Introduction
Liver fibrosis is a common wound-healing response of
the liver to a variety of chronic injuries, including infections,
particularly hepatitis B or C, alcoholic steatohepatitis,
non-alcoholic steatohepatitis and toxic agents, and is
characterized by an excessive deposition of extracellular matrix
(ECM) (1,2). It has been demonstrated that
activated hepatic stellate cells (HSCs) are the predominant cell
type responsible for ECM accumulation in the liver, and their
activation is associated with specific cytoskeletal and phenotypic
profiles (3,4). Therefore, the majority of
antifibrotic therapies are designed to inhibit the activation and
proliferation of HSCs. Platelet-derived growth factor (PDGF) is the
most potent mitogenic and proliferative cytokine described for HSCs
(5). PDGF-BB is the most potent
PDGF isoform and has been shown to be the most effective stimulator
of HSC proliferation (6). PDGF
receptor β (PDGFRβ) amplifies biological responses to PDGF-BB,
leading to the activation of downstream signaling pathways in HSCs
(7).
The Wnt signaling pathway is essential for
embryogenesis and adult tissue maintenance, and disturbance in this
signaling promotes neurodegenerative diseases and cancer (8–10).
There are two predominant Wnt signaling pathways: The canonical
Wnt/β-catenin pathway and the non-canonical Wnt/Ca2+
pathway. In previous decades, the canonical and non-canonical Wnt
signaling pathway have been reported to be important in liver
development and remodeling, and HSC activation (11–13).
Previously, it was reported that the Wnt/Ca2+ signaling
pathway can be activated in human HSCs induced by PDGF-BB, and is
involved in HSC activation and proliferation (14). However, the role of the
non-canonical Wnt signaling pathway in this specific
pathophysiological process has received little attention, and
remains to be fully elucidated, particularly concerning the
Wnt/Ca2+ signaling pathway (15).
Traditional Chinese herbs have been widely used as
hepatoprotective and antifibrotic drugs in humans (16) and animal models (17), with novel characteristics,
including being multi-ingredient, multi-targeting and with low
adverse effects. Gan-fu-kang (GFK) is a complex prescription
Chinese herbal medicine composed of 11 medical herbs, including
Salvia miltiorrhiza, Astragalus membranaceus, red
peony root, white peony root, Radix paeoniae alba and
Chinese thorowax root (18). This
herbal formula is considered to have effects in increasing blood
volume, energy and blood flow to the liver (18). Our pilot study showed that GFK has
markedly protective and therapeutic effects in an animal model of
hepatic injury induced by carbon tetrachloride (CCl4)
(18). Mechanistic investigations
have shown that GFK downregulates the mitogen-activated protein
kinase/activator protein 1 pathway (19) and canonical Wnt/β-catenin signaling
pathway (20) in the fibrotic
liver. However, the mechanism by which GFK inhibits liver fibrosis
remains to be fully elucidate.
The aim of the present study was to examine the
protective effect of GFK on hepatic fibrosis in liver tissues from
Sprague-Dawley rats and HSC-T6 cells. Furthermore, the present
study aimed to confirm whether GFK attenuates hepatic fibrosis via
the Wnt/Ca2+ pathway to elucidate the possible
underlying mechanism of its anti-fibrotic effect.
Materials and methods
Herbal medicine composition
GFK consists of 11 herbs, including 30 g each of
Salviae miltiorrhizae radix and Milkvetch root; 20 g each of
Fructus aurantii and Hoelen; 15 g each of Radix paeoniae rubra,
Radix paeoniae alba, Radix angelicae sinensis, Radix rehmanniae and
Rhizoma atractylodis macrocephalae; and 10 g each of Radix bupleuri
and Radix glycyrrhizae (Table I).
The 11 crude drugs were purchased from the pharmacy of the Second
Affiliated Hospital of Dalian Medical University (Dalian, China)
and extracted by the Department of Pathophysiology of Dalian
Medical University. The herbal decoction was stored at −20°C.
 | Table IComponents of the herbal prescription
Gan-fu-kang. |
Table I
Components of the herbal prescription
Gan-fu-kang.
| Herb name | Scientific
name | Local name | Place of
origin
(China) | Relative
quantity (g) |
|---|
| Salviae
miltiorrhizae radix | Salvia
miltiorrhiza Bunge | Dan Shen | Hebei | 30 |
| Milkvetch root | Astragalus
membranaceus (Fisch.) Bunge | Huang Qi | Shanxi | 30 |
| Fructus
aurantii | Citrus
aurantium L. | ZhiKe | Hubei | 20 |
| Hoelen | Poria cocos
(Schw.) Wolf | Fu Ling | Hubei | 20 |
| Radix paeoniae
rubra | Paeonia
veitchii Lynch | Chi Shao | Sichuan | 15 |
| Radix paeoniae
alba | Paeonia
emodi subsp. sterniana (H.R. Fletcher) Halda | Bai Shao | Anhui | 15 |
| Radix angelicae
sinensis | Angelica
sinensis (Oliv.) Diels | Dang Gui | Gansu | 15 |
| Radix
rehmanniae | Rehmannia
chingii H.L. Li | Di Huang | Liaoning | 15 |
| Rhizoma
atractylodis macrocephalae | Atractylodes
macrocephala Koidz. | Bai Zhu | Zhejiang | 15 |
| Radix bupleuri | Bupleurum
chinense DC. | Chai Hu | Hubei | 10 |
| Radix
glycyrrhizae | Glycyrrhiza
uralensis Fisch. | Gan Cao | Neimenggu | 10 |
Animals
Sprague-Dawley rats (n=53; 26 male and 27 female;
weight, 180–220 g; age, 6 weeks) were supplied by the Experimental
Animal Center of Dalian Medical University [confirmation no. SCXK
(Liao) 2004–0017]. The rats were housed in an air-conditioned room
at 22±1°C, with 50–60% relative humidity and a 12 h light-dark
cycle, and were fed a standard laboratory diet and tap water ad
libitum. The experiments were performed in accordance with the
principles and guidelines for the human treatment of animals set by
the National Institutes of Health Guide (NIH publication no. 85–23,
revised 1985) (21) and was
approved by the ethics committee of Dalian Medical University.
Experimental model and drug
treatment
The rats were randomly divided into five groups:
Normal control group (n=12), CCl4 (Tianjin Guangfu Fine
Chemical Research Institute, Tianjin, China) model group (n=8) and
three GFK groups (n=11). These rats were treated by subcutaneous
injection of CCl4 (0.5 mg/kg in a vehicle of olive oil;
twice/week) for 8 weeks, with the exception of the rats in the
normal control group, which were treated with vehicle only. In the
treatment groups, the rats received GFK via oral administration
(31.25, 312.5 and 3,125 mg/kg/day) between weeks 9 and 20. The
model group and the normal control group were administered with an
equal volume of normal saline. All rats were sacrificed by cervical
dislocation under diethyl ether (Nanjing Chemical Reagent Co.,
Ltd., Nanjing, China) anesthesia at the end of week 20. Blood (~4
ml) and complete liver samples were obtained for further
examination.
Preparation of drug-medicated serum
A total of 20 rats were randomly divided into two
groups. Sera was obtained from the rats, following administration
with normal saline or GFK at the middle dose (312.5 mg/kg/day) by
gavage for 7 days.
Biochemical determination
The levels of alanine aminotransferase (ALT),
aspartate aminotransferase (AST), albumin (ALB) and globulin in the
serum samples were determined using a commercial test reagent
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China). ALB
was determined in 0.02 ml serum using bromocresol green colorimetry
and total protein (TP) was determined by Coomassie brilliant blue
colorimetry in 0.05 ml serum. The samples were incubated with
either reagent for 10 min at room temperature prior to measuring
optical density at 628 nm (bromocresol green) or 595 nm (Coomassie
brilliant blue) on a spectrophotometer (721; Shanghai Optical
Instrument Factory, Shanghai, China).
