Introduction
Cell adhesion is crucial during the development and
adult life of multicellular organisms. There are principally two
types of adhesion: Cell-cell adhesion; and cell-extracellular
matrix (ECM) adhesion. The canonical receptors for cell-cell
adhesion are cadherins (1).
Cadherins are a multi-member glycoprotein family of transmembrane
Ca2+-dependent adhesion molecules, which maintain tissue
structure in normal and pathological settings (2,3).
They are major contributors to cell-cell adhesion. Numerous
cadherin superfamily members have been previously identified and
these are comprised of four different subfamilies (classical,
desmosomal, atypical and protocadherins) (4). Distinct members of the cadherin
family are important for morphogenesis during development,
formation of junctional complexes, induction of the polarized cell
type, development of cell-cell associations and the invasion of
tumor cells (5).
Liver-intestine cadherin (LI-cadherin), also termed
cadherin-17, is expressed in fetal livers and the gastrointestinal
tract during embryogenesis as one member of the 7D-cadherin
superfamily (6). LI-cadherin is
often upregulated in certain types of cancer, including
hepatocellular carcinoma, gastric, ductal pancreatic and colorectal
cancer, however, it has not been reported to be expressed in brain
tumors or blood malignancies (7).
LI-cadherin has been observed to be expressed in 96% of tumor
samples and is regarded as a useful diagnostic marker for
adenocarcinomas of the digestive system (8). Compared with classical cadherins,
including E-, N- and P-cadherin, LI-cadherin possesses several
unique features (9). For example,
it has seven extracellular cadherin domains, whereas classical
cadherins have five cadherin repeats. Additionally, LI-cadherin has
a short cytoplasmic domain composed of 20 amino acids, which shares
no homology with the corresponding regions of classical cadherins,
such as E-cadherin, which binds catenin proteins through their
cytoplasmic domains to initiate signaling cascades. Although
LI-cadherin is able to mediate Ca2+-dependent cell-cell
adhesion (10), the difference in
structure causes the adhesive function of LI-cadherin to be
independent of any interaction with catenins, the actin
cytoskeleton or other cytoplasmic components. The adhesive
mechanism of LI-cadherin remains unclear.
Previous studies have demonstrated that LI-cadherin
is associated with colorectal carcinoma (11), gastric cancer (12–16),
ductal adenocarcinoma of the pancreas (17) and hepatocellular carcinoma
(18–23). Furthermore, the expression level of
LI-cadherin is associated with lymph node metastasis, advanced pTNM
stage, early tumor recurrence and poor overall survival (15,16,24,25).
Together, these previous studies indicate that LI-cadherin is
associated with the ability of tumor cells to acquire an aggressive
phenotype in several types of cancer. However, the exact mechanisms
of LI-cadherin in the development of cancer remain unclear.
Galectin-3, a member of the β-galactoside-binding
lectin family, is involved in several biological processes,
including tumor cell proliferation, differentiation, angiogenesis,
adhesion, motility, invasion, cancer progression and metastasis
(26). Interaction of galectin-3
with adhesion and signaling receptors has been demonstrated to
promote tumor cell migration. For example, galectin-3 binding to
N-cadherin destabilizes cell-cell junctions by increasing the
turnover of N-cadherin and other glycoconjugates, which may
increase cell migration (27).
Whether LI-cadherin exhibits a similar function via binding
galectin-3 remains unclear. Additionally, a previous study
demonstrated that galectin-3 is cleaved by members of the matrix
metalloproteinase (MMP) family of enzymes. The 72-kDa (gelatinase
A, MMP-2) and 92-kDa (gelatinase B, MMP-9) proteinases cleave
galactin-3 to generate a 22-kDa fragment containing the
carbohydrate recognition domain and a 9-kDa fragment comprising of
the amino terminal of galectin-3. Thus, galectin-3 is considered as
a substrate for human MMP-2 and -9 (28). It is possible that galectin-3,
MMP-2 and MMP-3 are important regulating molecules in the
LI-cadherin signaling pathway.
In the current study, to classify the role of
LI-cadherin in regulating MMP-2, MMP3 and galectin-3 in colorectal
cancer cells, an RNA interference strategy was employed to
specifically knockdown LI-cadherin in LoVo cells. The results of
the present study demonstrate that a reduction in the galectin-3
expression levels is associated with the increased expression of
MMP-2 and MMP-9, which is mediated by LI-cadherin.
