Introduction
Sepsis poses a serious threat to the life of
patients, resulting in multiple organ failure syndrome. Treatment
options at present include surgery, antibiotics and other
supportive treatments, however, the therapeutic options available
remain unsatisfactory in terms of efficacy, with a mortality rate
of ~30% (1). In the United States,
~250,000 patients succumb to sepsis-associated mortality every year
(2). Sepsis predominantly results
in organic dysfunction due to the LPS, which is produced by
bacteria and interacts with the human inherent immune system,
resulting in its dysfunction (3,4) and
leading to activation of the inherent immune system, the inhibition
of adaptive immunity, mitochondrial dysfunction and hypermetabolism
accompanied by insulin resistance. Furthermore, the enhancement in
capillary permeability leads to tissue edema, which eventually
results in the dysfunction of tissue organs (4). One of the clinical manifestation of
sepsis is the impairment and insufficiency of cardiac function. A
previous study indicated that, among 235 patients who succumbed to
sepsis or septic shock-associated mortality, those exhibiting
cardiovascular system failure accounted for 35.3% (5). As the inflammatory reaction is
important in sepsis, previous studies have attempted to use
monoclonal antibodies raised against specific inflammatory factor
receptors to inhibit the inflammatory reaction (2,6).
However, sepsis results from interactions among various
inflammatory factors and, as a result, treatments based on
monoclonal antibodies lose their therapeutic potential due to the
targeting of a single receptor (2).
As the inflammatory reaction is closely associated
with immunological abnormalities in sepsis, effective treatments,
which are able inhibit excessive inflammatory reactions and
regulate immunological abnormality are of interest. Previous
studies have demonstrated that mesenchymal stem cells (MSCs) are
able to regulate the immune system, and MSCs have been used to
treat a range of conditions, including acute lung injury,
inflammatory bowel disease, graft versus-host reactions and
ischemic heart disease (7,8). Previous attempts have been made to
treat sepsis using MSCs, and MSCs have been successfully used in
animals to inhibit the systemic inflammation reaction through
interleukin (IL)-10, and thereby relieve the functional impairment,
which is associated with sepsis and endotoxemia (7–11).
MSCs have also elicited improved therapeutic effects
on the cardiac insufficiency associated with sepsis, accompanied by
a decreased expression of inflammatory factors, including tumor
necrosis factor-α (TNF-α) and IL-1 (11). However, at present, the treatment
for sepsis using MSCs is focused more on the inhibition of the
excessive inflammatory reaction caused by sepsis, and the effect of
MSCs on the immune system remain to be fully elucidated. As
lipopolysaccharide (LPS) is the predominant compound promoting the
involvement of Gram-negative bacteria in sepsis, and the severity
of sepsis is correlated with the concentration of LPS in the
circulatory system (12,13), LPS is commonly injected into an
animal model in order to simulate sepsis.
In the present study, MSCs were used to treat mice
with endotoxemia, with the aim to observe the effects of the MSCs
on cardiac function, and on the levels of cytokines in the serum
and myocardium of mice with endotoxemia. In addition, the effects
of MSCs on the immune system were investigated, in order to assist
in the development of novel therapies using MSCs for treating
sepsis.
Materials and methods
Bone marrow MSCs
All the animal experiments in the present study were
performed in accordance with the Experimental Animal Management
Regulations in Nanjing Medical University (Nanjing, China). The
present study was approved by the ethics committee of the First
Affiliated Hospital of Nanjing Medical University (Nanjing, China).
A total of 80 C57 BL/6 male mice (aged 8–10 weeks, weighing ~25 g,
12 h light/dark cycle), maintained at 24°C and 50% humidity were
purchased from the Animal Core Facility in Nanjing Medical
University. The mice were sacrificed by cervical vertebra
dislocation, and soaked in 75% alcohol for sterilization purposes
for 15 min. The bilateral shafts of the femurs were extracted under
sterile conditions. The cavitas medullaris was washed with
10 ml phosphate-buffered saline (PBS) using a sterile 5 ml syringe,
prior to the disposal of the femoral shafts. The washed bone marrow
was mixed by lashing and stirring, following which the marrow was
centrifuged at 750 × g for 5 min at 20°C, and the supernatant was
discarded. The remainder was resuspended in high-glucose Dulbecco's
modified Eagle's medium (DMEM; GE Healthcare Life Sciences, Logan,
UT, USA) containing 10% Gibco fetal bovine serum (FBS; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and was subsequently
inoculated in a Petri-dish (10 cm diameter) at 37°C in an incubator
containing 15% CO2. Following incubation for 4 days, the
medium was replaced for the first time with high-glucose DMEM
containing 10% FBS, following which the medium was replaced every
day until 80% of the cells were entering cell fusion at a 1:2
passage. The third generation cells were used.
Treatment of mice with endotoxemia using
MSCs
A total of 80 C57 BL/6 male mice (aged 8–10 weeks,
weighing ~25 g) were selected for the present study. The mice were
provided with free access to food (grain feed) and water, and were
housed at a temperature of 24°C and 50% humidity. The following
four groups of mice were established: Control; lipopolysaccharide
(LPS) treatment; LPS and MSC treatment (LPS + MSC group) and MSC
treatment group, with 20 mice in each group. LPS (Sigma-Aldrich,
St. Louis, MO, USA) was used at a concentration of 0.5 mg/ml, and
the concentration of MSCs used was 2×106/ml. In the
control group, 0.5 ml phosphate-buffered saline (PBS) was
administered through peritoneal injection and, after 1 h, 0.5 ml
PBS was administered via vena caudalis injection. In the LPS
group, 10 mg/kg LPS was administered via peritoneal injection and,
1 h later, 0.5 ml phosphate-buffered saline (PBS) was administered
via vena caudalis injection. In the LPS + MSC group, 10
mg/kg LPS was administered via peritoneal injection and, 1 h later,
106 bone marrow MSCs were administered via vena
caudalis injection. In the MSC group, 0.5 ml PBS was
administered via peritoneal injection and, 1 h later,
106 bone marrow MSCs were administered via vena
caudalis injection. No mice died throughout the course of the
experiment all were sacrificed by cervical vertebra dislocation.
The cardiac functions of the mice were investigated using a
two-dimensional echocardiogram (Vevo 2100; VisualSonics, Inc.,
Toronto, Canada) 2, 6 and 24 h, and 7 days following LPS injection.
Subsequently, five mice in each group were sacrificed in order to
examine the associated tissues.
Determination of the serum and the
myocardial inflammatory factor
A total of 500 μl of blood was collected from
each mouse and maintained at physiological temperature. Following
incubation for 1 h, the blood was centrifuged at 2,000 × g for 10
min to obtain the serum. Myocardial tissues were homogenized in 10
weight/volume of sodium chloride on ice and then centrifuged at
10,000 × g for 15 min at 4°C. The supernatants were collected for
IL-1β, IL-6, TNF-α and IL-10 assays with enzyme-linked
immunosorbent assay (ELISA) kits. An ELISA assay kit was purchased
from R&D Systems, Inc. (Minneapolis, USA). Aliquots (50
μl) of the standard, control or sample solution mixed with
50 μl assay dilutant were added to each well, and the
mixtures were incubated at room temperature for 2 h. Following the
removal of unbound material by washing five times, 100 μl
conjugate was added to each well and incubated at room temperature
for 2 h. Following rinsing, 100 μl substrate solution was
added and incubated at room temperature in the dark for 30 min, the
reaction was terminated by adding 100 μl stop solution to
each well, and the optical density was read at 450 nm within 30 min
using an SH-1000 microplate reader (Corona Electric Co., Ltd,
Hitachinaka, Japan).
