Introduction
Central nervous system complications occurring
following general anesthesia are receiving increased research
focus. Post-operative cognitive dysfunction (POCD) occurs within a
few days of anesthesia and affects cognitive ability, memory and
sleep in a reversible manner (1).
Sevoflurane is an inhaled anesthetic with rapid induction, and
minimal effects on the respiratory tract, circulation and metabolic
rate (2,3). In elderly patients, with brain oxygen
metabolism imbalance related to postoperative cognitive
dysfunction; Sevoflurane anesthesia in one-lung ventilation can
cause cerebral oxygen metabolism abnormalities, one-lung
ventilation after anesthesia in elderly patients with cognitive
function is ignored (4). In the
pathogenesis of POCD, inflammatory reactions and neuronal apoptosis
are suggested to serve important roles (5). Previous studies have indicated that
inflammatory reactions in POCD occur as a result of apoptotic
injury (6,7).
Tetrandrine is a bisbenzylisoquinoline alkaloid
extracted from Stephania tetrandra, a traditional Chinese
herbal medicine, and has been used for diseases including
hypertension, arrhythmia and pulmonary fibrosis (8). In recent years, a study indicated
that tetrandrine is able to inhibit the growth of a variety of
tumor cells, and as a result research has focused on its potential
use as an anticancer drug (9).
However, it remains to be elucidated whether tetrandrine is able to
affect sevoflurane-induced cognitive impairment in aged rats. In
the current study, the effect of tetrandrine on sevoflurane-induced
cognitive impairment was investigated in aged rats and the
potential mechanisms explored.
Materials and methods
Animals and surgery
Male 20-month-old Sprague-Dawley rats were obtained
from Shandong Hongli Medical Animal Experiment Research Co., Ltd.
(Shandong, China) and used in the present study, and were
acclimated in the animal care facility for 1 week prior to the
study. All rats were maintained in a 12:12 h light-dark cycle with
a constant room temperature (23±1°C) and allowed continuous access
to food and water. The experimental protocol was approved by the
Animal Ethics Committee of Zaozhuang Municipal Hospital (Shandong,
China). Rats were anesthetized in an environment containing 2%
sevoflurane (Sigma-Aldrich, St. Louis, MO, USA) for 5 h. The total
gas flow was 1.5 l/min, using 70% O2 as a carrier. The
oxygen and anesthetic agent fractions were measured using a gas
analysis system (TH-890B; Wuhan Tianhong Instruments Co., Ltd.,
Wuhan, China).
Experimental groups
A total of 32 rats were randomly divided into four
groups with 8 animals in each group: i) Control group (Con), normal
rats injected with saline (0.1 ml/100 g intraperitoneally, i.p.);
ii) Control-tetrandrine group (C-Tet), normal rats received
tetrandrine (30 mg/kg body weight, i.p., every 3 days;
Sigma-Aldrich) for 4 consecutive weeks; iii) Sevoflurane group
(Seo), sevoflurane-induced rats were anesthetized in an environment
containing 2% sevoflurane for 5 h, and received saline (0.1 ml/100
g, i.p.); iv) Sevoflurane-tetrandrine group (S-Tet),
sevoflurane-induced rats were anesthetized in an environment
containing 2% sevoflurane for 5 h, and received tetrandrine (30
mg/kg body weight, i.p., every 3 days) for 4 consecutive weeks. The
structure of tetrandrine (purity, >99%) is presented in Fig. 1.
Morris water maze (MWM)
Following treatment with tetrandrine, the spatial
memory ability of rats was investigated using the MWM test. A
circular, black painted pool (180 cm diameter, 50 cm deep) was
filled with water containing black non-toxic ink (temperature at
25±1°C) to a depth of 30 cm. A hidden platform (10 cm diameter) was
submerged 1 cm below the water line and placed in the center of the
northeast quadrant, which was determined with four starting
locations called north (N), east (E), south (S) and west (W) at
equal distance around the rim. The rats were tested 4 times/day
over 4 consecutive days. The time taken to escape to the hidden
platform was recorded as escape latency. Mean path length was
measured as the mean distance of movement for each rat. Time spent
in the target quadrant was recorded using a stopwatch. In turn,
each rat was placed in a random starting position, facing the pool
wall and allowed to swim and self-discover the hidden platform.
Once the rat arrived at the platform, it was allowed to stay on it
for 30 sec. On the 5th day, the hidden platform was removed, and
each rat was allowed to swim freely for 60 sec. The number of times
the rat crossed the platform was recorded within 60 sec and
swimming speed was calculated as the distance × number of times
crossing platform (n)/60 sec.
Western blot analysis
Following treatment with tetrandrine, the rats were
anesthetized with 2% sevoflurane (Sigma-Aldrich) and were
sacrificed by spinal dislocation, and the brain tissue was
obtained. The brain tissue was homogenized in
radioimmunoprecipitation assay lysis buffer (Sangon Biotech Co.,
Ltd., Shanghai, China) containing 1 mM ethylenediaminetetraacetic
acid, 50 mM Tris-HCl, 150 mM NaCl, 0.1% sodium dodecyl sulfate
(SDS; Nanjing Sunshine Biotechnology Co., Ltd., Nanjing, China),
0.25% sodium deoxycholate (Nanjing Sunshine Biotechnology Co.,
Ltd.), 1% NP-40 (Nanjing Sunshine Biotechnology Co., Ltd.), a
protease inhibitor cocktail and phosphatase inhibitors (both Sangon
Biotech Co., Ltd.). Following homogenization (12,000× g, 4°C, 10
min), the clear upper supernatants were collected, and the total
protein measured using a bicinchoninic acid (BCA) protein assay
(Beyotime Institute of Biotechnology, Shanghai, China). Equal
amounts of proteins were loaded onto a 10% SDS-PAGE gel (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) for
electrophoresis and then transferred onto nitrocellulose membranes
(EMD Millipore, Billerica, MA, USA). Membranes were blocked with 5%
(w/v) skimmed milk in 0.1% (v/v) Tween 20 (Nanjing Jiancheng
Bioengineering Institute) in phosphate-buffered saline (PBST) for 2
h. The membranes were then incubated with rabbit polyclonal
anti-cyclooxygenase-2 (COX-2; 1:1,000; cat. no. D151772; Sangon
Biotech Co., Ltd.), mouse monoclonal anti-inducible nitric oxide
synthase (iNOS; 1:1,000; cat. no. sc-5302; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), mouse monoclonal anti-Bcl-2
(1:500; cat. no. sc-509; Santa Cruz Biotechnology, Inc.) and rabbit
polyclonal anti-β-actin (1:5,000; cat. no. D110007; Sangon Biotech
Co., Ltd.) antibodies overnight at 4°C with agitation. Antibody
recognition was detected using anti-rabbit and anti-mouse antibody
(1:500 and 1:5,000 in PBS containing 1% normal goat serum,
respectively; cat. nos. sc-2491 and sc-358922, respectively; Santa
Cruz Biotechnology, Inc.). Antibody-bound proteins were detected
using an enhanced chemiluminescence western blotting analysis
system (Vector Laboratories, Inc., Burlingame, CA, USA).