Materials and methods
Cell lines and cell culture
Derivation of the pU6-LI-cadherin-short hairpin RNA
(shRNA)-transfected (Shanghai Genechem Co., Ltd., Shanghai, China)
LoVo cell (American Type Culture Collection, Manassas, VA, USA)
clone was performed as previously described (29). The
pU6-LI-cadherin-shRNA-transfected and pU6-control-shRNA-transfected
LoVo cell clones were maintained in Dulbecco's modified Eagle's
medium (GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal calf serum (ExCell, Shanghai, China) and G418 (350
µg/ml) (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), then 1 µg RNA
was reverse transcribed using the RevertAid First Strand cDNA
Synthesis kit (Fermentas; Thermo Fisher Scientific, Inc.,
Pittsburgh, PA, USA) according to the manufacturer's instructions.
RT-PCR was performed using standard methodology and Ex Taq DNA
polymerase and dNTPs were purchased from Takara Biotechnology Co.,
Ltd., (Dalian, China). Primers were designed according to the
sequences of MMP-2 (GenBank accession no. NM_004530), MMP-9
(GenBank accession no. NM_004994) and β-actin (GenBank accession
no. NM_001101). The primers used for MMP-2, MMP-9 and β-actin were
as follows: MMP-2, forward 5′-CCATCACTATGTGGGCTG-3′, reverse
5′-TGCTGGCTGCCTTAGAAC-3′ (168 bp); MMP-9, forward
5′-TTGACAGCGACAAGAAGT-3′, reverse 5′-AGTAGTGGCCGTAGAAGG-3′ (483
bp); and β-actin, forward 5′-AAAGACCTGTACGCCAACA-3′, reverse
5′-GGAGCAATGATCTTGATCTTC-3′ (125 bp). The PCR was conducted on an
S1000™ Thermal Cycler (Bio-Rad, Hercules, California, USA) and the
cycling conditions were as follows: For MMP-2 and β-actin, 94°C for
5 min, 30 cycles of 94°C for 30 sec, 58°C for 30 sec and 72°C for
30 sec, followed by an additional extension step of 10 min at 72°C;
and for MMP-9, 94°C for 5 min, 30 cycles of 94°C for 30 sec, 50°C
for 30 sec, and 72°C for 30 sec, followed by an additional
extension step of 10 min at 72°C. The RT-PCR products were
separated by electrophoresis in a 1.5% agarose gel and visualized
using ethidium bromide (Tiangen, Beijing, China) and a 2UV
Transilluminator (LM-26E; UVP Inc., Upland, CA, USA). The
expression ratio (MMP-2/β-actin and MMP-9/β-actin) measured by
densitometry (Gel-Pro Analyzer 4.0, Media Cybernetics, Inc.,
Rockville, MD, USA) was used to evaluate the mRNA levels of the
genes. The mRNA level of each sample was detected following at
least two independent experiments.
Western blot analysis
LoVo cells were lysed in radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology, Beijing, China)
containing 50 mM Tris-HCl, 150 mM NaCl, 1 mM
ethylenediaminetetraacetic acid, 1 mM sodium orthovanadate, 1 mM
NaF, 1% NP40, and 0.25% sodium deoxycholate supplemented with 1 mM
phenylmethylsulfonyl fluoride and quantified using the DC Protein
Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal
amounts of proteins from each sample were applied to a 10% sodium
dodecyl sulfate (SDS) polyacrylamide gel (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) and transferred to a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). The membranes were blocked in 5% non-fat milk and incubated
at 4°C overnight with diluted rabbit polyclonal anti-galectin-3
(1:1,000; cat. no. sc-20157; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) or mouse monoclonal anti-β-actin (1:3,000; cat.
no. sc-47778; Santa Cruz Biotechnology, Inc.) primary antibodies,
followed by incubation with horseradish peroxidase (HRP)-conjugated
goat anti-rabbit IgG (cat. no. ZDR-5403; ZSGB-Bio, Beijing, China).
Immunoreactive bands were visualized using Immobilon Western
Chemiluminescent HRP Substrate (EMD Millipore) and signals were
developed on X-ray film (Kodak, Rochester, NY, USA). The expression
ratio (galectin-3/β-actin) as measured by densitometry (Gel-Pro
Analyzer 4.0, Media Cybernetics, Inc.) was used to evaluate protein
levels.
