Introduction
MicroRNAs (miRNAs; miRs) are short, non-coding RNAs,
which regulate target mRNA by binding predominantly to the
3′-untranslated region (3′-UTR), inducing either translational
repression or the degradation of the mRNA target (1-3).
Previously, the involvement of miRNAs in the phenotypic modulation
of human glioma has been reported. For example, miRNA-21 knockdown
disrupts glioma growth in vivo and exhibits synergistic
cytotoxicity with neural precursor cell-delivered secretable tumor
necrosis factor-related apoptosis-inducing ligand in human glioma
(4); miRNA-34a acts as a tumor
suppressor in brain tumors and glioma stem cells (5); and miRNA-181a sensitizes human U87MG
malignant glioma cells to radiation by targeting B cell lymphoma 2
(6). Although miR-197 is
downregulated in glioblastoma, its roles in malignant tumor
progression remain to be elucidated (7).
Grb2-associated binding protein (GAB)2 belongs to a
family of evolutionarily conserved proteins consisting of three
mammalian paralogues: GAB1, GAB2 and GAB3; Drosophila
melanogaster homolog daughter of sevenless; and
Caenorhabditis elegans homolog suppressor of clear. Family
members exhibit 40–50% sequence homology, however they are
associated with unique cellular functions (8). GAB1 and GAB2 are expressed
ubiquitously, but are expressed at the highest levels in the brain,
kidney, lung, heart, testis and ovary (9). GAB3 also has a widespread expression
pattern, although its expression is highest in lymphoid tissues
(9). DNA amplification is a common
mechanism underlying oncogenic activation in human cancer. GAB2 is
located on chromosomal band 11q14.1, and amplification of
11q13-14.1 is frequently observed in human malignancies (10). The identification of GAB2 as a
potential oncogene has been reported in studies investigating
breast (11,12) and ovarian cancer (13), leukemia (14), melanoma (15) and gastric cancer (16). Previously, GAB2 was found to be
expressed at high levels in glioma and a subset of cancer cell
lines. Statistical analysis suggested that the upregulation of GAB2
was correlated with the World Health Organization (WHO) grade of
glioma, and that patients with high expression levels of GAB2
exhibited reduced survival rates (17). In a previous animal experiment,
knockdown of GAB2 inhibited the invasive ability of glioma cells in
the brains of mice with severe combined immunodeficiency (17). As demonstrated by cellular
investigations, the downregulation of GAB2 inhibits the migration
and invasion of glioma cells (17). Elucidating why the GAB2 gene is
overexpressed and determining how it may be downregulated will
assist in further understand the pathogenesis and progression of
glioma, and offer novel therapeutic targets.
Elucidation of the overexpression of GAB2 and
methods to downregulate the expression levels may aid in
understanding the pathogenesis and progression of the disease and
aid in development of novel targets for therapeutic strategies.
Materials and methods
Glioma tissue samples, cells and
GAB2-expressing plasmids
Human glioma tissue samples were obtained from the
Department of Neurosurgery, Shandong Provincial Hospital Affiliated
to Shandong University (Jinan, China). The patients included 38
World Health Organization (WHO) grade I; 39 WHO grade II; 44 WHO
grade III and 40 WHO grade IV cases) (18). The patients comprised 60 women and
111 men, with a mean age of 58 years (range, 31–78 years). The
human tissue samples were used according to internationally
recognized guidelines, as well as local and national regulations.
Investigations involving humans followed international and national
regulations. The medical ethics committee of Shandong Provincial
Hospital Affiliated to Shandong University (Shandong, China)
approved the experiments of the present study. Informed consent was
obtained from each individual. Human A172 glioblastoma cell lines
were donated by Dr Rosalind Segal (University of Heidelberg,
Heidelberg, Germany). The A172 cells were maintained at 37°C in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA). GAB2-expressing plasmids were donated by Dr Xiao Yao
(University of Heidelberg).
miRNA precursors
The miR-197 miRNA precursor (pre-miR-197) and a
control precursor (control miR) were purchased from Ambion (Thermo
Fisher Scientific, Inc.).
Transfection
For the transfection experiments, 5×104
A172 cells were cultured in serum-free medium without antibiotics
to 60% confluence for 24 h at 37°C, prior to being transfected with
Invitrogen Lipofectamine® 2000 transfection reagent
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Following incubation at 37°C for 6 h, the medium was
removed and replaced with normal culture medium for 48 h at 37°C,
unless otherwise specified.
Detection of miRNAs by in situ
hybridization in formalin-fixed paraffin-embedded (FFPE)
sections
Paraffin-embedded specimens of gliomas and adjacent
normal tissue were obtained from the Pathology Department of the
Shandong Provincial Hospital affiliated to Shandong University.
Sections (5 µm) were deparaffinized in 70% xylene (Tiangen
Biotech Co., Ltd., Beijing, China) and then rehydrated through a
series of graded ethanol dilutions (100–25%). The sections on
slides and submerged in diethyl pyrocarbonate-treated water
(Tiangen Biotech Co., Ltd.) and subjected to proteinase K (Tiangen
Biotech Co., Ltd.) (10 µg/ml) digestion and 0.2% glycine
treatment, refixed in 4% paraformaldehyde (Tiangen Biotech Co.,
Ltd.), and treated with acetylation solution (Tiangen Biotech Co.,
Ltd.) containing 66 mmol/l HCl, 0.66% (v/v) acetic anhydride and
1.5% (v/v) triethanolamine. The slides were rinsed three times with
1X phosphate-buffered saline (PBS) between treatments. The slides
were then prehybridized in hybridization solution (Tiangen Biotech
Co., Ltd.), containing 50% formamide, 5X saline sodium citrate
(SSC), 500 µg/ml yeast tRNA and 1X Denhardt's solution, at
50°C for 30 min. Subsequently, 5-10 pmol fluorescein
isothiocyanate-labeled, LNA-modified DNA probe (Exiqon A/S,
Vedbeak, Denmark) complementary to miR-197 was added to the 180
µl hybridization solution and hybridized for 2 h at a
temperature 20–25°C below the calculated melting temperature of the
LNA probe (37°C). Following washes in SSC at increasing stringency
(2-0.2X) at the same temperature as hybridization, a tyramide
signal amplification reaction was performed using a GenPoint
Fluorescein kit (Dako, Glostrup, Denmark), according to the
manufacturer's protocol. Finally, the slides were mounted with
Prolong Gold solution (Invitrogen; Thermo Fisher Scientific, Inc.).
The signals were visually quantified by Dr Li-Qiang Tian and Dr
En-Qin Liu using a quick score system (scores 0-5), which combines
the intensity of the signal and the percentage of positive cells
(signal: 0, no signal; 1, weak signal; 2, intermediate signal; 3,
strong signal; percentage: 0, 0%; 1, <30%; 2, >30%). The
tissue sections were then examined by Jian Li and Guang-Ming Xu in
a blinded-manner, who reported results in agreement with the
initial quantifications.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) of miRNA
Total RNA from the cultured A172 cells, with
efficient recovery of small RNAs, was isolated using an mirVana
miRNA Isolation kit (Ambion; Thermo Fisher Scientific, Inc.). In
addition, detection of the mature form of miRNAs was performed
using an mirVana qRT-PCR miRNA Detection kit, according to the
manufacturer's protocol. Reverse Transcription system (Promega
Corporation, Madison, WI, USA) was used with the miR-197 primer as
follows: 5′-TTC ACC ACC TTC TCC ACC CAGC-3′. The U6 small nuclear
RNA was used as an internal control. The RT-qPCR and quantification
were conducted as described previously (19).
Cell counting assay
At 48 h post-transfection, A172 cells were seeded in
96-well plates in triplicate at a density of 5×103
cells/well in 100 µl RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS and 1% antibiotics (Hyclone;
GE Healthcare Life Sciences). Cell proliferation was evaluated
using a Cell Counting kit-8 (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), according to the manufacturer's protocol, and the
absorbance value for each well was measured at 450 nm using a
microplate reader (Spectra Max 180; Molecular Devices, LLC,
Sunnyvale, CA, USA).
