔpH/min.
To evaluate which isoforms of the NHE family were
responsible for the pHi regulation induced by CoCl2
treatment, cells were perfused with Na+-free medium,
Na+-free medium with 10 nM cariporide (an NHE1-specific
inhibitor) or 25 mM EIPA (a non-specific NHE inhibitor). NHE
activity was recorded to identify cariporide-sensitive or
EIPA-sensitive components of acid extrusion.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
To measure the changes in NHE1 gene expression
levels induced by CoCl2 treatment, astrocytes were
incubated with 100 µM CoCl2 over a distinct time
course, and then harvested. Total RNA was extracted from astrocytes
using TRIzol reagent, according to the manufacturer's protocol, and
subjected to DNase treatment. RNA quantity and quality were
determined by an EU-2200R Ultraviolet spectrophotometer (Shanghai
Onlab Instruments Co., Ltd., Shanghai, China) at 260 and 280 nm.
Typically, the equivalent cDNA produced from reverse transcription
of 20 ng RNA using a commercial SuperScript III reverse
transcriptase kit was used for the RT-qPCR of each sample.
The expression of NHE1, HIF-1α and β-actin were
evaluated by RT-qPCR using Platinum SYBR Green qPCR Super Mix. The
cycling conditions used were as follows: 50°C for 2 min and 95°C
for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1
min. qPCR was performed using an ABI 7500 sequence detector
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Specific
primers are presented in Table I.
The quantities of gene-specific mRNA expression were determined by
the quantification cycle (Cq) method and Cq values for β-actin were
used as the internal control. The 2−ΔΔCq method was used
for relative quantitation (39).
 | Table ISpecific primers for the expression
of Na+/H+ exchanger 1, hypoxia inducible
factor-1α and β-actin. |
Table I
Specific primers for the expression
of Na+/H+ exchanger 1, hypoxia inducible
factor-1α and β-actin.
| Gene name | Primer sequence
(5′–3′) | Product size,
bp | Amplicon, bp |
|---|
|
Na+/H+ exchanger
1 | F:
CACCCTTTGAGATCTCCCTCT | 21 | 68 |
| R:
GGGGATCACATGGAAACCTA | 20 | |
| Hypoxia inducible
factor-1α | F:
AACAGAATGGAACGGAGCAA | 20 | 119 |
| R:
TTCACAATCGTAACTGGTCAGC | 22 | |
| β-actin | F:
CTAAGGCCAACCGTGAAAAG | 20 | 104 |
| R:
ACCAGAGGCATACAGGGACA | 20 | |
Western blot analysis
Following incubation of astrocytes with 100
µM CoCl2, cells were washed three times with
ice-cold PBS containing 50 mM Tris. Cells were collected and lysed
in 500 µl ice-cold lysis buffer (10 mM HEPES, 50 mM NaCl, 5
mM EDTA, 1 mM benzamidine, 0.5% Triton X-100). The cell lysate was
solubilized for 30 min at 4°C with end-over-end rotation and
subsequently homogenized 10 times using a 23-gauge needle (Jintan
FiveStar Health & Medical Co., Ltd., Jintan, China). Cellular
debris was cleared by centrifugation at 14,000 × g for 15 min. The
supernatant was collected, solubilized in loading buffer (5 mM
Tris-HCl, pH 6.8; 1% SDS; 10% glycerol; and 1% 2-mercaptoethanol)
and boiled for 10 min. Subsequently, samples were loaded onto and
size-fractionated by 10% SDS-PAGE, then electrophoretically
transferred to a nitrocellulose membrane. Following blocking with
5% non-fat milk in PBS-T (0.05% Tween/PBS) for 1 h at room
temperature, the membranes were incubated with rabbit anti-NHE1
(1:1,000; Santa Cruz Biotechnology, Inc.; cat. no. sc-28758), mouse
anti-HIF-1α (1:1,000; cat. no. sc-71247) or mouse anti-β-actin
(1:2,000; cat. no. sc-47778) antibodies. Membranes were washed with
PBS-T three times for 10 min then incubated with horseradish
peroxidase-conjugated bovine anti-mouse IgG (1:10,000; Santa Cruz
Biotechnology, Inc.; cat. no. sc-2371) and bovine anti-rabbit IgG
(1:10,000; cat. no. sc-2370) for 60 min at room temperature in the
dark. Blots were detected using an enhanced chemiluminescence
western blotting detection system (Beyotime Institute of
Biotechnology).