Determination of myocardial signal
pathway proteins
A total of 0.1 g of myocardial tissue was obtained,
to which 1 ml lysate and 10 μl phenylmethyl sulfonylfluoride
solution (Sigma-Aldrich) were added prior to homogenization. The
homogenate was centrifuged at 12,000 × g for 20 min (4 μl),
and the supernatant was collected to measure the concentration of
protein using the Bradford protein assay method (Jiancheng Biotech,
Nanjing, China), with bovine serum albumin as a standard (diluted
to a concentration of 5 mg/ml). The proteins were separated using
sodium dodecyl sulfate (SDS)-PAGE (5 μg/μl per well;
12%; Bio-Rad Laboratories, Inc., Hercules, CA, USA), and were
electrotransferred (300 mA) onto a polyvinylidene fluoride (PVDF)
membrane (EMD Millipore, Billerica, MA, USA). The myocardial
proteins were sealed off using 5% defatted milk powder as the
blocking agent for 2 h, and were subsequently separately incubated
overnight at 4°C with the corresponding primary antibody (1:5,000
dilution). The following primary antibodies were used: Rabbit
anti-Toll-like receptor (TLR)-4 (cat. no. bs-1021R; Bioss Biotech,
Woburn, MA, USA), rabbit anti-myeloid differentiation primary
response gene 88 (MyD88; cat. no. bs-1047R; Bioss Biotech), rabbit
anti-c-Jun N-terminal kinase (JNK; cat. no. ab179461; Abcam,
Cambridge, UK), rabbit anti-phosphorylated (p)-JNK (cat. no.
ab124956; Abcam) and rabbit anti-β-actin (cat. no. 4967; Cell
Signaling Biotechnology, Inc., Danvers, MA, USA). Additionally, the
NF-κB and the p38 MAPK Pathway Sampler kits were used (cat. nos.
9936 and 9913, respectively, Cell Signaling Biotechnology, Inc.).
The PVDF membrane was washed with 0.5% TBS-T solution (Beyotime
Institute of Biotechnology, Nantong, China) three times (5 min
each). Subsequently, goat anti-rabbit secondary antibody (1:6,000
dilution) conjugated to horseradish peroxidase (cat. no. 3056-1;
Epitomics, Inc., Hangzhou, China) was applied to the PVDF membrane,
prior to agitation on a rocking bed for 4 h at room temperature.
The membrane was subsequently washed with 0.5% TBS-T solution three
times (5 min each). TMB Membrane Peroxidase Substrate (Kirkegaard
& Perry Laboratories, Inc., Gaithersburg, MA, USA) was added
onto the membrane. The levels of target proteins were determined
using a gel imaging system (ChemiScope 2850, Clinx Science
Instruments Co., Ltd., Shanghai, China).
Determination of the growth rate of
splenic cells using a
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) assay
Concanavalin A (ConA), at a final concentration of 5
μg/ml, was added to RPMI-1640 culture medium to a 0.2
μm filter membrane and stored at 4°C. MTT (Sigma-Aldrich)
was dissolved in PBS to a final concentration of 5 mg/ml and
maintained at 4°C. The spleen was removed from the mice under the
sterile conditions and placed on a flat plate, followed by crushing
or cutting. The spleen was subsequently washed with Hank's solution
(Sigma-Aldrich) and filtered through four layers of sterile gauze
to obtain a suspension of single cells, which were further purified
through two successive centrifugation steps at 250 × g for 5 min at
4°C. The precipitated cells were resuspended in RPMI-1640 culture
medium, and the splenic cells obtained were cultured on a 96-well
culture plate, with 100 μl/well. The final concentration of
splenic cells was 1.5×106/ml, to which 100 μl
ConA solution (5 μg/ml) was added, and the cells were
cultured in a 5% CO2 incubator at 37°C for 16 h. An
aliquot of 10 μl MTT was added into each well prior to the
completion of the incubation period. Finally, the cell suspension
was centrifuged at 250 × g for 10 min at 4°C, the supernatant was
discarded, and 200 μl 10% SDS containing 0.04 mol/l HCl was
added. The culture plate was agitated on a decolorizing rocking bed
for 10 min. The photoabsorption of each well was measured at a
wavelength of 490 nm using a microplate reader (SH 1000; Corona
Electric Co., Ltd, Hitachinaka, Japan), the results were recorded
and a cell growth curve was constructed, with time on the x-axis
and light absorption on the y-axis. The incremental increases in
the growth rate of the splenic cells were calculated 24 h and 7
days following treatment with LPS.
Determination of the humoral immune
function
A total of 4 mice were used for this part of the
current study, all mice were sacrificed by cervical vertebra
dislocation. The mice were immunized with sheep red blood cells
(SRBCs; Cell-Bio Biotechnology Co., Ltd., Shanghai, China) and 50
mg of splenic tissues were extracted after 4 days, which were then
ground using a 100 mesh copper screen. Subsequently, the extracts
were washed twice in Hanks solution and made into a cell suspension
with PBS (5×106/ml). For the experimental groups, 1 ml
of 0.2% SRBCs and 1 ml of complement were added to 1 ml of the
splenic cell suspension, and the mixture was incubated at 37°C for
1 h. Centrifugation was performed at 250 × g for 5 min at 4°C, and
the supernatant was obtained. The photoabsorption was measured
using a SH-100 microplate reader at 413 nm (antibody optical
density value). In the control group, 1 ml 0.2% SRBCs was added to
1 ml splenic cell suspension in a blank tube without complement,
and the other procedures were identical with those detailed
above.
Assessment of macrophage phagocytosis in
mice
Venous blood was extracted from a chicken wing, to
which was added physiological saline, and the mixture was
centrifuged twice at 250 × g for 5 min at 4°C. Subsequently, an
appropriate volume of physiological saline was added, which was
determined by the volume of erythrocytes obtained, in order for the
concentration of chicken red blood cells (CRBCs; Cell-Bio
Biotechnology Co., Ltd.) to be 1%. At 3 days prior to the
experiment, 1 ml soluble amylum (Sigma-Aldrich) was administered to
the mice via peritoneal injection every day. At 30 min prior to the
experiment, 1 ml 1% CRBCs was administered to the mice via
intraperitoneal injection. After 3 days, the mice were sacrificed
and 200 μl of peritoneal fluid was extracted. A blob of
physiological saline solution was dropped onto the slide, to which
was added a blob of peritoneal fluid. The liquid was allowed to
stand for 10 min to enable cellular adherence of the ascites to
occur, following which the physiological saline solution was
discarded, and Wright's staining (Sigma-Aldrich) was performed when
the slide had been allowed to dry. A total of 100 mononuclear
macrophages were counted on each slide using a CX41 routine
microscope (Olympus Corporation, Tokyo, Japan), and the percentage
of cells phagocytosing CRBCs were calculated. The percentages of
phagocytosing cells were compared across the groups.
