Introduction
Chronic lymphocytic leukemia (CLL) is a disease that
results from apoptotic resistance and an aberration in the
accumulation of mature monoclonal B-cells. The prognosis of CLL has
been greatly improved by the application of a combined regimen of
fludarabine, cyclophosphamide and rituximab (FCR). However, there
are certain patients with relapsed/refractory CLL who do not
benefit from FCR treatment (1,2).
Patients who exhibit refractory disease or a poor prognosis require
novel therapeutic strategies.
Genetic deficiencies, dysregulation of environmental
factors and signaling pathways are involved in the pathogenesis of
CLL (3–5). Among them, dysregulation of the
B-cell receptor (BCR) signaling pathway is regarded as the most
important mechanism, in addition to signals from the
microenviroment, including interleukin 6, B-cell activating factor
and CXCL12/stromal cell-derived factor 1 (6). Certain inhibitors of critical kinases
involved in the BCR signaling pathway, including Bruton's tyrosine
kinase (BTK), spleen tyrosine kinase and phosphoinositide 3-kinase
(PI3K) have been suggested to improve the prognosis of patients
with CLL (7). Previously,
application of BTK inhibitors in CLL therapy has suggested that BTK
may be a therapeutic target in CLL. It has been reported that
patients with CLL that is previously untreated, has relapsed or is
refractory can obtain benefits from ibrutinib therapy (8). In the NCCN Guidelines of 2004, the
use of BTK inhibitors was listed in the treatment of CLL (9). Previous studies have focused upon the
combination treatment of ibrutinib with other
chemoimmunotherapeutic agents (10,11).
In CLL, BCR signaling has been demonstrated to be associated with
the PI3K, mitogen-activated protein kinase (MAPK) and nuclear
factor κB pathways (12). However,
the specific mechanisms involved in the use of BTK inhibitors in
CLL require further elucidation.
In normal B-cells, BTK has been demonstrated to be a
negative regulator of the Wnt signaling pathway by regulating CDC73
expression, however does not affect β-catenin expression (13). Another study on multiple myeloma
indicated that BTK is able to regulate activity of Wnt by
regulating CDC73, β-catenin, phosphorylated (p-) protein kinase B
and p-glycogen synthase kinase 3β (GSK3β) (14). Considering the alterations to
expression of Wnt signaling-associated molecules in tumors
originating from B lymphocytes, in addition to the complex
regulation of signaling pathways, it was suggested that
investigation of the role of the BTK inhibitor in regulation of Wnt
pathway of CLL may be beneficial.
Materials and methods
Cell culture and treatment reagents
The human MEC-1 CLL cell line was used in the
current study. Cells were suspended in Iscove's modified Dulbecco's
medium (IMDM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) with 10% fetal bovine serum (GE Healthcare Life Sciences,
Logan, UT, USA) cultured at 37°C with 5% carbon dioxide. BTK
inhibitor (ibrutinib) were purchased from Selleck Chemicals
(#S2680; Shanghai, China) and dissolved in dimethyl sulfoxide
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) to a concentration of 5 mM/l.
RNA isolation and reverse
transcription-quantitative poly- merase chain reaction
(RT-qPCR)
Total mRNA of samples was isolated using TRIzol
(Takara Biotechnology Co., Ltd., Dalian, China). Subsequently, the
NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.)
was used to measure the optical density (OD) value and the
concentration of RNA. The OD 260/280 values of all RNA samples were
read using the NanoDrop 2000 spectrophotometer (ratio, 1.8–2.0).
The reverse transcription system was comprised of reverse
transcription reagents, including 5X gDNA Eraser Buffer, gDNA
Eraser, 5X PrimeScript Buffer 2 (for qPCR), RT Primer Mix,
PrimeScript RT Enzyme Mix 1 and RNase Free dH2O from
Takara Biotechnology Co., Ltd. SYBR green-based RT-qPCR was
performed following according to the manufacturer's instructions
(Takara Biotechnology Co., Ltd.). The reaction system consisted of
10.0 μl SYBR Premix Ex Taq™, 0.4 μl PCR forward
primer (10 μM) and 0.4 μl PCR reverse primer (10
μM), 2.0 μl cDNA and 7.2 μl distilled water.
The RT-qPCR reaction was performed using the LightCycler 480
Instrument (Roche Diagnostics, Basel, Switzerland). The primers
used in the current study were as previously described (15,16).