Colony formation assay
For the colony formation assay, 5×104
A172 cells were transfected with pre-miR-197 or control miR for 24
h, prior to being seeded into a 6-well plate. A total of 0.2 ml FBS
was added per well on day 5. Following 9–10 days incubation at
37°C, the plates were washed with PBS and stained with 0.1% crystal
violet (Tiangen Biotech Co., Ltd.). Colonies with >50 cells were
manually counted (IX-70 inverted microscope; Olympus Corporation,
Tokyo, Japan). Plating efficiency was calculated by dividing the
number of colonies formed in the treated group by that in the
control.
Cell cycle analysis
The A172 cells (8.0×105 cells) were
seeded into a 100 mm culture plate and allowed to attach overnight.
The cells were transfected, as previously described, for 24 h at
37°C, washed twice with NaCl/Pi (Tiangen Biotech Co.,
Ltd.), and then centrifuged at 200 × g at room temperature for 10
min. The pellet was resuspended in 1 ml cold NaCl/Pi and
fixed in 70% ethanol for ≥12 h at 4°C. The fixed cells were
incubated with 100 µl DNase-free RNaseA (200 µg/ml;
Tiangen Biotech Co., Ltd.) for 30 min at 37°C, prior to the
addition of 1 mg/ml propidium iodide (Tiangen Biotech Co., Ltd.).
The stained cells were analyzed using a fluorescence-activated cell
sorter (BD Accuri C6; BD Biosciences, San Jose, CA, USA). The
percentages of cells in the G1, S and G2/M
phases of the cell cycle were determined using Cell Quest Pro
software (FlowJo; Tree Star, Inc., Ashland, OR, USA).
BrdU proliferation analysis
Cell proliferation was assessed using a colorimetric
BrdU Proliferation kit, according to the manufacturer's protocol
(Roche Diagnostics, Indianapolis, IN, USA). Briefly, the
transfected A172 cells were labeled with BrdU for 4 h at 37°C. The
genomic DNA was fixed and denatured by BrdU, and then incubated
with a rat peroxidase-conjugated anti-BrdU antibody (Sigma-Aldrich,
St. Louis, MO, USA; cat. no. 11202693001) for 90 min. A substrate
for the conjugated peroxidase, a component included in the kit, was
then added, and the reaction product was quantified by measuring
the absorbance at a wavelength of 270 nm using a SpectraMax M5
(Molecular Devices, LLC). The results were then normalized to the
number of total viable cells.
Western blot analysis
Confluent A172 cells were washed twice with cold PBS
and denatured in a radioimmunoprecipi-tation assay lysis buffer
(containing 20 mM Tris-HCl, 200 mM NaCl, 0.2% Nonidet P-40, 0.5%
Triton X-100 and protease inhibitors; Beijing Solarbio Science and
Technology Co., Ltd., Beijing, China) and boiled at 100°C for 10
min. Protein was quantified by BCA Protein assay kit (Beyotime
Institute of Biotechnology, Haimen, China) and western blot
analysis was performed, as described previously (20). Equal quantities of protein (5
µg) were separated by 10% sodium dodecyl sulfate
(SDS)-polyacrylamide gel electrophoresis (Beijing Solarbio Science
and Technology Co., Ltd.) and then transferred onto polyvinylidene
difluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Following blocking in Tris-buffered saline (TBS; Beijing
Solarbio Science and Technology Co., Ltd.) with 0.1% Triton X-100
and 5% milk, the membranes were incubated with the following
primary antibodies: Rabbit polyclonal anti-proliferating cell
nuclear antigen (PCNA; 1:500; Abcam, Cambridge, MA, USA; cat. no.
ab18197), rabbit polyclonal anti-retinoblastoma (Rb; 1:500; Abcam;
ab1765), rabbit polyclonal anti-Ki67 (1:500; Abcam; ab15580),
rabbit monoclonal anti-cyclin-dependent kinase (CDK)2 (1:500;
Abcam; ab32147), rabbit monoclonal anti-CDK4 (1:500; Abcam;
ab108357), rabbit monoclonal anti-E2F transcription factor 1 (E2F1;
1:500; Abcam; ab179445), rabbit monoclonal anti-GAB2 (1:500; Abcam;
ab32365) and mouse monoclonal anti-β-actin (1:500; Abcam; ab129348)
overnight at 4°C. Following washing with TBS three times, the
membranes were incubated with donkey anti-mouse Alexa Fluor 488
secondary antibodies (1:5,000; Abcam; cat. no. ab150105) and goat
anti-rabbit immunoglobulin G biotin-conjugated secondary antibodies
(1:5,000; Abcam; cat. no. ab6720) for 30 min at room temperature.
Signal detection was conducted with an enhanced chemiluminescence
system (GE Healthcare Life Sciences, Chalfont, UK). The specific
proteins were visualized and analyzed using an Odyssey™ Infrared
Imaging system (Gene Company, Ltd., Hong Kong, China).
Bioinformatics
Analysis of the potential miRNA target sites was
performed using the TargetScan algorithm (http://www.targetscan.org).
RT-qPCR for GAB2
Total RNA was isolated from A172 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). First-strand cDNA was synthesized from the total RNA using
Moloney Murine Leukemia Virus Reverse Transcriptase (Promega
Corporation), 2.5 µl 5X buffer and 2 µl random
hexamer primers (Sangon Biotech, Co., Ltd., Shanghai, China). The
concentration of the cDNA was quantified using a NanoDrop 1000
spectrophotometer (Thermo Fisher Scientific, Inc.). The reaction
was performed with the following thermo-cycling conditions:
Denaturation for 30 sec at 95°C, annealing for 45 sec at 52–58°C,
depending on the primers used, and extension for 45 sec at 72°C.
The PCR products were visual-ized on 2% agarose gels (Tiangen
Biotech Co., Ltd.) stained with 20% ethidium bromide (Tiangen
Biotech Co., Ltd.) under ultra violet transillumination. RT-qPCR
was performed using Power SYBR® Green PCR Master mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The primer sequences for GAB2 were
forward, 5′-CTG AGA CTG ATA ACG AGG AT-3′ and reverse, 5′-GAG GTG
TTT CTG CTT GAC-3′.
Immunofluorescence analysis
For immunofluorescence analysis, 5×104
A172 cells were plated on glass coverslips in six-well plates and
transfected with 50 nM pre-miR-197 or control miR. At 36 h
post-transfection, the coverslips were stained with the previously
mentioned anti-GAB2 antibodies. Alexa Fluor 488 goat anti-rabbit
IgG antibody was used as a secondary antibody (Invitrogen; Thermo
Fisher Scientific, Inc.). The coverslips were counterstained with
DAPI (Invitrogen; Thermo Fisher Scientific, Inc.) for visualization
of the nuclei. Microscopic analysis was performed using a confocal
laser-scanning microscope (FV300; Olympus Corporation).
Fluorescence intensity was measured in a small number of viewing
areas with 200–300 cells per coverslip, and analyzed using ImageJ
1.37 software (National Institutes of Health, Bethesda, MD,
USA).
Luciferase reporter assay
Luciferase reporter plasmids were purchased from
Tianjin Biotech Co., Ltd. (Tianjin, China). Briefly, the 3′-UTR of
human GAB2 mRNA was cloned into pRL-TK (Promega Corporation) using
three PCR-generated fragments, including positions of 3885–3891,
1151–1158, 2044–2050 in the 3′UTR of GAB2. Site-directed
mutagenesis of the miR-197 target sites in the GAB2-3′-UTR was
performed using a Quik Change Mutagenesis kit (Agilent Technologies
GmbH, Waldbronn, Germany), with GAB2-WT-luc (containing position
1151–1158) a template. For reporter assays, the A172 cells were
transiently transfected with wild-type or mutant reporter plasmid
and miRNA using Lipofectamine® 2000. Reporter assays
were performed 36 h post-transfection using a Dual Luciferase Assay
system (Promega Corporation), normalized for transfection
efficiency with co-transfected Renilla luciferase (Tiangen
Biotech Co., Ltd.).