Gelatin zymography
The culture supernatant was collected and the total
protein concentration of the supernatants of each sample was
determined using a Bradford Protein Assay kit (Beyotime Institute
of Biotechnology). Culture supernatants containing equal amounts of
total protein were mixed with SDS loading buffer (Beyotime
Insititute of Biotechnology) and electrophoresed on 10% denaturing
SDS polyacrylamide gels containing 1 mg/ml gelatin. Following
electrophoresis, the gels were soaked in 2.5% Triton X-100 on a
shaker for 1 h, the solution was changed after 30 min to eliminate
SDS. Following incubation in zymogen activation buffer (50 mM
Tris-HCl pH 7.5, 0.1% Triton X-100, 0.02% NaN3, 5 mM
CaCl2 and 1 µM ZnCl2) at 37°C for 12
h, the gels were rinsed in distilled water and stained for 5 h with
Coomassie blue R250 (Beyotime Institute of Biotechnology).
Gelatinolytic bands were observed as clear zones against the blue
background and the intensity of the bands was estimated using
ImageJ software, version 1.45 (imagej.nih.gov/ij/). The gelatinase expression level
of each sample was determined following a minimum of three
independent experiments.
Statistical analysis
All analyses were performed using SPSS software,
version 11.5 (SPSS, Inc., Chicago, IL, USA). The mRNA and protein
levels were analyzed by one-way analysis of variance followed by a
post-hoc least-significant difference test. P<0.05 was
considered to indicate a statistically significant difference.
Results
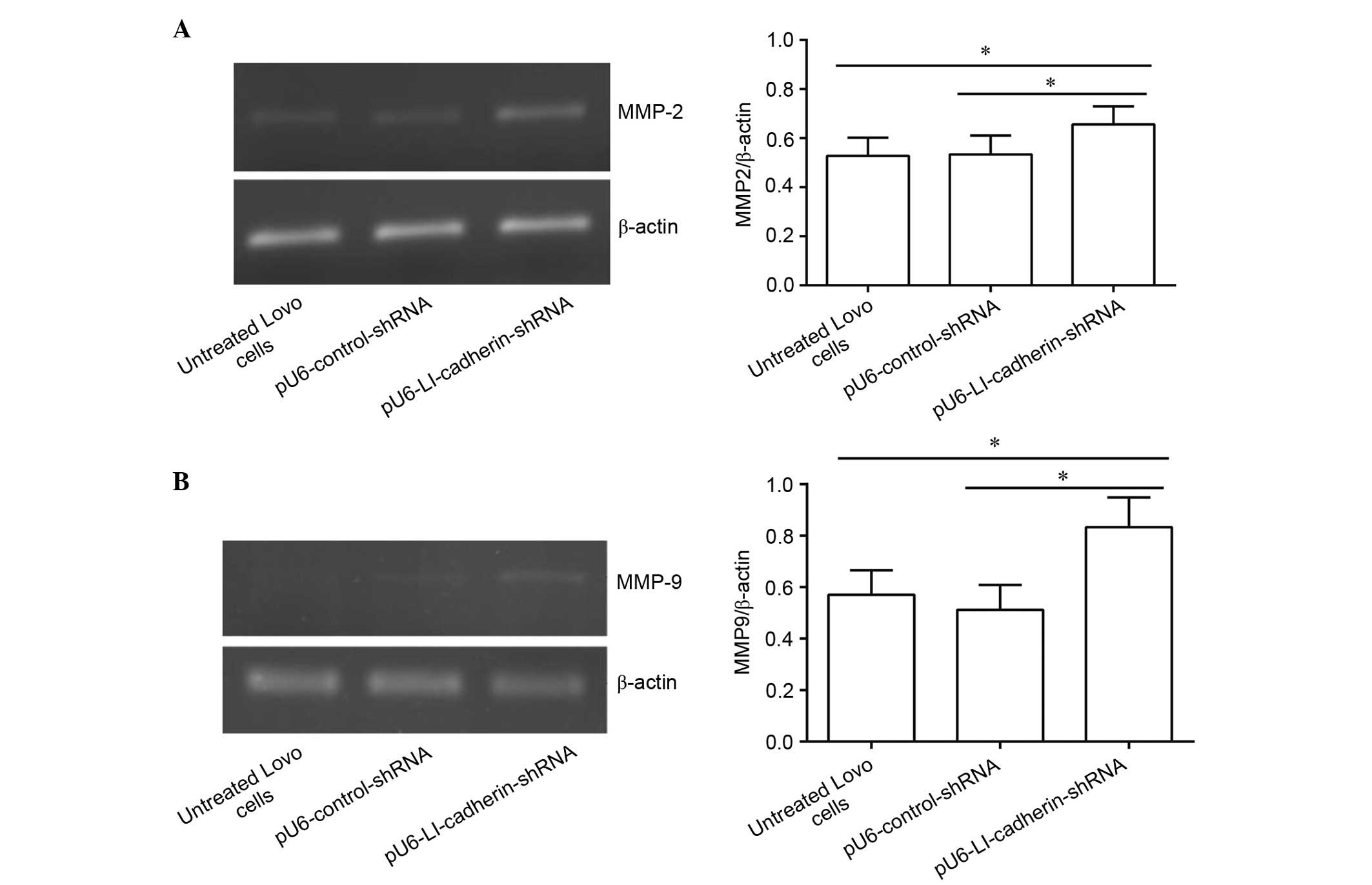