Statistical analysis
Data of multiple experiments are expressed as means
± standard deviation. Statistical differences among the different
time points were conducted using one-way analysis of variance
followed by the Student Newman-Keuls test. Statisticl analysis was
performed with the SPSS software, version 13.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
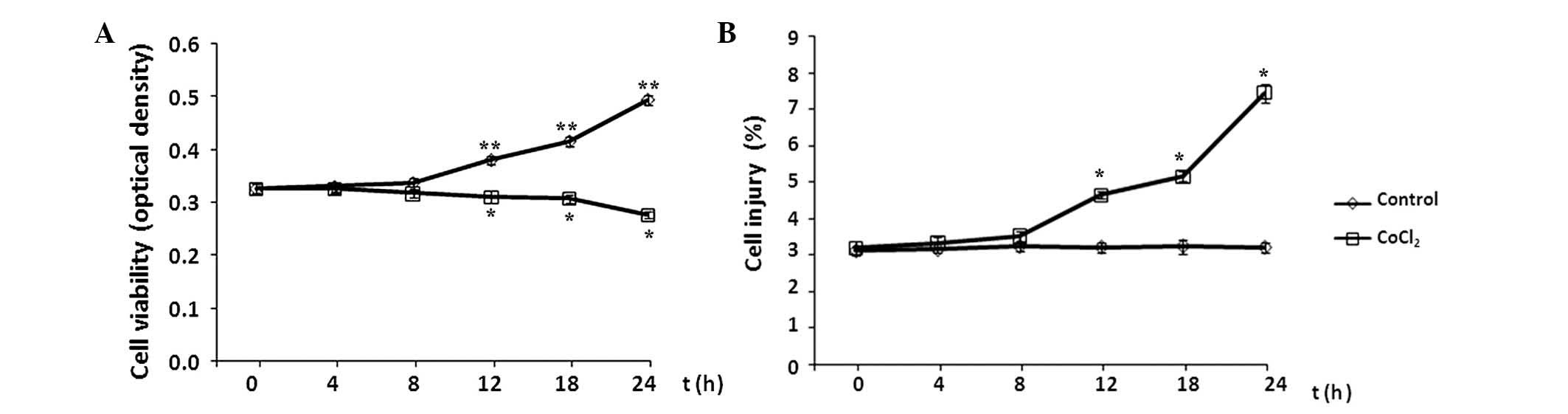
Effect of CoCl2 treatment on
astrocyte cell viability and injury
The effects of CoCl2 treatment on the
cell viability and injury of astrocytes were evaluated every 4 h
during a 24-h period by MTT assay and flow cytometry analysis,
respectively. Cell viability is presented as the optical density of
CoCl2-treated cells versus the control, and the level of
apoptosis is presented as the percentage of cells with FITC-Annexin
V binding excluding propidium iodide staining in total cell
numbers. Compared with the control group, the levels of cell
viability and injury remained at baseline levels in the initial 8 h
of CoCl2 treatment (P>0.05 vs. control; Fig. 2). Subsequently, cell viability
decreased significantly following exposure to 100 µM
CoCl2 for >8 h, while the level of cell injury
continued to increase in each subsequent time interval over the
24-h time course (P<0.05 vs. control).
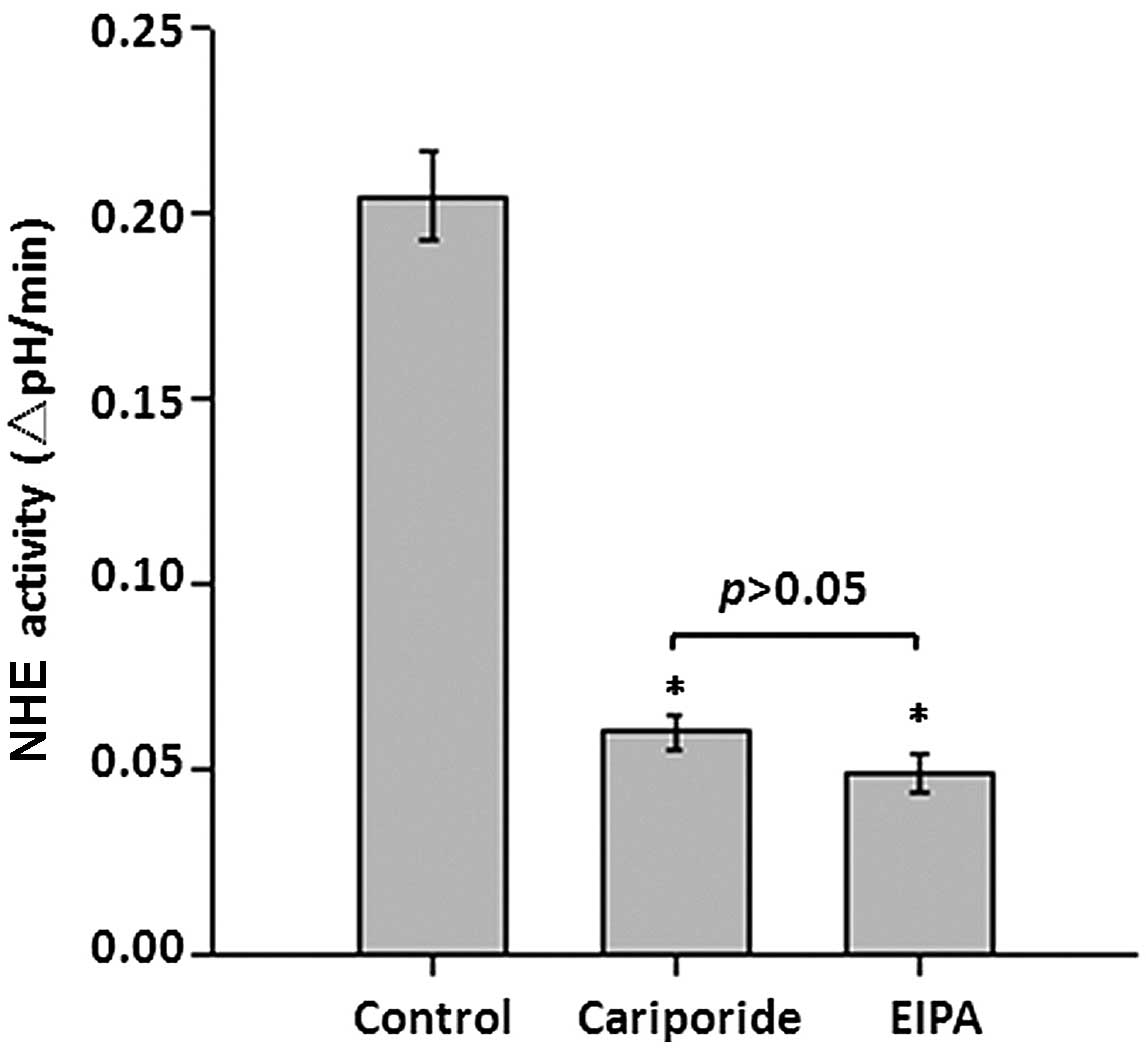
Role of NHE1 in pHi regulation in
astrocytes
To identify the role of NHE1 in the regulation of