Immunohistochemistry
Immunohistochemistry was performed using standard
laboratory techniques. Antigen retrieval was performed by
autoclaving at 121°C for 8 min (model, V8879l; Tiangen Biotech Co.,
Ltd.), following which the above-mentioned glioma tissue sample
sections were incubated with 10% normal goat serum in PBS for 15
min at 37°C to eliminate non-specific staining. Incubation with
anti-GAB2 antibody was then performed. Finally, the sections were
coun-terstained with 10% Mayer hematoxylin (Tiangen Biotech Co.,
Ltd.), dehydrated, mounted, and observed. Staining was evaluated by
a neuropathologist and an investigator in a blinded-manner (H-7000
electron microscope; Hitachi, Ltd., Tokyo, Japan). The sections
were classified as - (negative), + (focal and weak
immunoreactivity), ++ (diffuse and weak or focal and intense
immunoreactivity) or +++ (diffuse and intense immunoreactivity).
The data were analyzed using SPSS 11.5 (SPSS, Inc., Chicago, IL,
USA). Quantitative image analysis was performed, as previously
described (21). The comparison
between high expression levels was achieved using a χ2
test.
Examination of cell proliferation using
an MTT assay
The effect on cell proliferation was assessed using
MTT (Sigma-Aldrich). A172 cells were suspended in 200 µl
medium and seeded in 96-well plates (1×104/ml and grown
to 50% confluence and synchronized with serum-free medium for 24 h
prior to adding serum to a concentration of 10%. MTT reagent (20
µl; 5 mg/ml; Amresco, LLC, Solon, OH, USA) was added at 48
h. The cells were incubated at 37°C for 4 h and following
centrifugation at 500 × g for 5 min at room temperature, 150
µl dimethyl sulfoxide (Beijing Solarbio Science and
Technology Co., Ltd.) was added to halt the reaction. The cell
confluence was determined by absorbance at a wavelength of 570 nm
on a spectrophotometer (UV-16; Shimadzu Corporation, Kyoto, Japan)
and absorbance was directly proportional to the number of surviving
cells.
miRNA microarray
Total RNA from the cultured A172 cells, with
efficient recovery of small RNAs, was isolated using an mirVana
miRNA Isolation kit (Ambion; Thermo Fisher Scientific, Inc.). The
cRNA for each sample was synthesized using a 3′ IVT Express kit
(Affymetrix, Inc., Santa Clara, CA, USA), according to the
manufacturer's protocol. The purified cRNA was fragmented by
incubation in fragmentation buffer (provided in the 3′ IVT Express
kit) at 95°C for 35 min, and chilled on ice. The fragmented labeled
cRNA was applied to a microRNA 2.0 array (Affymetrix, Inc.) and
hybridized in a Genechip hybridization oven 640 (Affymetrix, Inc.)
at 45°C for 18 h. Following washing and staining in a Genechip
fluidics station 450 (Affymetrix, Inc.), the arrays were scanned
using a Genechip Scanner 3000 (Affymetrix, Inc.). The gene
expressions levels of the samples were normalized and compared
using Partek GS 6.5 (Partek, Inc, St. Louis, MO, USA). The
average-linkage hierarchical clustering of the data was applied
using Cluster (http://rana.lbl.gov) and the results
were displayed using TreeView (http://rana.lbl.gov) (22).
Northern blotting analysis
Northern blot analysis of miRNAs was performed, as
previously described (23). Total
RNA was fractionated on a denaturing 12% polyacrylamide gel
containing 8 M urea (Tiangen Biotech Co., Ltd.). The RNA was
transferred to a Nytran N membrane (Schleicher & Schuell
Bioscience GmbH, Dassel, Germany) by capillary method and fixed by
ultraviolet cross-linking. The membranes were probed with
32P-labelled standard DNA and LNA-modified
oligonucleotides, complementary to the mature miRNA-197 and U6
snRNA, 10 pmol of each probe was end-labeled with
[γ-32P] ATP using T4 polynucleotide kinase.
Prehybridization of the filters (Tiangen Biotech Co., Ltd.) was
conducted in 50% formamide, 0.5% SDS, 5X SSPE, 5X Denhardt's
solution (all from Tiangen Biotech Co., Ltd.) and 20 µg/ml
sheared and denatured salmon sperm DNA (Beijing Solarbio Science
and Technology Co., Ltd.). Hybridizations were performed in the
same solution at 34–45°C. The labeled probes were heated for 1 min
at 95°C prior to addition of the filters in the prehybridization
solution. Following hybridization, the membranes were washed at low
stringency in 2X SSC and 0.1% SDS at 34–45°C twice for 5 min each
time or at high stringency in 0.1 SSC and 0.1% SDS at 65°C twice
for 5 min. The results of the northern blot were visualized by
Quantity One software (Bio-Rad Laboratories, Inc.)
Statistical analysis
Each experiment was repeated at least three times.
All results are expressed as the mean ±standard deviation. The
difference between the means was analyzed using the Student's
t-test or the χ2 test. All statistical analyses were