Enzyme-linked immunosorbent assays
(ELISA)
Following treatment with tetrandrine, the brain
tissue was homogenized in radioimmunoprecipitation assay lysis
buffer. The homog-enate was centrifuged at 12,000 × g for 15 min at
4°C. The clear upper supernatants were collected for analysis of
interleukin (IL)-1β, tumor necrosis factor-α (TNF-α) and nuclear
factor-κB (NF-κB) activities according to the manufacturer's
instructions (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China).
Measurement of caspase-3 activity
Following treatment with tetrandrine, the brain
tissue was homogenized in radioimmuno-precipitation assay lysis
buffer. The homogenate was centrifuged at 12,000 × g for 15 min at
4°C, and the clear upper supernatants collected and the total
protein content was measured using a BCA protein assay (Beyotime
Institute of Biotechnology). Equal amounts of protein were added to
the reaction buffer containing Ac-DEVD-pNA (2 mM; Beyotime
Institute of Biotechnology) and incubated at 37°C for 6 h.
Caspase-3 activation was measured using a Microplate reader
(EMR500; Labomed, Inc., Los Angeles, CA, USA) at an absorbance of
405 nm.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Differences between groups were
investigated using SPSS software, version 19.0 (IBM SPSS, Armonk,
NY, USA) and a one-way analysis of variance followed by Dunnett's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Tetrandrine improves learning and memory
deficits in sevoflurane-treated aged rats
In order to investigate the effects of tetrandrine
on learning and memory in sevoflurane-treated aged rats, learning
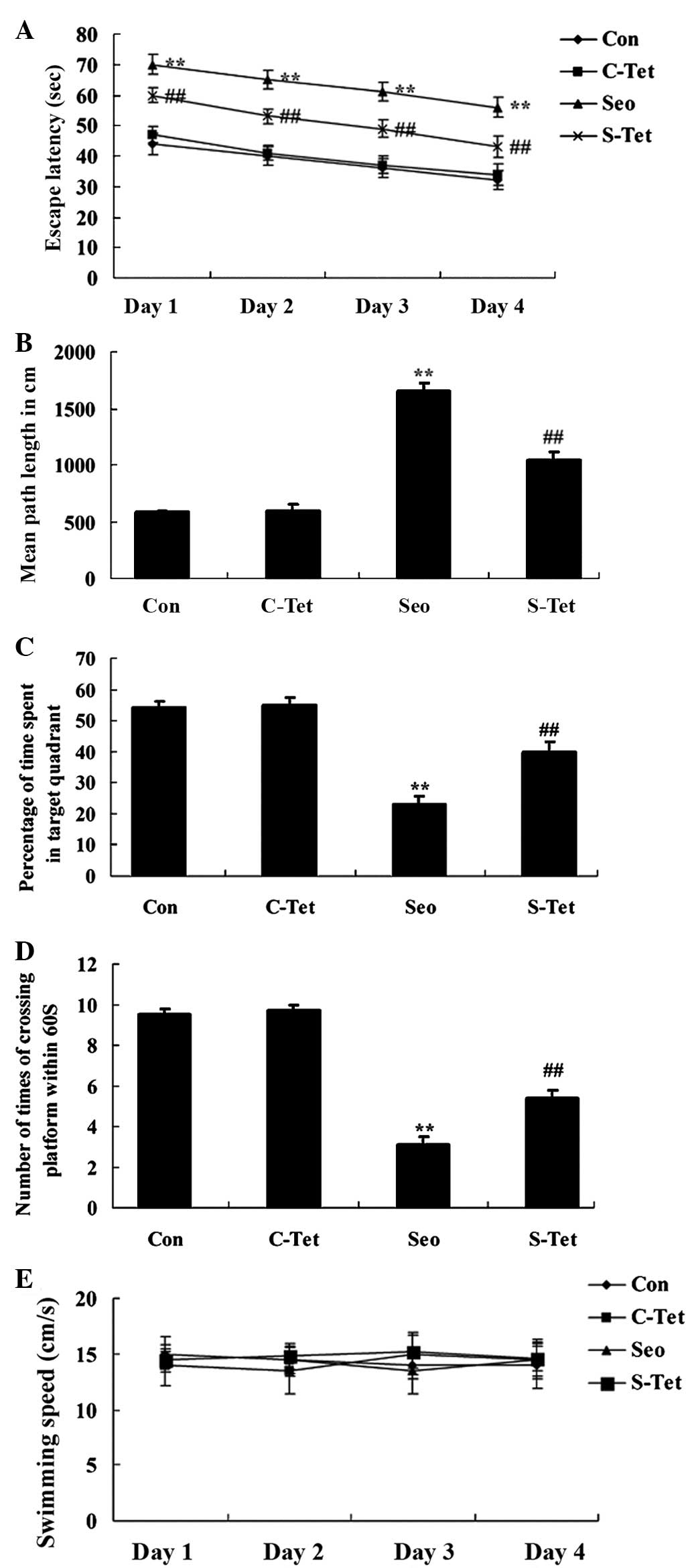
and memory were measured using MWM tests. Fig. 2A shows that the escape latency was
increased following treatment with sevoflurane, compared with the
control group. However, treatment with tetrandrine reduced the
escape latency in sevoflurane-treated aged rats (Fig. 2A). In addition, in the sevoflurane
group, the mean path length was increased compared with the control
group (Fig. 2B). Pretreatment with
tetrandrine significantly reduced the mean path length in
sevoflurane-treated aged rats (P<0.01; Fig. 2B).