pHi, NHE activity was determined in the presence of a specific NHE1
inhibitor and compared with overall NHE inhibition. The initial NHE
activity in astrocytes was 0.205±0.012 ΔpHi/min (Fig. 3). The recovery rate in the presence
of 10 nM cariporide, which specifically inhibits NHE-1 at this
concentration, was suppressed to 0.0599±0.004 ΔpHi/min (P<0.05
vs. control). However, treatment with 25 mM EIPA, which inhibits
all NHE isoforms at this concentration, demonstrated no further
suppression of NHE activity, compared with cariporide treatment
(0.0492±0.005 ΔpHi/min; P>0.05 vs. cariporide). These results
suggest that NHE1, not other NHEs isoforms, is important in
regulating the pHi homeostasis in astrocytes.
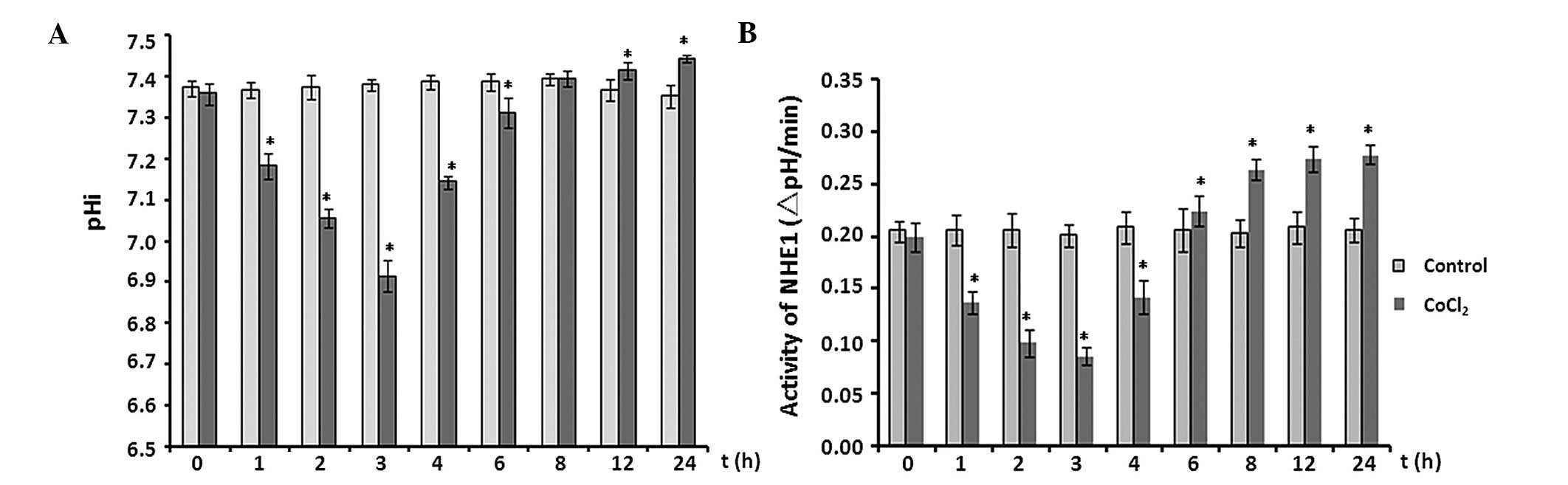
Effect of CoCl2 treatment on
pHi and activity of NHE1
The effects of CoCl2 treatment on pHi and
the activity of NHE1 in astrocytes were analyzed at multiple time
points over 24 h. In the initial 3 h, the pHi fell from 7.356±0.026
to 6.913±0.038 (P<0.05 vs. control). Subsequently, the pHi
increased steadily and reached 7.441±0.008 at the 24-h time point
(P<0.05; Fig. 4A).
Consistent with the time-dependent manner of pHi
changes, NHE1 activity behaved in a similar manner (Fig. 4B). NHE1 activity decreased by ~50%
after 3 h of exposure to 100 µM CoCl2 (P<0.05
vs. control; Fig. 4B), then
increased significantly throughout the remaining recording period
(P<0.05).