performed using SPSS 16.0 software (SPSS, Inc.) and P<0.05
indicated a statistically significant difference.
Results
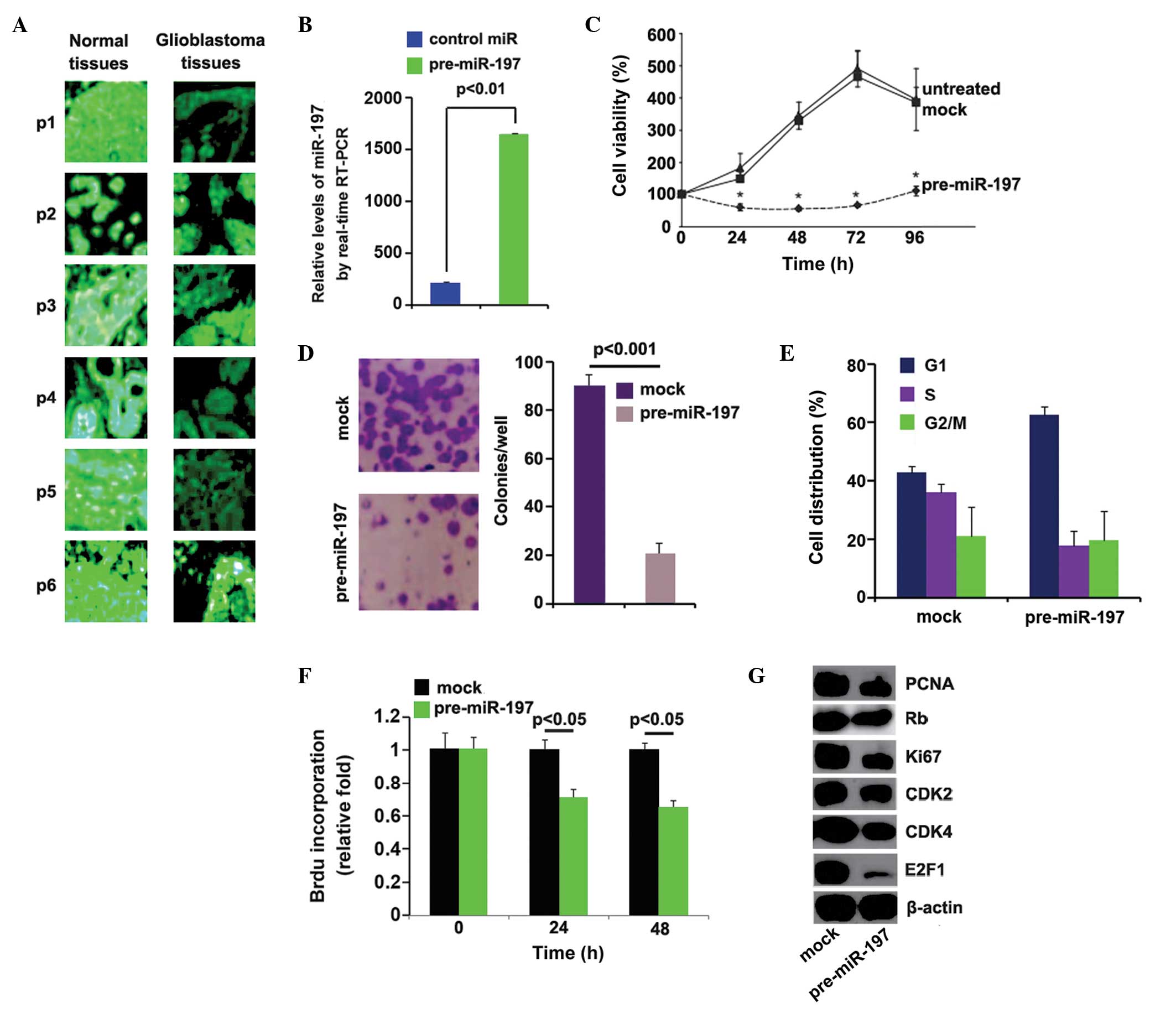
miR-197 functions as a tumor suppressor
gene in glioblastoma
In order to determine the expression levels of
miR-197 in glioblastoma, in situ hybridization was performed
to analyze the expression levels of miR-197 between glioblas-toma
tissues and adjacent normal tissues. An LNA-modified DNA probe
complementary to the indicated miRNA was used on consecutive 5
µm sections obtained from archived FFPE. The results of
in situ hybridization demonstrated that miR-197 was markedly
downregulated in the majority of the glioblastoma tissue samples,
compared with adjacent normal tissue samples (Fig. 1A). These results suggested that
miR-197 functioned as a tumor suppressor gene in glio-blastoma. The
roles of miR-197 in glioblastoma were then investigated in A172
cells. To examine whether the expression levels of miR-197 were
upregulated by pre-miR-197, the A172 cells were transfected with
pre-miR-197. Pre-miR-197 significantly upregulated the expression
levels of miR-197 in the A172 cells (Fig. 1B). The effects of miR-197 on
proliferation were also examined in the cells using a cell counting
assay. miR-197 inhibited proliferation 24–96 h post-transfection in
the A172 cells, compared with the untreated or control miR-treated
groups (Fig. 1C). A colony
formation assay was also performed for the A172 cells trans-fected
with pre-miR-197 or control miR. Consistent with the results of the
cell counting assay, the colony formation assay demonstrated that
miR-197 significantly inhibited colony formation in the A172 cells
(Fig. 1D). To further investigate
the effects of miR-197 on proliferation, cell-cycle analysis was
performed to analyze its effects on cell cycle. The A172 cells
transfected with pre-miR-197 exhibited lower S phase fractions
compared with the cells transfected with control miR (Fig. 1E). To examine whether the
inhibition of DNA synthesis contributed to lower S phase fractions
in the A172 cells transfected with pre-miR-197, a BrdU
incorporation assay was performed to detect DNA synthesis within
the cells. miR-197 significantly suppressed DNA synthesis in the
cells (Fig. 1F). In addition,
western blotting was performed to identify whether the protein
expression levels of the proliferation markers were affected by
miR-197 in the cells. The expression levels of PCNA, Rb, Ki67,
CDK2, CDK4 and E2F1 were downregulated by miR-197 in the cells
(Fig. 1G). These results suggested
that miR-197 inhibited the proliferation of A172 cells.
miR-197 downregulates protein expression
levels of GAB2 by targeting its 3′-UTR in A172 cells
miRNAs are short, non-coding RNAs, which regulate
the target mRNA by binding predominantly to the 3′-UTR,
inducing either translational repression or the degradation of the
target (1-3). The target genes of miR-197 were
screened using TargetScan (http://www.targetscan.org/vert_61/), which identified
a large number of target genes, however, GAB2 was examined as
several reports have demonstrated that GAB2 is an oncogene in
malignant tumors (10–16). Target sites on the 3′-UTR of
GAB2 are shown in Fig. 2A. It was
hypothesized that miR-197 downregulates the expression of GAB2 in
A172 cells. Western blot and RT-qPCR analyses were performed to
detect the expression levels of GAB2 in the A172 cells transfected
with pre-miR-197. The protein expression levels of GAB2 (Fig. 2B) were markedly downregulated in
the cells transfected with pre-miR-197, however, mRNA expression
levels remained unchanged (Fig. 2C and
D). Concordant with the results of the western blot analysis,
immunofluorescence analyses in the A172 cells transfected with
pre-miR-197 or control miR revealed that the protein expression
levels of GAB2 were significantly suppressed in the A172 cells
transfected with pre-miR-197 (Fig. 2E
and F). Fig. 2E shows
microscopy images of immunofluorescence staining of a
representative experiment (magnification, ×100), and Fig. 2F shows the mean fluorescence
intensities of three independent experiments.
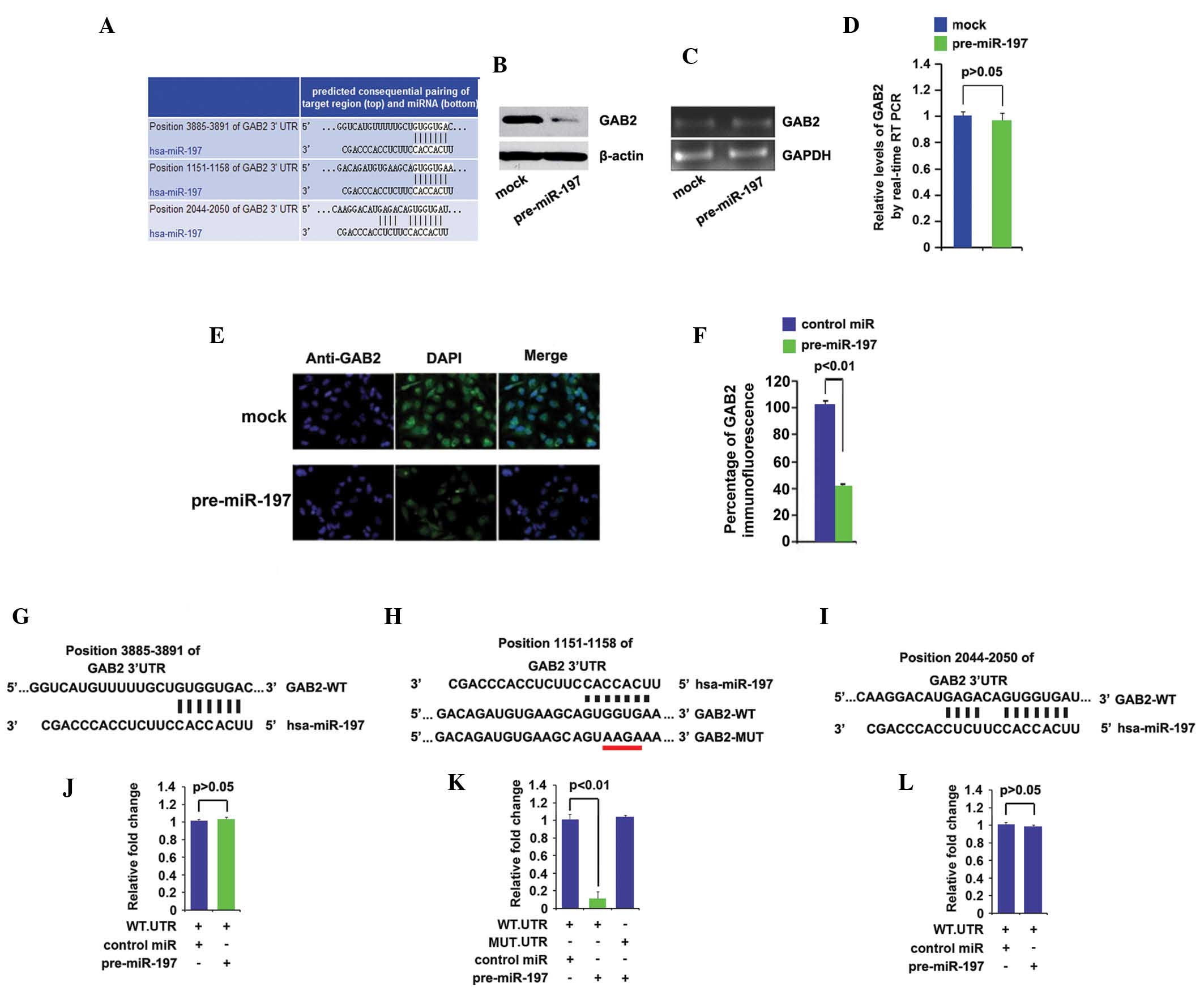 | Figure 2miR-197 downregulates the protein
expression levels of GAB2 by targeting its 3′-UTR in glioblastoma
cells. (A) Schematic of predicted miR-197 binding sites in the
3′-UTR of GAB2 mRNA. (B) Western blot analysis of the protein
expression levels of GAB2 in A172 cells. Mock groups were
trans-fected with control miR. β-actin was used as a loading
control (n=3). (C) RT-qPCR of the mRNA expression levels of GAB2 in
A172 cells transfected with pre-miR-197. Mock groups were
transfected with control miR. U6 served as a loading control (n=3).