The sequences used were as follows: Metadherin (MTDH), F
5′-TTACCACCGAGCAACTTACAAC-3′ and R 5′-ATTCCAGCCTCCTCCATTGAC-3′;
lymphoid-enhancing factor 1 (LEF-1), F 5′-GACGAGATGATCCCCTTCAA-3′
and R 5′-AGGGCTCCTGAGAGGTTTGT-3′; β-catenin, F
5′-TGGCAGCAACAGTCTTACCT-3′ and R 5′-CATAGCAGCTCGTACCCTCT-3′; and
β-actin, F 5′-TGACGTGGACATCCGCAAAG-3′ and R
5′-CTGGAAGGTGGACAGCGAGG-3′. Following preamplification (95°C
for 5 min), the PCRs were amplified for 40 cycles (95°C for
10 sec, 60°C for 10 sec and 72°C for 10 sec) on the
LightCycler 480. The data were analyzed using LightCycler 480
software, version 1.5 (Roche Diagnostics), using the
2−ΔΔCq method (17).
Protein isolation and western blot
analysis
Total proteins were collected from the cells using
radioimmunoprecipitation assay buffer and 1% phenylmethylsulfonyl
fluoride (Shenergy Biocolor Bioscience & Technology Co., Ltd.,
Shanghai, China). The protein concentrations were measured using
the Bicinchoninic Acid assay (Shenergy Biocolor Bioscience &
Technology Co., Ltd.). Proteins were electrophoresed using 10%
sodium dodecyl sulfate-polyacrylamide gels (Sangon Biotech Co.,
Ltd., Shanghai, China), and were then transferred onto immobilon-P
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Membranes were then blocked for 1 h with Tris-buffered saline
(Sangon Biotech Co., Ltd.) containing 5% non-fat dried milk and
0.1% Tween-20 (Solarbio Science & Technology Co., Ltd.).
Subsequent to incubation with primary antibodies at 4°C
overnight, the membranes were incubated with anti-rabbit or
anti-mouse IgGs conjugated to horseradish peroxidase (1:5,000;
#ZB-2301 or #ZB-2305; OriGene Technologies, Inc., Beijing, China).
The proteins of interest were detected with enhanced
chemiluminescence detection reagent according to the manufacturer's