Effect of CoCl2 treatment on
NHE1 and HIF-1α mRNA expression
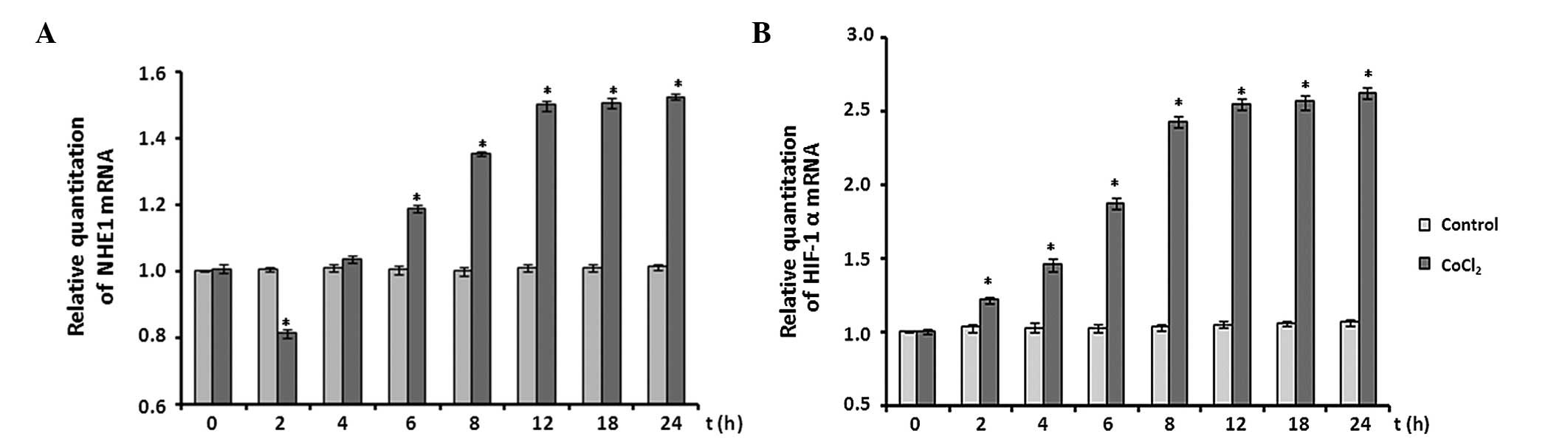
The effects of CoCl2 treatment on the
mRNA expression levels of NHE1 and HIF-1α were analyzed by RT-qPCR
assay. The expression levels of NHE1 mRNA were decreased by ~20% at
the 2 h time point following CoCl2 treatment (P<0.05
vs. control). Subsequently, NHE1 mRNA expression levels increased
significantly between 2 and 12 h, compared with the control levels
(P<0.05) and plateaued between 12 and 24 h at ~1.5-fold
expression (Fig. 5A).
Following CoCl2 treatment, HIF-1α mRNA
expression levels were also determined. When astrocytes were
exposed to 100 µM CoCl2, expression levels of
HIF-1α mRNA significantly increased immediately and reached an
~2.0-fold increase at the 12 h time point. The increase in HIF-1α
expression levels remained at ~2.6-fold throughout the remaining
recording period (P<0.05 vs. control; Fig. 5B).
Effect of CoCl2 treatment on
protein expression levels of NHE1 and HIF-1α
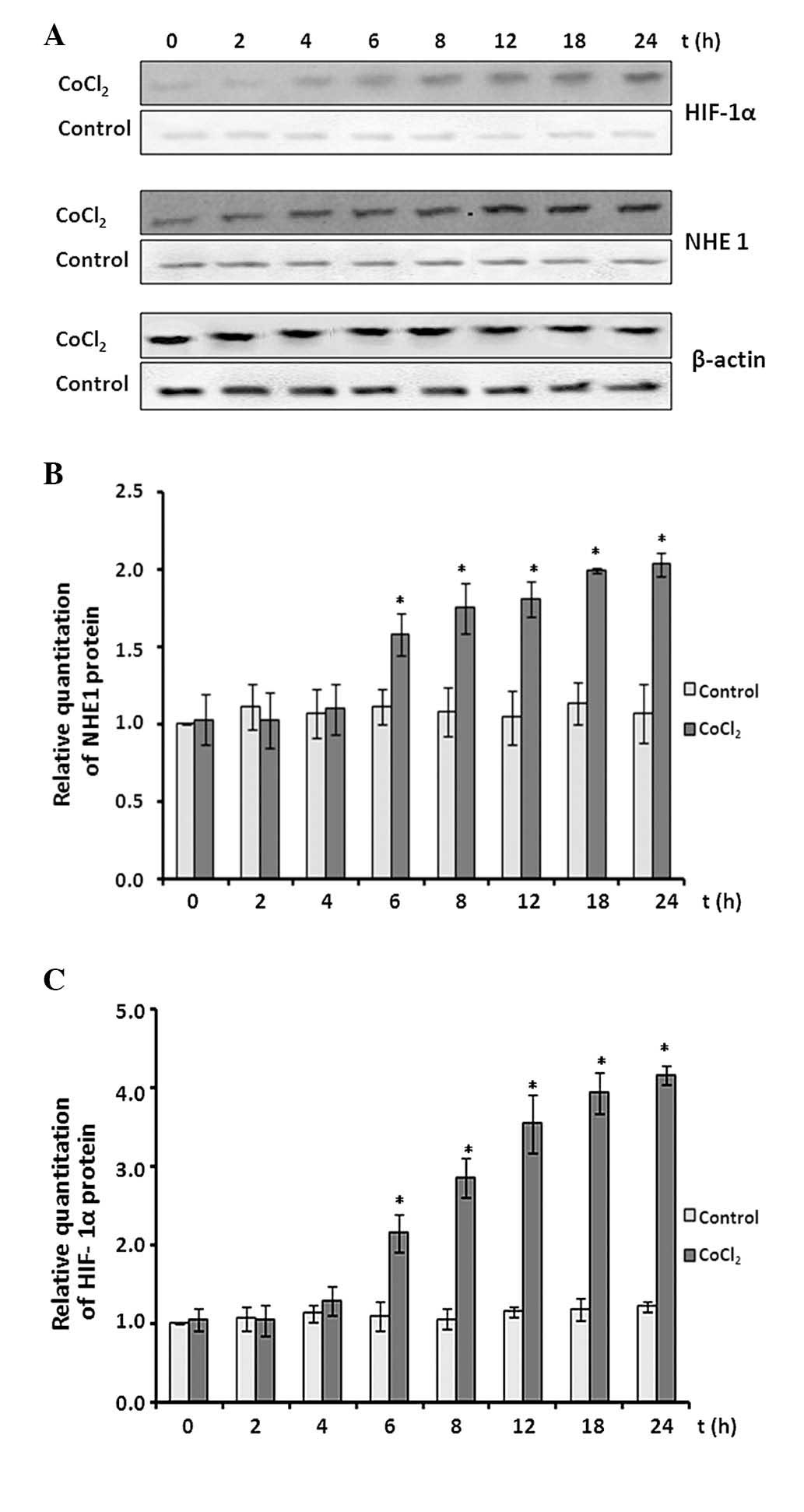
The protein expression levels of NHE1 and HIF-1α
were determined by western blot assay (Fig. 6A). CoCl2 treatment
demonstrated minor effects on NHE1 protein expression in the
initial 4 h of treatment (P>0.05 vs. control; Fig. 6B). Subsequently, NHE1 protein
expression levels increased significantly over the remaining
treatment time course and reached an ~2.1-fold increase at the 24-h
time point (P<0.05 vs. control).
HIF-1α protein expression levels also remained at
baseline levels in the initial 4 h of CoCl2 treatment
(P>0.05 vs. control). Subsequently, the expression levels began
to increase and reached ~4-fold at the 24-h time point (P<0.05
vs. control).
Discussion
The present study investigated the changes in cell
viability and injury at distinct time points following exposure of
astrocytes to 100 µM CoCl2. Cell viability and
injury remained unchanged in the initial 8 h of treatment followed
by a decrease in cell viability and an increase in cell injury
during the remaining recording period. Additionally, the current
study demonstrated that CoCl2 treatment significantly
affects pHi homeostasis and NHE1 expression in a time-dependent
manner. NHE1 protein directly interacts with various
apoptotic-related and other proteins involved in cell growth and