Statistical analysis
Data is presented as the mean ± standard error of
the mean, and statistically significant differences were assessed
by one-way analysis of variance followed by post-hoc analysis. All
statistical analyses were processed with Prism version 5 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Changes in the levels of cytokines in the
serum
The level of IL-1β in the LPS treatment group was
increased at the 2 h time point following LPS stimulation, with no
statistically significant difference following treatment with MSCs,
although more marked increases in the levels of IL-1β were observed
at the 6 h, 24 h and day 7 time points, with a marked decline
following treatment with MSCs. The levels of IL-6 and TNF-α in the
LPS treatment group were increased at the 2 h time point, although
without significant difference, compared with the control group.
The increase was more clearly discernible at 6 h, 24 h and day 7
time points, with a significant decline following treatment with
MSCs. The level of IL-10 in the LPS group exhibited no marked
change at the 2 h time point, and a marginal increase was observed
following treatment with MSCs, but without statistical
significance, compared with the control and LPS groups. However, a
significant increase (P<0.05) in the levels of IL-10 were
observed after 6 h, 24 h and 7 days, with a marked decrease
observed following treatment with MSCs. The level of IL-10 in the
MSC group declined after 24 h and 7 days, with a statistically
significant difference (P<0.05), compared with the LPS group.
Compared with the control group, no marked changes in cytokines
were observed in the MSC group (Fig.
1).
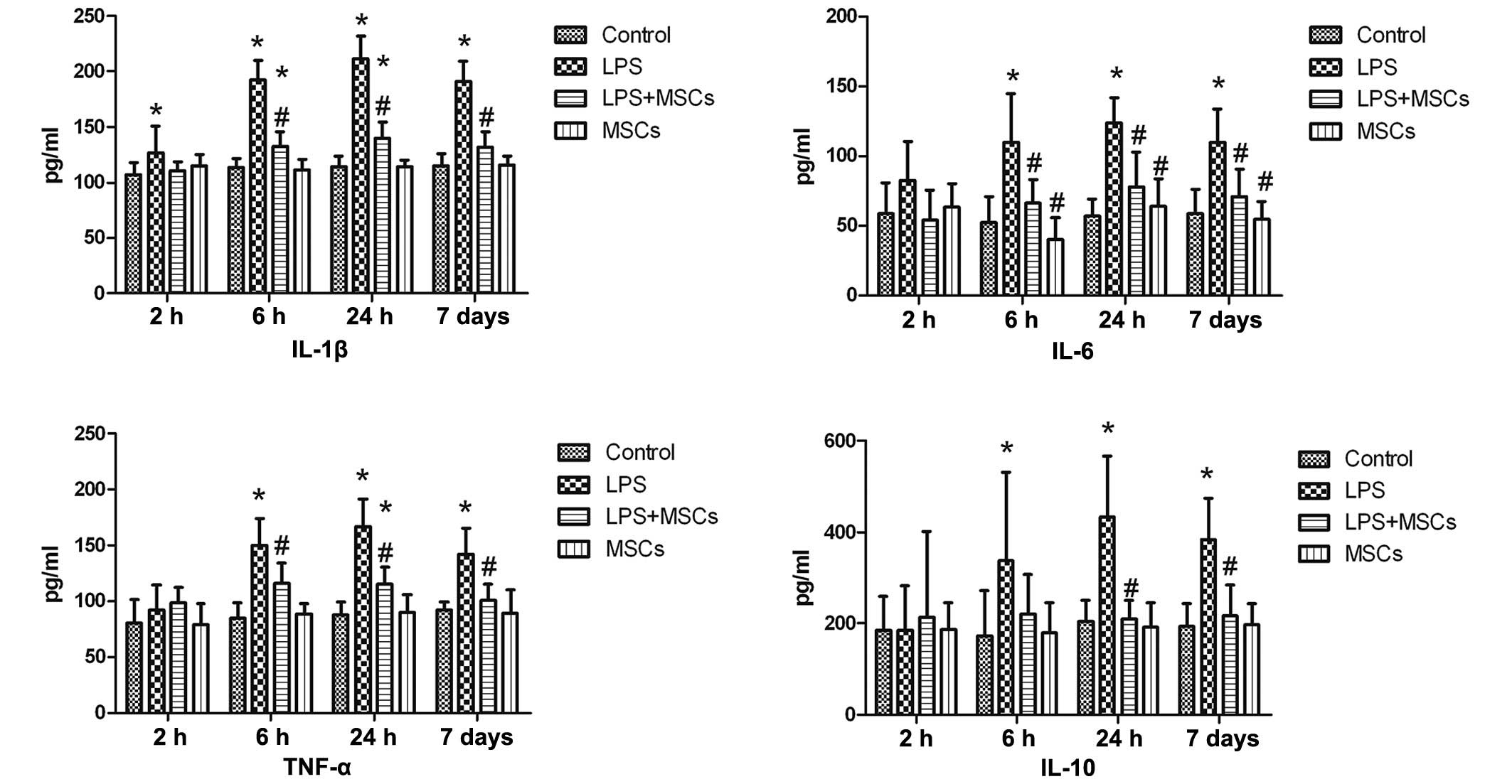 | Figure 1Changes in serum levels of cytokines.
The level of IL-1β in the LPS group increased at the 2 h time
point, and more marked increases in the levels of IL-1β were
observed at the 6 h, 24 h and 7 day time points, with a marked
decline following treatment with MSCs. The levels of IL-6 and TNF-α
in the LPS group were increased at the 6 h, 24 h and 7 day time
points, with a marked decline following treatment with MSCs.
Compared with the control, the level of IL-10 in the LPS group was
not significantly different at the 2 h time point, but levels were
significantly increased at the 6 h, 24 h and 7 day time points,
with a significant decline following treatment with MSCs.
*P<0.05, compared with the control group;
#P<0.05, compared with the LPS group. LPS,
lipopolysaccharide; MSC, mesenchymal stem cell; IL, interleukin;
TNF-α, tumor necrosis factor-α. |
Changes in levels of cytokines in the
myocardium
The level of IL-1β in the LPS group was marginally
increased at the 2 h time point following LPS stimulation, however,
the increase was not statistically significant, compared with the
control group. However, the levels of IL-1β were markedly increased
at the 6 h, 24 h and day 7 time points, with a decrease observed
following treatment with MSCs, although this was not statistically
significant. The levels of IL-6 and TNF-α in the LPS group
exhibited no marked changes at the 2 h time point, although a
marked increase was observed at the 6 h, 24 h and day 7 points,
with a significant (P<0.05) decline observed following MSC
treatment. The level of IL-10 in the LPS group did not exhibit a
marked change at the 2 h time point, however, the level was
significantly increased at the 6 h time point (P<0.05) following
MSC treatment. Marked increases in the levels of IL-10 were also
observed at the 24 h and 7 day times points, although the decrease
in levels following MSC treatment at these time points were not
statistically significant (Fig.
2).