As presented in Fig. 2C
and D, sevoflurane significantly reduced the time the rats
spent in the target quadrant and the number of times the rats
crossed the platform location, compared with the control group.
Notably, treatment with tetrandrine improved these alterations in
sevoflurane-treated rats (Fig. 2C and
D). However, no significant alterations in swimming speed were
observed in any of the groups (Fig.
2E). Therefore, tetrandrine at 30 mg/kg was observed to provide
protection against sevoflurane-induced cognitive impairment in
rats.
Tetrandrine reduces COX-2 expression in
sevoflurane-treated aged rats
In order to investigate the protective effect of
tetrandine in sevoflurane-treated aged rats, the expression levels
of COX-2 were measured using western blot analysis. Western
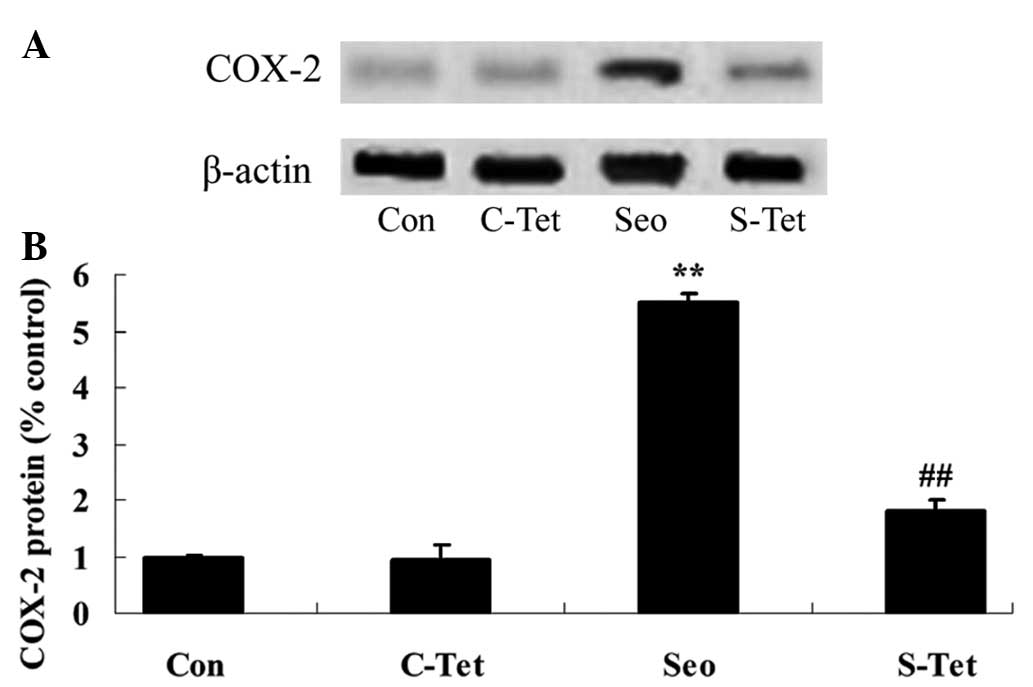
blotting indicated that COX-2 protein expression was upregulated
following sevoflurane treatment (Fig.
3A and B). Co-treatment of rats with tetrandrine significantly
reduced COX-2 protein expression (P<0.01; Fig. 3A and B).
Tetrandrine reduces the levels of IL-1β
and TNF-α in sevoflurane-treated aged rats
To investigate the protective effects of tetrandrine
on inflammation in sevoflurane-treated aged rats, the expression
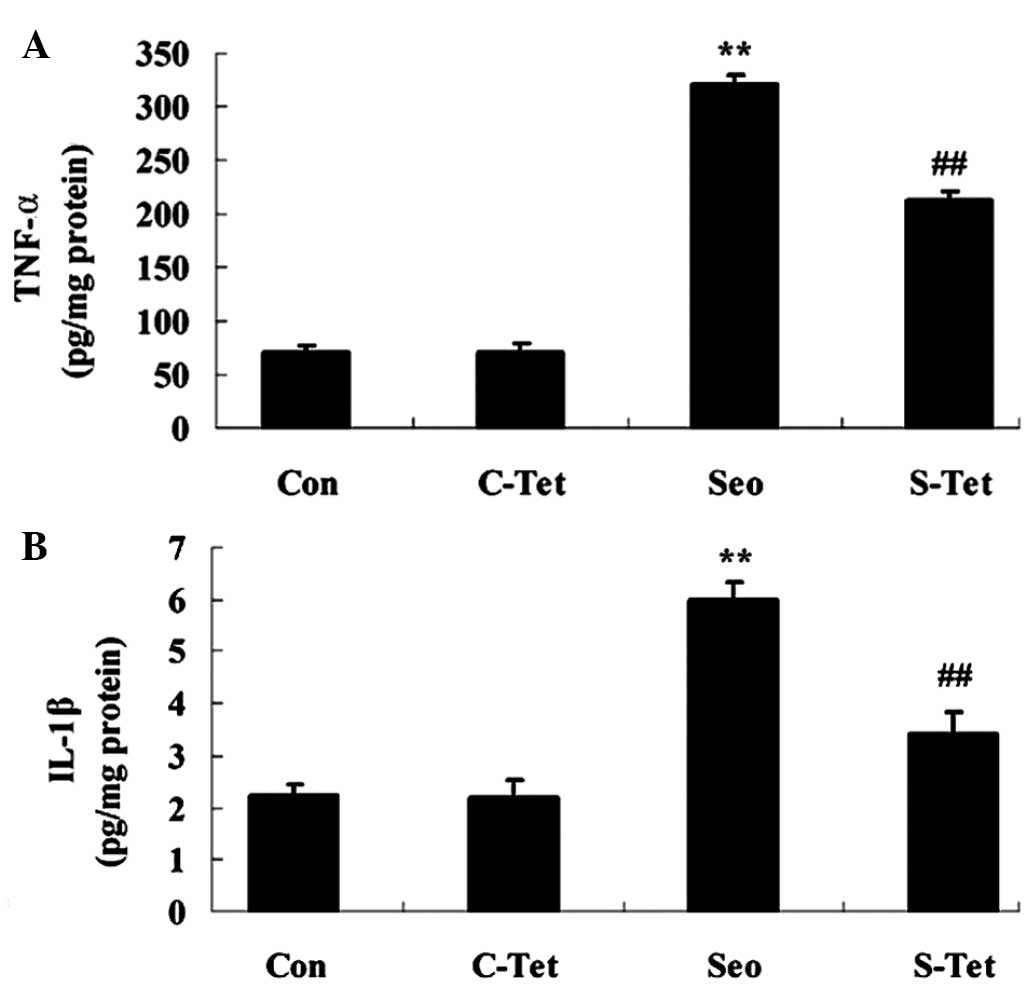
levels of IL-1β and TNF-α were measured. This indicated an increase
in IL-1β and TNF-α production in the sevoflurane-treated aged rats
compared with the control group (Fig.