proliferation (12), however, it
is controversial whether NHE1 is one of the key factors responsible
for the cell death induced by CoCl2 treatment (40,41).
Thus, the current study investigated whether the time-dependent
changes to cell viability and injury were associated with the
regulation of pHi and NHE activity following CoCl2
treatment.
The NHE gene family includes 9 different isoforms
(NHE1-9). NHE1 is expressed in the majority of cells and tissues,
and is by far the most abundant NHE isoform in the plasma membrane
of rat brains (42,43). However, there is currently little
information available on the functional expression of different
isoforms in astrocytes (44). To
evaluate which isoforms of the NHE family are responsible for pHi
regulation induced by CoCl2 treatment, the inhibitors
cariporide and EIPA were used. Cariporide, a potent and highly
selective NHE1 inhibitor (IC50: NHE1, 0.01 µM;
NHE2, 1.6 µM; NHE3, 1,000 µM) (45), specifically inhibits NHE1, not the
other NHE isoforms, at a concentration of 10 nM (43). In the present study, it was
observed that 10 nM cariporide successfully suppressed NHE1
activity, but 25 mM EIPA, which was previously demonstrated to
block all NHE isoforms at this concentration (46), had no further effect on the
recovery rate. This suggests that it is NHE1, rather than other NHE
isoforms, that is important in regulating pHi homeostasis in
astrocytes. Additionally, the recovery of pHi from acid loading
still occurred but at a slower pace in the presence of 25 mM EIPA,
indicating that other H+ extrusion systems may exist in
cultured astrocytes.
It has been previously reported that the effects of
CoCl2 treatment are very rapid, with pH and NHE activity
significantly decreased in parallel within 10 min when
O2 tension was reduced to <2% O2 (18,47–49).
The time-course experiment within the current study revealed that
the steady state pHi in astrocytes began to fall within the initial
3 h of CoCl2 treatment. Additionally, NHE1 activity was
downregulated over the same time period. These findings are in
agreement with previous studies (18,47–49).
The present study also observed that the pHi and
NHE1 activity increased between 4 and 8 h, and remained at high
values throughout the remaining time course. These results are
supported by other previous studies (9,18).