instructions (EMD Millipore). Primary antibodies were diluted using
primary antibody dilution buffer (Beyotime Institute of
Biotechnology, Shanghai, China) and secondary antibodies were
diluted using secondary antibody dilution buffer (Beyotime
Institute of Biotechnology). The antibodies used in the current
study were as follows: Rabbit polyclonal anti-MTDH antibody
(#13860-1-AP; 1:1,000; Proteintech Group, Inc., Chicago, IL, USA),
rabbit monoclonal anti-β-catenin (#8480; 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit polyclonal anti-LEF-1
(#14972-1-AP; 1:1,000; Proteintech Group, Inc.), mouse anti-human
monoclonal β-actin antibody (1:2,000) (#TA-09, OriGene
Technologies, Inc., Beijing, China).
Cell proliferation
The cell suspension (104 cells) in 100
μl IMDM culture medium were plated into 96-well plates.
Prior to the detection of proliferation, 10 μl Cell Counting
Kit-8 solution (Nanjing EnoGene Biotech Co., Ltd., Nanjing, China)
was added to each well, then the wells were cultured for 2 h at
37°C. Subsequently, a SpectraMax M2 microplate reader
(Molecular Devices, LLP, Sunnyvale, CA, USA) was used to detect the
absorbance at a wavelength of 450 nm, which was adjusted at 690
nm.
Statistics analysis
SPSS software, version 18.0 (SPSS, Inc., Chicago,
IL, USA) was used to perform statistical analysis. Student's t-test
was used, and P<0.05 was considered to indicate a statistically
significant difference.
Results
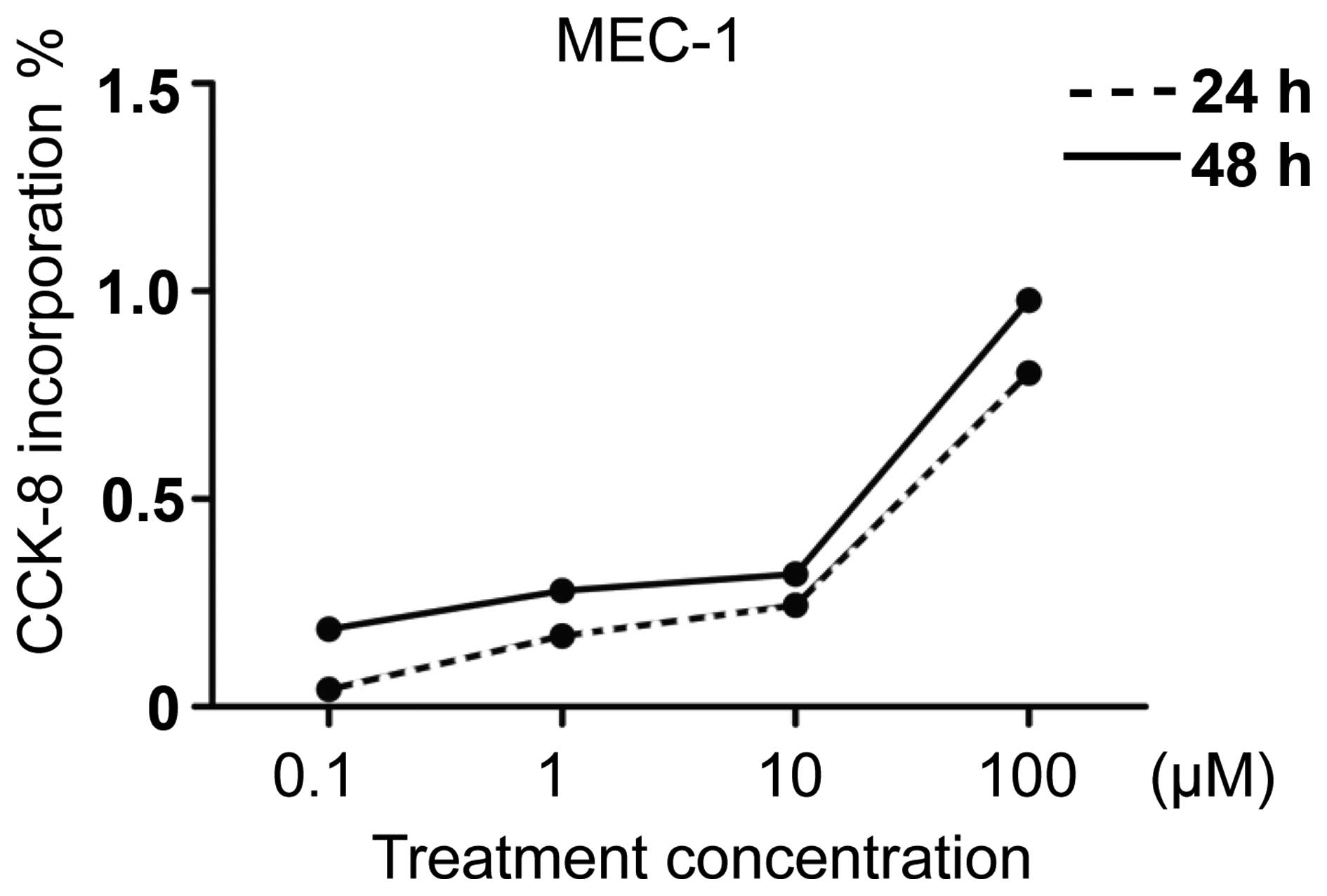
Ibrutinib inhibits the proliferation of
MEC-1
Ibrutinib has been previously identified to exhibit
a time- and concentration-dependent repressive effect on primary
B-cells in CLL (18). In addition,
the same study identified no significant difference in the
inhibition ratio in CLL cells from patients with