4A and B). Compared with the sevoflurane group rats, the levels
of IL-1β and TNF-α were significantly reduced in the
sevoflurane-tetrandrine group (P<0.01; Fig. 4A and B).
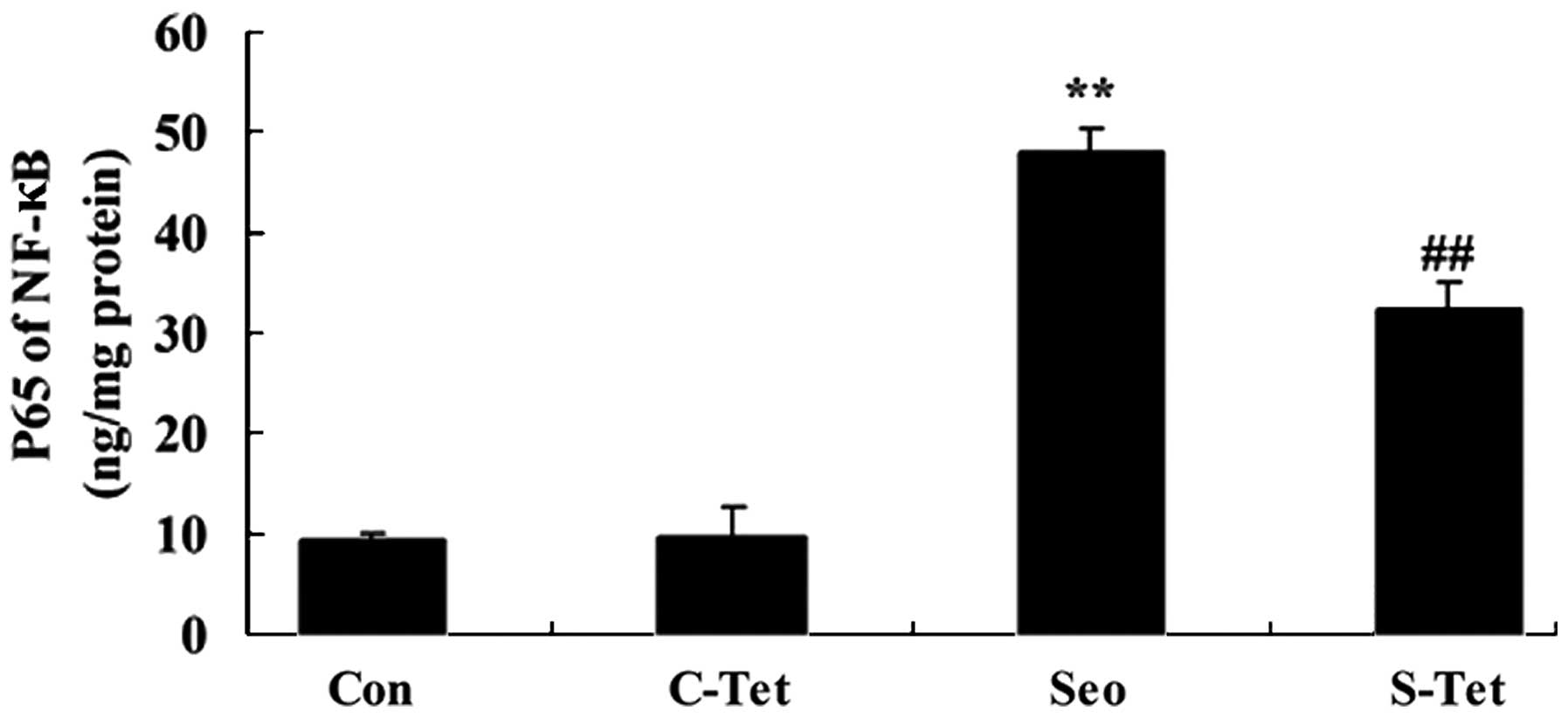
Tetrandrine reduces NF-κB in
sevoflurane-treated aged rats
To further investigate the protective effects of
tetrandrine on inflammation in sevoflurane-treated aged rats, the
activity of NF-κB was measured. As presented in Fig. 5, the activity of NF-κB was
increased by sevoflurane compared with the control. However,
tetrandrine significantly reduced the activity of NF-κB in
sevoflurane-treated aged rats (P<0.01; Fig. 5).