Considering that CoCl2 treatment significantly affects
pH homeostasis and that the upregulation of glycolysis to maintain
ATP production facilitates a decrease in pHi (9,18),
it is likely that NHE-1 may be activated by intracellular acidosis
to exchange intracellular H+ for extracellular
Na+. The current study evaluated the expression levels
of NHE1 mRNA and protein, and used HIF-1α as a marker of hypoxic
stress and a key regulator of hypoxia. It was observed that HIF-1α
protein increased gradually during the initial 12 h of
CoCl2 treatment and remained at high levels over the
remaining time course. These findings are supported by previous
studies (2,12). However, the NHE1 protein expression
did not change in the first 4 h, whereas NHE1 mRNA expression was
decreased by 20% in the initial 2 h of CoCl2 treatment.
Subsequently, the protein and mRNA levels of NHE1 increased
significantly throughout the time course.
Although the protein expression demonstrated no
detectable change, the function of NHE1 was inhibited and NHE1 mRNA
levels were decreased during the early period of CoCl2
treatment. Taken together, these findings may explain the drop in
pHi and NHE1 activity during the early period of CoCl2
treatment. Notably, despite reduced pHi and NHE1 activity, there
were no significant changes in cell viability and injury in the
early period of CoCl2 treatment. These findings are
consistent with previous reports (18,50).
It was suggested that hypoxia-induced acidification appears to be
beneficial for matching ATP production and consumption by
suppressing metabolic activity. By contrast, during the later
period, the mRNA and protein expression levels of NHE1 were
increased and remained at elevated levels. The activation of NHE1
may result in the acceleration of the Ca2+-mediated
signaling cascade to initiate deleterious events (22). The current study observed decreased
cell viability and increased injury after 8 h of CoCl2
treatment, and this appeared to be associated with enhanced pHi and
NHE1 activity.
In summary, NHE1, rather than other NHEs isoforms,
appeared to be dominant during the regulation of pHi homeostasis in
astrocytes under CoCl2 treatment. NHE1 activity and pHi
homeostasis changed over time under CoCl2 treatment, and
CoCl2-induced NHE-1 activity occurred independently of
HIF-1α activity. We propose, for the first time, that
CoCl2 treatment exerts early effects (in the initial 2–3
h of CoCl2 treatment) on pHi homeostasis, resulting in
cellular acidification associated with the inhibition of NHE1, and
later effects (after 2–3 h of CoCl2 treatment) on pHi
homeostasis, resulting in cellular alkalosis caused by the
stimulation of NHE1 activity. Additionally, it is likely that the
time-dependent changes in pHi and NHE activity induced by
CoCl2 treatment were responsible for the changes in cell
viability and injury. The findings of the current study emphasize
the relevance of CoCl2 treatment on the functional
regulation of NHE1. These results may be beneficial for the
elucidation of the mechanisms of human stroke injury, the
development of neuroprotective treatment strategies and the
determination of an optimal time lapse for the initiation of
therapy following stroke.
Acknowledgments
This work was supported by grants from the
Foundation from China Scholarship Council (no. 201207045015), the
'5451' project of Henan Health Department (no. 201201065), the
Foundation of Henan Educational Committee (nos. 12A310010 and
16A310003), the Foundation for University Key Teacher of Henan
Educational Committee (no. 2011GGJS-013), the Science and
Technology Planning Project of Henan (nos. 122300410338,
122300410082, 132300410029, 132102310138 and 142102310140), the
Science and Technology Planning Project of Zhengzhou (nos.
10PTGS484-7, 121PPTGG507-22, 2PPTSF302 and N201250105), and the
National Natural Science Foundation of China (no. 81500433). The
authors are grateful for the financial support to be able to
perform this work.
References
|
1
|
Weimar C, Goertler M, Harms L and Diener
HC: Distribution and outcome of symptomatic stenoses and occlusions
in patients with acute cerebral ischemia. Arch Neurol.
63:1287–1291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang Z, Zhao TZ, Zou YJ, Zhang JH and Feng
H: Hypoxia induces autophagic cell death through hypoxia-inducible
factor 1a in microglia. PLoS One. 9:e965092014. View Article : Google Scholar
|
|
3
|
Wang C, Wang Z, Zhang X, Zhang X, Dong L,
Xing Y, Li Y, Liu Z, Chen L, Qiao H, et al: Protection by silibinin
against experimental ischemic stroke: Up-regulated pAkt, pmTOR,
HIF-1α and Bcl-2, down-regulated Bax, NF-ĸB expression. Neurosci
Lett. 529:45–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang E, O'Donnell ME and Barakat AI:
Shear stress and 17beta-estradiol modulate cerebral microvascular
endothelial Na-K-Cl cotransporter and Na/H exchanger protein
levels. Am J Physiol Cell Physiol. 294:C363–C371. 2008. View Article : Google Scholar
|
|
5
|
Uria-Avellanal C and Robertson NJ:
Na+/H+ exchangers and intracellular pH in
perinatal brain injury. Transl Stroke Res. 5:79–98. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simon RP, Niro M and Gwinn R: Brain
acidosis induced by hypercarbic ventilation attenuates focal
ischemic injury. J Pharmacol Exp Ther. 267:1428–1431.