unmutated-immunoglobulin heavy chain variable (IgVH) or
mutated-IgVH (18). In the current
study, the effects of ibrutinib on the MEC-1 cell line were
investigated. According to the methods of a previous study, the
proliferation inhibition ratio was detected following ibrutinib
treatment with different concentrations (0, 0.1, 1, 10, 100
μmol) (Fig. 1). The results
indicated that in line with increases in treatment concentration of
the BTK inhibitor, the proliferation of MEC-1 was inhibited
compared with the control group. The proliferation inhibition was
more marked in MEC-1 cells with treatment for 48 h compared with 24
h; however, no significant difference was identified between the
two groups.
Ibrutinib inhibits the Wnt signaling
pathway
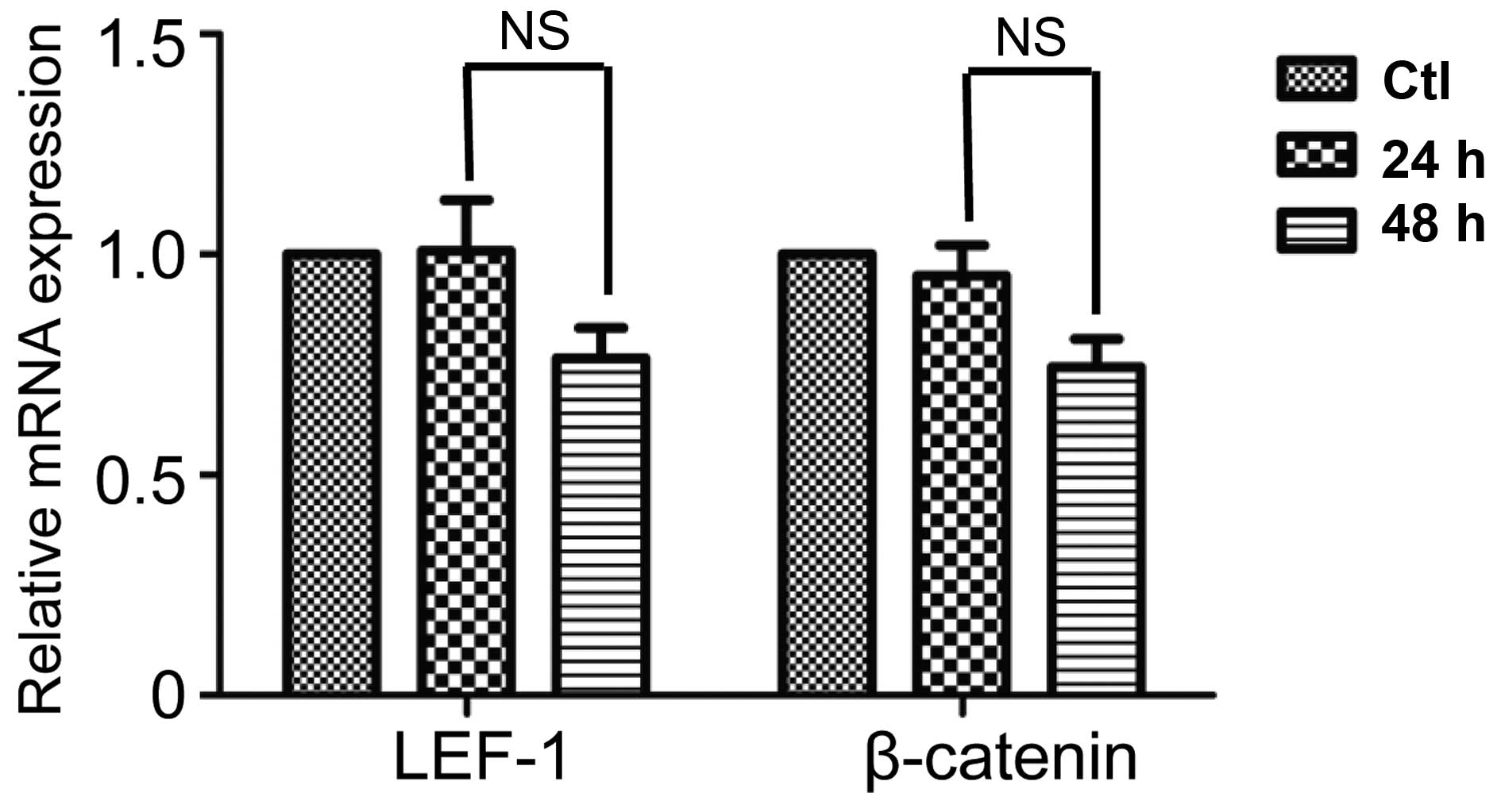
Susbequent to treatment with ibrutinib for 48 h, the
mRNA and protein expression levels of LEF-1 and β-catenin were
measured in order to evaluate the activation state of Wnt
signaling. The effects of ibrutinib on LEF-1 and β-catenin mRNA
expression subsequent to treatment with 10 μM ibrutinib for
24 and 48 h were evaluated. The results indicated that there was a
marginal time-dependent inhibitory effect of ibrutinib on LEF-1 and
β-catenin expression, however this was not statistically
significant between the two groups (P>0.05; Fig. 2). Accordingly, 48 h was selected
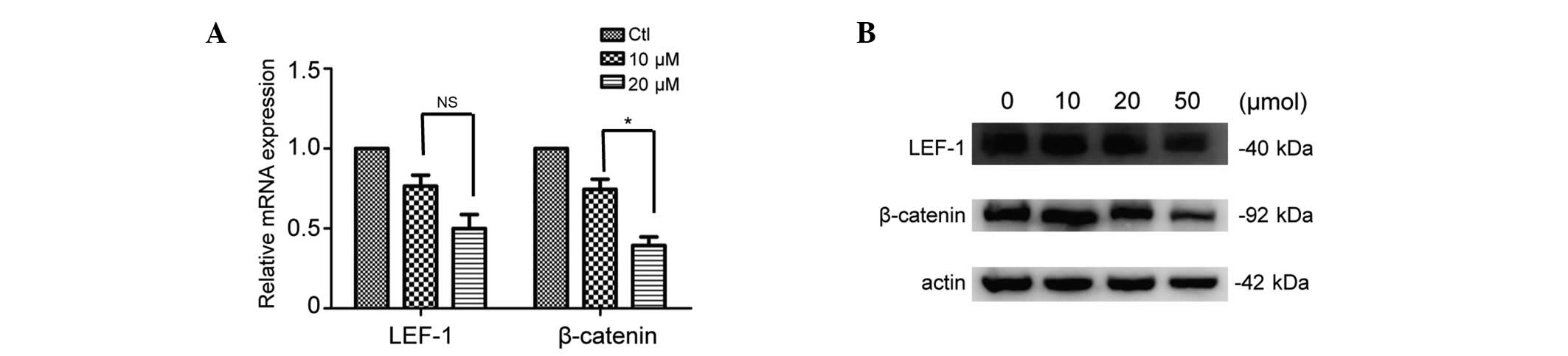
for further study. The results demonstrated that subsequent to
treatment with ibrutinib, the mRNA and protein expression levels of
LEF-1 (non-significant) and β-catenin (P<0.05) were
downregulated in a concentration-dependent manner (Fig. 3). The results indicate that
inhibition of BCR signaling activity with ibrutinib in CLL may
downregulate the activity of Wnt signaling in CLL.