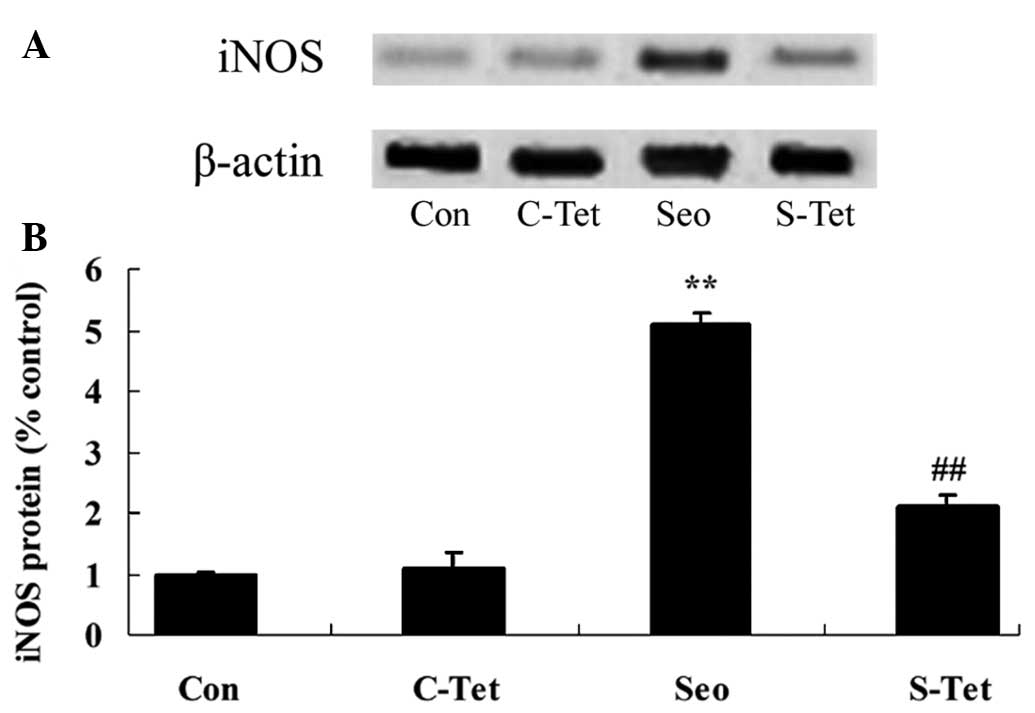
Tetrandrine reduces iNOS expression
levels in sevoflurane-treated aged rats
To further investigate the protective effects of
tetrandrine on sevoflurane-treated aged rats, iNOS protein
expression was measured by western blot analysis. The protein
expression of iNOS was increased in the sevoflurane group compared
with the control group (Fig. 6A and
B). Following tetrandrine treatment, iNOS protein expression
was significantly reduced in the sevoflurane-treated aged rats
(P<0.01; Fig. 6A and B).
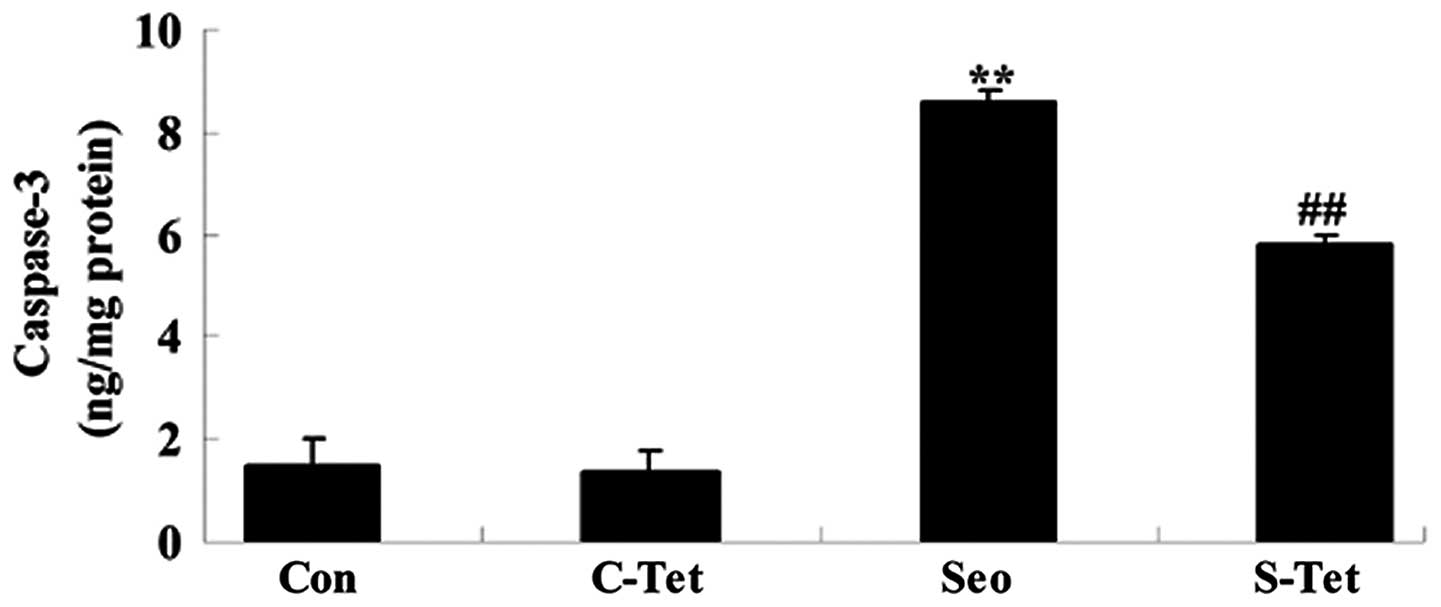
Tetrandrine reduces caspase-3 activity in
sevoflurane-treated aged rats
To investigate whether alterations in apoptosis may
serve a role in the protective effects of tetrandrine on
sevoflurane-treated aged rats, the activity of caspase-3 was
measured. As presented in Fig. 7,
sevoflurane significantly increased the activity of caspase-3
compared with the control group. However, treatment with
tetrandrine significantly reduced the increase in caspase-3
activity in sevoflurane-treated aged rats (P<0.01; Fig. 7).