1993.PubMed/NCBI
|
|
7
|
Vannucci RC, Towfighi J, Brucklacher RM
and Vannucci SJ: Effect of extreme hypercapnia on hypoxic-ischemic
brain damage in the immature rat. Pediatr Res. 49:799–803. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kendall GS, Robertson NJ, Iwata O, Peebles
D and Raivich G: N-methyl-isobutyl-amiloride ameliorates brain
injury when commenced before hypoxia ischemia in neonatal mice.
Pediatr Res. 59:227–231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaba NK, Schultz J, Law FY, Lefort CT,
Martel-Gallegos G, Kim M, Waugh RE, Arreola J and Knauf PA:
Inhibition of Na+/H+ exchanger enhances low
pH-induced L-selectin shedding and β-2-integrin surface expression
in human neutrophils. Am J Physiol Cell Physiol. 295:C1454–C1463.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Counillon L and Pouysségur J: The
expanding family of eukaryotic Na(+)/H(+)
exchangers. J Biol Chem. 275:1–4. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Orlowski J and Grinstein S: Diversity of
the mammalian sodium/proton exchanger SLC9 gene family. Pflugers
Arch. 447:549–565. 2004. View Article : Google Scholar
|
|
12
|
Xue J, Zhou D, Yao H, Gavrialov O,
McConnell MJ, Gelb BD and Haddad GG: Novel functional interaction
between Na+/H+ exchanger 1 and tyrosine
phosphatase SHP-2. Am J Physiol Regul Integr Comp Physiol.
292:R2406–R2416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi Y, Chanana V, Watters JJ, Ferrazzano P
and Sun D: Role of sodium/hydrogen exchanger isoform 1 in
microglial activation and proinflamatory responses in ischemic
brains. J Neurochem. 119:124–135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cengiz P, Kleman N, Uluc K, Kendigelen P,
Hagemann T, Akture E, Messing A, Ferrazzano P and Sun D: Inhibition
of Na+/H+ exchanger isoform 1 is
neuroprotective in neonatal hypoxic ischemic brain injury. Antioxid
Redox Signal. 14:1803–1813. 2011. View Article : Google Scholar :
|
|
15
|
Murphy E, Cross H and Steenbergen C:
Sodium regulation during ischemia versus reperfusion and its role
in injury. Circ Res. 84:1469–1470. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pedersen SF, O'Donnell ME, Anderson SE and
Cala PM: Physiology and pathophysiology of
Na+/H+ exchange and
Na+-K+-2Cl-cotransport in the heart, brain,
and blood. Am J Physiol Regul Integr Comp Physiol. 291:R1–R25.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rentsch ML, Ossum CG, Hoffmann EK and
Pedersen SF: Roles of Na+/H+ exchange in
regulation of p38 mitogen-activated protein kinase activity and
cell death after chemical anoxia in NIH3T3 fibroblasts. Pflugers
Arch. 454:649–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gibson JS, McCartney D, Sumpter J, Fairfax
TP, Milner PI, Edwards HL and Wilkins RJ: Rapid effects of hypoxia
on H+ homeostasis in articular chondrocytes. Pflugers
Arch. 458:1085–1092. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Azzopardi D, Wyatt JS, Cady EB, Delpy DT,
Baudin J, Stewart AL, Hope PL, Hamilton PA and Reynolds EO:
Prognosis of newborn infants with hypoxic-ischemic brain injury
assessed by phosphorus magnetic resonance spectroscopy. Pediatr
Res. 25:445–451. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wakabayashi S, Fafournoux P, Sardet C and
Pouysségur J: The Na+/H+ antiporter
cytoplasmic domain mediates growth factor signals and controls
'H(+)-sensing'. Proc Natl Acad Sci USA. 89:2424–2428.
1992. View Article : Google Scholar
|
|
21
|
Luo J, Chen H, Kintner DB, Shull GE and
Sun D: Decreased neuronal death in Na+/H+
exchanger isoform 1-null mice after in vitro and in vivo ischemia.
J Neurosci. 25:11256–11268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park SL, Lee DH, Yoo SE and Jung YS: The
effect of Na(+)/H(+) exchanger-1 inhibition
by sabiporide on blood-brain barrier dysfunction after
ischemia/hypoxia in vivo and in vitro. Brain Res. 1366:189–196.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valle-Casuso JC, González-Sánchez A,
Medina JM and Tabernero A: HIF-1 and c-Src mediate increased
glucose uptake induced by endothelin-1 and connexin43 in
astrocytes. PLoS One. 7:e324482012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang G, Hazra TK, Mitra S, Lee HM and
Englander EW: Mitochondrial DNA damage and a hypoxic response are
induced by CoCl(2) in rat neuronal PC12 cells. Nucleic Acids Res.
28:2135–2140. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karovic O, Tonazzini I, Rebola N, Edström
E, Lövdahl C, Fredholm BB and Daré E: Toxic effects of cobalt in
primary cultures of mouse astrocytes. Similarities with hypoxia and
role of HIF-1alpha. Biochem Pharmacol. 73:694–708. 2007. View Article : Google Scholar
|
|
26
|
Saxena S, Shukla D and Bansal A:
Augmentation of aerobic respiration and mitochondrial biogenesis in
skeletal muscle by hypoxia preconditioning with cobalt chloride.