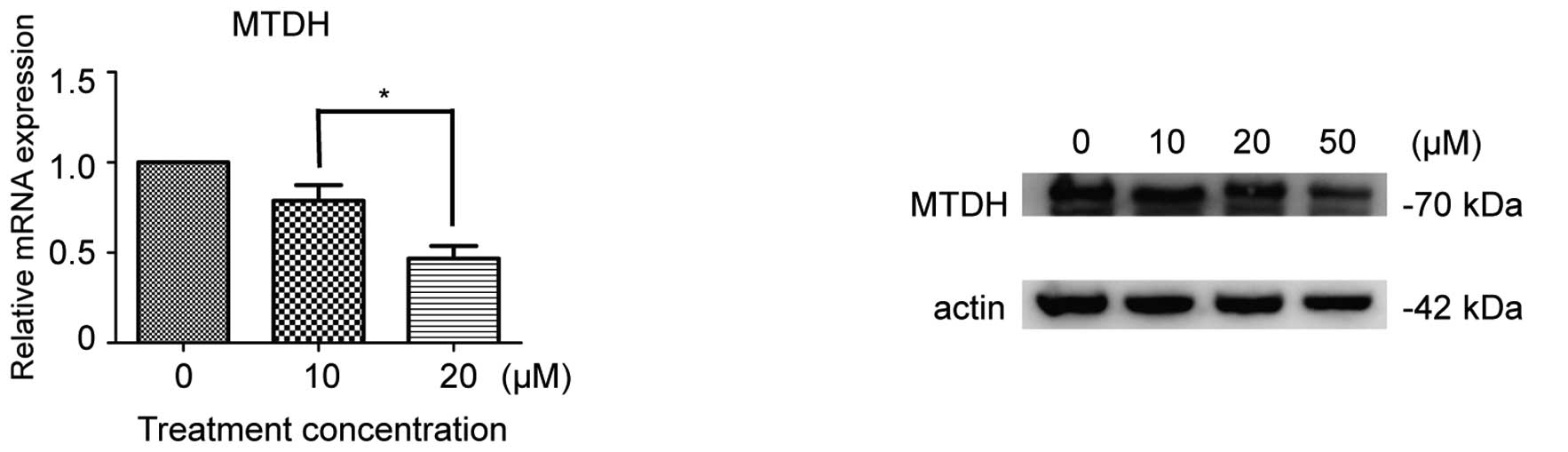
Ibrutinib inhibits MTDH expression
In a previous study, it was demonstrated that MTDH
is aberrantly overexpressed in CLL (19). Subsequent to interfering with MTDH
expression in CLL, the downregulation of LEF-1 expression was
detected, which was identified to be correlated with the downstream
factors c-Myc and cyclin D1 (19).
To detect whether the BTK inhibitor would inhibit CLL cell
viability via inhibition of MTDH expression, mRNA and protein MTDH
expression levels prior and subsequent to 48 h ibrutinib treatment
were measured. The results indicated that subsequent to ibrutinib
treatment, MTDH was downregulated, and the inhibitory rate was
associated with the concentration of ibrutinib (P<0.05; Fig. 4).
Discussion
As one of the most important signaling pathway in
CLL, BCR signaling is central to the development and maintenance of
B-cell malignancies. The BCR consists of immunoglobulin heavy and
light chains coupled to a CD79A-CD79B heterodimer that transduces
signals by engaging downstream nonreceptor kinases, including BTK
(12). BTK is a member of the Tec
family of kinases and is involved in the transduction of signals in
BCR-signaling and the mediation of B-cell activation. Ibrutinib,
which has undergone clinical trials, has been demonstrated to
exhibit clinical efficacy and safety in numerous B-cell
malignancies, including mantle cell lymphoma (MCL), diffuse large
B-cell lymphoma, follicular lymphoma and CLL, as a single agent in
addition to use in combination therapy (8,10,11,20,21).
Ibrutinib is an irreversible BTK inhibitor that has
been previously described as a covalent inhibitor of BTK able to
bind at cysteine 481 (22).
Ibrutinib was approved by the Food and Drug Administration in
November 2013 for the treatment of relapsed MCL and in February
2014 for relapsed/refractory CLL (23). It is suggested that ibrutinib may
be used in the future to complement traditional immunotherapy. In
addition, it has been demonstrated that ibrutinib possesses
potential to eliminate the need for chemoimmunotherapy (8,10).
An improved understanding of the mechanisms of ibrutinib will aid
in the development of effective combination therapy strategies to
improve the prognosis of patients with CLL (24,25).