Silencing of LI-cadherin increases the
mRNA level of MMP-2 and MMP-9 in LoVo cells
To investigate the effect of silencing LI-cadherin
on the mRNA levels of MMP-2 and MMP-9 in LoVo cells, RT-PCR
analysis was performed. The results demonstrated that there was no
significant difference between the mRNA levels of MMP-2 in
untreated LoVo cells and in LoVo cells stably expressing
pU6-control-shRNA (P>0.05). By contrast, the mRNA level of MMP-2
in LoVo cells expressing pU6-LI-cadherin-shRNA was significantly
increased compared with untreated (P<0.05) and
pU6-control-shRNA-expressing LoVo cells (P<0.05; Fig. 1A). Similarly, the mRNA level of
MMP-9 did not differ between untreated and LoVo cells stably
expressing pU6-control-shRNA (P>0.05). However, the mRNA level
of MMP-9 was significantly increased in LoVo cells following
silencing LI-cadherin compared with untreated and control
shRNA-transfected cells (P<0.05; Fig. 1B).
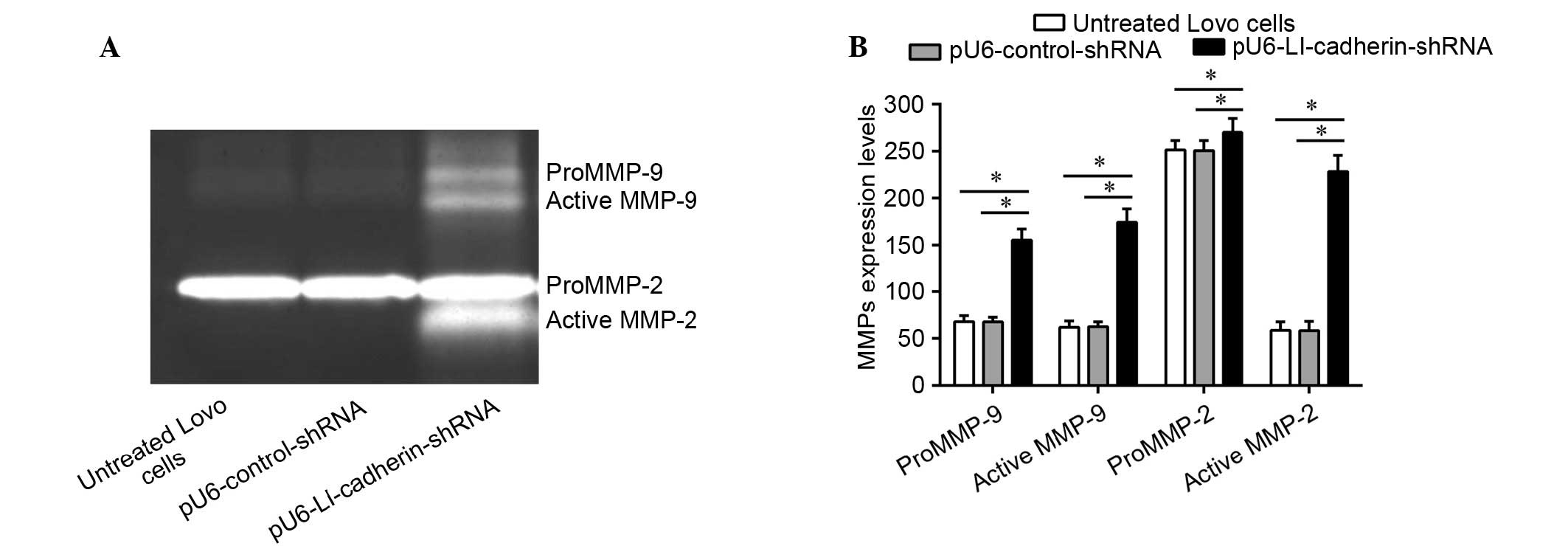
Silencing of LI-cadherin increases the
protein levels and activity of MMP-2 and MMP-9 in LoVo cells
To explore whether the protein expression levels and
activity of MMP-2 and MMP-9 were altered following knockdown of
LI-cadherin in LoVo cells, a gelatin zymography experiment was
performed (Fig. 2). The results of
the current study demonstrated that the protein levels of proMMP-2
and -9, and their active forms were significantly increased in LoVo
cells expressing pU6-LI-cadherin-shRNA compared with untreated and
control shRNA-transfected LoVo cells (P<0.05).