(D) Quantitative analysis of the RT-qPCR for GAB2 in A172 cells
transfected with pre-miR-197. Mock groups were transfected with
control miR. U6 served as a loading control (n=3). (E)
Immunofluorescence analyses of A172 cells transfected with
pre-miR-197 or control miR. Images of immunofluorescence staining
of a representative experiment (magnification, ×200; n=3). (F)
Quantitative presentation of mean immunofluorescence intensities of
GAB2 (n=3). (G) GAB2 3′-UTR position 3885–3891. (H) GAB2 3′-UTR
position 1151–1158. MUT contains a four base mutation (indicated by
the red line) at the miR-197 target region, inhibiting its binding.
(I) GAB2 3′-UTR position, 2044–2050. (J) Reporter assay conducted
with co-transfection of 500 ng WT-reporter and 50 nM control-miR
(mock), or pre-miR-197 (n=3). (K) Reporter assay following
cotransfection of 500 ng WT-or MUT-reporter and 50 nM control-miR
(mock), or pre-miR-197 (n=3). (L) Reporter assay following
co-transfection of 500 ng WT-reporter and 50 nM control-miR (mock),
or pre-miR-197 (n=3). Bars indicate the standard error of the mean.
miR, microRNA; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; GAB2, Grb2-associated binding protein 2;
3′-UTR, 3′ untranslated region; WT, wild-type; MUT, mutant. |
To further demonstrate the direct regulation of GAB2
by miR-197, luciferase reporters were constructed with three
targeting sequences: Wild-type-GAB2-WT-luc-1 (Fig. 2G), GAB2-WT-luc-2 (Fig. 2H) and GAB2-WT-luc-3 (Fig. 2I). Following GAB2-WT-luc-1
introduction into the A172 cells, miR-197 did not inhibit
GAB2-WT-luc-1 plasmids (Fig. 2J).
Therefore, GAB2-WT-luc-2 plasmids were investigated to determine
whether they targeted by miR-197 in the A172 cells. The luciferase
reporter assays demonstrated that the luciferase activities of the
GAB2-WT-luc-2 plasmids were significantly suppressed in the cells
(Fig. 2K). To further examine
whether miR-197 targeted the 3′-UTR of GAB2 in the predicted
sites, four bases were mutated (Fig.
2H). The mutant reporters were subsequently introduced into the
A172 cells and demonstrated that the luciferase activity levels of
GAB2-MUT-luc-2 were not suppressed in the cells (Fig. 2K). Having demonstrated that
GAB2-WT-luc-2 plasmids were inhibited by miR-197, luciferase
reporter assays were used to investigate whether miR-197 targeted
GAB2-WT-luc-3. miR-197 did not affect the luciferase activity
levels of GAB2-WT-luc-3 in the cells (Fig. 2L). These results suggested that
miR-197 suppressed the protein expression levels of GAB2 by
targeting its 3′-UTR at position 1151–1158.
GAB2 acts as an oncogene in
glioblastoma
To assess the expression levels of GAB2 in
glioblastoma, western blot analysis was performed in seven pairs of
glioblastoma tissue and matched adjacent normal tissue samples. The
expression levels of GAB2 were higher in the majority of the
glioblastoma tissue samples, compared with the normal tissue
samples (Fig. 3A).
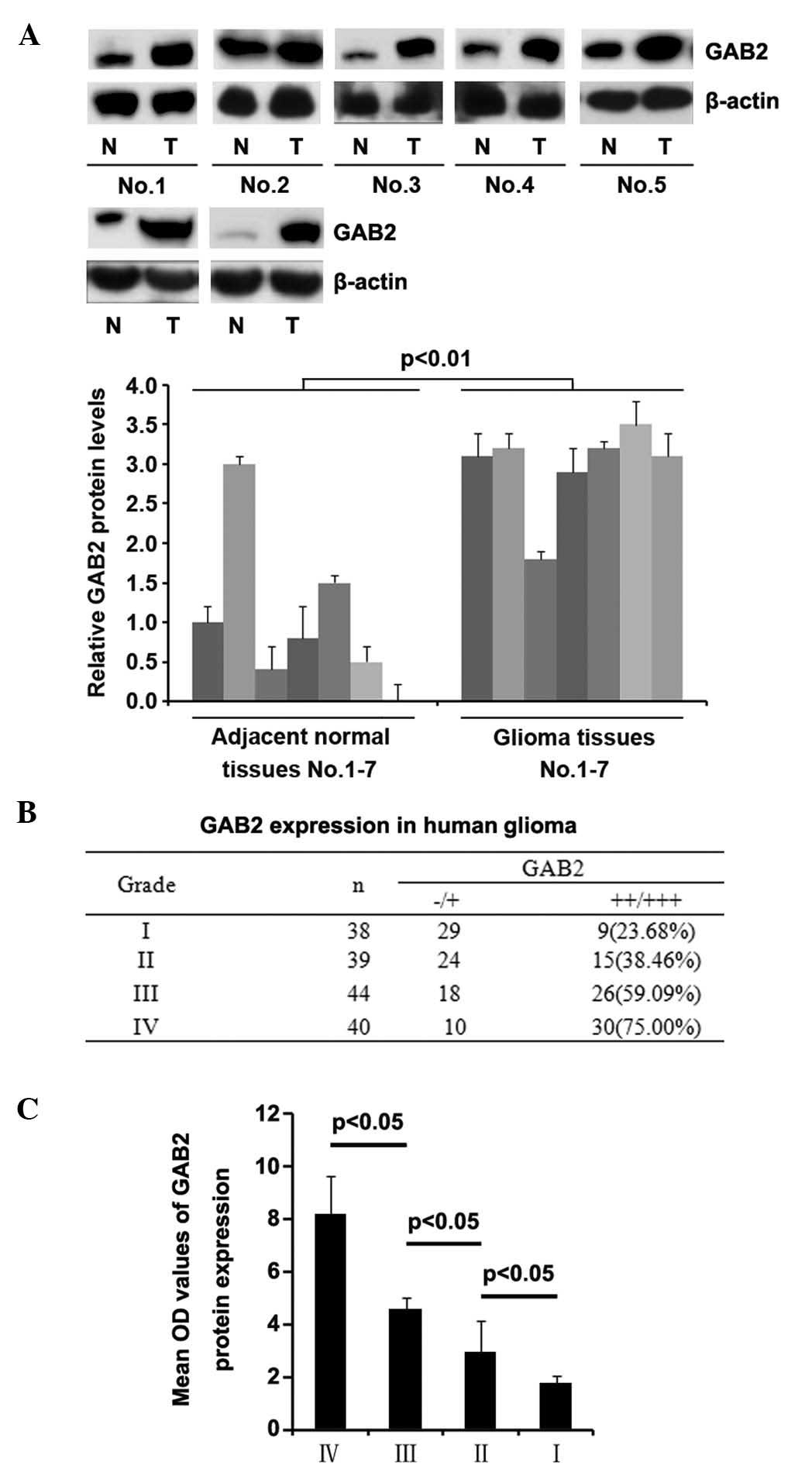 | Figure 3GAB2 is upregulated in glioblastoma
and associated with grade of glioma. (A) Western blot analysis of
the protein expression levels of GAB2 in seven pairs of
glioblastoma tissue and adjacent normal tissue samples. β-actin
served as a loading control (n=8). (B) Protein expression levels of
GAB2 (++/+++) in glioma grades I, II, III and IV.