Histopathological examination
The rat liver tissues were fixed in 10% formalin
(Nanjing Chemical Reagent Co., Ltd.), embedded in paraffin
(Sigma-Aldrich, St. Louis, MO, USA), cut (4 μm) and stained
with either hematoxylin and eosin (H&E; Nanjing Jiancheng
Bioengineering Institute) or with Picric Sirius red (Nanjing
Jiancheng Bioengineering Institute). The sections were examined by
light microscope (Eclipse 50i; Nikon Corporation, Tokyo, Japan),
and the collagen type I and type III staining by Picric Sirius red
were observed by polarizing microscope (CX31P; Olympus Corporation,
Tokyo, Japan). The liver fibrosis was evaluated by a pathologist in
a blinded-manner, and scored and graded according to the method of
Scheuer (22), as follows: Stage
0, no fibrosis; stage 1, fibrosis expansion of certain portal
areas; stage 2, formation of fibrous septa around the portal area;
stage 3, fibrosis with architectural distortion, complete septa
interconnecting with each; stage 4, early cirrhosis or
cirrhosis.
Hepatic hydroxyproline (Hyp) assay
The content of Hyp was measured
spectrophotometrically using a commercially available kit (Nanjing
Jiancheng Bioengineering Institute). The quantities of Hyp in the
rat liver are expressed as μg/g wet tissue.
Cell culture and
3–4,5-Dimethylthiazol-2-yl)-2,5-dipheny ltetrazolium bromide (MTT)
assay
The rat HSC-T6 cell line was provided by Professor
Lie-Ming Xu (Shanghai University of Chinese Traditional Medicine,
Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Tianjin
Haoyang Biological Manufacture Co., Ltd., Tianjin, China), 100 U/ml
penicillin and 100 U/ml streptomycin (Sigma-Aldrich), and incubated
at 37°C in 5% CO2 air. Following 2 weeks of culture on
plastic tissue-culture dishes, the cells were plated at a density
of 2×104 cells/well in 96-well plates. In this
experiment, these HSCs were randomly divided into three groups:
Control group, PDGF-BB (R&D Systems, Inc., Minneapolis, MN,
USA) group and PDGF-BB+GFK group. The concentration of PDGF-BB used
for treatment was 10 ng/ml for 24 h at 37°C, and the concentrations
of GFK-medicated serum (Dalian Medical University) were 2, 4, 6, 8,
10, 12, 14 and 16%. Cell viability was measured using an MTT assay
(Beyotime Institute of Biotechnology, Shanghai, China) at a
wavelength of 490 nm, and cellular morphology was observed using
phase-contrast microscopy (Olympus IX51; Olympus Corporation).
Immunohistochemistry
The liver tissue sections were deparaffinized using
xylene (Nanjing Chemical Reagent Co., Ltd.), rehydrated with graded
alcohols, treated with 0.3% endogenous peroxidase blocking solution
(Sigma-Aldrich) for 20 min. Following high pressure heating
retrieval (125°C and 103 kPa) and non-immune goat serum (ZSGB-BIO,
Beijing, China) blocking, the sections were incubated with one of
the following primary antibodies purchased from BIOSS (Beijing,
China): Rabbit anti-rat polyclonal anti-α-smooth muscle actin (SMA;
1:200; bs-0189R), anti-PDGF-BB (1:200; bs-1316R) and anti-PDGF
receptor β (PDGFRβ; 1:150; bs-0232R). The primary antibodies were
incubated overnight at 4°C. Following washing with
phosphate-buffered saline (PBS), goat anti-rabbit non-biotinylated
regents (ZSGB-BIO; cat. no. PV-9001) were used to react with the
primary antibody for 2 h at 37°C, followed by the addition of
diaminobenzidine (ZSGB-BIO) and monitoring of the staining. PBS was
used as a negative control. In each section, five randomly-selected
fields were examined using an Image-Pro Plus 6.0 analyzing system
(Media Cybernetics, Inc., Rockville, MD, USA).
Western blotting
The manually homogenized liver tissues and cell
lysates were treated with 150 μl radioimmunoprecipitation
assay extraction buffer (Beyotime Institute of Biotechnology), and
the supernatants were collected following centrifugation at 12,000
× g for 10 min at 4°C. The concentration of total protein was
estimated using a Bradford assay, with bovine serum albumin (BSA;
Sigma-Aldrich) as a standard. The protein samples (10 μg)
were subjected to 12% SDS-PAGE and were then transferred onto to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Following blocking with 5% BSA for 3 h at room temperature,
the membranes were treated with the following primary antibodies:
Rabbit anti-rat polyclonal antibodies against nuclear factor of
activated T cells (NFAT; 1:200; Wuhan Boster Biological Technology,
Ltd., Wuhan, China) and MMP7 (1:200; Wuhan Boster Biological
Technology, Ltd.) overnight at 4°C, washed with Tris-buffered
saline containing 0.05% Tween 20 (TBST), and then incubated with
peroxidase-conjugated secondary goat anti-rabbit antibody (1:2,000;
Santa Cruz Biotechnology, Inc.; cat. no. sc-2004) for 2 h at room
temperature. The membranes were washed with TBST and
Prolight-horseradish peroxidase (Tiangen Biotech, Co., Ltd.,
Beijing, China) was used for blot detection. Rabbit anti-rat
polyclonal β-actin antibodies (1:1,000; Santa Cruz Biotechnology,
Inc.; cat. no. 130657) served as a loading control. Densitometric
analysis was performed with LabWorks 4.6 (UVP, Inc., Upland, CA,
USA).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted from the liver tissues and
cell lysates using TRIzol reagent, according to the manufacturer's
protocol (Invitrogen; Thermo Fisher Scientific, Inc.). Reverse
transcription with oligo (dT) primers (Tiangen Biotech, Co., Ltd.),
Quant reverse transcriptase (Tiangen Biotech, Co., Ltd.) and dNTP
mixture were used to synthesize complementary DNA (cDNA) from the
total RNA. The primers were synthesized by Takara Biotechnology,
Co., Ltd. (Dalian, China) for this purpose, and are as listed in
Table II. A PCR reaction kit
(Tiangen Biotech Co., Ltd.) containing 2 μl cDNA template,
12.5 μl 2X Taq PCR Master mix, 1 μl primers and 8.5
μl double distilled water to a total volume of 25 μl.
Each sample had four replicates. The conditions for amplification
were as follows: Wnt5a, Collagen type I, α-SMA, Calcineurin: One
cycle of 94°C for 5 min, 32 cycles of 94°C for 30 sec, 64°C for 30
sec, 72°C for 30 sec, and a final extension of one cycle at 72°C
for 5 min; PDGF-BB and PDGFRβ: 30 cycles of 94°C for 30 sec, 57°C
for 30 sec and 72°C for 40 sec; Frizzled2: 35 cycles of 94°C for 30
sec, 57°C for 30 sec and 72°C for 35 sec; NFAT: 32 cycles of 94°C
for 30 sec, 58°C for 30 sec and 72°C for 30 sec;
calmodulin-dependent protein kinase II (CaMK II): 35 cycles of 94°C
for 30 sec, 56°C for 30 sec and 72°C for 40 sec; Collagen type III
and β-actin: 32 cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C
for 30 sec. The RT-PCR reaction was conducted using a T100 thermal
cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The PCR
products were electrophoresed on 2% agarose gels (Tiangen Biotech,
Co., Ltd.) and the band density was measured using LabWorks 4.6.