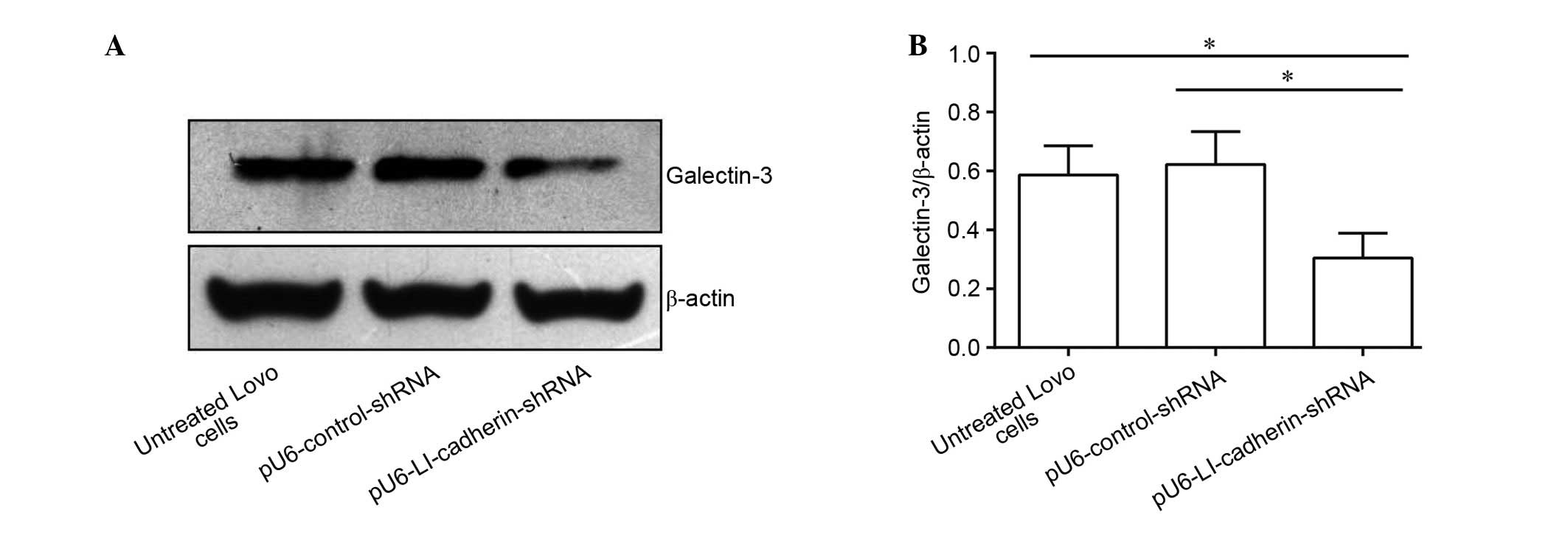
Silencing LI-cadherin reduces the protein
expression level of galectin-3
Western blot analysis was performed to detect the
effect of of LI silencing via transfection with
pU6-LI-cadherin-shRNA on the protein levels of galectin-3 in LoVo
cells. The protein level of galectin-3 exhibited no significant
difference between the untreated cells and cells stably expressing
pU6-control-shRNA. By contrast, following knockdown of LI-cadherin,
LoVo cells exhibited significantly reduced protein levels of
galectin-3 compared with untreated and control shRNA-transfected
cells (P<0.05; Fig. 3).