Immunohistochemical analysis of GAB2 in glioma. Tissue sections of
WHO grade I glioma (n=38), WHO grade II glioma (n=39), WHO grade
III glioma (n=44) and WHO grade IV glioma (n=40). (C) Mean optical
density values of immunohistochemical images for GAB2 in
glioblastoma grades I, II, III and IV. Tissue sections of WHO grade
I glioma (n=38), grade II glioma (n=39), WHO grade III glioma
(n=44) and WHO grade IV glioma (n=40). Bars indicate the standard
error of the mean. WHO, World Heath Organization; GAB2,
Grb2-associated binding protein 2; OD, optical density; T, tumor
tissue; N, normal tissue. |
To demonstrate the importance of GAB2 in the
pathogenesis and progression of glioblastoma, immunohistochemistry
was performed to detect the expression of GAB2 in human glioma
tissue specimens. The protein expression levels of GAB2 (++/+++) in
glioma grades I, II, III and IV were 23.68, 38.46, 59.09 and 75%,
respectively (Fig. 3B).
Statistical analysis demonstrated that the expression levels of
GAB2 were higher in the high-grade glioblastoma tissues, compared
with the low grade glioma tissues: Grade I, vs. IV, P<0.001;
grade II, vs. grade IV, P<0.001; grade I, vs. grade III,
P<0.001.
In order to further confirm that high expression
levels of GAB2 were associated with high-grade human glioblas-toma,
quantitative image analysis was performed to analyze the protein
expression of GAB2 in the tissue sections. The expression levels of
GAB2 were positively correlated with glioma grade (Fig. 3C).
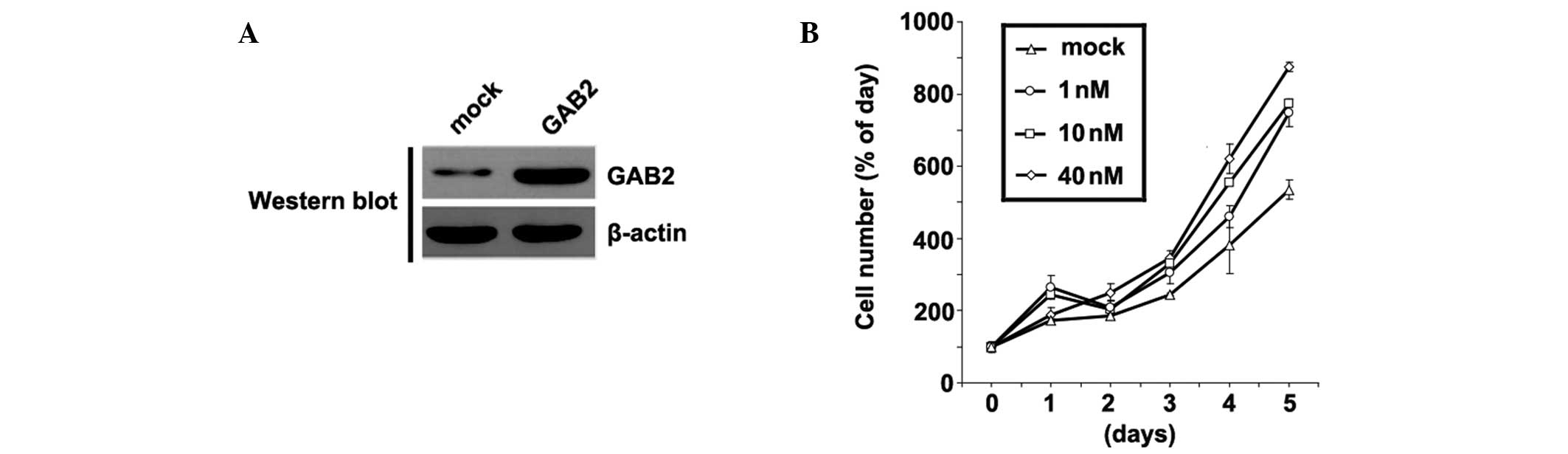
GAB2 promotes proliferation in A172
cells
In order to identify the role of GAB2 in the
regulation of A172 cell proliferation, the cells were transfected
with GAB2-expressing plasmids. Following stable transfection,
protein expression levels of GAB2 were quantified by western blot
analysis. The GAB2-expressing plasmids increased the protein
expression levels of GAB2 in the A172 cells (Fig. 4A). Furthermore, the proliferation
of the A172 cells was determined using an MTT assay. Overexpression
of GAB2 significantly increased the proliferation of A172 cells, in
a dose-dependent manner (Fig. 4B).
These data supported the hypothesis that GAB2 acts as a tumor
suppressor in glioblastoma.
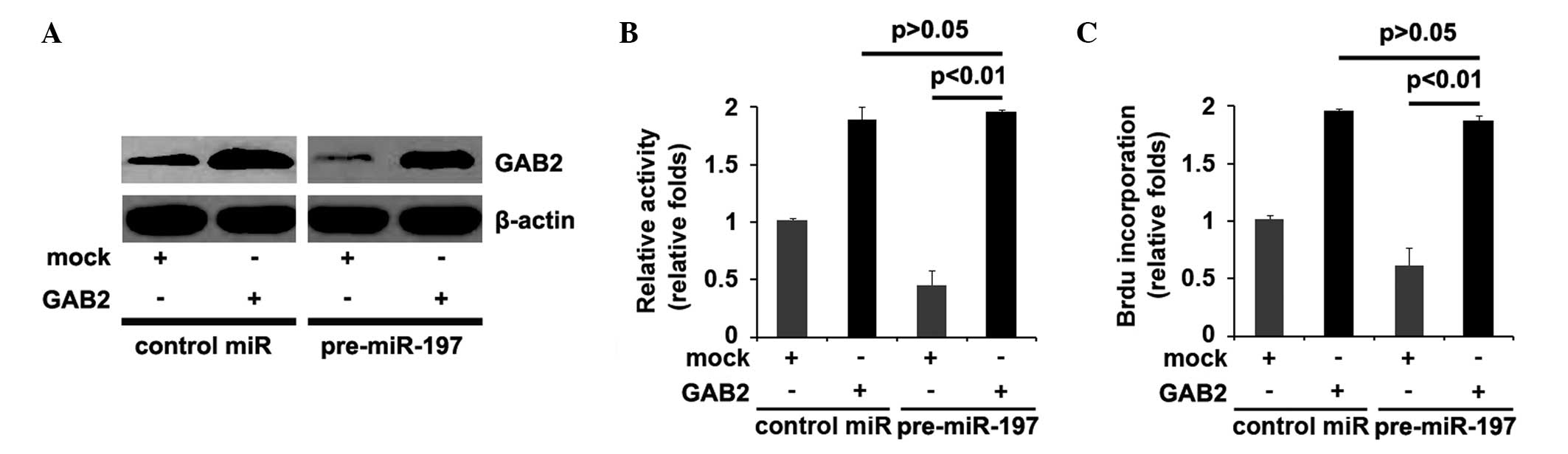
Introduction of GAB2 cDNA lacking
predicted 3′-UTR sites inhibits the cellular function of
miR-197
Due to the fact that miR-197 directly targets GAB2
through its 3′-UTR, the present study hypothesized that
ectopic expression of GAB2 by transfection with cDNA lacking the
predicted 3′-UTR target (in the present study, the GAB2
expression plasmids did not contain 3′-UTR position
1151–1158) can escape the regulation of miR-197, and thus attenuate
or decrease miR-197 function. The GAB2-expressing plasmid or empty
vector plasmid (pcDNA3.1) were transfected into the control miR or
pre-miR-197 treated-A172 cells. Immunoblotting revealed that
transfection with the GAB2 plasmids attenuated the effects of
miR-197 on GAB2 (Fig. 5A).