β-actin served as a loading control.
 | Table IIPrimer sequences and amplicon
sizes. |
Table II
Primer sequences and amplicon
sizes.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) | Product size
(bp) |
|---|
| Wnt5a |
AGGACTTACCTCGGGACTGG |
TGCGACCTGCTTCATTGTT | 171 |
| Frizzled2 |
CCTGGAGGTGCATCAATTCTAC |
CGCTCACCCAGAAACTTATAGC | 447 |
| CaMK II |
TTCTACTGTTGCCTCCAT |
AAAGTCCATCCCTTCCAC | 460 |
| Calcineurin |
GGACAGGGTGGTGAAAGC |
AGCGAGTGTTGGCAGGAG | 296 |
| NFAT |
GCCCAGCGATGAGTATGAA |
ATGCACCAGCACAGAACG | 310 |
| MMP-7 |
TGCCGGAGACTGGAAAGCTG |
GGTGCAAAGGCATGGCCTAG | 343 |
| Collagen type
I |
TGCCGTGACCTCAAGATGTG |
CACAAGCGTGCTGTAGGTGA | 461 |
| Collagen type
III |
AGATCATGTCTTGACTCAAGTC |
TTTACATTGCCATTGGCCTGA | 463 |
| α-SMA |
TGTGCTGGACTCTGGAGATG |
GATCACCTGCCCATCAGG | 291 |
| β-actin |
GGTATGGGTCAGAAGGACTCC |
TGATCTTCAGGTGCTAGGAGCC | 847 |
Statistical analysis
SPSS 19.0 statistical software (IBM SPSS, Armonk,
NY, USA) was used for statistical analysis. All data are expressed
as the mean ± standard deviation. Comparisons were performed using
one-way analysis of variance. Ordinal data were analyzed using a
Kruskal-Wallis test. P<0.05 was considered to indicate a
statistically significant difference.
Results
GFK treatment reduces
CCl4-induced liver injury in rats
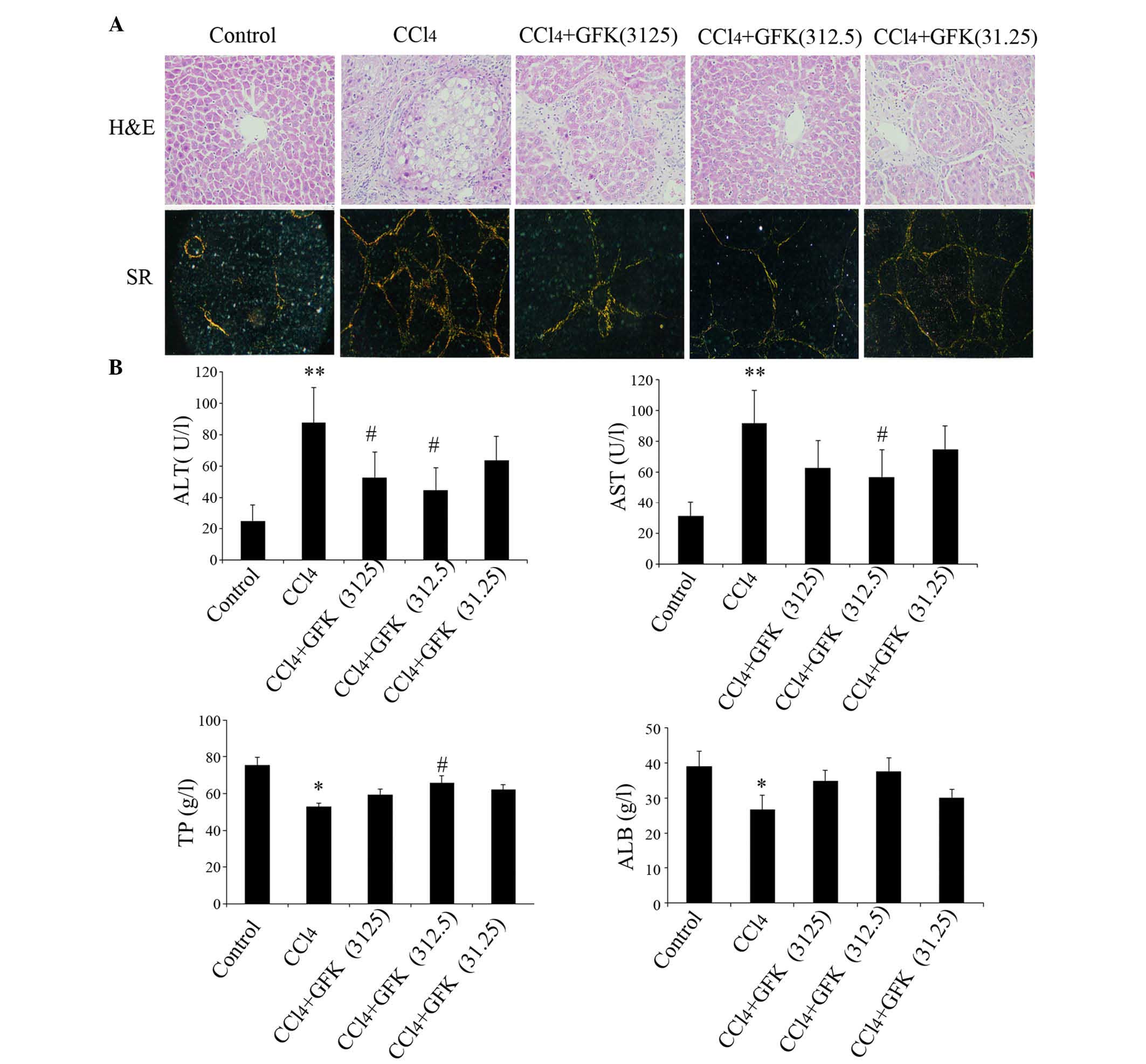
The degrees of hepatic damage in the rats were
assessed by histopathological examination of the liver sections
using H&E staining. As shown in Fig. 1A, compared with the control group,
the livers of the CCl4 treated rats exhibited marked
fatty infiltration, bridging necrosis, damage of liver lobules and
wide infiltration of inflammatory cells around the central vein.
The livers of the rats in the GFK (31.25 mg/kg) group showed
moderate widening and a fibrotic area with bridging fibrosis, which
were also observed in the GFK (3,125 mg/kg) group. However, in the
livers of the GFK (312.5 mg/kg)-treated rats, only mild focal
fibrotic changes of the portal area and mild bridging were
observed. The histopathological scores of liver fibrosis are shown
in Table III.
 | Figure 1Effect of GFK on
CCl4-induced liver fibrosis. (A) GFK (312.5 mg/kg)
attenuated pathological changes are shown by H&E
(magnification, ×400) and Sirius red staining (magnification,
×200). (B) GFK reduced the levels of ALT and AST, and increased the
level of TP at a dose of 312.5 mg/kg. Data are expressed as the
mean ± standard deviation (n=10–12). *P<0.05 and
**P<0.01, compared with the control group;
#P<0.05, compared with the CCl4 group.
GFK, Gan-fu-kang; CCL4, carbon tetrachloride; H&E,
hematoxylin and eosin; SR, Sirius red; ALT, alanine
aminotransferase; AST, aspartate aminotransferase; TP, total
protein; ALB, albumin. |
 | Table IIIHistopathological semi-quantitative
scores of rat liver tissues. |
Table III
Histopathological semi-quantitative
scores of rat liver tissues.
| Group | n | Hepatic fibrosis
score (n)
| Mean score |
|---|
| 0 | 1 | 2 | 3 | 4 |
|---|
| Control | 12 | 12 | 0 | 0 | 0 | 0 | 5.5b |
|
CCl4 | 8 | 0 | 0 | 2 | 3 | 3 | 44.82a |
| CCl4+GFK
(3,125) | 11 | 0 | 4 | 4 | 3 | 0 |
33.17a,b |
| CCl4+GFK
(312.5) | 11 | 0 | 7 | 4 | 0 | 0 |
22.96a,b |
| CCl4+GFK
(31.25) | 11 | 0 | 3 | 5 | 3 | 0 |
31.85a,b |
CCl4 treatment significantly increased
the serum levels of AST and ALT by four and three-fold,
respectively, and markedly decreased the serum levels of TP and
ALB, compared with the control group (P<0.05). The
administration of GFK markedly reduced the elevated levels in serum
AST (P<0.05; 3,125 and 312.5 mg/kg) and ALT (P<0.05; 312.5
mg/kg), compared with the CCl4 group. GFK treatment
(312.5 mg/kg) significantly enhanced the CCl4-induced
decrease in the serum level of TP (P<0.05). The serum level of
ALB showed a marginal increase in the GFK-treated groups (3,125 and
312.5 mg/kg), although without statistical significance (Fig. 1B).