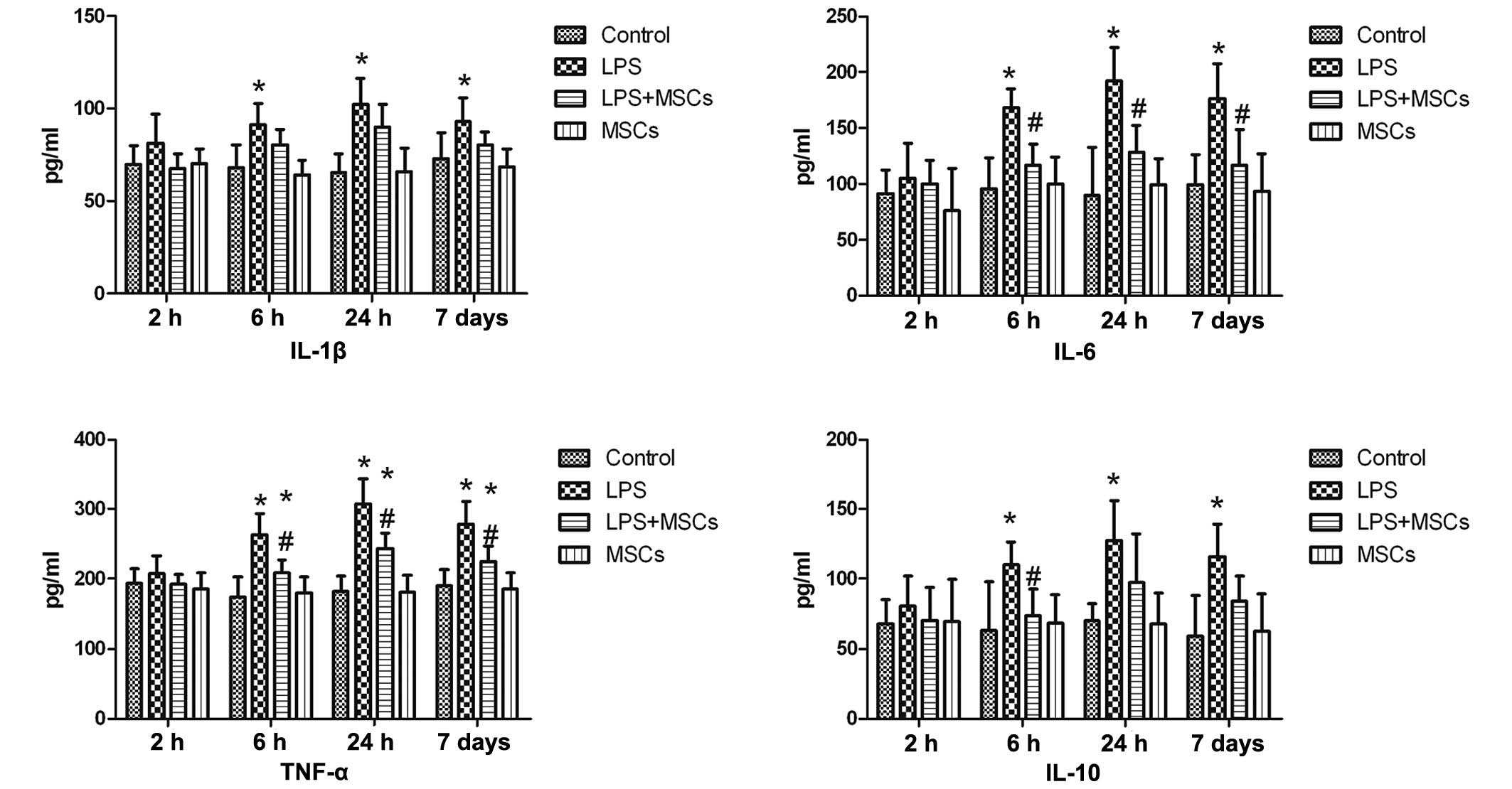 | Figure 2Changes in the levels of cytokines in
the myocardium. Compared with the control, the levels of IL-1β
increased at the 6 h, 24 h and 7 day time points, with a decline
following treatment with MSCs, which was not statistically
significant. The levels of IL-6 and TNF-α in the LPS group
exhibited no marked changes at the 2 h time point, although
significant increases in their levels were observed at the 6 h, 24
h and 7 day time points, with a significant decline following MSC
treatment. The level of IL-10 in the LPS group exhibited no marked
change at the 2 h time point, although a significant increase was
observed at the 6 h time point, which declined following MSC
treatment. A significant increase in the level of IL-10 was also
observed at the 24 h and 7 day time points, although the decline in
levels following MSC treatment was not statistically significant.
*P<0.05, compared with the control group;
#P<0.05, compared with the LPS group. LPS,
lipopolysaccharide; MSC, mesenchymal stem cell; IL, interleukin;
TNF-α, tumor necrosis factor-α. |
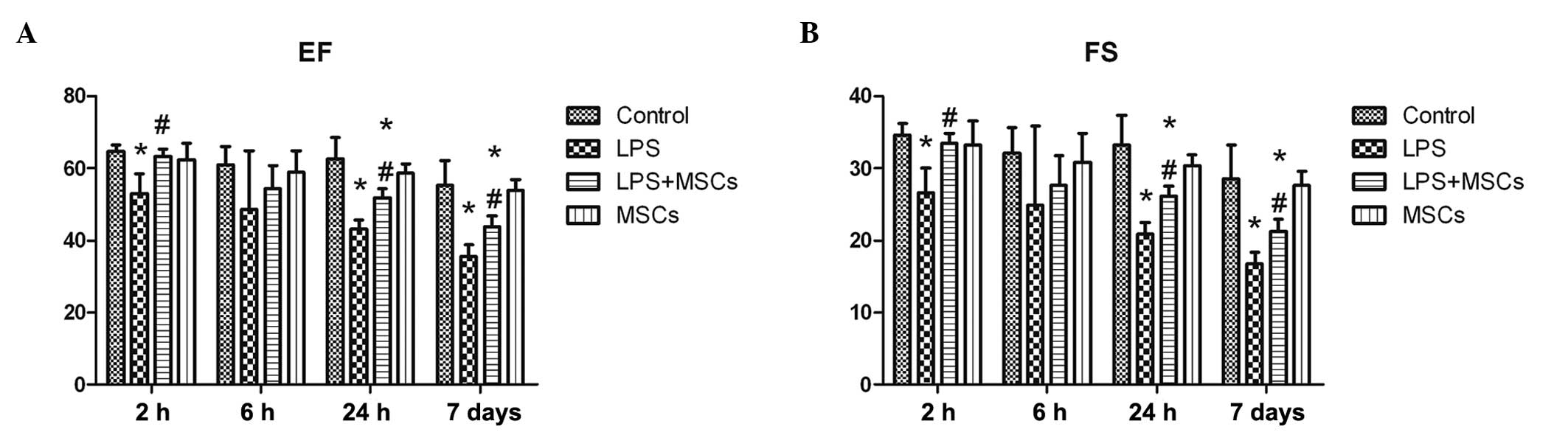
Changes in cardiac function
The level of the ejection fraction (EF) in the LPS
group markedly declined at the 2 h, 24 h and 7 day time points
following LPS stimulation, which recovered to a differing extent
following treatment with the MSCs, which was statistically
significant (P<0.05) for all the time points, with the exception
of the 6 h time point (Fig. 3). On
monitoring the changes in the fractional shortening, the results
obtained were similar to those obtained for the EF (Fig. 3).