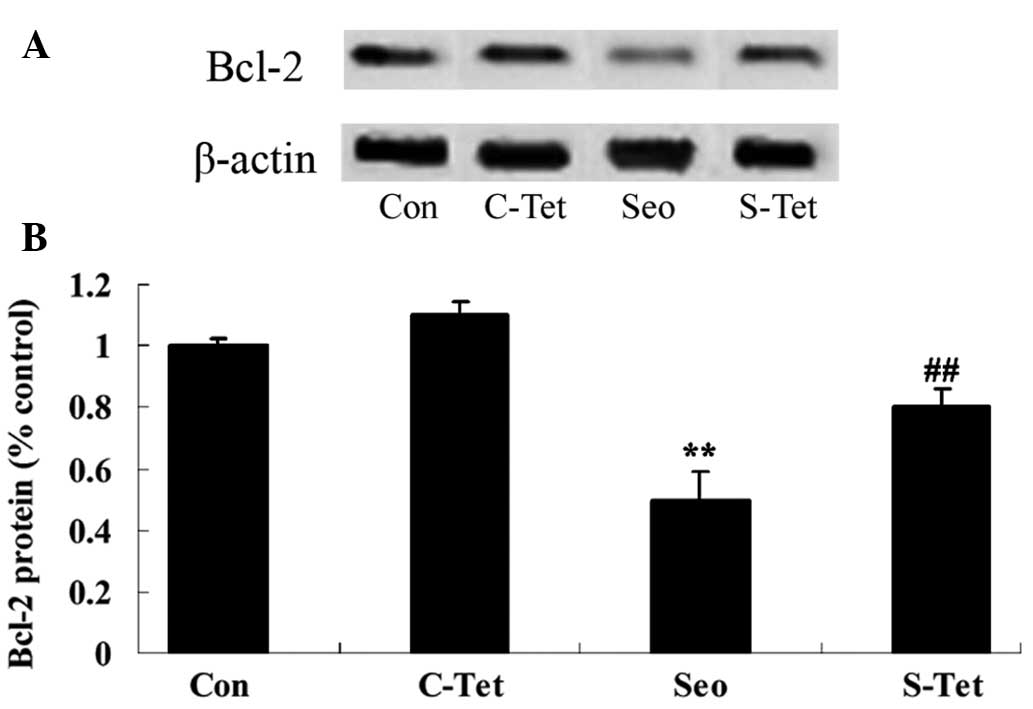
Tetrandrine reduces Bcl-2 expression
levels in sevoflurane-treated aged rats
To further investigate the protective effects of
tetrandrine on apoptosis in sevofluane-treated aged rats, Bcl-2
protein expression was measured using western blot analysis. As
presented in Fig. 8A and B,
sevoflurane reduced the expression levels of Bcl-2, compared with
the control group. However, tetrandrine resulted in an increase in
the expression levels of Bcl-2 in sevoflurane-treated aged rats
(P<0.01; Fig. 8A and B).
Discussion
POCD is a common complication in elderly patients
following surgery, and presents clinically as cognitive
dysfunction, memory impairment, personality alterations and
problems with social integration (10). POCD is associated with anesthesia.
Anesthesia results in central nervous system dysfunction, via
alterations in acetylcholine, dopamine and catechol-amines
(11). Sevoflurane is an inhaled
anesthetic which results in quick induction of anesthesia and has
been widely used clinically. However, studies have indicated that
sevoflurane anesthesia may lead to POCD in elderly patients
(12,13). Tetrandrine is a
bisbenzylisoquinoline alkaloid extracted from Stephania
tetrandra, a traditional Chinese herbal medicine, and has been
demonstrated to posses anti-inflammatory, analgesic and
antihypertensive effects, and to block calcium channels (14–17).
In the current study, tetrandrine was demonstrated to improve the
learning and memory deficits observed in sevoflurane-treated aged
rats. Chen et al (18)
reported that tetrandrine was able to ameliorate cognitive
impairment in a rat model of chronic cerebral hypoperfusion. In
addition, He et al (19)
demonstrated that tetrandrine attenuated spatial memory impairments
in a rat model of Alzheimer's disease (19). Thus, tetrandrine may represent a
potential candidate for the treatment of sevoflurane-induced
cognitive impairment.
Inflammatory reactions, the activation of COX-2, and
increased prostaglandin levels are closely associated processes in
dentate gyrus granular cells, hippocampal pyramidal neurons, the
pear-shaped zone, the new shallow cortex cell layer, the lower part
of the striatum, and the thalamus of POCD rats (20–22).
A previous study indicated that COX-2-dependent mechanisms may
serve a role in POCD (20). In the
present study, tetrandrine reduced COX-2 protein expression in
sevoflurane-treated aged rats. In support of this, Wu et al
(23) reported that tetrandrine
treatment reduced COX-2 expression in human monocytic cells. Kang
et al (24) indicated that
tetrandrine suppressed the production of pro-inflammatory mediators
via the suppression of COX-2 expression in stimulated mast cells
(24). However, the effect of
various doses of tetrandrine on sevoflurane-induced COX-2
expression requires further investigation.
POCD is a common complication in elderly patients
following surgery. The mechanism of POCD is not fully understood,
however a previous study suggests that inflammation within the
central nervous system may serve an important role (25). Peripheral inflammation is able to
result in inflammatory responses in the central nervous system via
a variety of mechanisms, leading to neuronal dysfunction,
inhibition of neuronal regeneration and the induction of
apop-tosis, ultimately resulting in reduced cognitive function
(26). In addition, the present
study demonstrated that tetrandrine is able to reduce the levels of
IL-1β, TNF-α and NF-κB in sevoflurane-treated aged rats. A study by
Lin et al (27) indicated
that tetrandrine suppressed LPS-induced astrocyte activation via
inhibition of IL-1β, TNF-α and NF-κB signaling. In addition, Zhang
et al (28) reported that
administration of tetrandrine significantly reduced IL-1β, TNF-α
and NF-κB levels in a mouse model of ulcerative colitis.
Nitric oxide (NO) is a gas with a short half-life,
which is able to affect the body in a variety of pathological
processes. Within the central nervous system, NO serves a dual
function of neuronal protection and neurotoxicity. The present
study indicated that the levels of iNOS increased significantly in
sevoflurane-induced rats, suggesting the production of NO may be
associated with POCD (29). In
addition, it has previously been indicated that apoptosis occurring
following POCD is associated with increased production of NO
(30). A previous study
demonstrated that using a selective iNOS inhibitor resulted in the
reduction in the expression of caspase-3 (31). Therefore, the expression of iNOS
may induce cell apoptosis, which is important in POCD (32). Notably, the current study observed
that treatment with tetrandrine inhibited the increase in the
levels of iNOS in the sevoflurane-treated aged rats. Previous
studies have suggested that tetrandrine inhibits the activation of
mesangial cells through a reduction in the expression of iNOS
(33). Wang et al (34) reported that tetrandrine treatment
reduced the production of iNOS and COX-2 expression in vitro
and in vivo.