GFK treatment attenuates
CCl4-induced collagen accumulation
Picro Sirius red staining was performed to observe
collagen synthesis in the liver tissues. Collagen I was red and
collagen III was green. Consistent with the H&E staining, the
fibrotic changes were substantially decreased following
administration of GFK at a dose of 312.5 mg/kg (Fig. 1A). Compared with the normal rats,
CCl4 administration caused a significant increase in the
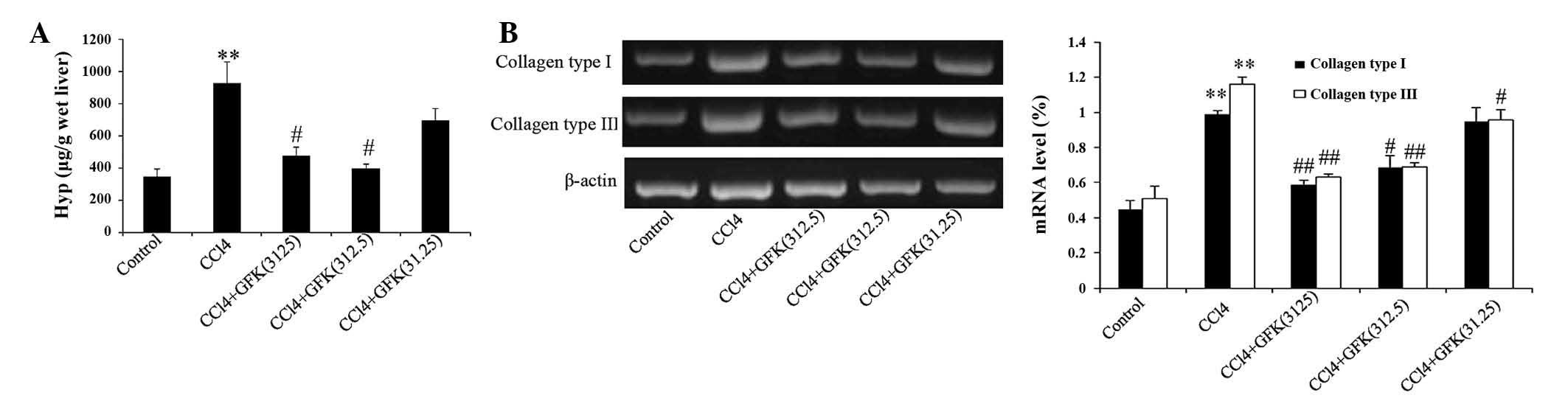
accumulation of collagen, as assessed by the hepatic Hyp content
and mRNA expression levels of collagen type I and III. In the
CCl4-treated model group, the hepatic Hyp content was
significantly increased, by three-fold, compared with the control
group. GFK treatment notably reduced the elevated levels of Hyp,
compared with the CCl4 group, when treated at GFK
concentrations of 312.5 and 3,125 mg/kg (P<0.05; Fig. 2A). The results of the RT-PCR
analysis showed that treatment with various doses of GFK reduced
the deposition of collagen type I and III in the
CCl4-induced fibrotic liver tissues (Fig. 2B).
GFK treatment suppresses α-SMA
activation, and the expression levels of PDGF-BB and PDGFRβ
α-SMA is a marker used to evaluate the degree of HSC
activation (23). In addition,
PDGF is one of the most potent mitogens for HSCs. Accordingly,
PDGFRβ is expressed in HSCs when transformed, and may be a marker
of HSC activation. In the present study, RT-PCR analysis revealed
that the upregulation in the mRNA levels of α-SMA, PDGF-BB and
PDGFRβ induced by CCl4 were significantly attenuated by
GFK (Fig. 3A). Image analysis of
the immunohistochemical staining of α-SMA, PDGF-BB and PDGFRβ
showed that, compared with the control group, CCl4
treatment significantly increased the accumulation of activated
HSCs (P<0.01; Fig. 3B).
Treatment with the different doses of GFK significantly decreased
HSC activation in the liver (P<0.01), with the medium dose
(312.5 mg/kg) having the most pronounced effect on HSC activation
(Fig. 3B).
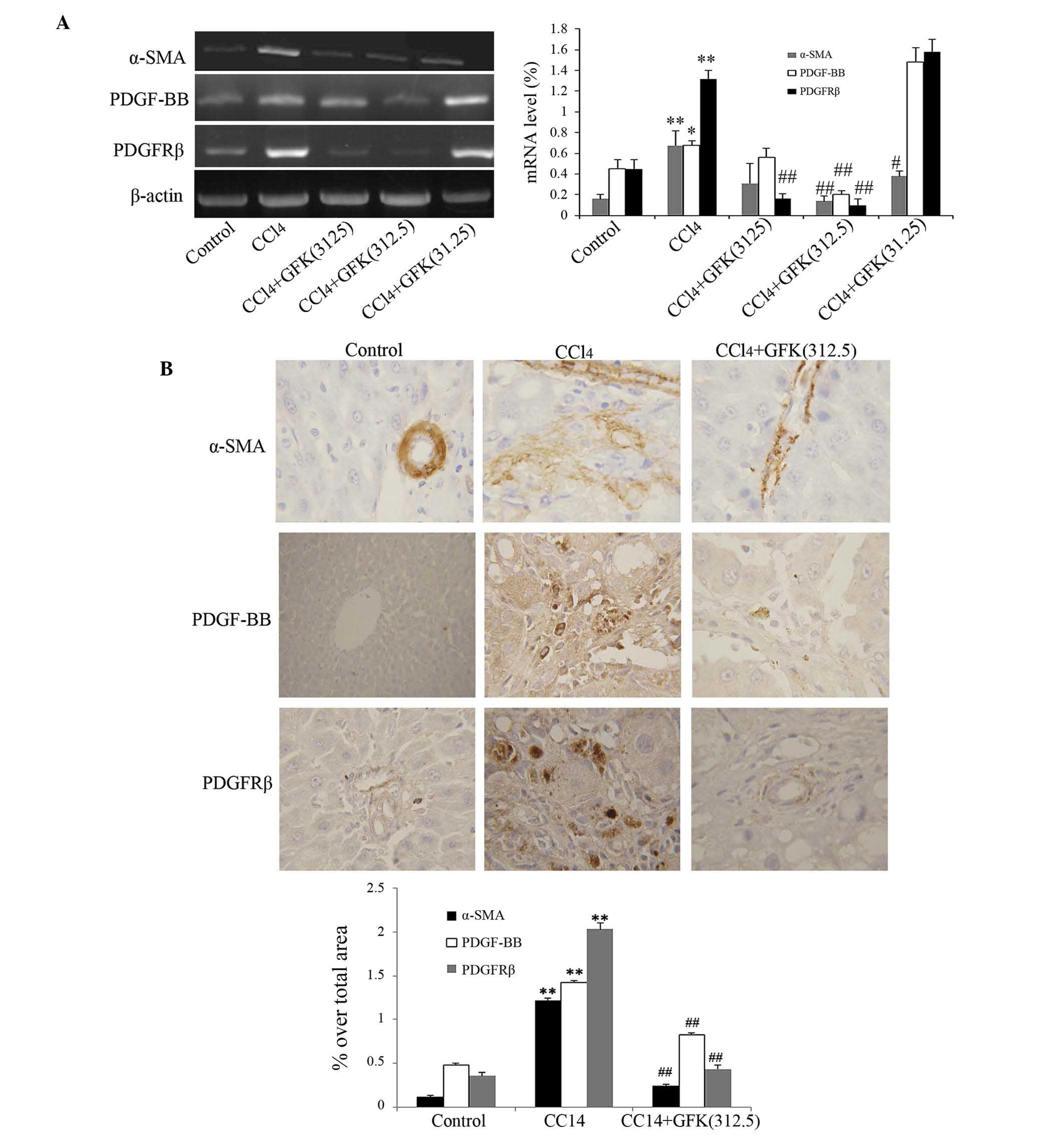 | Figure 3Effect of GFK on the activation of
HSCs. (A) mRNA levels of α-SMA, PDGF-BB and PDGFRβ were
significantly downregulated by GFK (312.5 mg/kg). Data are
expressed as the mean ± standard deviation (n=5). (B)
Immunohistochemical detection and evaluation of the expression
levels of α-SMA, PDGF-BB and PDGFRβ in the rat liver tissues
(magnification, ×400). The areas of cells positive for α-SMA,
PDGF-BB and PDGFRβ were reduced by GFK (312.5 mg/kg).