Discussion
A previous study demonstrated that knockdown of
LI-cadherin promotes cell migration, invasion and adhesion
(29). However, the mechanisms
that mediate these changes remain unclear. The present study
demonstrated that silencing of LI-cadherin increases the expression
levels (mRNA and protein) and activation of MMP-2 and -9, and
downregulates the protein level of galectin-3, which is a substrate
for human MMP-2 and MMP-9. Based on the present data, it is
proposed that knockdown of LI-cadherin expression facilitates the
invasion of cancer cells by degrading ECM components via enhanced
expression and activation of MMP-2 and -9, and increases cancer
cell adhesion and migration via altered expression of
galectin-3.
The MMPs are a tightly regulated family of enzymes
that degrade ECM and basement membrane components, thus allowing
cancer cells access to subepithelial structures (30). MMP-2 and -9, in particular, are
important for cleaving major components of the basement membrane,
such as type IV collagen. They interact with αvβ3 integrin, which
contributes to collagen degradation, cell migration and cell
invasion (31,32). A previous study demonstrated that
silencing LI-cadherin promotes cell invasion (29). The present study demonstrated that
knockdown of LI-cadherin increased the expression levels and
activation of MMP-2 and -9. Thus, it is concluded that
LI-cadherin-associated invasion may contribute to
LI-cadherin-induced alteration and activation of MMP-2 and -9
A previous study demonstrated that galectin-3 (31
kDa) is cleaved by MMP-2 and -9 to generate 22-kDa and 9-kDa
fragments (28,33). In the current study, silencing
LI-cadherin in LoVo cells significantly increased the mRNA levels
of MMP-2 and -9, whereas the protein level of galectin-3 was
downregulated under the same conditions. Together these studies
suggest that the reduction in the protein levels of galectin-3 (31
kDa) is induced via enhanced cleavage resulting from increased
expression levels of MMP-2 and -9 meditated by LI-cadherin.
In the present study, silencing LI-cadherin reduced
the protein levels of galectin-3, a protein that is closely
involved in tumor cell transformation, migration, invasion and
metastasis. A previous study indicates that
Ca2+-dependent cell-cell adhesion mediated by
LI-cadherin is independent of interaction with cytoplasmic
components (10). Thus, it is
possible that LI-cadherin inhibits LoVo cell migration and adhesion
via galectin-3 in an indirect manner. However, galectin-3
predominantly promotes tumor development in cancer, however, it
acts as a tumor-suppressor in certain types of cancer (34). Additionally, Bartolomé et al
(7) reported a different mechanism
when they investigated the association between LI-cadherin and cell
proliferation and adhesion. They demonstrated that cell adhesion,
proliferation and liver metastasis were reduced following knockdown
of LI-cadherin in KM12 metastatic colorectal cancer cells, and the
effects are regulated by interaction between LI-cadherin and α2β1
integrin. The previous study also observed a significant
correlation between LI-cadherin overexpression and poor survival in
colorectal cancer, whereas, other studies had previously
demonstrated that reduced expression of LI-cadherin is associated
with lymph node metastasis (11),
or tumor dedifferentiation and poor overall survival (35) based on immunohistochemical
analysis. The conflicting results are possibly caused by
differences in samples number, the ratio of cancer stage and cell
types. The association of LI-cadherin expression with cell adhesion
requires further elucidation.
In summary, previous studies have demonstrated that
LI-cadherin has an important function in migration, invasion and
adhesion (13,19,29,36).
LI-cadherin acts via various molecular mechanisms depending on the
cancer cell type. In the current study, it was identified that in
colorectal cancer cells, there was an association between
LI-cadherin, gelatinases and galectin-3, providing insight into the
functional regulation of LI-cadherin and a better understanding of
the molecular mechanisms of the LI-cadherin. Further study of
LI-cadherin may be important to advance the understanding of human
cancer progression and developing novel diagnostic markers.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81460462
and 31460696), the Technology Pedestal and Society Development
Project of Jiangxi Province (grant no. 20141BBG70040) and the
Science Foundation of Educational Department of Jiangxi Province
(grant no. 86283702).
References
|
1
|
Bulgakova NA, Klapholz B and Brown NH:
Cell adhesion in Drosophila: Versatility of cadherin and integrin
complexes during development. Curr Opin Cell Biol. 24:702–712.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leckband D and Sivasankar S: Cadherin
recognition and adhesion. Curr Opin Cell Biol. 24:620–627. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan X, Yan L, Liu S, Shan Z, Tian Y and
Jin Z: N-cadherin, a novel prognostic biomarker, drives malignant
progression of colorectal cancer. Mol Med Rep. 12:2999–3006.