As the overexpression of miR-197 in glioblastoma
A172 cells inhibited proliferation, the present study investigated
whether GAB2 suppressed the function of miR-197 in proliferation by
treating the control miR or pre-miR-197-transfected A172 cells with
either GAB2-expressing plasmids or empty vector (pcDNA3.1). MTT and
BrdU incorporation assays were performed, which demonstrated that
the pre-miR-197-treated A172 cells exhibited a ~30–50% decrease in
proliferation (Fig. 5B) and DNA
synthesis (Fig. 5C), compared with
the control miR-treated cells. Overexpression of GAB2 sufficed to
reverse the loss of proliferation (Fig. 5B) and DNA synthesis (Fig. 5C) observed in the
pre-miR-197-treated cells.
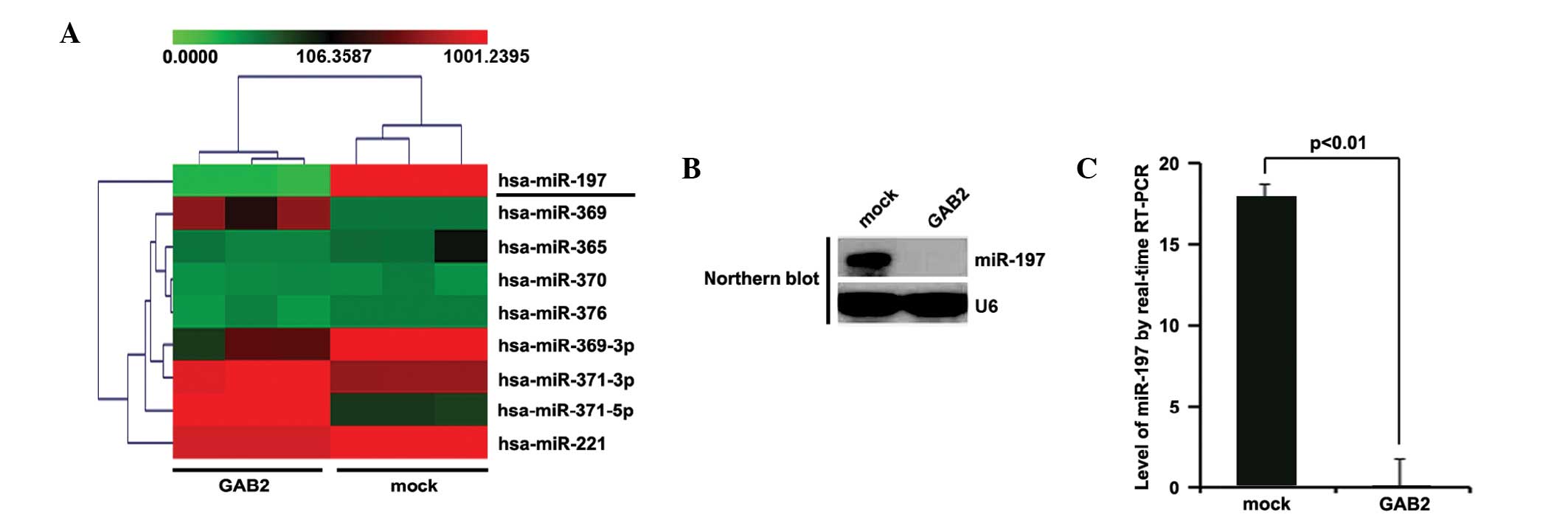
GAB2 significantly downregulates the
expression levels of miR-197 in A172 cells
Tumor suppressor genes can exert their functions by
regulating miRNA expression in glioma (24), several of which function as tumor
suppressor genes or oncogenes (5,25,26).
The present study hypothesized that GAB2 functions as an oncogene
by regulating relevant miRNAs. An miRNA microarray was performed,
in which RNAs isolated from A172 cells transfected with GAB2 or
empty vectors were hybridized to a custom miRNA microarray
platform. Following three cycles of hybridization, quantification
and normalization, several miRNAs, specifically miR-197, were
downregulated >1,000-fold in the cells (Fig. 6A). To further examine the
regulation of GAB2, northern blotting was performed to detect the
expression levels of miR-197. Concordant with the previous results,
the results of the northern blot (Fig.
6B) demonstrated that GAB2 significantly downregulated the
expression of miR-197. Furthermore, RT-qPCR was performed to
quantify the expression levels of miR-197 in the A172 cells
transfected with GAB2-expressing plasmids or empty vectors. As with
the results of the northern blot analysis, the RT-qPCR analysis
demonstrated that the expression levels of miR-197 were
significantly suppressed by GAB2 (Fig.
6C).
Discussion
The expression levels of certain miRNAs are
upregulated or downregulated, and function either as oncogenes or
tumor suppressor genes by regulating target genes in malignant
tumors (27–34). For example, miR-10b has been found
to be markedly upregulated in breast cancer tissue samples, and
initiated tumor invasion and metastasis (27). miR-214 is frequently downregulated
in ovarian cancer, and induces cell survival and cisplatin
resistance by targeting the 3′-UTR of the phosphatase and
tensin homolog (PTEN), which leads to downregulation of PTEN
protein and activation of the Akt signaling pathway (28). The polycistronic miRNA cluster,
miR-17-92, is overexpressed in human lung cancer, enhancing cell
proliferation (29). miRNA-101 is
downregulated in gastric cancer and is involved in cell migration
and invasion (30). miR-96 is
significantly downregulated (>5-fold) in pancreatic cancer
tissues, compared with normal tissues (31), suppresses Kirsten rat sarcoma viral
oncogene homolog and functions as a tumor suppressor gene in the
disease (32). miR-184 functions
as a potential oncogenic miRNA in squamous cell carcinoma of the
tongue (33). miR-125b exerts
tumor-suppressive effects in hepatic carcinogenesis via suppression
of the expression of the LIN28B oncogene, suggesting a therapeutic
application for miR-125b in hepatocellular carcinoma (34). Although the expression of miR-197is
downregulated in glioblastoma, its roles in the malignant tumor
remain to be elucidated (7). The
present study investigated why the gene is downregulated and
whether it functions as a tumor suppressor gene to further
understand the pathogenesis and progression of the disease, and
offer novel therapeutic targets.
The results of the present study demonstrated that
the expression of miR-197 was downregulated in glioblastoma tissue
samples. The roles of miR-197 were examined in the disease, and
demonstrated that it inhibited colony formation and proliferation
in the glioblastoma cells. The mechanism underlying miR-197-induced
suppression proliferation was also investigated. The results
suggested that miR-197 down-regulated the expression of GAB2 in
glioblastoma cells by targeting its 3′-UTR, and the
introduction of GAB2 cDNA lacking predicted 3′-UTR sites
inhibited miR-197 cellular function. This suggested that miR-197
functions as a tumor suppressor by targeting GAB2. The results also
showed that GAB2 acted as an oncogene in glioblastoma, was
associated with grades of glioma and promoted proliferation in the
A172 cells. Furthermore, the expression of miR-197 was
significantly suppressed by GAB2. These results suggested that, due
to the downregulated expression of miR-197 in glioblastoma, GAB2
was overexpressed, and this overexpression further promoted
tumorigenesis and progression by suppressing the expression of
miR-197. The miR-197-mediated GAB2 regulation in glioblastoma A172
cells, demonstrated in the present study, has potential basic and
clinical implications. miR-197 may be used as a potent tumor
suppressor by inhibiting proliferation and regulating various
oncogenes in human glioblastoma, therefore, the pharmacological
restoration of miR-197 may represent a promising therapeutic
strategy. GAB2 acts as an oncogene, therefore, restoration of
miR-197 may decrease its expression levels. Future investigations
are required to further examine the role of miR-197 in
glioblastoma.