*P<0.05 and **P<0.01, compared with the
control group; #P<0.05 and ##P<0.01,
compared with the CCl4 groupGFK, Gan-fu-kang;
CCL4, carbon tetrachloride; PDGF, platelet-derived
growth factor; α-SMA, α-smooth muscle actin; PDGFRβ, PDGF receptor
β. |
GFK treatment inhibits activation of the
Wnt/Ca2+ signaling pathway in vivo
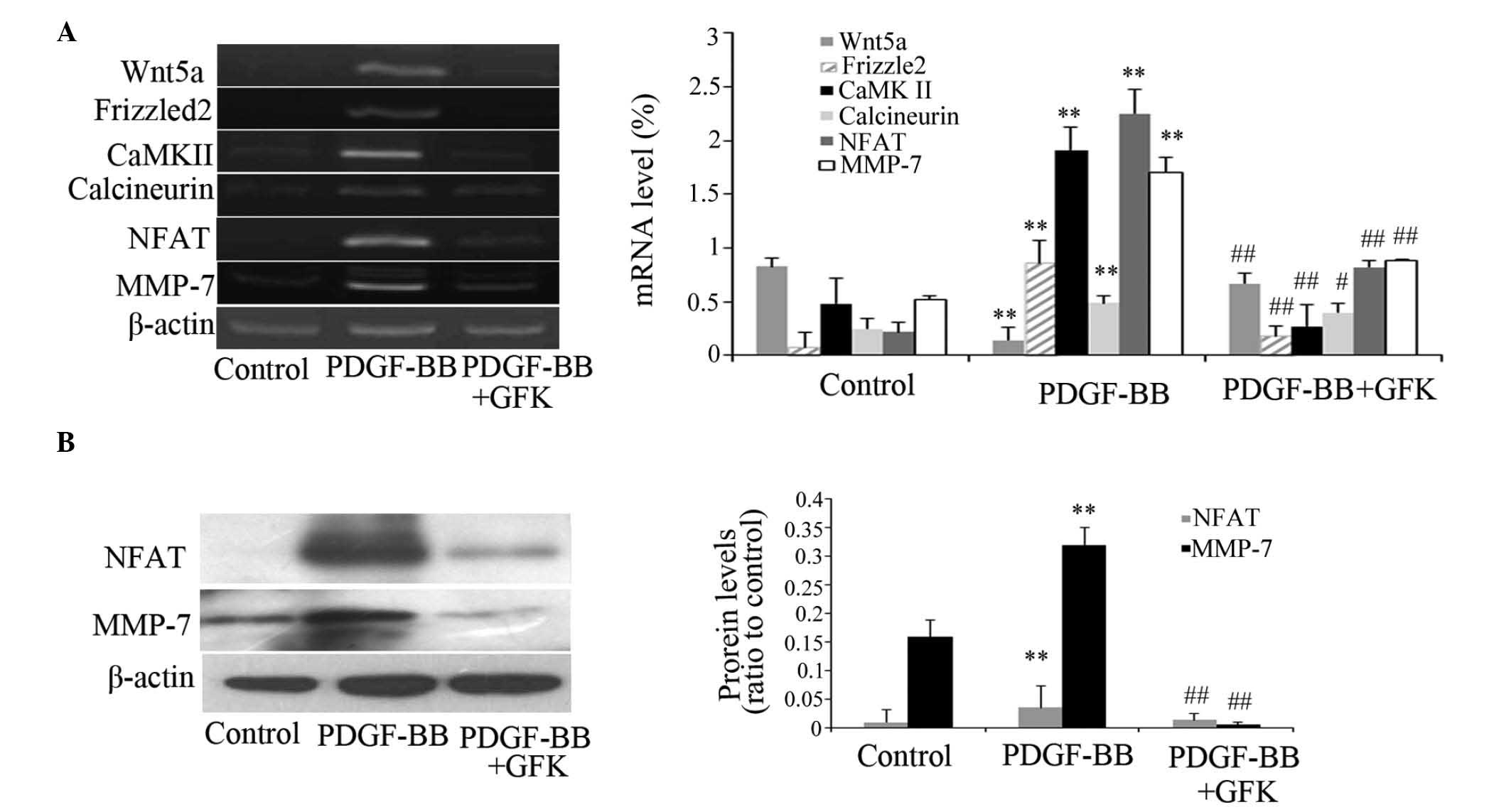
To elucidate the possible molecular pathway by which
GFK suppressed liver fibrosis, the present study examined the mRNA
and protein expression levels of several associated genes in the
Wnt/Ca2+ signaling pathway. The mRNA expression levels
of Wnt5a, Frizzled2, CaMK II, calcineurin, NFAT and MMP-7 were
increased in the CCl4-treated livers, compared with the
control group. Following administration of GFK, the mRNA expression
levels decreased at GFK dose of 312.5 mg/kg, compared with the
CCl4 group (Fig. 4A).
Western blot analysis indicated a significant reduction in the
protein levels of NFAT and MMP-7 (P<0.01; Fig. 4B). This suggested that GFK inhibits
the Wnt/Ca2+ signaling pathway via protein synthesis
levels rather than by repressing mRNA expression.