Changes in the protein expression level
of myocardial signaling proteins
The protein expression levels of TLR-4 and
p65-nuclear factor-κB (NF-κB) in the LPS group exhibited no marked
changes at the 2 h time point, however. the observed levels were
markedly increased at the 6 h, 24 h and 7 day time points, with
lower levels of expression observed following treatment with the
MSCs. The protein expression of MyD88 in the LPS group was
increased at the 24 h time point, with a decrease observed
following treatment with MSCs. The levels of p-p38 in the LPS group
were markedly increased at the 2 h, 6 h, 24 h and 7 day time
points, with a decrease observed for all the time points following
treatment with MSCs (Fig. 4).
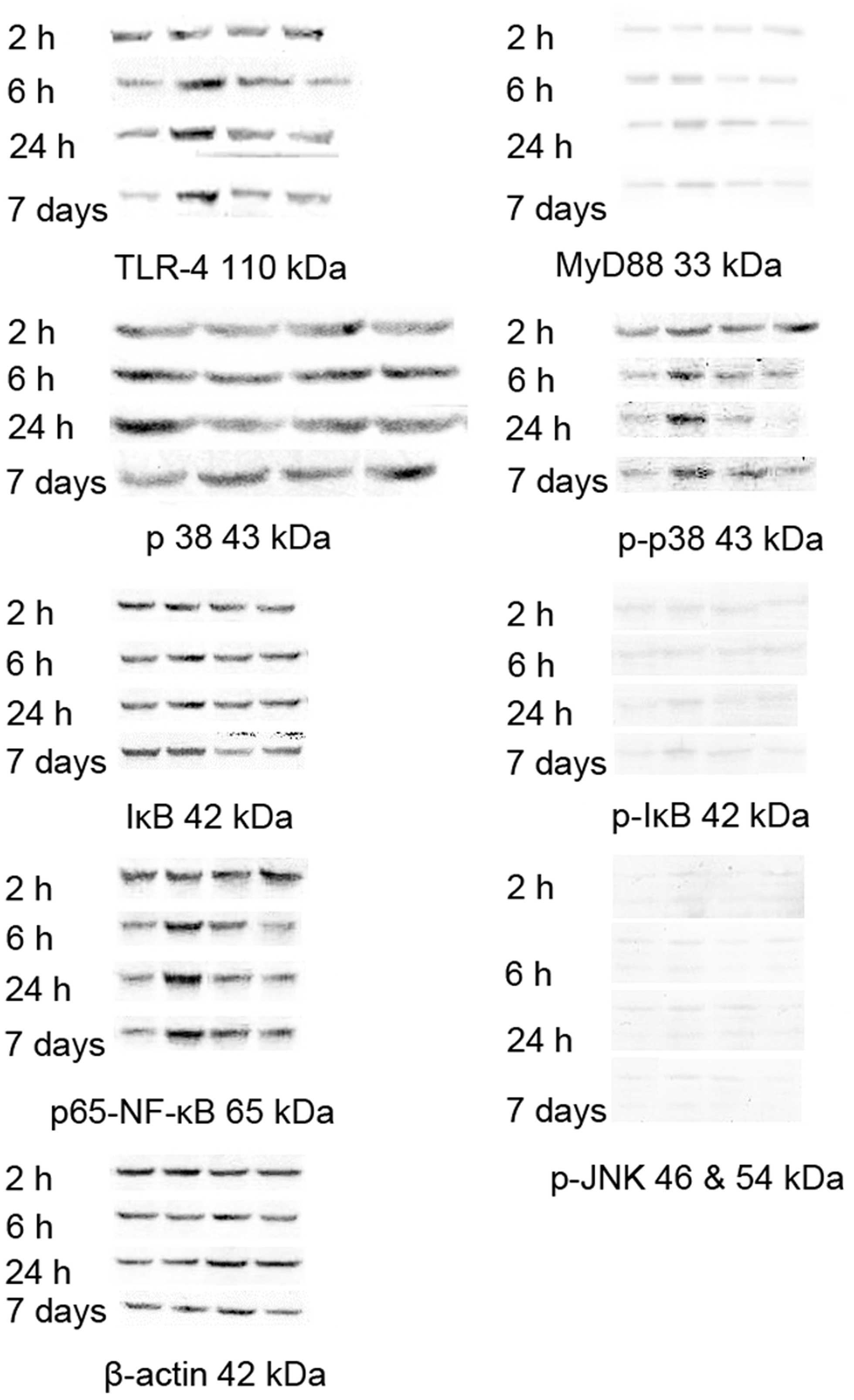 | Figure 4Changes in the levels of signaling
proteins in the myocardium. The protein expression levels of TLR-4
and p65-NF-κB in the LPS group exhibited no marked changes at the 2
h time point, although the levels were markedly increased at the 6
h, 24 h and 7 day time points, and decreased following treatment
with MSCs. The expression of MyD88 in the LPS group increased at
the 24 h time point and declined following MSC treatment. The level
of p-p38 in the LPS group was markedly increased at the 2 h, 6 h,
24 h and 7 day time points, and decreased following MSC treatment.
β-actin was used as an experimental control. p-, phosphorylated;
TLR-4, Toll-like receptor 4; NF-κB, nuclear factor-κB; MyD88,
myeloid differentiation primary response gene 88; IκB, inhibitor of
κB; JNK, c-Jun N-terminal kinase. |
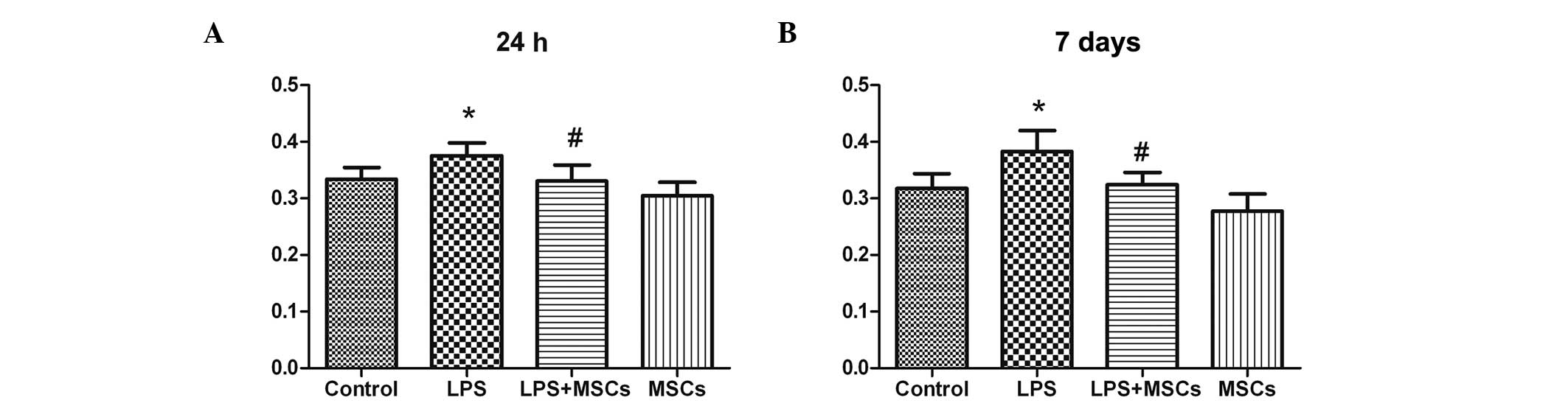
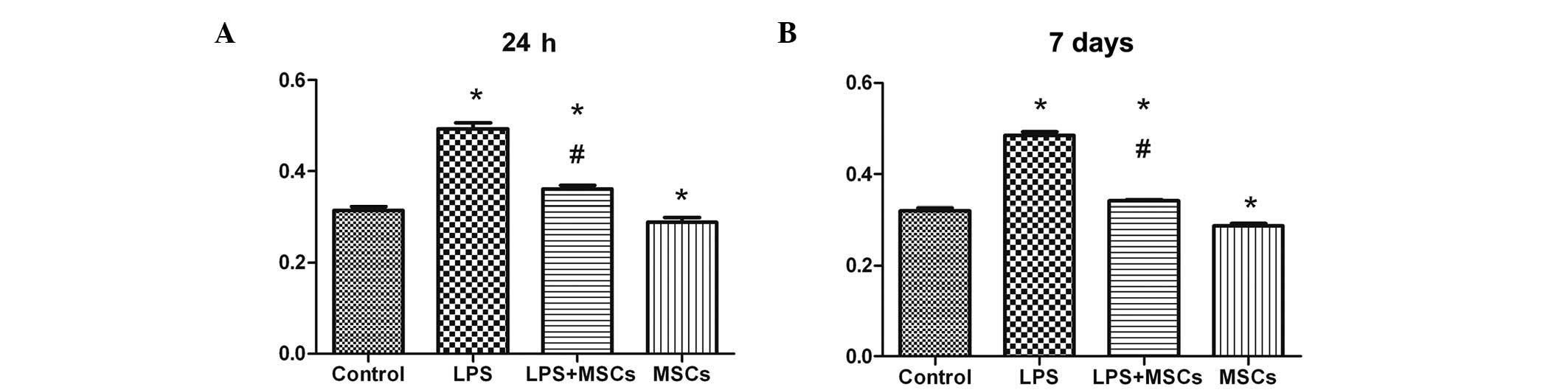
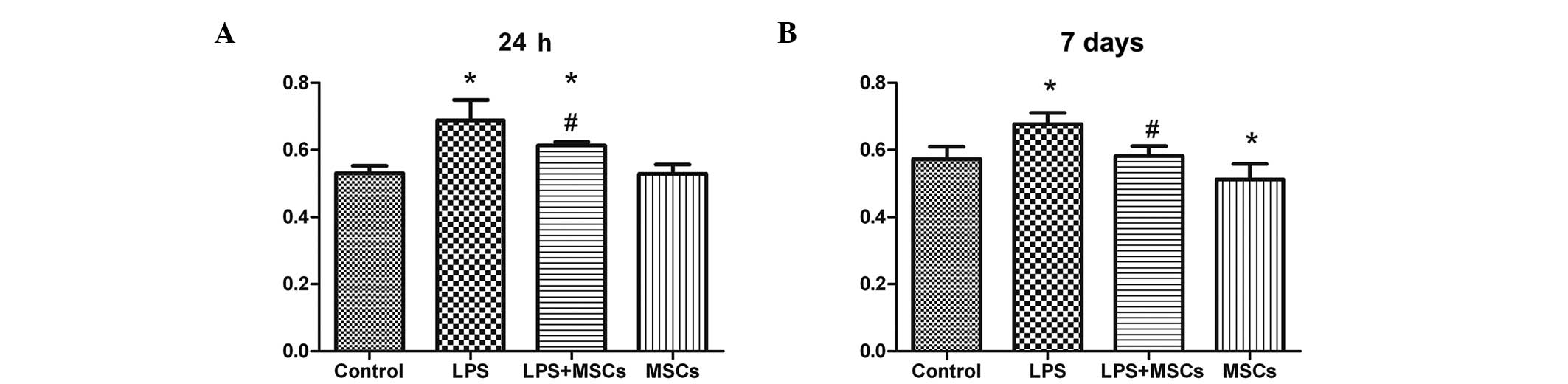
Changes in immunological function
The growth rate of the splenic cells in the LPS
group were markedly increased at the 24 h and 7 day time points,
with a marked decease observed following MSC treatment (Fig. 5). No significant differences were
identified between the MSC and control group. The humoral immune
function in the LPS group was markedly increased at the 24 h and 7
day time points, with marked decreases observed following treatment
with MSCs (Fig. 6). The humoral
immune function in the MSCs group declined, compared with the
control group. Phagocytosis of the macrophages in the LPS group
occurred to a greater extent at the 24 h and 7 day time points,
with marked decreases following treatment with MSCs, although the
level of phagocytosis remained higher following MSC treatment,
compared with the control group at the 24 h time point (Fig. 7). Phagocytosis in the MSCs group
occurred to a lesser extent than that in the control group after 7
days (Fig. 7).