2015.PubMed/NCBI
|
|
4
|
Shapiro L and Weis WI: Structure and
biochemistry of cadherins and catenins. Cold Spring Harb Perspect
Biol. 1:a0030532009. View Article : Google Scholar :
|
|
5
|
van Roy F: Beyond E-cadherin: Roles of
other cadherin superfamily members in cancer. Nat Rev Cancer.
14:121–134. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takamura M, Yamagiwa S, Matsuda Y, Ichida
T and Aoyagi Y: Involvement of liver-intestine cadherin in cancer
progression. Med Mol Morphol. 46:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartolomé RA, Barderas R, Torres S,
Fernandez-Aceñero MJ, Mendes M, García-Foncillas J, Lopez-Lucendo M
and Casal JI: Cadherin-17 interacts with α2β1 integrin to regulate
cell proliferation and adhesion in colorectal cancer cells causing
liver metastasis. Oncogene. 33:1658–1669. 2014. View Article : Google Scholar
|
|
8
|
Su MC, Yuan RH, Lin CY and Jeng YM:
Cadherin-17 is a useful diagnostic marker for adenocarcinomas of
the digestive system. Mod Pathol. 21:1379–1386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berndorff D, Gessner R, Kreft B, Schnoy N,
Lajous-Petter AM, Loch N, Reutter W, Hortsch M and Tauber R:
Liver-intestine cadherin: Molecular cloning and characterization of
a novel Ca(2+)-dependent cell adhesion molecule expressed in liver
and intestine. J Cell Biol. 125:1353–1369. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kreft B, Berndorff D, Böttinger A,
Finnemann S, Wedlich D, Hortsch M, Tauber R and Gessner R:
LI-cadherin-mediated cell-cell adhesion does not require
cytoplasmic interactions. J Cell Biol. 136:1109–1121. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takamura M, Ichida T, Matsuda Y, Kobayashi
M, Yamagiwa S, Genda T, Shioji K, Hashimoto S, Nomoto M, Hatakeyama
K, et al: Reduced expression of liver-intestine cadherin is
associated with progression and lymph node metastasis of human
colorectal carcinoma. Cancer Lett. 212:253–259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakamoto N, Oue N, Sentani K, Anami K,
Uraoka N, Naito Y, Oo HZ, Hinoi T, Ohdan H, Yanagihara K, et al:
Liver-intestine cadherin induction by epidermal growth factor
receptor is associated with intestinal differentiation of gastric
cancer. Cancer Sci. 103:1744–1750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu QS, Zhang J, Liu M and Dong WG:
Lentiviral-mediated miRNA against liver-intestine cadherin
suppresses tumor growth and invasiveness of human gastric cancer.
Cancer Sci. 101:1807–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong WG, Yu QF, Xu Y and Fan LF:
Li-cadherin is inversely correlated with galectin-3 expression in
gastric cancer. Dig Dis Sci. 53:1811–1817. 2008. View Article : Google Scholar
|
|
15
|
Park SS, Kang SH, Park JM, Kim JH, Oh SC,
Lee JH, Chae YS, Kim SJ, Kim CS and Mok YJ: Expression of
liver-intestine cadherin and its correlation with lymph node
metastasis in gastric cancer: Can it predict N stage
preoperatively? Ann Surg Oncol. 14:94–99. 2007. View Article : Google Scholar
|
|
16
|
Dong W, Yu Q and Xu Y: Altered expression
of a Li-cadherin in gastric cancer and intestinal metaplasia. Dig
Dis Sci. 52:536–542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takamura M, Sakamoto M, Ino Y, Shimamura
T, Ichida T, Asakura H and Hirohashi S: Expression of
liver-intestine cadherin and its possible interaction with
galectin-3 in ductal adenocarcinoma of the pancreas. Cancer Sci.
94:425–430. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan ZJ, Fang XJ, Wang JX, Xue JF and Zhang
Y: Expression of liver-intestine cadherin and its significance in
hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi. 91:2546–2548.