Bcl-2, Bax and caspase 3 are important
apoptosis-related proteins in the hippocampus, which are associated
with POCD. In the Bcl-2 family of proteins, Bcl-2 is an important
anti-apoptotic protein whilst Bax promotes apoptosis (35). Bcl-2 is predominantly localized to
the nuclear membrane, endoplasmic reticulum and the mitochondrial
membrane. Under normal physiological conditions, Bax is located in
the cytoplasm, however, following apoptotic stimuli it undergoes
conformational alterations and becomes associated with the
mitochondrial membrane. Bcl-2 functions as an anti-apoptotic
protein by binding to Bax and preventing this process from
occurring (30). Caspase-3 is a
key executioner of apoptosis and its activation serves an important
role in triggering downstream apoptotic processes (36). The current study indicates that
tetrandrine reduced the increase in caspase-3 activity observed in
sevoflurane-treated rats. In addition, tetrandrine-treated
sevoflurane-induced rats were observed to have increased expression
levels of Bcl-2 compared with untreated sevoflurane-treated rats.
In support of this, Gong et al (37) indicated that tetrandrine attenuated
lipopolysaccharide-induced fulminant hepatic failure through a
reduction in the levels of caspase-3.
In conclusion, the current study indicates that
tetrandrine is able to ameliorate sevoflurane-induced cognitive
impairment via the suppression of inflammation and apoptosis. This
suggests that tetrandrine may be a potential candidate treatment to
ameliorate cognitive impairment and alleviate neurodegeneration in
sevoflurane-treated elderly patients. Further in vivo or
in vitro studies are required to investigate the effects of
tetrandrine on cognitive function following sevoflurane exposure,
and whether there is an effect upon the interaction between neurons
and microglia following treatment with tetrandrine.
References
|
1
|
Liu J, Wang P, Zhang X, Zhang W and Gu G:
Effects of different concentration and duration time of isoflurane
on acute and long-term neurocognitive function of young adult
C57BL/6 mouse. Int J Clin Exp Pathol. 7:5828–5836. 2014.PubMed/NCBI
|
|
2
|
Boost KA, Leipold T, Scheiermann P, Hoegl
S, Sadik CD, Hofstetter C and Zwissler B: Sevoflurane and
isoflurane decrease TNF-alpha-induced gene expression in human
monocytic THP-1 cells: Potential role of intracellular IkappaBalpha
regulation. Int J Mol Med. 23:665–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le Freche H, Brouillette J,
Fernandez-Gomez FJ, Patin P, Caillierez R, Zommer N, Sergeant N,
Buée-Scherrer V, Lebuffe G, Blum D and Buée L: Tau phosphorylation
and sevoflurane anesthesia: An association to postoperative
cognitive impairment. Anesthesiology. 116:779–787. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y, Pan N, Ma Y, Zhang S, Guo W, Li H,
Zhou J, Liu G and Gao M: Inhaled sevoflurane may promote
progression of amnestic mild cognitive impairment: A prospective,
randomized parallel-group study. Am J Med Sci. 345:355–360. 2013.
View Article : Google Scholar
|
|
5
|
Wan Y, Xu J, Ma D, Zeng Y, Cibelli M and
Maze M: Postoperative impairment of cognitive function in rats: A
possible role for cytokine-mediated inflammation in the
hippo-campus. Anesthesiology. 106:436–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen W, Liu B, Zhang F, Xue P, Cui R and
Lei W: The effects of dexmedetomidine on post-operative cognitive
dysfunction and inflammatory factors in senile patients. Int J Clin
Exp Med. 8:4601–4605. 2015.PubMed/NCBI
|
|
7
|
Su X, Feng X, Terrando N, Yan Y, Chawla A,
Koch LG, Britton SL, Matthay MA and Maze M: Dysfunction of
inflammation-resolving pathways is associated with exaggerated
postoperative cognitive decline in a rat model of the metabolic
syndrome. Mol Med. 18:1481–1490. 2012. View Article : Google Scholar
|
|
8
|
Fang DC and Jiang MX: Studies on
tetrandrine calcium antagonistic action. Chin Med J (Engl).
99:638–644. 1986.
|
|
9
|
Shi C, Ahmad Khan S, Wang K and Schneider
M: Improved delivery of the natural anticancer drug tetrandrine.
Int J Pharm. 479:41–51. 2015. View Article : Google Scholar
|
|
10
|
Wang Y, He H, Li D, Zhu W, Duan K, Le Y,
Liao Y and Ou Y: The role of the TLR4 signaling pathway in
cognitive deficits following surgery in aged rats. Mol Med Rep.
7:1137–1142. 2013.PubMed/NCBI
|
|
11
|
Leijten FS, Alpherts WC, Van Huffelen AC,
Vermeulen J and Van Rijen PC: The effects on cognitive performance
of tailored resection in surgery for nonlesional mesiotemporal lobe
epilepsy. Epilepsia. 46:431–439. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin Y, Zhao X, Li H, Wang Z and Wang D:
Effects of sevoflurane and propofol on the inflammatory response
and pulmonary function of perioperative patients with one-lung
ventilation. Exp Ther Med. 6:781–785. 2013.PubMed/NCBI
|
|
13
|
Cremer J, Stoppe C, Fahlenkamp AV, Schälte
G, Rex S, Rossaint R and Coburn M: Early cognitive function,
recovery and well-being after sevoflurane and xenon anaesthesia in
the elderly: A double-blinded randomized controlled trial. Med Gas
Res. 1:92011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He FQ, Qiu BY, Li TK, Xie Q, Cui J, Huang
XL and Gan HT: Tetrandrine suppresses amyloid-β-induced
inflammatory cytokines by inhibiting NF-κB pathway in murine BV2
microglial cells. Int Immunopharmacol. 11:1220–1225. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park PH, Nan JX, Park EJ, Kang HC, Kim JY,
Ko G and Sohn DH: Effect of tetrandrine on experimental hepatic
fibrosis induced by bile duct ligation and scission in rats.
Pharmacol Toxicol. 87:261–268. 2000. View Article : Google Scholar
|
|
16
|
Zhang J, Yu B, Zhang XQ, Sheng ZF, Li SJ,
Wang ZJ, Cui XY, Cui SY and Zhang YH: Tetrandrine, an
antihypertensive alkaloid, improves the sleep state of
spontaneously hypertensive rats (SHRs). J Ethnopharmacol.