Discussion
In the late 1980s, it was hypothesized that
inflammatory factors are important in sepsis, and aimed to identify
a biomarker to improve diagnostic and prognostic purposes and
interfere with inflammatory factors, in order to improve the
treatment of sepsis (14).
Subsequently, the modes of seizure of sepsis were identified and
delineated, with the liberation of several inflammatory factors
upon stimulation by LPS at an early stage of disease, and patients
with sepsis acquiring immunosuppression at more advanced stages of
the disease (15). Therefore,
attempts have been made to improve the effectiveness of strategies
to treat sepsis clinically, by inhibiting the excessive
inflammatory reaction. As LPS is important in sepsis,
investigations of the mechanism underlying its action have revealed
that LPS activates the inherent (or native) immune system,
predominantly through TLR-4 (16),
and, following the formation of a complex between TLR-4 and myeloid
differentiation factor (2MD-2), LPS activates NF-κB via MyD88 to
elicit the production of various types of inflammatory factors. The
results in the present study revealed that, following stimulation
of the mice with LPS, the protein expression levels of TLR-4, MyD88
and NF-κB in the myocardium increased, with corresponding increases
in the levels of inflammatory factors in the serum and myocardium.
Therefore, the present study hypothesized that an antagonist of
TLR-4 may be used to treat sepsis through the interruption of the
stimulatory role exerted by LPS on the immune system (2). Although, in early investigations, the
antagonist of TLR-4, eritoran, exhibited an apparent tendency to
increase the survival rate of patients with sepsis (17), a subsequent large three-stage
clinical trial indicated that the mortality rates of patients with
sepsis were not lowered as a consequence of treatment with eritoran
(6). The possible explanation for
this is that the signaling pathway mediated by TLR-4 is not the
only route by which LPS acts on the human body. Caspase-11,
independently of TLR-4, is also involved in apoptosis induced by
LPS (18,19). LPS acts in association with
caspase-11, producing an inflammasome following entry to
macrophages, and active IL-1 is liberated by the inflammasome under
the stimulation of caspase-11 (20,21).
In the present study, the level of p-p38 was markedly increased in
the LPS group, which was decreased following treatment with MSCs.
These results suggested that, as the complex pathogenesis of sepsis
remains to be fully elucidated, it is difficult for a treatment
regimen aimed at a single factor to exert a marked therapeutical
effect on sepsis.