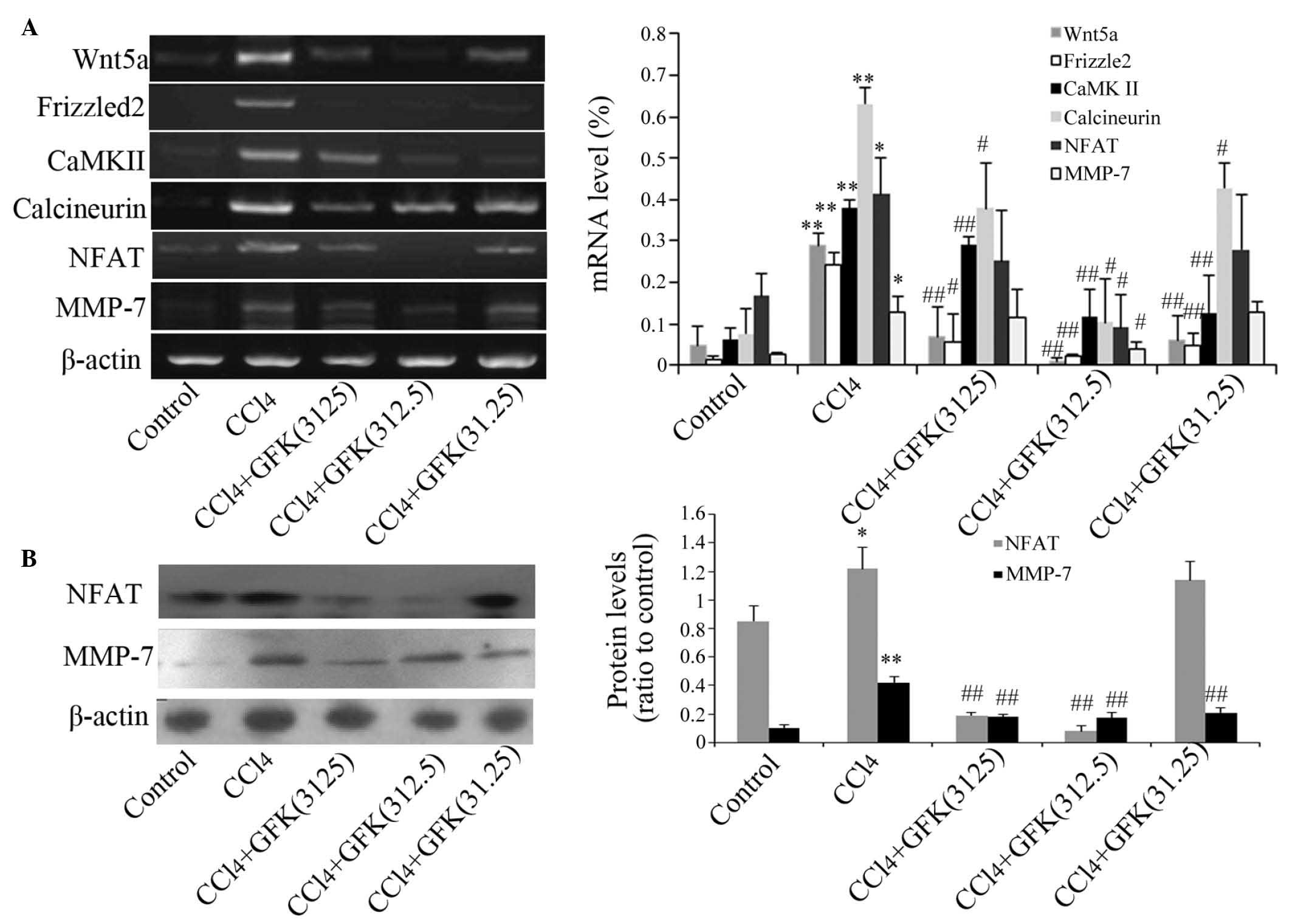 | Figure 4Effect of GFK on genes of the
Wnt/Ca2+ signaling pathway. (A) Effect of GFK on the
mRNA expression levels of genes in the Wnt/Ca2+
signaling pathway. (B) Effect of GFK on the expression levels of
cytosolic NFAT and MMP-7, determined using Western blotting.
CCl4 treatment decreased the expression levels of NFAT
and MMP-7, compared with those of the control group. Treatment with
GFK at concentrations of 3,125 or 312.5 mg/kg increased the levels
of NFAT and MMP-7. Data are expressed as the mean ± standard
deviation (n=5). *P<0.05 and **P<0.01,
compared with the control group; #P<0.05 and
##P<0.01, compared wirh the CCl4 group.
GFK, Gan-fu-kang; CCL4, carbon tetrachloride; NFAT,
Nuclear factor of activated T cells; MMP-7, matrix
metalloproteinase-7; CaMKII, calmodulin-dependent protein kinase
II. |
GFK treatment attenuates the
proliferation and activation of HSC-T6 cells
To evaluate the effects of GFK on HSCs in the liver,
HSC-T6 cells were treated with increasing concentrations of GFK.
The MTT assay showed that GFK treatment caused a dose-dependent
reduction in the number of HSC-T6 cells (Fig. 5A). The results revealed that GFK
inhibited the viability, which was stimulated by PDGF-BB. The
present study then calculated the half maximal inhibitory
concentration (IC50), which was used in the subsequent
experiment. As shown in Fig. 5B,
the HSCs cultured with PDGF-BB for 24 h exhibited an intermediate
stage of the activation process. The HSCs exposed to GFK at the
IC50 maintained a quiescent morphology. In addition, GFK
treatment at the IC50 markedly decreased the expression
levels of PDGFRβ, α-SMA, and collagen type I and III (Fig. 5C).
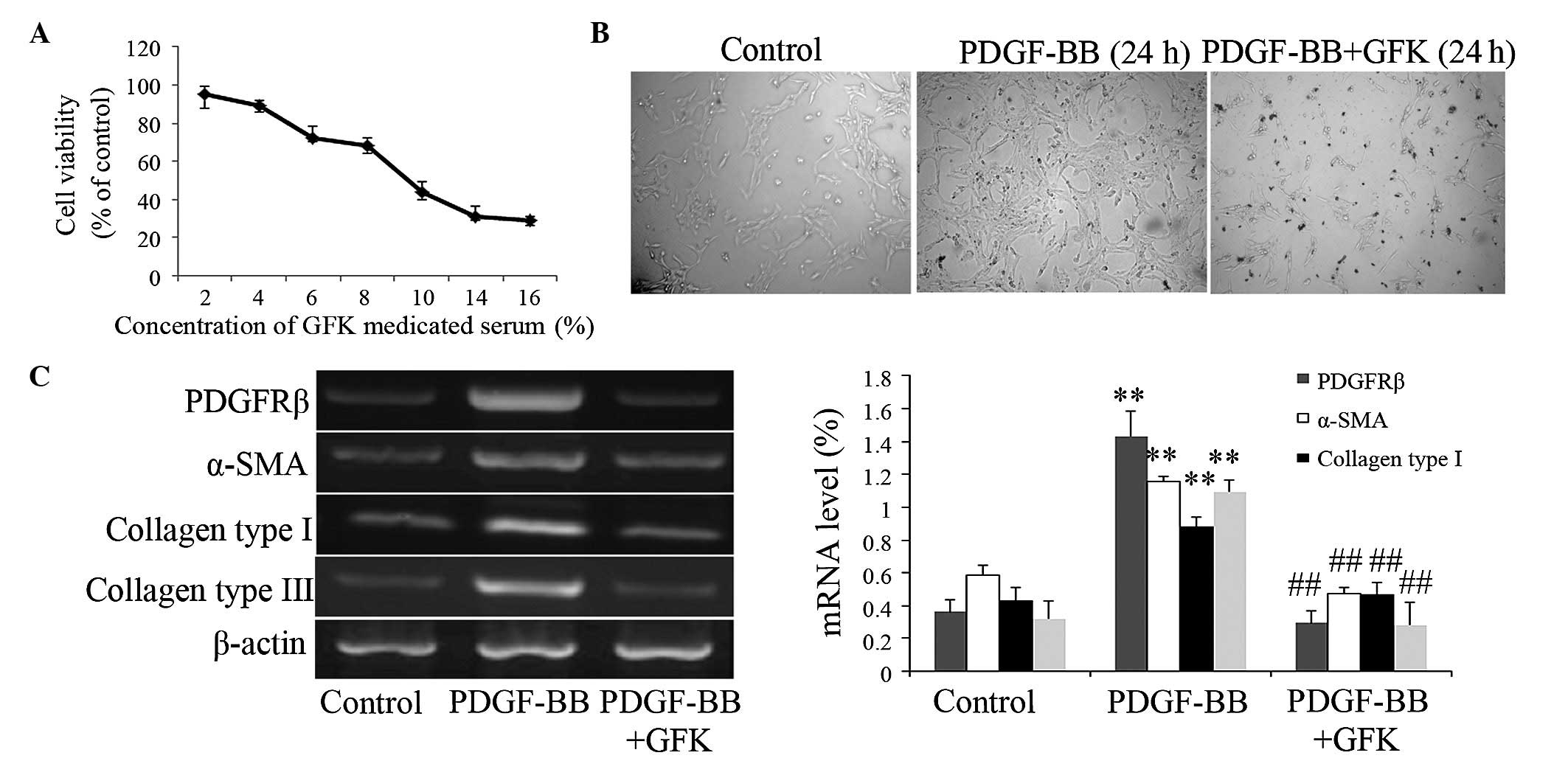 | Figure 5Effect of GFK on HSC-T6 cells. (A)
Cell viability was detected using a
3–4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
The results are expressed as the percentage of control cell
viability at 24 h. (B) Effect of GFK treatment for 24 h on HSC-T6
cell morphology (magnification, ×100). (C) Effect of GFK on the
expression levels of PDGFRβ, α-SMA, and collagen type I and III in
HSC-T6 cells. Data are expressed as the mean ± standard deviation
(n=5). **P<0.01, compared with the control group;
##P<0.01, compared with the PDGF-BB treatment group.