151:729–732. 2014. View Article : Google Scholar
|
|
17
|
Bickmeyer U, Weinsberg F, Müller E and
Wiegand H: Blockade of voltage-operated calcium channels, increase
in spontaneous catecholamine release and elevation of intracellular
calcium levels in bovine chromaffin cells by the plant alkaloid
tetrandrine. Naunyn Schmiedebergs Arch Pharmacol. 357:441–445.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen L, Chen L, Lv Y, Cui Z, Bei G, Qin G,
Zhou J and Ge T: Tetrandrine ameliorates cognitive impairment via
inhibiting astrocyte-derived S100B activation in a rat model of
chronic cerebral hypoperfusion. Neurol Res. 35:614–621. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He FQ, Qiu BY, Zhang XH, Li TK, Xie Q, Cui
DJ, Huang XL and Gan HT: Tetrandrine attenuates spatial memory
impairment and hippocampal neuroinflammation via inhibiting NF-κB
activation in a rat model of Alzheimer's disease induced by
amyloid-β(1–42). Brain Res. 1384:89–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamer AR, Galoyan SM, Haile M, Kline R,
Boutajangout A, Li YS and Bekker A: Meloxicam improves object
recognition memory and modulates glial activation after splenectomy
in mice. Eur J Anaesthesiol. 29:332–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li YC, Xi CH, An YF, Donh WH and Zhou M:
Perioperative inflammatory response and protein S-100β
concentrations - relationship with post-operative cognitive
dysfunction in elderly patients. Acta Anesthesiol Scand.
56:595–600. 2012. View Article : Google Scholar
|
|
22
|
Cheng Q, Wang J, Wu A, Zhang R, Li L and
Yue Y: Can urinary excretion rate of 8-isoprostrane and
malonaldehyde predict postoperative cognitive dysfunction in aging?
Neurol Sci. 34:1665–1669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu SJ and Ng LT: Tetrandrine inhibits
proinflammatory cytokines, iNOS and COX-2 expression in human
monocytic cells. Biol Pharm Bull. 30:59–62. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang OH, An HJ, Kim SB, Mun SH, Seo YS,
Joung DK, Choi JG, Shin DW and Kwon DY: Tetrandrine suppresses
pro-inflammatory mediators in PMA plus A23187-induced HMC-1 cells.
Int J Mol Med. 33:1335–1340. 2014.PubMed/NCBI
|
|
25
|
Hu Z, Ou Y, Duan K and Jiang X:
Inflammation: A bridge between postoperative cognitive dysfunction
and Alzheimer's disease. Med Hypotheses. 74:722–724. 2010.
View Article : Google Scholar
|
|
26
|
Windham BG, Simpson BN, Lirette S, Bridges
J, Bielak L, Peyser PA, Kullo I, Turner S, Griswold ME and Mosley
TH: Associations between inflammation and cognitive function in
African Americans and European Americans. J Am Geriatr Soc.
62:2303–2310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin ST, Wang Y, Xue Y, Feng DC, Xu Y and
Xu LY: Tetrandrine suppresses LPS-induced astrocyte activation via
modulating IKKs-IkappaBalpha-NF-kappaB signaling pathway. Mol Cell
Biochem. 315:41–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang DK, Cheng LN, Huang XL, Shi W, Xiang
JY and Gan HT: Tetrandrine ameliorates
dextran-sulfate-sodium-induced colitis in mice through inhibition
of nuclear factor-kappaB activation. Int J Colorectal Dis. 24:5–12.
2009. View Article : Google Scholar
|
|
29
|
Tang N, Ou C, Liu Y, Zuo Y and Bai Y:
Effect of inhalational anaesthetic on postoperative cognitive
dysfunction following radical rectal resection in elderly patients
with mild cognitive impairment. J Int Med Res. 42:1252–1261. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qian XL, Zhang W, Liu MZ, Zhou YB, Zhang
JM, Han L, Peng YM, Jiang JH and Wang QD: Dexmedetomidine improves
early postoperative cognitive dysfunction in aged mice. Eur J
Pharmacol. 746:206–212. 2015. View Article : Google Scholar
|
|
31
|
Kiang JG, Aggravante NG, Smith JT and
Bowman PD: 17-DMAG diminishes hemorrhage-induced small intestine
injury by elevating Bcl-2 protein and inhibiting iNOS pathway,
TNF-α increase, and caspase-3 activation. Cell Biosci. 11:212011.
View Article : Google Scholar
|
|
32
|
Jia M, Liu WX, Sun HL, Chang YQ, Yang JJ,
Ji MH, Yang JJ and Feng CZ: Suberoylanilide hydroxamic acid, a
histone deacetylase inhibitor, attenuates postoperative cognitive
dysfunction in aging mice. Front Mol Neurosci. 8:522015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu CJ, Wang YH, Lin CJ, Chen HH and Chen
YJ: Tetrandrine down-regulates ERK/NF-κB signaling and inhibits
activation of mesangial cells. Toxicol In Vitro. 25:1834–1840.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang QS, Cui YL, Gao LN, Guo Y, Li RX and
Zhang XZ: Reduction of the pro-inflammatory response by
tetrandrine-loading poly(L-lactic acid) films in vitro and in vivo.
J Biomed Mater Res A. 102:4098–4107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li SY, Xia LX, Zhao YL, Yang L, Chen YL,
Wang JT and Luo AL: Minocycline mitigates isoflurane-induced
cognitive impairment in aged rats. Brain Res. 1496:84–93. 2013.
View Article : Google Scholar
|
|
36
|
Chen R, Liu S, Piao F, Wang Z, Qi Y, Li S,
Zhang D and Shen J: 2,5-hexanedione induced apoptosis in
mesenchymal stem cells from rat bone marrow via
mitochondria-dependent caspase-3 pathway. Ind Health. 53:222–235.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gong X, Luo FL, Zhang L, Li HZ, Wu MJ, Li
XH, Wang B, Hu N, Wang CD, Yang JQ and Wan JY: Tetrandrine
attenuates lipopolysaccharide-induced fulminant hepatic failure in
D-galactosamine-sensitized mice. Int Immunopharmacol. 10:357–363.
2010. View Article : Google Scholar
|