It has been reported that in vitro, apoptosis can be induced
in primary CLL cells by administration of a high concentration of
ibrutinib (18). In the current
study, the CLL cell line MEC-1 was treated with ibrutinib, in order
to identify the optimal culture conditions for the subsequent
experiments. The results indicated that the activity of ibrutinib
in MEC-1 cells was time- and concentration-dependent. For the
further evaluation of the effect of ibrutinib, 48 h was selected
for the following experiments. Subsequently, the effect of
ibrutinib on the Wnt signaling pathway in CLL was evaluated.
The canonical Wnt signaling pathway is associated
with a variety of cellular processes and is involved in
carcinogenesis (26–28). Its activation is mediated by the
inhibition of the activity of GSK3β, and leads to the stabilization
of β-catenin, thus promoting nuclear translocation. In the nucleus,
β-catenin interacts with DNA-binding protein T-cell
factor/lymphoid-enhancing factor family members to drive the
transcription of various target genes including cyclin D1 and c-Myc
(29). Wnt signaling-associated
genes including WNT-3, WNT4, WNT-5B, WNT-7B, WNT-9A, WNT-10A,
WNT-16B and LEF-1 have been demonstrated to be overexpressed in CLL
(16,30,31).
Previous studies have demonstrated that inhibition of the WNT
signaling pathway by small-interfering RNA of LEF-1 or inhibitors
such as CGP049090 and PKF115-584 can effectively reduce the
proliferation of CLL and induce apoptosis of CLL cells (32,33).
This indicates that the Wnt signaling pathway may be a potential
therapeutic target in CLL (16,33–35).
BTK, which has been previously demonstrated to be a
downregulator of the Wnt signaling pathway in normal B-cells, can
also promote the activity of the Wnt pathway in multiple myeloma
(14,15). The current study aimed to
investigate the association between ibrutinib and the Wnt signaling
pathway. In the current study, LEF-1 and β-catenin were selected
for the evaluation of the function of Wnt signaling. The results
indicated that subsequent to treatment with ibrutinib for 48 h,
LEF-1 and β-catenin were correspondingly inhibited. This indicated
that BTK may promote the activity of the Wnt pathway in CLL.
In a previous study, it was demonstrated that
over-activation of the Wnt signaling pathway is partially
associated with overexpression of MTDH (36). With the inhibition of MTDH
expression, CLL cells were identified to exhibit apoptosis in
addition to downregulation of Wnt signaling pathway molecules,
including LEF-1, with no clear downregulation of β-catenin in the
levels of total protein (19).
Under conditions of BCR activation, MTDH has previously been
identified to be upregulated in CLL cells. (36). In addition, it has been observed
that LEF-1 is upregulated in line with increases of MTDH (data not
shown).
Whether inhibition of BTK expression would affect
MTDH expression was investigated in CLL in the current study. A
downregulation in the MTDH mRNA and protein expression levels was
identified in MEC-1 cells subsequent to treatment with ibrutinib
for 48 h. These results indicate that BTK is an upstream regulator
in CLL, and that its inhibition leads to the downregulation of
MTDH. Further studies should focus upon elucidating the precise
mechanisms of the regulatory role of BTK in MTDH and LEF-1
regulation. This would aid in increasing the understanding of the
pathogenesis of CLL.
Multiple signaling pathways have been identified to
be dysregulated in CLL, including the Wnt, Notch, BCR, MAPK and
PI3Kδ pathways. The complex regulation of different signaling
pathways is key in the molecular mechanisms of refractory and
chemoresistant tumors. Further investigation into the regulatory
mechanisms of signaling in CLL and other tumors would aid in
improving understanding of the pathogenesis of these diseases, thus
benefiting the development of effective therapeutative
strategies.
Acknowledgments
The current study was partly supported by grants
from the following funding bodies: The National Natural Science
Foundation of China (grant nos. 81270598, 81473486 and 81302044);
the National Public Health Grand Research Foundation (grant no.
201202017); the Natural Science Foundations of Shandong Province
(grant nos. 2009ZRB14176 and ZR2012HZ003); the Technology
Development Projects of Shandong Province (grant nos.
2008GG2NS02018, 2010GSF10250 and 2014GSF118021); the Promotive
Research Fund for Excellent Young and Middle-aged Scientists of
Shandong Province (grant nos. BS2013YY003 and BS2013YY009); the
Program of Shandong Medical Leading Talent; and the Taishan Scholar
Foundation of Shandong Province.