GFK, Gan-fu-kang; CCL4, carbon tetrachloride; α-SMA, α-smooth
muscle actin; PDGF, platelet-derived growth factor; PDGFR, PDGF
receptor β. |
GFK treatment inhibits the
Wnt/Ca2+ signaling pathway in HSC-T6 cells
The levels of principal elements in the
Wnt/Ca2+ signaling pathway, including Wnt5a, Frizzled2,
CaMK II, calcineurin, NFAT and MMP-7, were detected in HSC-T6 cells
using RT-PCR and Western blot analyses. The results showed that the
expression levels of these components were all attenuated in the
presence of GFK at the IC50 (Fig. 6).
Discussion
At present, the major obstacles in the treatment of
liver fibrosis are the unsatisfactory effects and various side
effects due to immune suppression and cytotoxicity (24). Medicinally, herbal drugs often
provide alternative treatment options for liver diseases. GFK is a
multi-ingredient herbal drug, which contains 11 crude plant
ingredients. These 11 plants offer certain synergistic effects in
hepatic injury therapy. One of the important components of GFK is
Astragalus membranaceus, which has been found to
significantly delay the formation of liver fibrosis induced by
CCl4 in vivo (25). The other major component in GFK,
Salvia miltiorrhiza, can induce HSC apoptosis or inhibit the
proliferation of HSCs (26). The
remaining components of GFK, including Atractylodes macrocephala,
Rehmannia glutinosa and licorice root, have been confirmed to have
hepatoprotective effects (27–29).
In the present study, the results showed that GFK suppresses the
progress of CCl4-induced liver fibrosis in rats. The
data also indicated that GFK inhibited the proliferation of
HSCs.
In the present study, as expected, histopathological
examination (H&E staining) revealed that an 8-week period of
subcutaneous injection of CCl4 (0.5 mg/kg; twice per
week) markedly induced hepatofibrotic changes, whereas GFK
administration substantially ameliorate these alternations. These
findings were also confirmed by the detection of the biochemical
indicators, ALT, AST, TP and ALB. The progressive accumulation of
ECM results in liver fibrosis. Collagen, particularly collagen type
I and type III is the predominant component of the ECM, and Hyp is
a degradation product of collagen, which has been used as an
indicator for evaluating collagen deposition (30). The results of the present study
showed that CCl4 notably induced the accumulation of
collagen, as determined by Hyp concentrations and Picric Sirius red
staining, and that collagen deposition was markedly lowered by GFK
treatment. It was also found that GFK administration significantly
downregulated the expression levels of collagen type I and type III
in vivo and in vitro, suggesting that GFK may enhance
collagenolysis in the fibrotic liver.
It is generally accepted that HSCs are pivotal in
the development of liver fibrosis, and that α-SMA is a key marker
of HSC activation. In the present study, GFK was found to decrease
HSC viability in a dose-dependent manner. Additionally, the data
confirmed that GFK treatment (312.5 mg/kg) considerably inhibited
the activation of HSCs, as observed in the immunohistochemical
staining of α-SMA. The suppression of the gene expression levels of
α-SMA in liver fibrosis and in the PDGF-BB-treated HSC-T6 cells
were also more prominent in the GFK administration group.
Consistent with the changes in levels of α-SMA, the expression
levels of the profibrogenic cytokines, PDGF-BB and PDGFRβ, were
marked in the fibrotic liver tissues, whereas they were attenuated
following GFK treatment. In addition, GFK decreased the protein and
mRNA levels of PDGF-BB and PDGFRβ in vivo and in
vitro, demonstrating that the antifibrotic effects of GFK
exerted its inhibitory effects on HSC activation via modulation of
the profibrogenic cytokines, PDGF-BB and PDGFRβ.
The activation of Wnt signaling has been implicated
in the conversion of numerous fibrotic diseases, including lung,
heart, kidney and liver fibrosis (31–34).
It has been demonstrated that the canonical and non-canonical Wnt
signaling pathways are involved in the pathogenesis of liver
fibrosis (35). The non-canonical
Wnt/Ca2+ pathway includes the binding of Wnt5a to its
receptor, Frizzled2, which then leads to elevated intracellular
Ca2+ concentrations via a G-protein-dependent mechanism
(36). Elevated Ca2+
can activate Ca2+/CaMK II via calcineurin/NFAT pathways
(37–39). The transcription factor, NFAT,
translocates from the cytosol to the nucleus, thereby activating
downstream target gene transcription (40). In the present study, the expression
levels of components of the Wnt/Ca2+ signaling pathway,
including Wnt5a, Frizzled2, CaMK II, calcineurin, NFAT and MMP-7,
were markedly upregulated in the rats with hepatic fibrosis and the
PDGF-BB-treated HSC-T6 cells. However, they were markedly decreased
by GFK treatment. Furthermore, the effects on the protein levels of
NFAT and MMP-7 were in accordance with the alterations in their
mRNA expression levels. Taken together, these results suggested
that GFK ameliorated liver fibrosis and activated HSCs by
regulating the Wnt/Ca2+ signaling pathway.
Acknowledgments
This study was financially supported by the Natural
Science Foundation of Liaoning Province, China (grant no.
201102055).
References
|
1
|
Friedman SL, Maher JJ and Bissell DM:
Mechanisms and therapy of hepatic fibrosis: Report of the AASLD
single topic basic research conference. Hepatology. 32:1403–1408.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pellicoro A, Ramachandran P, Iredale JP
and Fallowfield JA: Liver fibrosis and repair: Immune regulation of
wound healing in a solid organ. Nat Rev Immunol. 14:181–194. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Puche JE, Saiman Y and Friedman SL:
Hepatic stellate cells and liver fibrosis. Compr Physiol.
3:1473–1492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duval F, Moreno-Cuevas JE, González-Garza
MT, Rodríguez-Montalvo C and Cruz-Vega DE: Liver fibrosis and
protection mechanisms action of medicinal plants targeting
apoptosis of hepatocytes and hepatic stellate cells. Adv Pharmacol
Sci. 2014:3732952014.PubMed/NCBI
|
|
5
|
Breitkopf K, Roeyen CV, Sawitza I, Wickert
L, Floege J and Gressner AM: Expression patterns of PDGF-A, -B, -C
and -D and the PDGF-receptors alpha and beta in activated rat
hepatic stellate cells (HSC). Cytokine. 31:349–357. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fang L, Zhan S, Huang C, Cheng X, Lv X, Si
H and Li J: TRPM7 channel regulates PDGF-BB-induced proliferation
of hepatic stellate cells via PI3 K and ERK pathways. Toxicol Appl
Pharmacol. 272:713–725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shah R, Reyes-Gordillo K,
Arellanes-Robledo J, Lechuga CG, Hernández-Nazara Z, Cotty A,
Rojkind M and Lakshman MR: TGF-β 1 up-regulates the expression of
PDGF-β receptor mRNA and induces a delayed PI3K-, AKT- and p70
S6K-dependent proliferative response in activated hepatic stellate
cells. Alcohol Clin Exp Res. 37:1838–1848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miao CG, Yang YY, He X, Huang C, Huang Y,
Zhang L, Lv XW, Jin Y and Li J: Wnt signaling in liver fibrosis:
Progress, challenges and potential directions. Biochimie.