References
|
1
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pasquinelli AE, Reinhart BJ, Slack F,
Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B,
Müller P, et al: Conservation of the sequence and temporal
expression of let-7 heterochronic regulatory RNA. Nature.
408:86–89. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Corsten MF, Miranda R, Kasmieh R,
Krichevsky AM, Weissleder R and Shah K: MicroRNA-21 knockdown
disrupts glioma growth in vivo and displays synergistic
cytotoxicity with neural precursor cell delivered S-TRAIL in human
gliomas. Cancer Res. 67:8994–9000. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guessous F, Zhang Y, Kofman A, Catania A,
Li Y, Schiff D, Purow B and Abounader R: microRNA-34a is tumor
suppressive in brain tumors and glioma stem cells. Cell Cycle.
9:1031–1036. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen G, Zhu W, Shi D, Lv L, Zhang C, Liu P
and Hu W: MicroRNA-181a sensitizes human malignant glioma U87MG
cells to radiation by targeting Bcl-2. Oncol Rep. 23:997–1003.
2010.PubMed/NCBI
|
|
7
|
Sana J, Hajduch M, Michalek J, Vyzula R
and Slaby O: MicroRNAs and glioblastoma: Roles in core signalling
pathways and potential clinical implications. J Cell Mol Med.
15:1636–1644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu H and Neel BG: The 'Gab' in signal
transduction. Trends Cell Biol. 13:122–130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishida K and Hirano T: The role of Gab
family scaffolding adapter proteins in the signal transduction of
cytokine and growth factor receptors. Cancer Sci. 94:1029–1033.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwab M: Amplification of oncogenes in
human cancer cells. Bioessays. 20:473–479. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bentires-Alj M, Gil SG, Chan R, Wang ZC,
Wang Y, Imanaka N, Harris LN, Richardson A, Neel BG and Gu H: A
role for the scaffolding adapter GAB2 in breast cancer. Nat Med.
12:114–121. 2006. View
Article : Google Scholar
|
|
12
|
Bocanegra M, Bergamaschi A, Kim YH, Miller
MA, Rajput AB, Kao J, Langerød A, Han W, Noh DY, Jeffrey SS, et al:
Focal amplification and oncogene dependency of GAB2 in breast
cancer. Oncogene. 29:774–779. 2010. View Article : Google Scholar
|
|
13
|
Brown LA, Kalloger SE, Miller MA, Shih
IeM, McKinney SE, Santos JL, Swenerton K, Spellman PT, Gray J,
Gilks CB and Huntsman DG: Amplification of 11q13 in ovarian
carcinoma. Genes Chromosomes Cancer. 47:481–489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zatkova A, Schoch C, Speleman F, Poppe B,
Mannhalter C, Fonatsch C and Wimmer K: GAB2 is a novel target of
11q amplification in AML/MDS. Genes Chromosomes Cancer. 45:798–807.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Horst B, Gruvberger-Saal SK, Hopkins BD,
Bordone L, Yang Y, Chernoff KA, Uzoma I, Schwipper V, Liebau J,
Nowak NJ, et al: Gab2-mediated signaling promotes melanoma
metastasis. Am J Pathol. 174:1524–1533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SH, Jeong EG, Nam SW, Lee JY, Yoo NJ
and Lee SH: Increased expression of Gab2, a scaffolding adaptor of
the tyrosine kinase signalling, in gastric carcinomas. Pathology.
39:326–329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi L, Sun X, Zhang J, Zhao C, Li H, Liu
Z, Fang C, Wang X, Zhao C, Zhang X, et al: Gab2 expression in
glioma and its implications for tumor invasion. Acta Oncol.
52:1739–1750. 2013. View Article : Google Scholar
|
|
18
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmittgen TD, Lee EJ, Jiang J, Sarkar A,
Yang L, Elton TS and Chen C: Real-time PCR quantification of
precursor and mature microRNA. Methods. 44:31–38. 2008. View Article : Google Scholar
|
|
20
|
Liao XH, Lu DL, Wang N, Liu LY, Wang Y, Li
YQ, Yan TB, Sun XG, Hu P and Zhang TC: Estrogen receptor α mediates
proliferation of breast cancer MCF-7 cells via a
p21/PCNA/E2F1-dependent pathway. FEBS J. 281:927–942. 2014.
View Article : Google Scholar
|
|
21
|
Bacus SS, Zelnick CR, Plowman G and Yarden
Y: Expression of the erbB-2 family of growth factor receptors and
their ligands in breast cancers. Implication for tumor biology and
clinical behavior. Am J Clin Pathol. 102(4 Suppl 1): S13–S24.
1994.PubMed/NCBI
|
|
22
|
Eisen MB, Spellman PT, Brown PO and
Botstein D: Cluster analysis and display of genome-wide expression
patters. Proc Natl Acad Sci USA. 95:14863–14868. 1998. View Article : Google Scholar
|
|
23
|
Yu J, Ryan DG, Getsios S,
Oliveira-Fernandes M, Fatima A and Lavker RM: MicroRNA-184
antagonizes microRNA-205 to maintain SHIP2 levels in epithelia.
Proc Natl Acad Sci USA. 105:19300–19305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He L, He X, Lim LP, de Stanchina E, Xuan
Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Novakova J, Slaby O, Vyzula R and Michalek
J: MicroRNA involvement in glioblastoma pathogenesis. Biochem
Biophys Res Commun. 386:1–5. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sasayama T, Nishihara M, Kondoh T, Hosoda
K and Kohmura E: MicroRNA-10b is overexpressed in malignant glioma
and associated with tumor invasive factors, uPAR and RhoC. Int J
Cancer. 125:1407–1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang H, Kong W, He L, Zhao JJ, O'Donnell
JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV and Cheng JQ:
MicroRNA expression profiling in human ovarian cancer: miR-214
induces cell survival and cisplatin resistance by targeting PTEN.
Cancer Res. 68:425–433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hayashita Y, Osada H, Tatematsu Y, Yamada
H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y and
Takahashi T: A polycistronic microRNA cluster, miR-17-92, is
overexpressed in human lung cancers and enhances cell
proliferation. Cancer Res. 65:9628–9632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang HJ, Ruan HJ, He XJ, Ma YY, Jiang XT,
Xia YJ, Ye ZY and Tao HQ: MicroRNA-101 is down-regulated in gastric
cancer and involved in cell migration and invasion. Eur J Cancer.
46:2295–2303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Szafranska AE, Davison TS, John J, Cannon
T, Sipos B, Maghnouj A, Labourier E and Hahn SA: MicroRNA
expression alterations are linked to tumorigenesis and
non-neoplastic processes in pancreatic ductal adenocarcinoma.
Oncogene. 26:4442–4452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu S, Lu Z, Liu C, Meng Y, Ma Y, Zhao W,
Liu J, Yu J and Chen J: miRNA-96 suppresses KRAS and functions as a
tumor suppressor gene in pancreatic cancer. Cancer Res.
70:6015–6025. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wong TS, Liu XB, Wong BY, Ng RW, Yuen AP
and Wei WI: Mature miR-184 as potential oncogenic microRNA of
squamous cell carcinoma of tongue. Clin Cancer Res. 14:2588–2592.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang L, Wong CM, Ying Q, Fan DN, Huang S,
Ding J, Yao J, Yan M, Li J, Yao M, et al: MicroRNA-125b suppresses
human liver cancer cell proliferation and metastasis by directly
targeting oncogene LIN28B2. Hepatology. 52:1731–1740. 2010.
View Article : Google Scholar : PubMed/NCBI
|