2011.In Chinese.
|
|
19
|
Ding ZB, Shi YH, Zhou J, Shi GM, Ke AW,
Qiu SJ, Wang XY, Dai Z, Xu Y and Fan J: Liver-intestine cadherin
predicts microvascular invasion and poor prognosis of hepatitis B
virus-positive hepatocellular carcinoma. Cancer. 115:4753–4765.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen XT, Du HY, Yuan SF, Wang SM and Li M:
Effect of monoclonal antibodies against LI-cadherin on the
proliferation of human hepatocellular carcinoma cells. Nan Fang Yi
Ke Da Xue Xue Bao. 29:880–883. 2009.In Chinese. PubMed/NCBI
|
|
21
|
Wang XQ, Luk JM, Garcia-Barcelo M, Miao X,
Leung PP, Ho DW, Cheung ST, Lam BY, Cheung CK, Wong AS, et al:
Liver intestine-cadherin (CDH17) haplotype is associated with
increased risk of hepatocellular carcinoma. Clin Cancer Res.
12:5248–5252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang XQ, Luk JM, Leung PP, Wong BW,
Stanbridge EJ and Fan ST: Alternative mRNA splicing of liver
intestine-cadherin in hepatocellular carcinoma. Clin Cancer Res.
11:483–489. 2005.PubMed/NCBI
|
|
23
|
Wong BW, Luk JM, Ng IO, Hu MY, Liu KD and
Fan ST: Identification of liver-intestine cadherin in
hepatocellular carcinoma - a potential disease marker. Biochem
Biophys Res Commun. 311:618–624. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ryu KH, Shim KN, Jung SA, Yoo K, Joo YH
and Lee JH: Significance of preoperative tissue levels of
vascular-endothelial cadherin, liver-intestine cadherin and
vascular endothelial growth factor in gastric cancer. Korean J
Gastroenterol. 60:229–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ko S, Chu KM, Luk JM, Wong BW, Yuen ST,
Leung SY and Wong J: Overexpression of LI-cadherin in gastric
cancer is associated with lymph node metastasis. Biochem Biophys
Res Commun. 319:562–568. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fortuna-Costa A, Gomes AM, Kozlowski EO,
Stelling MP and Pavão MS: Extracellular galectin-3 in tumor
progression and metastasis. Front Oncol. 4:1382014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boscher C, Zheng YZ, Lakshminarayan R,
Johannes L, Dennis JW, Foster LJ and Nabi IR: Galectin-3 protein
regulates mobility of N-cadherin and GM1 ganglioside at cell-cell
junctions of mammary carcinoma cells. J Biol Chem. 287:32940–32952.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ochieng J, Fridman R, Nangia-Makker P,
Kleiner DE, Liotta LA, Stetler-Stevenson WG and Raz A: Galectin-3
is a novel substrate for human matrix metalloproteinases-2 and -9.
Biochemistry. 33:14109–14114. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu QF, Dong WG and Ren JL: Knockdown of
Li-cadherin increases metastatic behaviors of LoVo cells. J Cancer
Res Clin Oncol. 136:1641–1649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hadler-Olsen E, Winberg JO and
Uhlin-Hansen L: Matrix metalloproteinases in cancer: Their value as
diagnostic and prognostic markers and therapeutic targets. Tumour
Biol. 34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brooks PC, Strömblad S, Sanders LC, von
Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP and
Cheresh DA: Localization of matrix metalloproteinase MMP-2 to the
surface of invasive cells by interaction with integrin alpha v beta
3. Cell. 85:683–693. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rolli M, Fransvea E, Pilch J, Saven A and
Felding-Habermann B: Activated integrin alphavbeta3 cooperates with
metalloproteinase MMP-9 in regulating migration of metastatic
breast cancer cells. Proc Natl Acad Sci USA. 100:9482–9487. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ochieng J, Green B, Evans S, James O and
Warfield P: Modulation of the biological functions of galectin-3 by
matrix metalloproteinases. Biochim Biophys Acta. 1379:97–106. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song L, Tang JW, Owusu L, Sun M-Z, Wu J
and Zhang J: Galectin-3 in cancer. Clin Chim Acta. 431:185–191.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kwak JM, Min BW, Lee JH, Choi JS, Lee SI,
Park SS, Kim J, Um JW, Kim SH and Moon HY: The prognostic
significance of E-cadherin and liver intestine-cadherin expression
in colorectal cancer. Dis Colon Rectum. 50:1873–1880. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baumgartner W: Possible roles of
LI-Cadherin in the formation and maintenance of the intestinal
epithelial barrier. Tissue Barriers. 1:e238152013. View Article : Google Scholar : PubMed/NCBI
|