In subsequent studies on sepsis, it has been
revealed that the hypothesis suggesting patients with sepsis suffer
an excessive inflammatory reaction in the early stages of the
disease, and immunosuppression in the advanced stage, was not
supported by genomic analyses (22). Thereafter, it has been suggested
that the onset of sepsis is accompanied by the activation and
inhibition of the immune system (23), with characteristics of abnormal
activation predominantly in the early stage, and immunosuppression
predominantly in the advanced stage. In addition, the timing and
intensity of early immune activation of the body was associated
with a range of factors, including the patients' physical status,
virulence of pathogenic bacteria and other complicating factors
(24). Among patients with
refractory sepsis, due to the immunosuppression resulting from a
deficiency in the inflammatory factors to activate the adaptive
immune system, it is difficult for persistent infections to be
controlled (25). The
immunosuppression of patients with sepsis is predominantly
characterized by the apoptosis of lymphocytes and dendritic cells,
decreased expression levels of the cell-surface antigen-presenting
complex and human leucocyte antigen-death receptor, and an increase
in the expression level of inhibitory immune regulatory molecules,
including programmed death 1 and cytotoxic T-lymphocyte-associated
antigen 4. In targeting the immunosuppressive condition,
immunostimulatory therapies have been suggested for the treatment
of sepsis (26), however, this
remains a preliminary stage of investigation, with concerns that
they may aggravate the inflammatory reaction and provoke
autoimmunity.
There has been increased interest in the ability of
MSCs to adjust the function of the immune system extensively, the
action of which is twofold and differs from previous treatment
regimens. A wealth of evidence has established that MSCs exert a
suppressive effect on the innate and adaptive immune systems
(27–29). MSCs have been revealed to stimulate
the immune system (30).
γ-Interferon stimulates MSCs, generating their antigen-presenting
capability, which leads to the stimulation of the adaptive immune
system (31). MSCs also stimulate
natural B cells and their differentiation and proliferation during
transit times, and promote B cells to differentiate into plasma
cells following antigenic stimulation (32). As sepsis is associated with an
excessive inflammatory reaction, as well as immunosuppression, the
appropriateness of using MSCs in treatment strategies for sepsis
remains to be fully elucidated.
In the present study, the inflammatory reaction
resulting from LPS stimulation led to a decrease in left cardiac
function, similar to sepsis. MSCs have more marked therapeutic
effects on cardiac insufficiency in rats with endotoxemia; and they
also reduce the expression levels of host of inflammatory factors,
including IL-1, TNF-α and IL-6 (7). However, in the present study, the
expression of the inflammatory factor, IL-10, following stem cell
therapy produced a different experimental result. Németh et
al (9) demonstrated that,
through stimulating the expression of IL-10, stem cells alleviate
the organic damage caused by sepsis. Weil et al (11) demonstrated that, following stem
cell therapy, the level of IL-10 in the serum increases. Notably,
the present study revealed that, following LPS stimulation, no
significant change in the serum level of IL-10 was observed in the
LPS group, however, an increase was observed in the MSC group. At 6
h, 24 h and 7 days following treatment, the level of IL-10 in the
LPS group increased significantly, and the level of IL-10 declined
following treatment with MSCs. The level of IL-10 in the myocardium
increased markedly after 6 h, 24 h and 7 days, and declined
following treatment with MSCs. The above-mentioned experimental
results are in agreement with the findings of previous studies
(33,34). Differences in results among studies
may be associated with the different experimental conditions used.
Although the expression of IL-10 following treatment with MSCs
requires further analysis, the present study hypothesized that MSCs
exert a bifunctional role in endotoxemia, by inhibiting
inflammatory factors, including IL-1 and IL-6, and inhibiting the
compensatory expression of IL-10 following LPS stimulation. This
avoids excessive inhibition of immunological function, as
excessively inhibiting the inflammatory reaction results in
immunosuppression, and a higher ratio of IL-10 to TNF-α is
indicative of a poor prognosis in patients with sepsis (35).
In the assessment of the immunological function, the
present study demonstrated that the mice in the LPS group
manifested an abnormal reinforcement of cellular immunity, humoral
immunity and phagocytosis of macrophages during the first week,
which declined following treatment with MSCs. The humoral immune
function in the MSC group was lower, compared with that in the
control group. By contrast, no significant differences were
identified in humoral or cellular immune function among the LPS,
MSC or control groups, which indicated that the presence of MSCs in
the mice with endotoxemia did not markedly suppress their adaptive
immune response. In the present study, during the monitoring of the
mice for 1 week, the immunological function remained in the stage
of reinforcement, although immunosuppression did not manifest
itself during sepsis, which may have been associated with the
experimental approach used. Therefore, further investigations are
required to examine the effects of MSCs on mice with sepsis
predominantly accompanied by immunosuppression.
In conclusion, the present study indicated that MSCs
exert regulatory roles in the immune system in several diverse
ways, by inhibiting the excessive inflammatory reaction and
reinforcing the immunological function, and by avoiding the
abnormal increase in anti-inflammatory IL-10. Using MSCs to treat
sepsis may circumvent the difficulties associated with therapies
are aimed at single cytokines or other molecules. MSCs are readily
obtained and easy to culture in vitro, with rapid and simple
amplification. Previous studies have also shown that MSCs may exert
antibiotic action by excreting antimicrobial peptide LL-37, thereby
stimulating neutrophil granulocytes (36,37).
Another advantage is that their immunogenicity is low, which makes
it possible to treat sepsis using allogeneic stem cells. Taken
together, the results of the present study confirmed that the
therapeutic effects and underlying mechanism of MSCs in the
treatment of sepsis require further investigation, in order to
develop novel approaches for the treatment for sepsis.
References
|
1
|
Levy MM, Dellinger RP, Townsend SR,
Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay
G, Beale R, et al: The Surviving Sepsis Campaign: Results of an
international guideline-based performance improvement program
targeting severe sepsis. Intensive Care Med. 36:222–231. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ianaro A, Tersigni M and D'Acquisto F: New
insight in LPS antagonist. Mini Rev Med Chem. 9:306–317. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gioannini TL and Weiss JP: Regulation of
interactions of Gram-negative bacterial endotoxins with mammalian
cells. Immunol Res. 39:249–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marshall JC, Charbonney E and Gonzalez PD:
The immune system in critical illness. Clin Chest Med. 29:605–616.
vii2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Torgersen C, Moser P, Luckner G, Mayr V,
Jochberger S, Hasibeder WR and Dünser MW: Macroscopic postmortem
findings in 235 surgical intensive care patients with sepsis.
Anesth Analg. 108:1841–1847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Opal SM, Laterre PF, Francois B, LaRosa
SP, Angus DC, Mira JP, Wittebole X, Dugernier T, Perrotin D,
Tidswell M, et al ACCESS Study Group: Effect of eritoran, an
antagonist of MD2-TLR4, on mortality in patients with severe
sepsis: The ACCESS randomized trial. JAMA. 309:1154–1162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gonzalez-Rey E, Anderson P, González MA,
Rico L, Büscher D and Delgado M: Human adult stem cells derived
from adipose tissue protect against experimental colitis and
sepsis. Gut. 58:929–939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JW, Fang X, Gupta N, Serikov V and
Matthay MA: Allogeneic human mesenchymal stem cells for treatment
of E. coli endotoxin-induced acute lung injury in the ex vivo
perfused human lung. Proc Natl Acad Sci USA. 106:16357–16362. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Németh K, Leelahavanichkul A, Yuen PS,
Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller
BH, Brown JM, et al: Bone marrow stromal cells attenuate sepsis via
prostaglandin E(2)-dependent reprogramming of host macrophages to
increase their interleukin-10 production. Nat Med. 15:42–49. 2009.