95:2326–2335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Harthi L: Wnt/β-catenin and its diverse
physiological cell signaling pathways in neurodegenerative and
neuropsychiatric disorders. J Neuroimmune Pharmacol. 7:725–730.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holland JD, Klaus A, Garratt AN and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Curr
Opin Cell Biol. 25:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ge WS, Wang YJ, Wu JX, Fan JG, Chen YW and
Zhu L: β-catenin is overexpressed in hepatic fibrosis and blockage
of Wnt/β-catenin signaling inhibits hepatic stellate cell
activation. Mol Med Rep. 9:2145–2151. 2014.PubMed/NCBI
|
|
12
|
Rashid ST, Humphries JD, Byron A, Dhar A,
Askari JA, Selley JN, Knight D, Goldin RD, Thursz M and Humphries
MJ: Proteomic analysis of extracellular matrix from the hepatic
stellate cell line LX-2 identifies CYR61 and Wnt-5a as novel
constituents of fibrotic liver. J Proteome Res. 11:4052–4064. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
MadanKumar P, NaveenKumar P, Manikandan S,
Devaraj H and NiranjaliDevaraj S: Morin ameliorates chemically
induced liver fibrosis in vivo and inhibits stellate cell
proliferation in vitro by suppressing Wnt/β-catenin signaling.
Toxicol Appl Pharmacol. 277:210–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shin HW, Park SY, Lee KB, Shin E, Nam SW,
Lee JY and Jang JJ: Transcriptional profiling and Wnt signaling
activation in proliferation of human hepatic stellate cells induced
by PDGF-BB. Korean J Hepatol. 15:486–495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saneyoshi T, Kume S, Amasaki Y and
Mikoshiba K: The Wnt/calcium pathway activates NF-AT and promotes
ventral cell fate in Xenopus embryos. Nature. 417:295–299. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei F, Lang Y, Gong D and Fan Y: Effect of
Dahuang zhechong formula on liver fibrosis in patients with chronic
hepatitis B: A meta-analysis. Complement Ther Med. 23:129–138.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin HJ, Chen JY, Lin CF, Kao ST, Cheng JC,
Chen HL and Chen CM: Hepatoprotective effects of Yi Guan Jian, an
herbal medicine, in rats with dimethylnitrosamine-induced liver
fibrosis. J Ethnopharmacol. 134:953–960. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu TT, Jiang MN, Li C, Che Y and Jia YJ:
Effect of Chinese traditional compound, Gan-fu-kang, on
CCl(4)-induced liver fibrosis in rats and its probable molecular
mechanisms. Hepatol Res. 37:221–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lou JL, Jiang MN, Li C, Zhou Q, He X, Lei
HY, Li J and Jia YJ: Herb medicine Gan-fu-kang attenuates liver
injury in a rat fibrotic model. J Ethnopharmacol. 128:131–138.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang C, Wang Y, Chen H, Yang G, Wang S,
Jiang M, Cong L, Yuan L, Li H and Jia Y: Protective effect of the
herbal medicine Gan-fu-kang against carbon tetrachloride-induced
liver fibrosis in rats. Mol Med Rep. 8:954–962. 2013.PubMed/NCBI
|
|
21
|
National Institutes of Health: Guide for
the Care and Use of Laboratory Animals. 6th edition. National
Academies Press; Washington, DC: 1985
|
|
22
|
Scheuer PJ: Classification of chronic
viral hepatitis: A need for reassessment. J Hepatol. 13:372–374.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zakaria S, Youssef M, Moussa M, Akl M,
El-Ahwany E, El-Raziky M, Mostafa O, Helmy AH and El-Hindawi A:
Value of α-smooth muscle actin and glial fibrillary acidic protein
in predicting early hepatic fibrosis in chronic hepatitis C virus
infection. Arch Med Sci. 6:356–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schiavon F: Transient joint effusion: A
forgotten side effect of high dose corticosteroid treatment. Ann
Rheum Dis. 62:491–492. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun WY, Wang L, Liu H, Li X and Wei W: A
standardized extract from Paeonia lactiflora and Astragalus
membranaceus attenuates liver fibrosis induced by porcine serum in
rats. Int J Mol Med. 29:491–498. 2012.
|
|
26
|
Lee HS, Son WC, Ryu JE, Koo BA and Kim YS:
Standardized Salvia miltiorrhiza extract suppresses hepatic
stellate cell activation and attenuates steatohepatitis induced by
a methionine-choline deficient diet in mice. Molecules.
19:8189–8211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin C, Zhang PJ, Bao CQ, Gu YL, Xu BH, Li
CW, Li JP, Bo P and Liu XN: Protective effects of Atractylodes
macrocephala polysaccharide on liver ischemia-reperfusion injury
and its possible mechanism in rats. Am J Chin Med. 39:489–502.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu PS, Wu SJ, Tsai YH, Lin YH and Chao JC:
Hot water extracted Lycium barbarum and Rehmannia glutinosa inhibit
liver inflammation and fibrosis in rats. Am J Chin Med.
39:1173–1191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hajiaghamohammadi AA, Ziaee A and Samimi
R: The efficacy of licorice root extract in decreasing transaminase
activities in non-alcoholic fatty liver disease: A randomized
controlled clinical trial. Phytother Res. 26:1381–1384. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hofman K, Hall B, Cleaver H and Marshall
S: High-throughput quantification of hydroxyproline for
determination of collagen. Anal Biochem. 417:289–291. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song P, Zheng JX, Xu J, Liu JZ, Wu LY and
Liu C: β-catenin induces A549 alveolar epithelial cell mesenchymal
transition during pulmonary fibrosis. Mol Med Rep. 11:2703–2710.
2015.
|
|
32
|
Ye B, Ge Y, Perens G, Hong L, Xu H,
Fishbein MC and Li F: Canonical Wnt/β-catenin signaling in
epicardial fibrosis of failed pediatric heart allografts with
diastolic dysfunction. Cardiovasc Pathol. 22:54–57. 2013.
View Article : Google Scholar
|
|
33
|
Li X, Yamagata K, Nishita M, Endo M,
Arfian N, Rikitake Y, Emoto N, Hirata K, Tanaka Y and Minami Y:
Activation of Wnt5a-Ror2 signaling associated with
epithelial-to-mesenchymal transition of tubular epithelial cells
during renal fibrosis. Genes Cells. 18:608–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li W, Zhu C, Li Y, Wu Q and Gao R: Mest
attenuates CCl4-induced liver fibrosis in rats by inhibiting the
Wnt/β-catenin signaling pathway. Gut Liver. 8:282–291. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang F, Parsons CJ and Stefanovic B: Gene
expression profile of quiescent and activated rat hepatic
stellate-cells implicates Wnt signaling pathway in activation. J
Hepatol. 45:401–409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sheldahl LC, Park M, Malbon CC and Moon
RT: Protein kinase C is differentially stimulated by Wnt and
Frizzled homologs in a G-protein-dependent manner. Curr Biol.
9:695–698. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
De A: Wnt/Ca2+ signaling
pathway: A brief overview. Acta Biochim Biophys Sin (Shanghai).
43:745–756. 2011. View Article : Google Scholar
|
|
38
|
Buchholz M, Schatz A, Wagner M, Michl P,
Linhart T, Adler G, Gress TM and Ellenrieder V: Overexpression of
c-myc in pancreatic cancer caused by ectopic activation of NFATc1
and the Ca2+/calcineurin signaling pathway. EMBO J.
25:3714–3724. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kohn AD and Moon RT: Wnt and calcium
signaling: Beta-catenin-independent pathways. Cell Calcium.
38:439–446. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim J, Kim DW, Chang W, Choe J, Kim J,
Park CS, Song K and Lee I: Wnt5a is secreted by follicular
dendritic cells to protect germinal center B cells via
Wnt/Ca2+/NFAT/NF-κB-B cell lymphoma 6 signaling. J
Immunol. 188:182–189. 2012. View Article : Google Scholar
|