References
|
1
|
Keating MJ, O'Brien S, Albitar M, Lerner
S, Plunkett W, Giles F, Andreeff M, Cortes J, Faderl S, Thomas D,
et al: Early results of a chemoimmunotherapy regimen of
fludarabine, cyclophos-phamide and rituximab as initial therapy for
chronic lymphocytic leukemia. J Clin Oncol. 23:4079–4088. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Badoux XC, Keating MJ, Wang X, O'Brien SM,
Ferrajoli A, Faderl S, Burger J, Koller C, Lerner S, Kantarjian H
and Wierda WG: Fludarabine, cyclophosphamide and rituximab
chemoimmunotherapy is highly effective treatment for relapsed
patients with CLL. Blood. 117:3016–3024. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li PP and Wang X: Role of signaling
pathways and miRNAs in chronic lymphocytic leukemia. Chin Med J.
126:4175–4182. 2013.PubMed/NCBI
|
|
4
|
Chen J and McMillan NA: Molecular basis of
pathogenesis, prognosis and therapy in chronic lymphocytic
leukaemia. Cancer Biol Ther. 7:174–179. 2008. View Article : Google Scholar
|
|
5
|
Ten Hacken E and Burger JA:
Microenvironment dependency in chronic lymphocytic leukemia: The
basis for new targeted therapies. Pharmacol Ther. 144:338–348.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shain KH and Tao J: The B-cell receptor
orchestrates environment-mediated lymphoma survival and drug
resistance in B-cell malignancies. Oncogene. 33:4107–4113. 2014.
View Article : Google Scholar
|
|
7
|
Robak T and Robak P: BCR signaling in
chronic lymphocytic leukemia and related inhibitors currently in
clinical studies. Int Rev Immunol. 32:358–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farooqui MZ, Valdez J, Martyr S, Aue G,
Saba N, Niemann CU, Herman SE, Tian X, Marti G, Soto S, et al:
Ibrutinib for previously untreated and relapsed or refractory
chronic lymphocytic leukaemia with TP53 aberrations: A phase 2,
single-arm trial. Lancet Oncol. 16:169–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zelenetz AD, Gordon LI, Wierda WG,
Abramson JS, Advani RH, Andreadis CB, Bartlett N, Byrd JC, Czuczman
MS, Fayad LE, et al National comprehension cancer network: Chronic
lymphocytic leukemia/small lymphocytic lymphoma, version 1.2015. J
Natl Compr Canc Netw. 13:326–362. 2015.PubMed/NCBI
|
|
10
|
Alinari L, Quinion C and Blum KA: Bruton's
tyrosine kinase inhibitors in B-cell non-Hodgkin's lymphomas. Clin
Pharmacol Ther. 97:469–477. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Da Roit F, Engelberts PJ, Taylor RP, Breij
EC, Gritti G, Rambaldi A, Introna M, Parren PW, Beurskens FJ and
Golay J: Ibrutinib interferes with the cell-mediated anti-tumor
activities of therapeutic CD20 antibodies: Implications for
combination therapy. Haematologica. 100:77–86. 2015. View Article : Google Scholar
|
|
12
|
Herman SE, Mustafa RZ, Gyamfi JA,
Pittaluga S, Chang S, Chang B, Farooqui M and Wiestner A: Ibrutinib
inhibits BCR and NF-kB signaling and reduces tumor proliferation in
tissue-resident cells of patients with CLL. Blood. 123:3286–3295.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
James RG, Biechele TL, Conrad WH, Camp ND,
Fass DM, Major MB, Sommer K, Yi X, Roberts BS, Cleary MA, et al:
Bruton's tyrosine kinase revealed as a negative regulator of Wnt-
beta- catenin signaling. Sci Signal. 2:2009. View Article : Google Scholar
|
|
14
|
Yang Y, Shi J, Gu Z, Salama ME, Das S,
Wendlandt E, Xu H, Huang J, Tao Y, Hao M, et al: Bruton tyrosine
kinase is a therapeutic target in stem-like cells from multiple
myeloma. Cancer Res. 75:594–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gutierrez A Jr, Tschumper RC, Wu X,
Shanafelt TD, Eckel-Passow J, Huddleston PM III, Slager SL, Kay NE
and Jelinek DF: LEF-1 is a prosurvival factor in chronic
lymphocytic leukemia and is expressed in the preleukemic state of
monoclonal B-cell lymphocytosis. Blood. 116:2975–2983. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ge X, Lv X, Feng L, Liu X, Gao J, Chen N
and Wang X: Metadherin contributes to the pathogenesis of diffuse
large B-cell lymphoma. PLoS One. 7:e394492012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Herman SE, Gordon AL, Hertlein E,
Ramanunni A, Zhang X, Jaglowski S, Flynn J, Jones J, Blum KA, Buggy
JJ, et al: Bruton tyrosine kinase represents a promising
therapeutic target for treatment of chronic lymphocytic leukemia
and is effectively targeted by PCI-32765. Blood. 117:6287–6296.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li P, Feng LL, Chen N, Ge XL, Lv X, Lu K,
Ding M, Yuan D and Wang X: Metadherin contributes to the
pathogenesis of chronic lymphocytic leukemia partially through
Wnt/β-catenin pathway. Med Oncol. 32:4792015. View Article : Google Scholar
|
|
20
|
Maddocks K, Christian B, Jaglowski S,
Flynn J, Jones JA, Porcu P, Wei L, Jenkins C, Lozanski G, Byrd JC
and Blum KA: A phase 1/1b study of rituximab, bendamustine and
ibrutinib in patients with untreated and relapsed/refractory
non-Hodgkin lymphoma. Blood. 125:242–248. 2015. View Article : Google Scholar
|
|
21
|
Hallek M, Kay NE, Osterborg A, Chanan-Khan
AA, Mahler M, Salman M, Wan Y, Sun S, Zhuang SH and Howes A: The
helios trial protocol: A Phase III study of ibrutinib in
combination with bendamustine and rituximab in relapsed/refractory
chronic lymphocytic leukemia. Future Oncol. 11:51–59. 2015.