View Article : Google Scholar
|
|
10
|
Weil BR, Manukyan MC, Herrmann JL, Wang Y,
Abarbanell AM, Poynter JA and Meldrum DR: Mesenchymal stem cells
attenuate myocardial functional depression and reduce systemic and
myocardial inflammation during endotoxemia. Surgery. 148:444–452.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weil BR, Herrmann JL, Abarbanell AM,
Manukyan MC, Poynter JA and Meldrum DR: Intravenous infusion of
mesenchymal stem cells is associated with improved myocardial
function during endotoxemia. Shock. 36:235–241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Opal SM, Scannon PJ, Vincent JL, White M,
Carroll SF, Palardy JE, Parejo NA, Pribble JP and Lemke JH:
Relationship between plasma levels of lipopolysaccharide (LPS) and
LPS-binding protein in patients with severe sepsis and septic
shock. J Infect Dis. 180:1584–1589. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marshall JC, Foster D, Vincent JL, Cook
DJ, Cohen J, Dellinger RP, Opal S, Abraham E, Brett SJ, Smith T, et
al: MEDIC study: Diagnostic and prognostic implications of
endotoxemia in critical illness: Results of the MEDIC study. J
Infect Dis. 190:527–534. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bone RC, Balk RA, Cerra FB, Dellinger RP,
Fein AM, Knaus WA, Schein RM and Sibbald WJ; The ACCP/SCCM
Consensus Conference Committee; American College of Chest
Physicians/Society of Critical Care Medicine: Definitions for
sepsis and organ failure and guidelines for the use of innovative
therapies in sepsis. Chest. 101:1644–1655. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hotchkiss RS and Karl IE: The
pathophysiology and treatment of sepsis. N Engl J Med. 348:138–150.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Medzhitov R: Approaching the asymptote: 20
years later. Immunity. 30:766–775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tidswell M, Tillis W, Larosa SP, Lynn M,
Wittek AE, Kao R, Wheeler J and Gogate J; Opal SM; Eritoran Sepsis
Study Group: Phase 2 trial of eritoran tetrasodium (E5564), a
Toll-like receptor 4 antagonist, in patients with severe sepsis.
Crit Care Med. 38:72–83. 2010. View Article : Google Scholar
|
|
18
|
Hagar JA, Powell DA, Aachoui Y, Ernst RK
and Miao EA: Cytoplasmic LPS activates caspase-11: Implications in
TLR4-independent endotoxic shock. Science. 341:1250–1253. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kayagaki N, Wong MT, Stowe IB, Ramani SR,
Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP,
Muszyński A, et al: Noncanonical inflammasome activation by
intracellular LPS independent of TLR4. Science. 341:1246–1249.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ng TM and Monack DM: Revisiting caspase-11
function in host defense. Cell Host Microbe. 14:9–14. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lamkanfi M and Dixit VM: Inflammasomes:
Guardians of cytosolic sanctity. Immunol Rev. 227:95–105. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang BM, Huang SJ and McLean AS:
Genome-wide transcription profiling of human sepsis: A systematic
review. Crit Care. 14:R2372010. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Monneret G, Venet F, Pachot A and Lepape
A: Monitoring immune dysfunctions in the septic patient: A new skin
for the old ceremony. Mol Med. 14:64–78. 2008. View Article : Google Scholar
|
|
24
|
Skrupky LP, Kerby PW and Hotchkiss RS:
Advances in the management of sepsis and the understanding of key
immunologic defects. Anesthesiology. 115:1349–1362. 2011.PubMed/NCBI
|
|
25
|
Salomao R, Brunialti MK, Rapozo MM,
Baggio-Zappia GL, Galanos C and Freudenberg M: Bacterial sensing,
cell signaling, and modulation of the immune response during
sepsis. Shock. 38:227–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hotchkiss RS and Opal S: Immunotherapy for
sepsis - a new approach against an ancient foe. N Engl J Med.
363:87–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beyth S, Borovsky Z, Mevorach D,
Liebergall M, Gazit Z, Aslan H, Galun E and Rachmilewitz J: Human
mesenchymal stem cells alter antigen-presenting cell maturation and
induce T-cell unresponsiveness. Blood. 105:2214–2219. 2005.
View Article : Google Scholar
|
|
28
|
Aggarwal S and Pittenger MF: Human
mesenchymal stem cells modulate allogeneic immune cell responses.
Blood. 105:1815–1822. 2005. View Article : Google Scholar
|
|
29
|
Ramasamy R, Fazekasova H, Lam EW, Soeiro
I, Lombardi G and Dazzi F: Mesenchymal stem cells inhibit dendritic
cell differentiation and function by preventing entry into the cell
cycle. Transplantation. 83:71–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stagg J: Immune regulation by mesenchymal
stem cells: Two sides to the coin. Tissue Antigens. 69:1–9. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chan JL, Tang KC, Patel AP, Bonilla LM,
Pierobon N, Ponzio NM and Rameshwar P: Antigen-presenting property
of mesenchymal stem cells occurs during a narrow window at low
levels of interferon-gamma. Blood. 107:4817–4824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Traggiai E, Volpi S, Schena F, Gattorno M,
Ferlito F, Moretta L and Martini A: Bone marrow-derived mesenchymal
stem cells induce both polyclonal expansion and differentiation of
B cells isolated from healthy donors and systemic lupus
erythematosus patients. Stem Cells. 26:562–569. 2008. View Article : Google Scholar
|
|
33
|
Shin S, Kim Y, Jeong S, Hong S, Kim I, Lee
W and Choi S: The therapeutic effect of human adult stem cells
derived from adipose tissue in endotoxemic rat model. Int J Med
Sci. 10:8–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mei SH, Haitsma JJ, Dos Santos CC, Deng Y,
Lai PF, Slutsky AS, Liles WC and Stewart DJ: Mesenchymal stem cells
reduce inflammation while enhancing bacterial clearance and
improving survival in sepsis. Am J Respir Crit Care Med.
182:1047–1057. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Dissel JT, van Langevelde P,
Westendorp RG, Kwappenberg K and Frölich M: Anti-inflammatory
cytokine profile and mortality in febrile patients. Lancet.
351:950–953. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Krasnodembskaya A, Song Y, Fang X, Gupta
N, Serikov V, Lee JW and Matthay MA: Antibacterial effect of human
mesenchymal stem cells is mediated in part from secretion of the
antimicrobial peptide LL-37. Stem Cells. 28:2229–2238. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hall SR, Tsoyi K, Ith B, Padera RF Jr,
Lederer JA, Wang Z, Liu X and Perrella MA: Mesenchymal stromal
cells improve survival during sepsis in the absence of heme
oxygenase-1: the importance of neutrophils. Stem Cells. 31:397–407.
2013. View Article : Google Scholar :
|