Toxicol Appl Pharmacol. 264:324–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caltana L, Merelli A, Lazarowski A and
Brusco A: Neuronal and glial alterations due to focal cortical
hypoxia induced by direct cobalt chloride (CoCl2) brain
injection. Neurotox Res. 15:348–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martin LJ, Brambrink AM, Lehmann C,
Portera-Cailliau C, Koehler R, Rothstein J and Traystman RJ:
Hypoxia-ischemia causes abnormalities in glutamate transporters and
death of astroglia and neurons in newborn striatum. Ann Neurol.
42:335–348. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giffard RG and Swanson RA:
Ischemia-induced programmed cell death in astrocytes. Glia.
50:299–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li L, Zhang B, Tao Y, Wang Y, Wei H, Zhao
J, Huang R and Pei Z: DL-3-n-butylphthalide protects endothelial
cells against oxidative/nitrosative stress, mitochondrial damage
and subsequent cell death after oxygen glucose deprivation in
vitro. Brain Res. 1290:91–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takano T, Oberheim N, Cotrina ML and
Nedergaard M: Astrocytes and ischemic injury. Stroke. 40:S8–S12.
2009. View Article : Google Scholar :
|
|
32
|
Badawi Y, Ramamoorthy P and Shi H:
Hypoxia-inducible factor 1 protects hypoxic astrocytes against
glutamate toxicity. ASN Neuro. 4:231–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Efrati S, Fishlev G, Bechor Y, Volkov O,
Bergan J, Kliakhandler K, Kamiager I, Gal N, Friedman M, Ben-Jacob
E and Golan H: Hyperbaric oxygen induces late neuroplasticity in
post stroke patients - randomized, prospective trial. PLoS One.
8:e537162013. View Article : Google Scholar :
|
|
34
|
Björklund O, Shang M, Tonazzini I, Daré E
and Fredholm BB: Adenosine A1 and A3 receptors protect astrocytes
from hypoxic damage. Eur J Pharmacol. 596:6–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McCarthy KD and de Vellis J: Preparation
of separate astroglial and oligodendroglial cell cultures from rat
cerebral tissue. J Cell Biol. 85:890–902. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kato H, Kogure K, Araki T and Itoyama Y:
Astroglial and microglial reactions in the gerbil hippocampus with
induced ischemic tolerance. Brain Res. 664:69–76. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Roos A and Boron WF: Intracellular pH.
Physiol Rev. 61:296–434. 1981.PubMed/NCBI
|
|
38
|
Zhao H, Shiue H, Palkon S, Wang Y,
Cullinan P, Burkhardt JK, Musch MW, Chang EB and Turner JR: Ezrin
regulates NHE3 translocation and activation after Na-glucose
cotransport. Proc Natl Acad Sci USA. 101:9485–90. 2004. View Article : Google Scholar
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
40
|
Wang Y, Luo J, Chen X, Chen H, Cramer SW
and Sun D: Gene inactivation of Na+/H+
exchanger isoform 1 attenuates apoptosis and mitochondrial damage
following transient focal cerebral ischemia. Eur J Neurosci.
28:51–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Harley W, Floyd C, Dunn T, Zhang XD, Chen
TY, Hegde M, Palandoken H, Nantz MH, Leon L, Carraway KL III, et
al: Dual inhibition of sodium-mediated proton and calcium efflux
triggers non-apoptotic cell death in malignant gliomas. Brain Res.
1363:159–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu L, Quinn DA, Garg HG and Hales CA:
Deficiency of the NHE1 gene prevents hypoxia-induced pulmonary
hypertension and vascular remodeling. Am J Respir Crit Care Med.
177:1276–1284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cengiz P, Kleman N, Uluc K, Kendigelen P,
Hagemann T, Akture E, Messing A, Ferrazzano P and Sun D: Inhibition
of Na+/H+ exchanger isoform 1 is
neuroprotective in neonatal hypoxic ischemic brain injury. Antioxid
Redox Signal. 14:1803–1813. 2011. View Article : Google Scholar :
|
|
44
|
Wada M, Miyakawa S, Shimada A, Okada N,
Yamamoto A and Fujita T: Functional linkage of
H+/peptide transporter PEPT2 and
Na+/H+ exchanger in primary cultures of
astrocytes from mouse cerebral cortex. Brain Res. 1044:33–41. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Orlowski J and Grinstein S:
Na+/H+ exchangers of mammalian cells. J Biol
Chem. 272:22373–22376. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Son EJ, Moon IS, Kim SH, Kim SJ and Choi
JY: Interferon-gamma suppresses Na+-H+
exchanger in cultured human endolymphatic sac epithelial cells. J
Cell Biochem. 107:965–972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wilkins RJ and Hall AC: Measurement of
intracellular pH in isolated bovine articular chondrocytes. Exp
Physiol. 77:521–524. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Browning JA and Wilkins RJ: The
characterisation of mechanisms regulating intracellular pH in a
transformed human articular chondrocyte cell line C-20/A4. J
Physiol. 513P:54P1998.
|
|
49
|
Tattersall AL, Browning JA and Wilkins RJ:
Modulation of H+ transport mechanisms by interleukin-1
in isolated bovine articular chondrocytes. Cell Physiol Biochem.
16:43–50. 2005. View Article : Google Scholar
|
|
50
|
Hand SC: Oxygen, pHi and arrest of
biosynthesis in brine shrimp embryos. Acta Physiol Scand.
61:543–551. 1997. View Article : Google Scholar
|