View Article : Google Scholar
|
|
22
|
Cheng S, Guo A, Lu P, Ma J, Coleman M and
Wang YL: Functional characterization of BTK (C481S) mutation that
confers ibrutinib resistance: Exploration of alternative kinase
inhibitors. Leukemia. 29:885–900. 2015. View Article : Google Scholar
|
|
23
|
Zelenetz AD, Gordon LI, Wierda WG,
Abramson JS, Advani RH, Andreadis CB, Bartlett N, Byrd JC, Czuczman
MS, Fayad LE, et al National comprehensive cancer network:
Non-Hodgkin's lymphomas, version 4.2014. J Natl Compr Canc Netw.
12:1282–1303. 2014.PubMed/NCBI
|
|
24
|
Woyach JA, Furman RR, Liu TM, Ozer HG,
Zapatka M, Ruppert AS, Xue L, Li DH, Steggerda SM, Versele M, et
al: Resistance mechanisms for the bruton's tyrosine kinase
inhibitor ibrutinib. N Engl J Med. 370:2286–2294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Burger JA, Keating MJ, Wierda WG, Hartmann
E, Hoellenriegel J, Rosin NY, de Weerdt I, Jeyakumar G, Ferrajoli
A, Cardenas-Turanzas M, et al: Safety and activity of ibrutinib
plus rituximab for patients with high-risk chronic lymphocytic
leukaemia: A single-arm, phase 2 study. Lancet Oncol. 15:1090–1099.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nusse R: Wnt signaling in disease and in
development. Cell Res. 15:28–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gough NR: Focus issue: Wnt and β- catenin
signaling in development and disease. Sci Signal. 5:2012.
View Article : Google Scholar
|
|
29
|
Gordon MD and Nusse R: Wnt signaling:
Multiple pathways, multiple receptors and multiple transcription
factors. J Biol Chem. 281:22429–22433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Memarian A, Hojjat-Farsangi M,
Asgarian-Omran H, Younesi V, Jeddi-Tehrani M, Sharifian RA,
Khoshnoodi J, Razavi SM, Rabbani H and Shokri F: Variation in Wnt
genes expression in different subtypes of chronic lymphocytic
leukemia. Leuk Lymphoma. 50:2061–2070. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu D, Zhao Y, Tawatao R, Cottam HB, Sen M,
Leoni LM, Kipps TJ, Corr M and Carson DA: Activation of the Wnt
signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad
Sci USA. 101:3118–3123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gandhirajan RK, Staib PA, Minke K, Gehrke
I, Plickert G, Schlösser A, Schmitt EK, Hallek M and Kreuzer KA:
Small molecule inhibitors of Wnt/beta-catenin/lef-1 signaling
induces apoptosis in chronic lymphocytic leukemia cells in vitro
and in vivo. Neoplasia. 12:326–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gandhirajan RK, Poll-Wolbeck SJ, Gehrke I
and Kreuzer KA: Wnt/β-catenin/LEF-1 signaling in chronic
lymphocytic leukemia (CLL): A target for current and potential
therapeutic options. Curr Cancer Drug Targets. 10:716–727. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu D, Liu JX, Endo T, Zhou H, Yao S,
Willert K, Schmidt-Wolf IG, Kipps TJ and Carson DA: Ethacrynic acid
exhibits selective toxicity to chronic lymphocytic leukemia cells
by inhibition of the Wnt/beta-catenin pathway. PLoS One.
4:e82942009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu D, Choi MY, Yu J, Castro JE, Kipps TJ
and Carson DA: Salinomycin inhibits Wnt signaling and selectively
induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl
Acad Sci USA. 108:13253–13257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li P, Yao QM, Zhou H, Feng LL, Ge XL, Lv
X, Chen N, Lu K and Wang X: Metadherin contribute to BCR signaling
in chronic lymphocytic leukemia. Int J Clin Exp Pathol.
7:1588–1594. 2014.PubMed/NCBI
|